Abstract
Macrophages, a key cell in the inflammatory cascade, have been associated with poor prognosis in cancers, including breast cancer. In this study, we investigated the relationship of a subset of macrophages—proliferating macrophages (promacs)—with clinicopathologic characteristics of breast cancer, including tumor size, grade, stage, lymph node metastases, hormone receptor status, subtype, as well as early recurrence, and survival. This study included a discovery and validation set that was conducted at two institutions and laboratories (University of California, San Francisco and University of Chicago) using two independent cohorts of patients with breast cancer. Formalin-fixed, paraffin-embedded sections and/or tissue microarrays were double-stained with anti-CD68 (a macrophage marker) and anti-PCNA (a proliferation marker) antibodies. The presence of intratumoral promacs was significantly correlated with high grade, hormone receptor negative tumors, and a basal-like subtype. In contrast, there was no correlation between promacs and tumor size, stage, or the number of the involved lymph nodes. These findings were consistent between the two study cohorts. Finally, promac numbers were a significant predictor of recurrence and survival. In the pooled analysis, elevated promac levels were associated with a 77% increased risk of dying (P = 0.015). The presence of promacs in human breast cancer may serve as a prognostic indicator for poor outcomes and early recurrence and serve as a potential cellular target for novel therapeutic interventions.
Keywords: Breast cancer, Proliferating macrophages, Promac, Tumor-associated macrophage, Basal-like breast cancer, Hormone receptor negative tumor, Prognosis
Introduction
Macrophages are derived from hematopoietic progenitor cells in the bone marrow that enter the circulation and become blood monocytes. Monocytes migrate into tissues to undergo final differentiation, with accompanying loss of proliferative capacity, into resident macrophages. Macrophages perform a multitude of functions essential for tissue remodeling, inflammation, and immunity, including phagocytosis, cytotoxicity, and secretion of a wide array of cytokines, growth factors, lysozymes, proteases, complementary components, coagulation factors, and prostaglandins [1].
Chronic inflammation has been implicated in the initiation and promotion of various cancers [2]. Recruitment of leukocytes to a site of chronic inflammation triggers changes in the microenvironment that include production of reactive oxygen and nitrogen species, and the enrichment of cytokines and growth factors that may encourage proliferation of premalignant and malignant cells [3]. In the majority of malignant tumors, macrophages are a major component of the host leukocytic infiltrate. These tumor-associated macrophages (TAM) have been shown to be associated with poor prognosis in a variety of cancers, including breast cancer [4–11].
Although TAM are primarily derived from peripheral blood monocytes recruited into the tumor mass, there is also evidence of local proliferation of TAM. Proliferating macrophages, termed promacs, have been isolated from a variety of murine tumors [12–14]. In human lymphoma, promacs were defined by the expression of proliferating cell nuclear antigen (PCNA), a marker of proliferation [15]. PCNA has also been shown to be expressed in macrophages associated with glomerulonephritis [16], atherosclerosis [17], and AIDS-related dementia [18].
In this study, we investigated the role of promacs in human breast cancer. We performed the initial study at the University of California, San Francisco (UCSF) and performed a validation study using a completely independent set of patients and an independent laboratory at the University of Chicago (U of C). We sought to correlate the presence of infiltrating promacs with clinicopathologic characteristics such as tumor size, grade, hormone status, lymph node metastasis, and subtype. We also wanted to determine whether promacs were associated with early recurrence and survival.
Materials and methods
Sample collection
University of California, San Francisco (discovery cohort)
One hundred and ten archived paraffin blocks (obtained between 1991 and 1994) were retrieved from the UCSF breast oncology program tumor bank with IRB approval. Pathologic characteristics including tumor stage, grade, lymph node involvement, and receptor status (ER, PR, and HER2/Neu) were obtained from the UCSF Cancer Registry Database. Samples were collected to acquire approximately equal numbers of grade 1, grade 2, and grade 3 tumors. Patient characteristics including age, and follow-up information (recurrence and mortality) were recorded.
University of Chicago (validation cohort)
Breast tumor blocks for tissue microarray (TMA) construction were obtained from the surgical pathology archive at the U of C with IRB approval. The validation cohort included 106 patients diagnosed with breast cancer at the U of C Medical Center between 1996 and 2004. Tumor size, grade, and lymph node metastasis were determined by pathologic examination. Pathologic features, including histological diagnosis and grade were evaluated separately, and tumor size, and axillary lymph node metastasis were abstracted from pathologic reports.
