Abstract
Guinea pigs are important reservoirs of Trypanosoma cruzi, the causative parasite of Chagas disease. Interestingly, captured colonies of T. infestans, the main vector of T. cruzi in the Southern Cone of South America, sporadically present with infection prevalence rates above 80%. Such high values are not consistent with the relatively short 7–8 week parasitemic period that has been reported in the literature. We experimentally measured the infectious periods of a group of T. cruzi-infected guinea pigs with two methods: xenodiagnosis and direct microscopy, each performed weekly for a year. Another group of infected guinea pigs received only direct microscopy weekly, to control for the effect that inoculation by triatomine saliva may have on parasitemia in the host. We observed infectious periods longer than those previously reported in a number of guinea pigs from both the xenodiagnosis and control groups. While some guinea pigs were infectious for a short time, other “super-shedders” were parasitemic up to 22 weeks after infection, and/or positive by xenodiagnosis for a year after infection. This heterogeneity in infectiousness has strong implications for T. cruzi transmission dynamics and control, as super-shedder guinea pigs may play a disproportionate role in pathogen spread.
Keywords: Guinea pig, infectiousness, super-shedder, Trypanosoma cruzi, Triatoma infestans, xenodiagnosis
Graphical abstract
Chagas disease, a zoonotic and vector-borne disease, is one of the most important parasitic diseases in the Americas. The disease has several domestic and wild animal reservoirs [1,2], and different mathematical models have included domestic animal reservoirs in the transmission of Trypanosoma cruzi, the causative parasite of Chagas disease. Most of these models use population average parameters [3–5] or sample from aggregate measures [6] for inference on larger epidemiological processes. However, heterogeneity in transmission parameters is often an important component of vector-borne disease dynamics. Infectiousness is the “capacity of an infected host to transmit the pathogen following contact with a susceptible host [7]”, or with a vector in the case of vector-borne diseases. Inter- and intra-individual heterogeneity in infectiousness can have profound implications for emergence of disease, pathogen transmission dynamics and control strategies [1,8,9]. Super-shedders, defined as individuals with the potential to be highly infectious, [10] can be disproportionately important in the emergence and maintenance of infectious diseases [9].
In Peru, guinea pigs (Cavia porcellus) are important reservoirs of T. cruzi [2–4,11,12], and Triatoma infestans is either the main or sole triatomine vector, depending on the area [12]. Some studies suggest that parasitemia in guinea pigs is detectable for only 7 to 8 weeks after T. cruzi infection [13–16]; such brief parasitemia would suggest limited transmission from guinea pigs to insect vectors. However, in our field observations, some T. infestans colonies collected from guinea pig pens have an infection prevalence greater than 80%, which supports prior observations that high rates of transmission can occur in these ecotopes [2].
Xenodiagnosis and the evaluation of parasitemia by direct microscopy are two ways to experimentally assess host infectiousness. Importantly, saliva from a number of arthropod vectors has been shown to alter immune mechanisms in vitro. [17]. More recently, experiments performed by Mesquita et al. demonstrated that inoculating laboratory mice with the saliva of triatomine vector Rhodinus prolixus in the presence of T. cruzi significantly increases blood parasitemia [18]. These results suggest that exposing guinea pigs to triatomine saliva, as necessitated by xenodiagnosis, may affect the behavior of T. cruzi within its mammalian host. The objectives of the present study were to characterize the temporal patterns of infectiousness among T. cruzi-infected guinea pigs, and determine if these patterns are affected by exposure to vector saliva..
