Abstract
Background & Aims
Pancreatic acinar cells have an expanded apical endosomal system, the physiologic and pathophysiologic significance of which is still emerging. Phosphatidylinositol-3,5-bisphosphate [PI(3,5)P2] is an essential phospholipid generated by phosphatidylinositol 3-phosphate 5-kinase (PIKfyve), which phosphorylates phosphatidylinositol-3-phosphate (PI3P). PI(3,5)P2 is necessary for maturation of early endosomes (EE) to late endosomes (LE). Inhibition of EE to LE trafficking enhances anterograde endosomal trafficking and secretion at the plasma membrane by default through a recycling endosome (RE) intermediate. We assessed the effects of modulating PIKfyve activity on apical trafficking and pancreatitis responses in pancreatic acinar cells.
Methods
Inhibition of EE to LE trafficking was achieved using pharmacologic inhibitors of PIKfyve, expression of dominant negative PIKfyve K1877E, or constitutively active Rab5-GTP Q79L. Anterograde endosomal trafficking was manipulated by expression of constitutively active and dominant negative Rab11a mutants. The effects of these agents on secretion, endolysosomal exocytosis of lysosome associated membrane protein (LAMP1), and trypsinogen activation in response to supramaximal cholecystokinin (CCK-8), bile acids, and cigarette toxin was determined.
Results
PIKfyve inhibition increased basal and stimulated secretion. Adenoviral overexpression of PIKfyve decreased secretion leading to cellular death. Expression of Rab5-GTP Q79L or Rab11a-GTP Q70L enhanced secretion. Conversely, dominant-negative Rab11a-GDP S25N reduced secretion. High-dose CCK inhibited endolysosomal exocytosis that was reversed by PIKfyve inhibition. PIKfyve inhibition blocked intracellular trypsin accumulation and cellular damage responses to supramaximal CCK-8, tobacco toxin, and bile salts in both rodent and human acini.
Conclusions
These data demonstrate that EE-LE trafficking acutely controls acinar secretion and the intracellular activation of zymogens, leading to the pathogenicity of acute pancreatitis.
Keywords: Endosome, Pancreatitis, PIKfyve, Trypsin
Abbreviations used in this paper: AP, adaptor protein; BFA, brefeldin A; CCK, cholecystokinin; CLP, constitutive-like pathway; DMEM, Dulbecco’s minimal essential medium; DMSO, dimethyl sulfoxide; EE, early endosome; GDP, guanosine diphosphate; GFP, green fluorescent protein; GTP, guanosine triphosphate; HA, hemagglutinin; LAMP1, lysosome-associated membrane protein; LDH, lactate dehydrogenase; LE, late endosome; LY294002, 2-morpholin-4-yl-8-phenylchromen-4-one; MRP, minor-regulated pathway; PI, phosphatidylinositol; PIKfyve, phosphatidylinositol 3-phosphate 5-kinase; PI(3)P, phosphatidylinositol 3-phosphate; PI(3,5)P2, phosphatidylinositol-3,5-bisphosphate; RE, recycling endosome; TGN, trans-Golgi network; VAMP8, vesicle-associated membrane protein 8; Vps34, phosphatylinositol 3-kinase; WT, wild type; YM201636, 6-amino-N-[3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin-2-yl)phenyl]pyridine-3-carboxamide; ZG, zymogen granule
Summary.
In addition to zymogen granule secretion, acinar cells express an anterograde endosomal secretory pathway coordinated in the early endosome (EE). Altered trafficking from EEs to late endosomes/lysosomes or apical membrane during acute pancreatitis controls secretion and zymogen activation.