Tissue microarrays were constructed from formalin-fixed, paraffin-embedded (FFPE) tumor samples and adjacent histological normal epithelium, which served as an internal positive control. Cores were precisely arrayed into a new recipient paraffin block using the automated tissue microarrayer ATA-27 (Beecher Instruments, Silver Spring, MD) with the method described by Kononen et al. [19].
Immunohistochemistry
UCSF
Tissue immunohistochemistry was performed on FFPE tissue sections using a standard streptavidin–biotin peroxidase method (details in Supplementary Table 1). 5-μm sections were deparaffinized in xylene and rehydrated using graded ethanol. Antigen retrieval was performed using microwave-heated 10 mM citrate buffer for 10 min. Double staining of CD68/PCNA was performed using dual endogenous enzyme block (Dako #3006) for 10 min, and incubation with anti-CD68 mouse monoclonal antibody (Dako #M0876, 1:50 dilution) for 30 min, followed by a second incubation with anti-PCNA mouse monoclonal antibody (Dako #M0879, 1:500 dilution) overnight. For anti-CD68, DAB plus (Dako #K1395) was used as a substrate, and for anti-PCNA, BCIP/NPT substrate (Dako #K0598) was used. The slides were counterstained with periodic acid Schiff reagent (American Master Tech Scientific #KTPAS) for 10 min at room temperature. Two pathologists independently evaluated the immunostains without knowledge of clinical outcomes or the results of previous immunostains. Slides were scanned at low power (20×) to determine three “hotspots” of positive staining. Positive staining cells (brown CD8+ cells with blue nuclear PCNA staining) were then counted in three high-power fields (HPF) (100×), and the mean number was calculated. Representative sections stained for promacs from grade 1, 2, and 3 tumors are shown in Fig. 1.
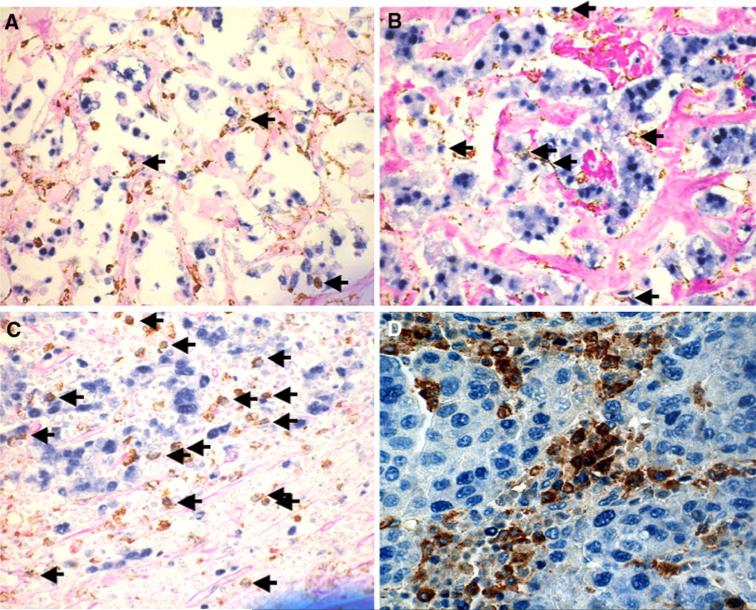
Fig. 1.
