Abstract
To shed light on the epidemiology of ascariasis in Ecuador and Zanzibar, 177 adult worms retrieved by chemo-expulsion from either people or pigs were collected, measured and subjected to polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) analysis of the ribosomal internal transcribed spacer (ITS) region. Upon double digestion with RsaI and HaeIII, PCR-RFLP analysis revealed the presence of A. lumbricoides in people and A. suum in pigs in Ecuador. In contrast, while there are no pigs on Zanzibar, of the 56 worms obtained from people, one was genotyped as A. suum. No additional genetic variation was detected upon further PCR-RFLP analysis with several other restriction enzymes. Upon measurement, worm mass and length differed by location and by species, A. suum being lighter and longer. While there is no evidence to suggest zoonotic transmission in Ecuador, an enduring historical signature of previous zoonotic transmission remains on Zanzibar.
Introduction
Ascariasis, caused by infection with the giant roundworm Ascaris lumbricoides, is common throughout the developing world and is estimated to affect ~14.6% of the world's population (Brooker & Pullan, 2013). Although the majority of infections are asymptomatic, many millions suffer chronic morbidity (de Silva et al., 1997) with the greatest burden on children aged 5–15 years (Bethony et al., 2006). Ascaris lumbricoides is closely related to Ascaris suum, the large roundworm of pigs, from which it is morphologically indistinguishable, differing by only 1.9% sequence divergence in the mitochondrial genome (Liu et al., 2012). Laboratory studies have shown that Ascaris eggs from infected humans can infect pigs and vice versa (Takata, 1951; Galvin, 1968). Consequently, it is uncertain whether these are two true species (Leles et al., 2012) or how transmission might incorporate pigs as a zoonotic reservoir. To distinguish worms and investigate transmission cycles, sequence analysis of mitochondrial DNA markers, particularly cox1 and nad1 genes, has been used, as well as sizing of microsatellite-containing loci (Betson et al., 2013). A simpler method involving polymerase chain reaction–restriction length polymorphism (PCR-RFLP) analysis of the ribosomal internal transcribed spacer region (ITS) with HaeIII has revealed a polymorphic restriction site that can broadly differentiate the two species upon distinctive RFLP profiles (Anderson et al., 1995; Nejsum et al., 2005).
To date, molecular epidemiology studies have shown it is highly likely that ascariasis is a zoonosis in North America, Denmark, Brazil, Japan, the United Kingdom and other European countries (Anderson, 1995; Nejsum et al., 2005; Arizono et al., 2010; Bendall et al., 2011; Iñiguez et al., 2012; Cavallero et al., 2013). In addition, zoonotic transmission has been demonstrated in China and Uganda (Zhou et al., 2012; Betson et al., 2014). The global view is far from complete, however, and many countries with high Ascaris prevalence have received no attention. Despite a high prevalence of Ascaris (Cooper et al., 2003; Moncayo et al., 2008) and widespread pig farming in rural parts of Ecuador, no studies have been published on the molecular epidemiology of Ascaris in these areas. In rural areas, where humans live in close contact with pigs and sanitation is poor, there is a clear risk of zoonotic transmission. The objective of the present study was to investigate evidence of cross-transmission of Ascaris in Ecuador and Zanzibar by PCR-RFLP analysis of the ITS, using double digestion with HaeIII and RsaI, further screening for diversity with several other restriction enzymes.
Materials and methods
A parasitological survey of children and pigs was conducted in May/June of 2013 in Esmeraldas Province, northern coastal Ecuador (fig. 1A) and had been undertaken previously in children in Zanzibar (Stothard et al., 2008) (fig. 1B). Spatial co-ordinates, as taken by a hand-held GPS unit (e-trex, Garmin Ltd, Olathe, Kansas, USA), in decimal degrees, for the villages surveyed were in Ecuador: Quinindé (+0.330618o, − 79.464481o) and Súa (+0.860764o, − 79.875254o), and in Zanzibar: Tumbatu-Jongowe ( − 5.855866o, − 39.229831o), Kandwi ( − 5.943357o, − 39.334667o), Ghana ( − 6.002032o, − 39.260545o) and Kizimbani ( − 6.057987o, − 39.245278o). Infection status with Ascaris was determined using direct saline examinations and duplicate standard 41.7 mg Kato–Katz smears. Children with infection intensities of >500 eggs/g of stool were treated with pyrantel pamoate at 10 mg/kg of body weight (Combantrin®, Pfizer, Quito, Ecuador).