For T. cruzi infection we used Arequipa TC-35, a strain isolated in our Zoonotic Disease Field Laboratory in the city of Arequipa, Peru. This strain was isolated from a T. infestans (biological origin) collected in a guinea pig corral in La Joya, Arequipa (geographical origin). The feces from that T. cruzi-infected triatomine were inoculated intra-peritoneally into a female guinea pig. T. cruzi from the guinea pig’s blood was isolated and kept in LIT medium at 28oC since December 2008. The strain was typified at the Molecular Biology Lab for Chagas Disease at “Research Institute of Genetic Engineering and Molecular Biology Dr. Hector N. Torres”, Buenos Aires, Argentina and its discrete typing unit is TcI. Trypomastigotes were produced in cell culture with RPMI culture media, 10% FBS and cellular line LLC-MK2, and obtained from the culture supernatant, concentrated through centrifugation, and counted using a Neubauer chamber. We sourced 12 two-month old female Andean guinea pigs, weighing 650 to 750g, from a commercial farm. The Animal Use and Welfare Committee of Universidad Peruana Cayetano Heredia approved all the experiments and protocols related to these animals (Approval Identification Number 57822). Before experimental infection, we took baseline blood samples from each animal by saphenous venipuncture. The sera were tested for the presence of anti–T. cruzi antibodies using the enzyme-linked immunosorbent assay (ELISA) described elsewhere [13]. All the animals used in this study were determined to be negative for T. cruzi by ELISA. We inoculated all twelve guinea pigs through intraperitoneal injection, with 106 parasites in 100 μL of RPMI medium. The guinea pigs were subsequently split into two group: 8 of the 12 guinea pigs were subjected to both xenodiagnosis and parasitemia evaluation by direct microscopy, while the remaining 4 were only evaluated by direct microscopy.
To track infectiousness, every week over the course of a year, starting one week after parasite inoculation, we carried out xenodiagnoses. We carried out xenodiagnosis with 20 fifth-instar nymphs of T. infestans to each guinea pig and allowed the insects to feed for 20 minutes. Insects were evaluated for T. cruzi infection between 39 and 42 days after feeding. A few drops of triatomine rectal contents were obtained by applying pressure to the insect abdomen, and then diluted with saline solution and compressed between a glass slide and cover slip. The presence of mobile parasites in all microscopic fields of a 22mm x 22mm slide cover was determined at 400X magnification.
To evaluate parasitemia by direct microscopy, every week for a year, we collected blood from each animal by venipuncture in three heparinized capillary tubes, centrifuged the capillary tubes at 4,000 × g for 2 minutes, and extracted the buffy coat and spread it on a slide. The amount of blood collected in each capillary tube varied between 33.9 to 35.8ul. We examined 100 fields on the slides by light microscopy to detect parasites. The number of parasites was expressed in terms of parasites per milliliter of blood. We evaluated parasitemia weekly during the first 27 weeks. We did not detect parasitemia in any animal after week 22; therefore, after week 27 we evaluated parasitemia every other week and stopped evaluating parasitemia at week 43.