Immunohistochemical staining for promacs in breast cancer tissue. Sections were double stained with anti-CD68 (macrophage marker; DAB) and anti-PCNA (proliferation marker; BCIP/NPT (a, b, and c), or ferangi blue (d)). Proliferating macrophages (promacs) show brown cytoplasmic and blue nuclear staining (see arrows in panels a, b, and c). Quantitation of proliferating macrophages was done as described in text. a UCSF case, grade 1; b UCSF case, grade 2; c UCSF case, grade 3; d U of C case
U of C
TMA paraffin specimens were cut into 4-μm sections and mounted on positively charged slides. The slides were deparaffinized and rehydrated in xylene followed by graded alcohols, then washed in Tris-buffered saline. Immunohistochemical assays were performed using a DAKO immunostainer with antibodies and antigen unmasking as detailed in Supplementary Table 1. Slides were incubated in 0.03% hydrogen peroxide for 5 min to block endogenous peroxidase activity, followed by incubation for 20 min in a protein-blocking solution (Protein Block Serum-free solution, DAKO Corp.) to reduce nonspecific background. Envision+ reagents (DAKO) were used as a detection system. Slides were then treated for 5 min with 3–3′-diaminobenzidine chromogen, counterstained with hematoxylin, and coverslipped. Appropriate negative controls for the immunostaining were prepared by omitting the primary antibody step. The results of immunostainings were scored semi-quantitatively by two observers using Reiner's four-point scale based on intensity and percentage of IHC reaction [20]. EGFR and HER2 stainings were evaluated according to manufacturer's instructions (DAKO). For promacs, anti-CD68 antibody (DAKO, M0814) and anti-PCNA antibody (DAKO, M0879) were used for dual staining. Anti-PCNA was detected with Ferangi Blue Chromogen Kit (Biocare Medical, FB812S) without counterstaining (Fig. 1).
Consistent with previous publication [21] breast cancer subtypes were defined as luminal A (ER+ and/or PR+, HER2−), luminal B (ER+ and/or PR+, HER2+), basal-like (ER−, PR−, HER2−, CK5/6+, and/or EGFR+), HER2+/ER− (HER2+, ER−, PR−), and unclassified (negative for all five markers).
Statistical analysis
Initially, two pathologists independently counted numbers of promacs in each tumor sample. The independent counts were averaged and used in further analyses. The R program maxstat was used to find an optimal cutpoint for promacs as a predictor of recurrence. We found, in the UCSF cohort, five promacs per HPF was the optimal cutpoint that provided a good separation of the time-to-recurrence curves (Fig. 2). Then, we examined the relationship between promacs and clinico-pathologic variables using t test or Wilcoxon rank-sum test for continuous or ordinal variables (e.g., age, tumor size, grade, stage, etc.) and Fisher's exact test for categorical variables (e.g., ER).
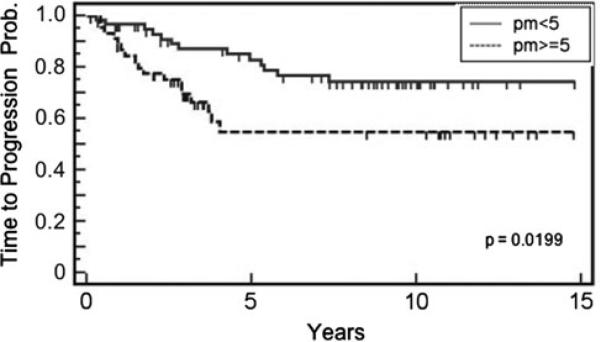
Fig. 2.
Time-to-recurrence according to promac levels in human breast cancer. Patients in the discovery cohort with ≥5 promacs/HPF recurred earlier compared to patients with <5 promacs/HPF (p = 0.0199, log rank test)
Time-to-recurrence, overall survival, and recurrence-free survival rates were estimated using the Kaplan–Meier method, and the comparison between high promacs and low promacs was performed using the generalized Wilcoxon test for equality of survival function. The Wilcoxon test weights early events (death or recurrence) more than late events and thus is sensitive to early differences. Cox proportional hazard models were used to estimated hazard ratios and 95% confidence interval (CI). To examine whether promacs were an independent prognostic factor, we adjusted for age at diagnosis, ER status, histological grade, and tumor stage in multivariate Cox models. Separate analyses were conducted for each study cohort, and then a pooled analysis was conducted, in which the study indicator variable was adjusted for.
Results
Patient characteristics
There were 110 breast cancer patients from UCSF (the discovery cohort), and 106 patients from U of C (the validation cohort) included in this study. The age distribution (mean ± SD) was similar between the two cohorts (55.5 ± 14.2 years and 56.0 ± 15.7 years, respectively). The proportion of patients with lymph node metastatic tumors was also similar between the discovery (40%) and validation cohort (49%). There were significantly more low-grade tumors in the discovery cohort (30%) than in the validation cohort (4%), most likely due to the selection of equal numbers of cases across all the three grades in the UCSF cohort. The tumors were larger in the validation cohort (median 2.9 cm) than in the discovery cohort (2 cm). Finally, patients in the discovery cohort were more likely to have HER2κ tumors, whereas patients in the validation cohort were more likely to have hormone receptor positive tumors (details see Table 1).