Fig. 1.
Sources of Ascaris used in the study: (A) location of Quinindé where human and pig sampling was carried out and Súa where pig sampling was carried out within Ecuador; and (B) the location of the four villages (Kandwi, Ghana, Kizimbani and Tumbatu-Jongowe) on Unguja, Zanzibar.
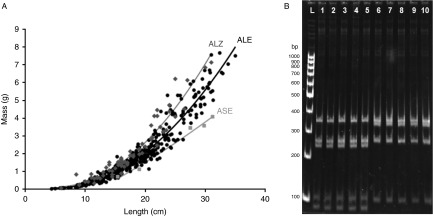
Within Quinindé and Súa, pigs were also examined for infection using duplicate Kato–Katz smears and then treated with piperazine citrate (retailed as S.P. VERMES soluble powder, Qalian, Segre, France) at 0.2 g/kg by delivery in food or water. All expelled stools were collected from treated children and pigs. Worms were removed from stools and washed thoroughly. After blotting dry, the mass and length of each worm was measured twice and data were analysed by regression (fig. 2A). Worms were placed in 70% ethanol for long-term storage for later genotyping.
Fig. 2.
The morphological and molecular characterization of Ascaris from Ecuador and Zanzibar. (A) The relationship between worm mass and length of 380 human-derived worms from Ecuador (ALE) of which 172 were subjected to PCR-RFLP, 5 pig-derived worms from Ecuador (ASE) and 68 worms from Zanzibar (ALZ), all with quadratic regression lines. One outlier from the Ecuador data set was removed due to measurement error. (B) PAGE gel image following digestion of ITS PCR products of Ecuadorian Ascaris samples with HaeIII and RsaI. Lanes: L, 100 bp ladder; 1–5, pig-derived worms; 6–10, human-derived worms.
Genomic DNA was extracted previously from 56 Ascaris worms from Zanzibar, originally collected from children in 2006 (Stothard et al., 2008; Betson et al., 2011). The ribosomal ITS was amplified from all worms according to a published PCR protocol (Nejsum et al., 2005). To maximize detection of variation within ITS amplicons, double digestions with HaeIII and RsaI were performed, and products were separated by non-denaturing polyacrylamide gel electrophoresis (PAGE) with ethidium bromide staining.
Results and discussion
In Ecuador 381 worms were collected from 22 children, and 5 worms from pigs; a total of 68 worms were collected from 14 individuals in Zanzibar. The mean worm length and mass of human-derived worms was 18.3 cm and 1.97 g in Ecuador and 17.1 cm and 2.07 g in Zanzibar. A clear relationship between worm mass and length was found (r s= 0.968, n= 453, P< 0.001; fig. 2A) and it appeared that worms could be differentiated by location: worms from Zanzibar appeared to be heavier than worms from Ecuador. In addition, pig-derived worms appeared to be lighter than human-derived worms from Ecuador. A test for the equality of slopes of log weight on log length (Draper & Smith, 1998) found that the regression lines of each group were parallel (F 2,447= 0.85, P= 0.426) but had significantly different intercepts, with pig-derived worms being lighter, and human-derived worms from Zanzibar being heavier, at any given length (F 2,447= 56.18, P< 0.001).
Ascaris ITS sequences were digested with multiple restriction enzymes, but only HaeIII was found to have a polymorphic restriction site. A double digestion with restriction enzymes HaeIII and RsaI was performed because this approach created fragment sizes amenable for PAGE analysis. This double digest produced a four-band human-derived Ascaris-like genotype or a five-band pig-derived Ascaris-like genotype (fig. 2B). In total, 121 Ascaris worms (31.3%) were transported to the UK and genotyped. All 116 worms from children produced the human-derived Ascaris-like genotype profile, while all 5 worms from pigs produced the pig-derived Ascaris-like genotype profile. In contrast, of the 56 worms from children in Zanzibar that were genotyped using PCR-RFLP, one worm from Kizimbani was found to have a pig-derived Ascaris-like genotype.