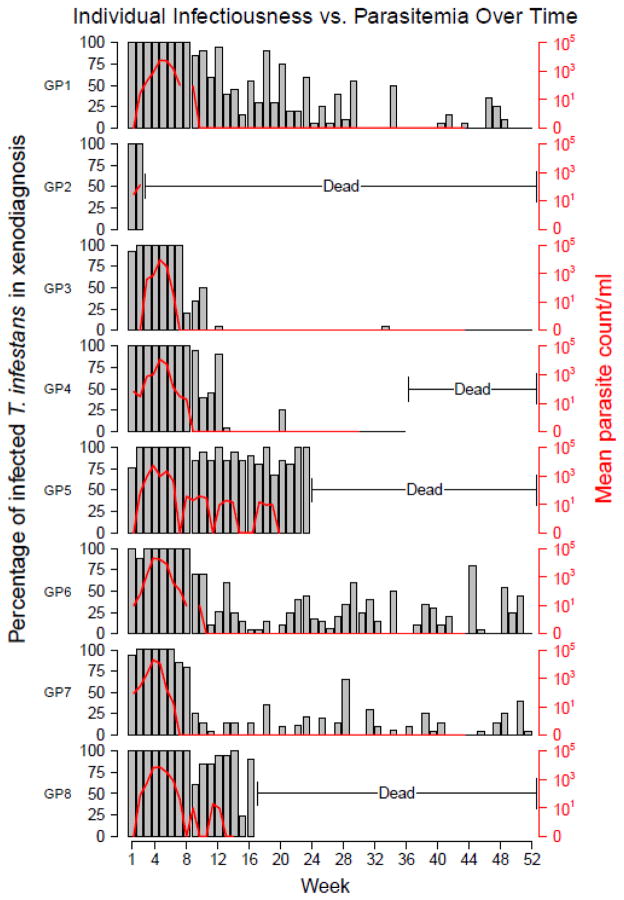
The bar plots in figure 1 show the temporal patterns of infectiousness of the eight guinea pigs to T. infestans (xenodiagnosis). Infectiousness is expressed as the percentage of insects infected by feeding on a guinea pig at a given time after the start of the experiment. Guinea pigs 1, 6, and 7 were infectious to feeding vectors for one year after inoculation. Generally, the infectiousness of these animals was extremely high (≥ 75%) for the first 8 weeks post-infection, and then oscillated (even dipping down to 0 some weeks) for the remainder of the year. Other guinea pigs, namely 3 and 4, became essentially noninfectious by week 12, with the exception of a couple isolated episodes of transmission. Guinea pigs 2, 5, and 8, died too early to characterize their long-term trends, though the persistently high infectiousness of guinea pig 5 (≥70%) for 23 weeks before death suggests a third pattern of persistent infectiousness with no or delayed oscillations. Figure 1 also shows the parasitemia of these animals, as evaluated by direct microscopy. The relationship between parasitemia and infectiousness varied in each guinea pig, though in each case, parasitemia as assessed by microscopy fell to 0 weeks before the animal ceased being infectious to vectors. Guinea pig 6 displayed a particularly high discordance between infectiousness and parasitemia. The parasite count oscillated, with troughs to 0, which would suggest changing infectiousness over time. However, the animal was highly infectious (>75%) during that period.
Figure 1.
Patterns of infectiousness heterogeneity as assessed through infection of insects (xenodiagnosis) and parasite counts (microscopy) among eight guinea pigs (GP).
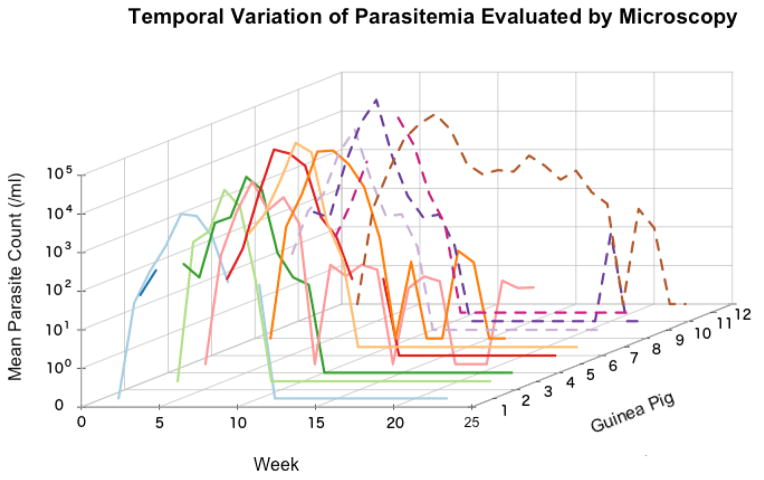
To evaluate the potential effect of saliva on T. cruzi levels within its mammalian host, the eight infected guinea pigs examined by xenodiagnosis (and hence exposed to triatomine saliva) concurrently had their levels of parasitemia measured by direct microscopy, along with a control group of four T. cruzi-infected guinea pigs never exposed to triatomine saliva (not evaluated with xenodiagnoses). Figure 2 shows the temporal pattern of parasitemia of guinea pigs exposed and not exposed to triatomine saliva. Qualitatively, these patterns do not appear different between the two groups. Parasitemia was low during weeks 1 and 2 post-inoculation (median = 28 parasite/ml; Inter-Quartile Range = 0–72), increased substantially between weeks 3 and 7 (median = 1,032 parasites/ml; IQR = 206–5,749), and then declined fast so that between weeks 8 and 10, the median was 0 parasites per ml (IQR = 0–28). Between weeks 11 and 43 parasitemia remained very low (median = 0 parasites/ml, IQR = 0–0) with a few weeks in which some animals showed values above 0. While most animals ceased being parasitemic between weeks 9 and 15, we observed prolonged parasitemia (up to weeks 20–22) in one guinea pig exposed to triatomine saliva and in two guinea pigs not exposed to triatomine saliva (Figure 2). Among the animals not exposed to triatomine saliva only one guinea pig, which was parasitemic between weeks 1 and 7, died at week 20.