Table 1.
Relationship between clinical factors and proliferating macrophages in the UCSF and U of C cohorts
| Characteristics | UCSF cohort |
U of C cohort |
||||
|---|---|---|---|---|---|---|
| Mean ± SD |
P value | Mean ± SD |
P value | |||
| Promacs low (<5) | Promacs high (≥5) | Promacs low (<5) | Promacs high (≥5) | |||
| Age at diagnosis, year | 58.0 ± 14.4 | 52.5 ± 13.5 | 0.046 | 60.0 ± 14.0 | 53.3 ± 15.4 | 0.032 |
| Tumor size, cm | 2.5 ± 1.9 | 2.5 ± 1.6 | 0.90 | 2.9 ± 2.2 | 3.4 ± 2.5 | 0.22 |
| N (%) | P value | N (%) | P value | |||
|---|---|---|---|---|---|---|
| Lymph node | ||||||
| Negative | 34 (56) | 32 (65) | 0.33 | 23 (56) | 28 (47) | 0.42 |
| Positive | 27 (44) | 17 (35) | 18 (44) | 31 (53) | ||
| AJCC stage | ||||||
| 0 | 0 | 0 | 0.43 | 4 (10) | 2 (3) | 0.12 |
| 1 | 21 (35) | 21 (44) | 12 (30) | 13 (21) | ||
| 2 | 31 (52) | 21 (44) | 17 (42) | 32 (52) | ||
| 3 | 7 (12) | 5 (10) | 6 (15) | 12 (20) | ||
| 4 | 1 (2) | 1 (2) | 1 (2) | 2 (3) | ||
| Histological grade | ||||||
| 1 | 25 (41) | 8 (16) | 0.0005 | 3 (8) | 1 (2) | 0.049 |
| 2 | 19 (31) | 12 (24) | 19 (51) | 23 (39) | ||
| 3 | 17 (29) | 29 (59) | 14 (41) | 35 (59) | ||
| Estrogen receptor | ||||||
| Negative | 12 (20) | 23 (48) | 0.002 | 8 (19) | 36 (58) | <0.001 |
| Positive | 49 (80) | 25 (52) | 35 (81) | 26 (42) | ||
| Progesterone receptor | ||||||
| Negative | 19 (31) | 22 (46) | 0.16 | 14 (33) | 39 (63) | 0.005 |
| Positive | 42 (69) | 26 (54) | 28 (67) | 23 (37) | ||
| HER2, n (%) | ||||||
| Negative | 16 (57) | 25 (73) | 0.19 | 37 (88) | 49 (79) | 0.30 |
| Positive | 12 (43) | 9 (26) | 5 (12) | 13 (21) | ||
| Molecular subtype | ||||||
| Luminal A | 31 (74) | 25 (40) | <0.001 | |||
| Luminal B | 4 (10) | 2 (3) | ||||
| Basal-like | 6 (14) | 23 (37) | ||||
| HER2+/ER– | 1 (2) | 11 (18) | ||||
| Unclassified | 0 | 1 (2) |
UCSF University of California at San Francisco (the discovery cohort), U of C University of Chicago (the validation cohort), promacs proliferating macrophages calculated as the number per high-power field, SD standard deviation, AJCC American joint committee on cancer
Immunohistochemical identification of proliferating macrophages
Breast cancer tissue sections were examined for the presence of promacs by double-staining with anti-CD68 (a macrophage marker) and anti-PCNA (a marker of proliferation). Promacs were enumerated as described above and concordance of promac counts per high-power field (HPF) between two pathologists was high at a κ of 0.87. Figure 1 shows representative stained sections from grade 1, 2, and 3 tumors from the discovery cohort (panels a, b, and c, respectively) and a representative stained section from the validation cohort (panel d). Background differences are due to the differences in counterstains, periodic acid Schiff and hematoxylin, at UCSF and U of C, respectively. There was no significant difference in the number of promacs between the two cohorts (P = 0.20).