Genotyping adult worms using an ITS PCR-RFLP assay did not provide any evidence of cross-transmission of Ascaris in this sympatric setting in Quinindé, Ecuador, and there appears to be a clear separation of transmission cycles of A. lumbricoides in people and A. suum in pigs. In Zanzibar, however, only one worm was characterized by PCR-RFLP analysis to be A. suum. Previous molecular characterization of these worms by Betson et al. (2011), using cox1 haplotype analysis, found five worms with pig-derived Ascaris-like genotypes, while microsatellite data found no zoonotic worms or hybrids (Betson et al., 2014), illustrating that differing conclusions may have been drawn with other markers (Anderson, 2001). As the population of Zanzibar is Muslim and pigs are no longer present, it is unlikely that pigs are a source of infection in this area. Instead Betson et al. (2011) hypothesized that these genotypes are an ancestral genotype which was retained in Zanzibar, or they crossed by introgression from A. suum to A. lumbricoides worms when pigs were more common in the early twentieth century.
The use of molecular epidemiology is crucial in determining the source of Ascaris infections and implementing effective control strategies. In areas where pigs and humans co-exist and where cross-transmission has been found to be a significant source of infections, control programmes must also focus on targeting treatment of pigs. This is important not just in terms of health but because transmission of ascariasis between humans and pigs could allow the spread of drug resistance and virulence alleles into human parasites (Iñiguez et al., 2012). In other parts of the world, such as our study area in Ecuador, where pig holdings are common but where there does not appear to be cross-transmission, control programmes could be simplified to exclude infection control in pigs.
Acknowledgements
The authors would like to thank the field teams in Ecuador and Zanzibar for assistance.
Financial support
Financial support was received from the Liverpool School of Tropical Medicine. The work reported here constitutes the MSc research project findings of A.M.S. Sample collection in Ecuador was supported by a Wellcome Trust grant 088862/Z/09/Z.
Ethical standards
The study protocol was approved by the Ethical Committees of Liverpool School of Tropical Medicine and Pontificia Universidad Catolica del Ecuador.
References
- Anderson T.J.C. (1995) Ascaris infections in humans from North America: molecular evidence for cross-infection. Parasitology 110, 215–219. [DOI] [PubMed] [Google Scholar]
- Anderson T.J.C. (2001) The dangers of using single locus markers in parasite epidemiology: Ascaris as a case study. Trends in Parasitology 17, 183–188. [DOI] [PubMed] [Google Scholar]
- Arizono N., Yoshimura Y., Tohzaka N., Yamada M., Tegoshi T., Onishi K. & Uchikawa R. (2010) Ascariasis in Japan: is pig-derived Ascaris infecting humans? Japanese Journal of Infectious Diseases 63, 447–448. [PubMed] [Google Scholar]
- Bendall R.P., Barlow M., Betson M., Stothard J.R. & Nejsum P. (2011) Zoonotic ascariasis, United Kingdom. Emerging Infectious Diseases 17, 1964–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethony J., Brooker S., Albonico M., Geiger S.M., Loukas A., Diemert D. & Hotez P.J. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. The Lancet 367, 1521–1532. [DOI] [PubMed] [Google Scholar]
- Betson M., Halstead F.D., Nejsum P., Imison E., Khamis I.S., Sousa-Figueiredo J.C., Rollinson D. & Stothard J.R. (2011) A molecular epidemiological investigation of Ascaris on Unguja, Zanzibar using isoenzyme analysis, DNA barcoding and microsatellite DNA profiling. Transactions of the Royal Society of Tropical Medicine & Hygiene 105, 370–379. [DOI] [PubMed] [Google Scholar]