Figure 2.
Parasitemia levels assessed by direct microscopy. Each line represents the trajectory of mean parasitemia from three capillary tubes of one animal. Disjoint lines represent weeks in which the parasitemia could not be evaluated. Parasitemia levels measured after week 22 were 0 for all animals and not shown in the graph.
Also in figure 2, it is important to note that shortly after inoculation, direct microscopy is very useful to detect infection – and infectiousness – in guinea pigs, but this usefulness decreases rapidly after week 7 post-infection, and becomes useless (sensitivity zero or close to zero) after week 16. After week 22, direct microscopy was unable to detect any infectious animal, even though 3 out of 5 were infectious for 30 more weeks, as determined by xenodiagnosis. Overall, there was a moderate correlation in the results obtained by xenodiagnosis and direct microscopy. The Spearman correlation between these two values in the eight guinea pigs receiving both evaluations was 0.79, and ranged individually from 0.56 to 0.87.
In this experiment we evaluated the temporal patterns of infectiousness of 12 guinea pigs for a year after T. cruzi infection, with 8 receiving xenodiagnosis and parasitemia evaluation by direct microscopy, and 4 subject to direct microscopy alone. While some guinea pigs ceased being parasitemic or infectious 10 to 16 weeks after infection, others were infectious for a much longer period. These super-shedders include 3 animals that were parasitemic 20 to 22 weeks after infection, and 3 animals that were positive by xenodiagnosis a whole year after infection (5 animals total, with 1 overlap). This wide range of values suggests a high degree of heterogeneity in the infectious period of T. cruzi infected guinea pigs. The extreme variation in infectiousness and parasitemia observed in twelve animals suggests that at least the same degree of heterogeneity would likely be present in a larger experimental group. Also, we used only one high infective dose to increase the chances of infection in each of the experimental animals. However, a variety of low and high doses could have produced different infectiousness patterns.
Our results differ from those of Castro-Sesquen et al. [13], who observed parasitemia of Trypanosoma cruzi in guinea pigs between day 15 and 55 (only up to 8 weeks) post-infection. The objective of their study was to develop an animal model for human pathology; therefore, parasitemia was evaluated to differentiate acute and chronic phases, and central tendencies of inflammatory and immunological markers were evaluated in those phases in 72 guinea pigs. One notable methodological difference between their experiment and ours is that, in addition to directly monitoring parasitemia in all guinea pigs by the hematocrit method, we also evaluated the infectiousness of a subset of guinea pigs by xenodiagnosis, exposing some guinea pigs and T. cruzi to triatomine saliva. We found no evidence that exposure to triatomine saliva prolongs parasitemia, since we observed extended parasitemia not only in guinea pigs subjected to xenodiagnosis, but also in guinea pigs never exposed to triatomines. A second, and perhaps more significant, difference is that, for experimental infection, we used Arequipa strain TC-35, a strain isolated in our lab in 2008, while Castro-Sesquen et al. used Y strain, an older strain that has been suggested to have undergone loss of virulence since at least 1997 [19]. The strain-dependence of parasitemia duration is supported by Basombrio [14], who found that 10% of guinea pigs infected with the Tulahuen strain exhibited high and persistent parasitemia. These results may be explained by the Tulahuen strain’s faster growth rate and greater resistance to oxidative stress over the Y strain [20].