Promacs and clinical characteristics
For our initial analysis on the discovery cohort, we used time to recurrence as an endpoint to evaluate whether promac numbers were associated with early recurrence. Cases were dichotomized into high or low promacs at various cutpoints (number of promacs per HPF) to find an optimal value. As shown in Fig. 2, dichotomizing cases into <5 and ≥5 promacs per HPF yielded an optimal and significant (P = 0.0199) separation of cases based on time to recurrence. Patients with high promac counts recurred earlier compared to those with low counts. Promac counts were dichotomized into low (<5 promacs per HPF) or high (≥5 promacs per HPF) in the subsequent presentation for better clinical interpretation.
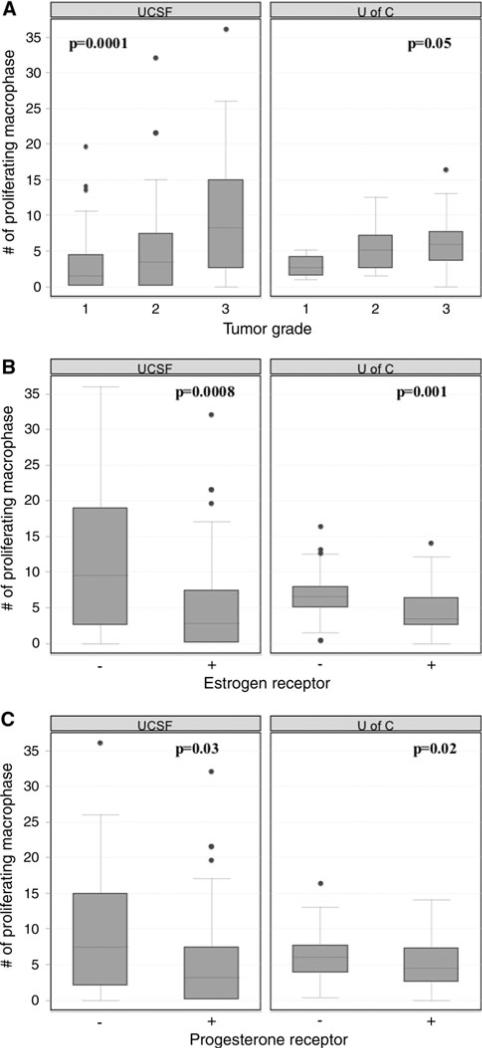
As shown in Table 1, a high level of promacs was associated with approximately 6-year earlier onset of breast cancer in both cohorts (pooled analysis: P = 0.003). High promacs were also positively correlated with histological grade in both cohorts (Fig. 3a) as well as in the pooled cohort (pooled analysis: P < 0.0001). In addition, in both cohorts, hormone receptor negative tumors were associated with higher promac counts (Fig. 3b and c). This association between hormone receptor status and promacs was also significant in the pooled cohort (P < 0.0001 for estrogen receptor; P = 0.002 for progesterone receptor). Neither lymph node involvement, tumor size, AJCC stage, nor HER2 status was correlated with promacs in either cohort. In the validation cohort, we also assessed breast cancer subtypes (determined by IHC) in relation to proliferating macrophages and found that promacs were over-represented in basal-like and HER2+/ER− tumors.
Fig. 3.