- Betson M., Nejsum P. & Stothard J.R. (2013) From the twig tips to the deeper branches: new insights into evolutionary history and phylogeography of Ascaris . pp. 266–287 in Holland C. (Ed.) Ascaris: the neglected parasite. Oxford, Elsevier. [Google Scholar]
- Betson M., Nejsum P., Bendall R.P., Deb R.M. & Stothard J.R. (2014) Molecular epidemiology of ascariasis: A global perspective on the transmission dynamics of Ascaris in people and pigs. Journal of Infectious Diseases [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S.J. & Pullan R.L. (2013) Ascaris lumbricoides and ascariasis: estimating number infected and burden of disease. pp. 343–362 in Holland C. (Ed.) Ascaris: the neglected parasite. Oxford, Elsevier. [Google Scholar]
- Cavallero S., Snabel V., Pacella F., Perrone V. & D'Amelio S. (2013) Phylogeographical studies of Ascaris spp. based on ribosomal and mitochondrial DNA sequences. PLoS Neglected Tropical Diseases 7, e2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.J., Chico M.E., Bland M., Griffin G.E. & Nutman T.B. (2003) Allergic symptoms, atopy, and geohelminth infections in a rural area of Ecuador. American Journal of Respiratory and Critical Care Medicine 168, 313–317. [DOI] [PubMed] [Google Scholar]
- de Silva N.R., Chan M.S. & Bundy D.A.P. (1997) Morbidity and mortality due to ascariasis: re-estimation and sensitivity analysis of global numbers at risk. Tropical Medicine and International Health 2, 519–528. [DOI] [PubMed] [Google Scholar]
- Draper N.R. & Smith H. (1998) Applied regression analysis. 3rd edn. 706 pp. Chichester, Wiley. [Google Scholar]
- Galvin T.J. (1968) Development of human and pig Ascaris in the pig and rabbit. The Journal of Parasitology 54, 1085–1091. [PubMed] [Google Scholar]
- Iñiguez A.M., Leles D., Jaeger L.H., Carvalho-Costa F.A., Araújo A. & Amazonas Research Group (2012) Genetic characterisation and molecular epidemiology of Ascaris spp. from humans and pigs in Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 106, 604–612. [DOI] [PubMed] [Google Scholar]
- Leles D., Gardner S.L., Reinhard K., Iñiguez A. & Araujo A. (2012) Are Ascaris lumbricoides and Ascaris suum a single species? Parasite & Vectors 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.H., Wu C.Y., Song H.Q., Wei S.J., Xu M.J., Lin R.Q., Zhao G.H., Huang S.Y. & Zhu X.Q. (2012) Comparative analyses of the complete mitochondrial genomes of Ascaris lumbricoides and Ascaris suum from human and pigs. Gene 492, 110–116. [DOI] [PubMed] [Google Scholar]
- Moncayo A.L., Vaca M., Erazo S., Oviedo G., Rodriguez A., Chico M.E., Gomez E., Barreto M.L. & Cooper P.J. (2008) Impact of long-term treatment with ivermectin on geohelminth infections in Esmeraldas Province, Ecuador. PLoS Neglected Tropical Diseases 2, e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum P., Parker E.D. Jr, Frydenberg J., Roepstorff A., Boes J., Haque R., Astrup I., Prag J. & Sørensen S. (2005) Ascariasis is a zoonosis in Denmark. Journal of Clinical Microbiology 43, 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard J.R., Imison E., French M.D., Sousa-Figueiredo J.C., Khamis I.S. & Rollinson D. (2008) Soil-transmitted helminthiasis among mothers and their pre-school children on Unguja Island, Zanzibar with emphasis upon ascariasis. Parasitology 135, 1447–1455. [DOI] [PubMed] [Google Scholar]
- Takata I. (1951) Experimental infection of man with Ascaris of man and the pig. Kitasato Archives of Experimental Medicine 23, 151–159. [PubMed] [Google Scholar]
- Zhou C., Li M., Yuan K., Deng S. & Peng W. (2012) Pig Ascaris: an important source of human ascariasis in China. Infection, Genetics and Evolution 12, 1172–1177. [DOI] [PubMed] [Google Scholar]