Guinea pigs have long been recognized as important reservoirs of T. cruzi [2,12]. The high degree of heterogeneity we observed in the duration and patterns of infectiousness could have important consequences for the transmission dynamics of T. cruzi. The 20/80 rule [8] suggests that 20% of the infected population contributes at least 80% of the parasite transmission. This ratio suggests that control programs would be highly efficient if they targeted that 20% of the host population. Our results suggest the possibility of the presence of super shedders in guinea pig populations, and under specific circumstances it is possible that these highly infectious animals would produce ‘superspreading’ events [10]. Lloyd-Smith et al. [9] highlighted the importance of individual variation in disease emergence. In the fine-scale system of triatomine vectors and small mammals, such variation could mean a sharp rise of T. cruzi infection levels in vector colonies. The production system of guinea pigs in the Andes is based on small household breeders with backyard corrals that contain few individuals [21]. In areas with triatomine infestation, discrete colonies of T. infestans are found associated with these corrals, and can be isolated enough to be considered demes. The guinea pigs found in those corrals, if infected, could exhibit any of the distinctive patterns of infectiousness we observed in our experiment, and might create very different transmission dynamics between different T. infestans colonies. In addition, guinea pig population dynamics, such as those imposed by slaughter during guinea pig roasts, can also impact transmission dynamics and even promote ‘superspreading’ events by increasing the contact rate between super-shedder hosts and triatomine vectors [10,22].
Despite the importance of guinea pigs in T. cruzi transmission, little efforts have been made to focus control measures on these animals. Galvani and May [23] assert that “control measures require better knowledge of variability in individual infectiousness.” For Chagas disease, a complex system with several animal species reservoirs, understanding such heterogeneities will inform control strategies. Lloyd-Smith et al. emphasized [9] that it is important to find predictive factors of higher infectiousness. We found a high degree of heterogeneity in infectiousness, but we did not look for host or strain intrinsic factors associated with this variability. Nor did we include variability in age, sex, or body condition of the hosts, all of which are factors that have been associated with variability in infectiousness [24]. Genetic differences and differences in behavioral avoidance have also been associated with infectiousness heterogeneity [24,25], but given the study design, we could not assess the influence of these parameters. Isolated studies have tried to find strategies to prevent infection in guinea pigs without taking into account individual infectiousness. In 1990, Basombrio reported partial protection of an experimental vaccine to prevent T. cruzi infection in guinea pigs [14] and more recently Basso et al. reported cross protection with a T. rangeli-based vaccine [16]. Levy et al. (2006) used insecticide-impregnated nets to protect guinea pigs from triatomine bites and Schwarz et al. [26] developed an immunoassay to detect exposure of guinea pigs to triatomine saliva and determine the efficacy of impregnated nets. These new tools for control and surveillance could have more impact if paired with information about infectiousness.
Our results suggest that triatomine saliva does not play a role in lengthening the duration of parasitemia, at least in guinea pigs, but it might play an important role in other aspects of the transmission of T. cruzi. We also found direct microscopy to be highly dependent on the time since infection and insensitive in detecting T. cruzi several weeks post-infection, compared to xenodiagnosis. Xenodiagnosis is a better tool for longitudinal studies of infectiousness and high variability of infectiousness was observed in an experimental group of guinea pigs. Such variability might be related to T. cruzi strain and idiosyncratic immune response and could be present in guinea pig populations.
HIGHLIGHTS.
Some guinea pigs infected with T. cruzi are infectious for up to a year.