Promac association with grade and hormone receptor status in human breast cancer. a Promacs positively correlated with grade; b increased promacs in ER negative tumors; c increased promacs in PR negative tumors
Promacs and clinical outcomes
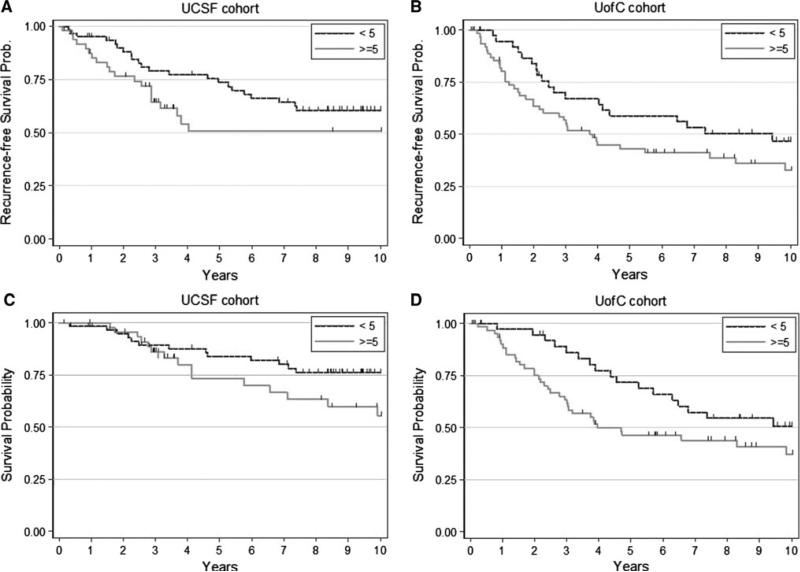
After a median follow-up of about 9 years in both cohorts, 28 breast cancer patients in the discovery cohort and 52 in the validation cohort died. There were 19 survivors with recurrent disease. As depicted in Fig. 4, the recurrence-free survival rate was lower in patients with high promacs in both cohorts. The unadjusted hazard ratio comparing high with low promacs was very similar between the two cohorts, though both were marginally significant (Table 2). The pooled hazard ratio for recurrence-free survival and promacs was statistically significant (P = 0.034). After adjusting for age, estrogen receptor status, grade, stage, and study cohort, high promac level was associated with 45% increased risk of recurrence/death, though the association was not statistically significant. Similarly, high promacs were associated with worse overall survival in both cohorts (Fig. 4c, d). In the univariate analysis, the strength of association was similar between the two cohorts though only marginally significant and reached statistically significant levels in the pooled samples (P = 0.015). After adjusting for age, estrogen receptor status, grade, stage, and study cohort, high promacs were associated with 75% increased risk of death (P = 0.048).
Fig. 4.
Promac levels in human breast cancer are associated with early recurrence and death. Patients were split into two groups: those whose tumors contained <5 promacs/HPF and those whose tumors had ≥5 promacs/HPF. a and b Kaplan–Meier analyses showing recurrence-free survival over time for the discovery and validation cohorts, respectively. c and d Kaplan–Meier analyses showing overall survival for the discovery and validation cohorts, respectively
Table 2.
Clinical outcomes and proliferating macrophages in the UCSF and U of C cohorts
| Endpoints | UCSF cohort |
U of C cohort |
Pooled cohortsa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Promacs low (<5) | Promacs high (≥5) | P value | Promacs low (<5) | Promacs high (≥5) | P value | Promacs low (<5) | Promacs high (≥5) | P value | |
| Relapse-free survival | |||||||||
| Events/patients | 22/61 | 20/48 | 19/41 | 38/62 | 41/102 | 58/110 | |||
| 5-year probability | 73% | 51% | 0.091 | 59% | 43% | 0.054 | 68% | 46% | 0.004 |
| Unadjusted HR (95% CI) | 1.0 (ref) | 1.56 (0.85–2.88) | 0.15 | 1.0 (ref) | 1.59 (0.91–2.76) | 0.10 | 1.0 (ref) | 1.56 (1.03–2.34) | 0.034 |
| Adjusted HR (95% CI)b | 1.0 (ref) | 1.60 (0.76–3.34) | 0.21 | 1.0 (ref) | 1.31 (0.68–2.54) | 0.42 | 1.0 (ref) | 1.45 (0.89–2.34) | 0.14 |
| Overall survival | |||||||||
| Deaths/patients | 13/61 | 15/48 | 17/41 | 35/62 | 41/101 | 58/110 | |||
| 5-year probability | 84% | 73% | 0.18 | 72% | 46% | 0.021 | 79% | 57% | 0.002 |
| Unadjusted HR (95% CI) | 1.0 (ref) | 1.87 (0.88–3.95) | 0.10 | 1.0 (ref) | 1.71 (0.96–3.06) | 0.07 | 1.0 (ref) | 1.77 (1.12–2.57) | 0.015 |
| Adjusted HR (95% CI)b | 1.0 (ref) | 2.84 (0.99–8.13) | 0.052 | 1.0 (ref) | 1.48 (0.73–2.97) | 0.28 | 1.0 (ref) | 1.75 (1.00–3.04) | 0.048 |
Additionally adjusted for the indicator variable of cohort in multivariate Cox models
Adjusted for age, estrogen receptor status, histological grade, and tumor stage in multivariate Cox models
UCSF University of California at San Francisco (the discovery cohort), U of C University of Chicago (the validation cohort), promacs proliferating macrophages calculated as the number per high-power field, HR hazard ratio, CI confidence interval
Discussion
In this study, we examined the role of CD68+/PCNA+ proliferating macrophages (promacs) in breast cancer. These experiments were carried out at two independent institutions and laboratories (the UCSF and the U of C) using two independent cohorts of patients with breast cancer. We found increased numbers of infiltrating promacs were significantly associated with high grade, hormone receptor negative tumors in both cohorts. There was no correlation between promacs and tumor size, stage, or the number of the involved lymph nodes. From the U of C study, tissue cores with very high number of promacs were disproportionately represented in the basal subtype, as well as the HER2+/ER− subtype.