Prolonged T. cruzi parasitemia detected by microscopy occurred in a fraction of guinea pigs.
Super-shedder guinea pigs might be related to high T. cruzi levels in triatomines.
Acknowledgments
We gratefully acknowledge the following institution working in Peru on the control of Chagas disease: Ministerio de Salud del Perú (MINSA), the Dirección General de Salud de las Personas (DGSP), the Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxenicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), the Dirección General de Salud Ambiental (DIGESA), the Gobierno Regional de Arequipa, the Gerencia Regional de Salud de Arequipa (GRSA), the Pan American Health Organization (PAHO/OPS) and the Canadian International Development Agency (CIDA). Funding for these studies came from National Institutes of Health NIAID P50 AI074285 and 5R01 AI101229.
LIST OF ABBREVIATIONS
- ELISA
enzyme-linked immunosorbent assay
- LIT medium
Liver infusion tryptose medium
- T. cruzi
Trypanosoma cruzi
- T. infestans
Triatoma infestans
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ricardo Castillo-Neyra, Email: rcastillo@jhu.edu, Center for Clinical Epidemiology and Biostatistics, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, PA, USA. Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Peru.
Katty Borrini Mayorí, Email: yttakbm@gmail.com, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Peru.
Renzo Salazar Sánchez, Email: rendaths@gmail.com, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Peru.
Jenny Ancca Juárez, Email: jenyma14@gmail.com, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Peru.
Sherrie Xie, Email: xiex@vet.upenn.edu, Center for Clinical Epidemiology and Biostatistics, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, PA, USA.
Cesar Náquira Velarde, Email: cesar.naquira@gmail.com, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Peru.
Michael Z Levy, Email: mzlevy@mail.med.upenn.edu, Center for Clinical Epidemiology and Biostatistics, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, PA, USA.
References
- 1.Gürtler RE, Cécere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrer A. Trypanosomiasis americana en el Perú: III. Importancia del cobayo como reservorio de la enfermedad de Chagas en la región sudoccidental. Rev Perú Med Exp Salud Publica. 1955;9:45–55. [Google Scholar]
- 3.Coffield DJ, Spagnuolo AM, Shillor M, Mema E, Pell B, Pruzinsky A, et al. A model for Chagas disease with oral and congenital transmission. PLoS ONE. 2013;8:e67267. doi: 10.1371/journal.pone.0067267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science (New York, NY) 2001;293:694–8. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 5.Nouvellet P, Cucunubá ZM, Gourbière S. Ecology, Evolution and Control of Chagas Disease: A Century of Neglected Modelling and a Promising Future. Advances in Parasitology. 2015 doi: 10.1016/bs.apar.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Basombrio MA, Gorla D, Catalá S, Segura MA, Mora MC, Gómez L, et al. Number of vector bites determining the infection of guinea pigs with Trypanosoma cruzi. Memórias Do Instituto Oswaldo Cruz. 1996;91:421–3. doi: 10.1590/s0074-02761996000400006. [DOI] [PubMed] [Google Scholar]
- 7.Beldomenico PM, Begon M. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol Evol (Amst) 2010;25:21–7. doi: 10.1016/j.tree.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USa. 1997;94:338–42. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–9. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lass S, Hudson PJ, Thakar J, Saric J, Harvill E, Albert R, et al. Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. J R Soc Interface. 2013;10:20120588. doi: 10.1098/rsif.2012.