Using the UCSF cohort as a discovery set, we found that at a cutpoint of five promacs per HPF, there was a significant difference in time-to-progression between patients with high vs. low promac infiltration. Using this cutpoint in both cohorts, a trend (albeit not significant) was observed between higher number of promacs and poor relapse-free survival. We also observed trends between high promacs and poor overall survival in both cohorts, and this was significant in the pooled cohort analysis even after adjusting for age, estrogen receptor status, histological grade, and tumor stage.
Solid tumors are infiltrated with leukocytes (predominately lymphocytes and macrophages) and the cross-talk between these cells and the cancer cells are likely to have profound effects on tumor progression. The presence of tumor-associated macrophages (TAM) represents one of the hallmarks of cancer-associated inflammation. TAM produce a variety of cytokines and chemokines, as well as growth factors for both epithelial and endothelial cells, which play a vital role in tumor growth and metastasis [2, 22].
TAM have been identified as a major negative prognostic indicator on clinical outcome in patients with lymphoma and breast cancer [5–7, 10, 11]. In addition, CD68+ macrophages have been shown to be strongly associated with high grade ductal carcinoma in situ (DCIS) breast cancer and associated comedonecrosis. In particular, increased size and density of DCIS lesions, and clumped appearance on MRI enhancement correlated with greater numbers of CD68+ macrophages [23].
Normally, tissue macrophages derived from circulating blood monocytes lose their proliferative capacity. However, local proliferation of TAM has been observed in some cancers. Proliferating TAM have been isolated from mouse mammary tumors, melanoma, and lung carcinoma [13, 14] as well as methylcholanthrene-induced sarcomas in C57BL/6 J mice [12]. Human lymphoma-associated macrophages have been shown to express the proliferation-associated marker PCNA [15]. PCNA has also been shown to be expressed in macrophages associated with other diseases [16–18].
Early recurrence is a harbinger for poor survival. A characteristic feature of triple negative cancers and HER2+/hormone receptor negative cancers is that the risk of recurrence is in the first 5 years (ASCO poster Esserman et al. 2010, manuscript submitted). Promacs are most commonly present in these tumor types; thus, it is not surprising that tumors with a high level of proliferating macrophages have a higher risk for early recurrence.
We have demonstrated the presence of PCNA+ macrophages (promacs) in breast cancer and examined their association with various clinical parameters. These findings, corroborated at two independent institutions, suggest that the presence of promacs may serve as a prognostic indicator for poor outcome. Perhaps more importantly, these cells may serve as a potential cellular target for novel therapeutic interventions. Owing to their multifaceted role in tumor progression, macrophages offer a variety of therapeutic targets including inhibition of their recruitment, activation, and/or survival at the tumor site, reversal of their polarization/immune suppression activity, and inhibition of their angiogenic and matrix remodeling activities.
Supplementary Material
Acknowledgments
This study was supported by the Breast Cancer Research Foundation, the National Cancer Institute (grants CA-RO1 89085-01A, P50-CA58223-09A1, P50 CA125183, P50 ES012382, and P30 CA14599-32), the UC SPORE, and the Avon Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-010-1154-y) contains supplementary material, which is available to authorized users.
Contributor Information
Michael J. Campbell, Department of Surgery, University of California, San Francisco, USA
Nathan Y. Tonlaar, Pritzker School of Medicine, University of Chicago, Chicago, USA
Elisabeth R. Garwood, Department of Surgery, University of California, San Francisco, USA
Dezheng Huo, Department of Health Studies, University of Chicago, Chicago, USA.
Dan H. Moore, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA
Andrey I. Khramtsov, Department of Medicine, University of Chicago, Chicago, USA
Afred Au, Department of Pathology, University of California, San Francisco, USA.
Frederick Baehner, Department of Pathology, University of California, San Francisco, USA.
Yinghua Chen, Department of Medicine, University of Chicago, Chicago, USA.
David O. Malaka, Department of Medicine, University of Chicago, Chicago, USA
Amy Lin, Department of Surgery, University of California, San Francisco, USA.
Oyinlolu O. Adeyanju, Department of Medicine, University of Chicago, Chicago, USA
Shihong Li, Department of Pathology, University of Chicago, Chicago, USA.
Can Gong, Department of Pathology, University of Chicago, Chicago, USA.
Michael McGrath, Department of Laboratory Medicine and Medicine, San Francisco General Hospital, University of California, San Francisco, USA.
Olufunmilayo I. Olopade, Department of Medicine, University of Chicago, Chicago, USA
Laura J. Esserman, Department of Surgery, University of California, San Francisco, USA Carol F. Buck Breast Care Center, 1600 Divisadero St, Box 1710, San Francisco, CA 94115, USA.
References
- 1.Auger MJ, Ross JA. The biology of the macrophage. In: Lewis CE, McGee JOD, editors. The macrophage. Oxford University Press; New York: 1992. pp. 3–56. [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res. 2006;25:365–372. [PubMed] [Google Scholar]
- 5.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, Miller TP, LeBlanc M, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Connors JM, Lansdorp PM, Ouyang Q, Lister TA, Davies AJ, Norton AJ, Muller-Hermelink HK, Ott G, Campo E, Montserrat E, Wilson WH, Jaffe ES, Simon R, Yang L, Powell J, Zhao H, Goldschmidt N, Chiorazzi M, Staudt LM. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 6.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 7.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 8.Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 9.Takanami I, Takeuchi K, Kodaira S. Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology. 1999;57:138–142. doi: 10.1159/000012021. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angio-genesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- 11.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 12.Evans R, Cullen RT. In situ proliferation of intratumor macrophages. J Leukoc Biol. 1984;35:561–572. doi: 10.1002/jlb.35.6.561. [DOI] [PubMed] [Google Scholar]
- 13.Stewart CC. Local proliferation of mononuclear phagocytes in tumors. J Reticuloendothel Soc. 1983;34:23–27. [PubMed] [Google Scholar]
- 14.Stewart CC, Beetham KL. Cytocidal activity and proliferative ability of macrophages infiltrating the EMT6 tumor. Int J Cancer. 1978;22:152–159. doi: 10.1002/ijc.2910220208. [DOI] [PubMed] [Google Scholar]
- 15.Zenger E, Abbey NW, Weinstein MD, Kapp L, Reis J, Gofman I, Millward C, Gascon R, Elbaggari A, Herndier BG, McGrath MS. Injection of human primary effusion lymphoma cells or associated macrophages into severe combined immunodeficient mice causes murine lymphomas. Cancer Res. 2002;62:5536–5542. [PubMed] [Google Scholar]
- 16.Yang N, Isbel NM, Nikolic-Paterson DJ, Li Y, Ye R, Atkins RC, Lan HY. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 1998;54:143–151. doi: 10.1046/j.1523-1755.1998.00978.x. [DOI] [PubMed] [Google Scholar]
- 17.Shiomi M, Yamada S, Ito T. Atheroma stabilizing effects of simvastatin due to depression of macrophages or lipid accumulation in the atheromatous plaques of coronary plaque-prone WHHL rabbits. Atherosclerosis. 2005;178:287–294. doi: 10.1016/j.atherosclerosis.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164:2089–2099. doi: 10.1016/S0002-9440(10)63767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 20.Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–7061. [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 23.Esserman LJ, Kumar AS, Herrera AF, Leung J, Au A, Chen YY, Moore DH, Chen DF, Hellawell J, Wolverton D, Hwang ES, Hylton NM. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. 2006;24:4603–4610. doi: 10.1200/JCO.2005.04.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.