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy MZ, Quispe Machaca VR, Ylla-Velasquez JL, Waller LA, Richards JM, Rath B, et al. Impregnated netting slows infestation by Triatoma infestans. Am J Trop Med Hyg. 2008;79:528–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Levy MZ, Bowman NM, Kawai V, Waller LA, Cornejo del Carpio JG, Cordova Benzaquen E, et al. Periurban Trypanosoma cruzi – infected Triatoma infestans, Arequipa, Peru. Emerg Infect Dis. 2006;12:1345–52. doi: 10.3201/eid1209.051662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, Velásquez DE, et al. Cavia porcellus as a model for experimental infection by Trypanosoma cruzi. Am J Pathol. 2011;179:281–8. doi: 10.1016/j.ajpath.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basombrio MA. Trypanosoma cruzi: partial prevention of the natural infection of guinea pigs with a killed parasite vaccine. Experimental Parasitology. 1990;71:1–8. doi: 10.1016/0014-4894(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 15.Nargis M, Chisty MM, Ihama Y, Sato H, Inaba T, Kamiya H. Kinetics of Trypanosoma cruzi infection in guinea-pigs, with special reference to the involvement of epidermal Langerhans' cells in the induction of immunity. Parasitology. 2001;123:373–80. doi: 10.1017/s0031182001008551. [DOI] [PubMed] [Google Scholar]
- 16.Basso B, Moretti E, Fretes R. Vaccination with Trypanosoma rangeli induces resistance of guinea pigs to virulent Trypanosoma cruzi. Vet Immunol Immunopathol. 2014;157:119–23. doi: 10.1016/j.vetimm.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Titus RG, Ribeiro JM. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today (Regul Ed) 1990;6:157–60. doi: 10.1016/0169-4758(90)90338-5. [DOI] [PubMed] [Google Scholar]
- 18.Mesquita RD, Carneiro AB, Bafica A, Gazos-Lopes F, Takiya CM, Souto-Padron T, et al. Trypanosoma cruzi infection is enhanced by vector saliva through immunosuppressant mechanisms mediated by lysophosphatidylcholine. Infect Immun. 2008;76:5543–52. doi: 10.1128/IAI.00683-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto PL, Takami R, Nunes EV, Guilherme CS, Oliveira OC, Gama-Rodrigues J, et al. Life cycle of Trypanosoma cruzi (Y strain) in mice. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:141–6. doi: 10.1590/s0041-87811999000500002. [DOI] [PubMed] [Google Scholar]
- 20.Mielniczki-Pereira AA, Chiavegatto CM, López JA, Colli W, Alves MJM, Gadelha FR. Trypanosoma cruzi strains, Tulahuen 2 and Y, besides the difference in resistance to oxidative stress, display differential glucose-6-phosphate and 6-phosphogluconate dehydrogenases activities. Acta Tropica. 2007;101:54–60. doi: 10.1016/j.actatropica.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Morales E. The guinea pig in the Andean economy: from household animal to market commodity. Latin American Research Review. 1994;29:129–42. [Google Scholar]
- 22.Levy MZ, Tustin A, Castillo-Neyra R, Mabud TS, Levy K, Barbu CM, et al. Bottlenecks in domestic animal populations can facilitate the emergence of Trypanosoma cruzi, the aetiological agent of Chagas disease. Proc Biol Sci. 2015:282. doi: 10.1098/rspb.2014.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvani AP, May RM. Epidemiology: Dimensions of superspreading. Nature. 2005;438:293–5. doi: 10.1038/438293a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, et al. Social Organization and Parasite Risk in Mammals: Integrating Theory and Empirical Studies. Annual Review of Ecology, Evolution, and Systematics. 2003;34:517–47. [Google Scholar]
- 25.Bousema T, Baidjoe A. Heterogeneity in malaria transmission: underlying factors and implications for disease control. In: Takken W, Koenraadt CJM, editors. Ecology of parasite-vector interactions. Vol. 3. Wageningen; the Netherlands: 2013. pp. 197–220. [Google Scholar]
- 26.Schwarz A, Juarez JA, Richards J, Rath B, Machaca VQ, Castro YE, et al. Anti-triatomine saliva immunoassays for the evaluation of impregnated netting trials against Chagas disease transmission. International Journal for Parasitology. 2011;41:591–4. doi: 10.1016/j.ijpara.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]