Acute pancreatitis is an inflammatory disease of the exocrine pancreas initiated in part by the premature activation of proteolytic zymogens by lysosomal hydrolases in an unidentified intracellular compartment.1 The coincident activation of nuclear factor-κB promotes inflammation and cellular damage in this disease.2 Ductal obstruction, retrograde perfusion of bile salts, chronic ethanol exposure, hypercalcemia, hyperlipidemia, or infection can lead to acute experimental pancreatitis. These treatments are relevant to the etiologies of clinical disease. In addition to zymogen activation, a common feature of acute pancreatitis is the pronounced inhibition of digestive enzyme secretion from the acinar cell.3 Acinar preparations stimulated with high-dose cholecystokinin (CCK), bile salts, or cigarette smoke toxin are useful to induce early events of pancreatitis in the absence of an inflammatory response. Stimulation of acinar cells with the high-affinity CCK agonist JMV-180 or bombesin show no high-dose secretory inhibition or accumulation of activated zymogens, suggesting that secretory inhibition may play a key role in accumulating activated zymogens in the acinar cell and causing injury during pancreatitis.3
Phosphatidylinositols (PI) regulate membrane trafficking events based on their reversible phosphorylation of the inositol ring.4 Class III PI3-kinase Vps34 (phosphatylinositol 3-kinase) phosphorylates PI to generate PI3 phosphate [PI(3)P]. which is essential for formation of the early endosomal (EE) compartment.5 The PI3-kinase inhibitors wortmannin and LY294002 (2-morpholin-4-yl-8-phenylchromen-4-one) were shown to fully block intracellular trypsinogen activation in isolated acini and in vivo.6, 7 However, high concentrations of these inhibitors also block class I PI3-kinases, which generate phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3] from phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] (see Figure 1). Genetic ablation of the p110 γ-subunit of class I PI3-kinase significantly alters intracellular calcium mobilization, a necessary signal for zymogen activation, making the inhibitory mechanism of these agents uncertain.6, 7, 8
Figure 1.
Inhibition of phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) enhances secretion in rodent and human acini. (A) Diagram depicting the classic zymogen granule pathway (ZGP) versus the constitutive-like pathway (CLP), which uses anterograde endosomal trafficking through early (EE) and recycling endosomes (RE) to secrete small amounts of zymogens. The EE also gives rise to the late endosome (LE). Synthesis of phosphatidylinositol-3,5-bisphosphate [PI(3,5)P2] via PIKfyve is required for EE to LE trafficking and is inhibited by YM201636 and apilimod. (B) PI3 kinase pathways: phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] is phosphorylated by class I PI 3-kinases to produce phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3]. The class III PI 3-kinase Vps34 (phosphatylinositol 3-kinase) generates PI(3)P (phosphatidylinositol 3-phosphate). Class I and II enzymes are inhibited by wortmannin and LY294002. PI(3)P is phosphorylated by PIKFyve to generate PI(3,5)P2. (C) Rat pancreas fractionated to obtain postnuclear supernatant (PNS), zymogen granule (ZG), and crude microsomes (CM) remaining after ZG removal was analyzed by immunoblotting. PIKfyve was not found in ZG fractions. (D) Isolated rat acini pretreated 30 minutes as control (0.1% dimethyl sulfoxide) or with indicated concentrations of YM201636 showed enhanced basal secretion. (E) Stimulated secretion (Total − Basal) by maximal (100 pM) or supramaximal (10 nM) cholecystokinin-1 (CCK-8) (30 minutes) was enhanced at 1 μM but not 10 μM YM201638. (F) Immunofluorescence analysis of acinar PI(3,5)P2 levels. Analysis was conducted on cryostat sections of pancreatic lobules that were treated 30 minutes with YM201636 ± CCK-8 (30 minutes). PIKfyve inhibition by 10 μM YM201636 strongly reduced PIP(3,5)P2 immunoreactivity under basal and CCK-8 stimulated conditions. (G) Pretreatment of rat acini for 30 minutes with 100 μM LY294002 or 1 μM wortmannin had no effect on basal or CCK-8-stimulated secretion. However, YM201636, apilimod (apil), and bafilomycin A1 (BafA1) enhanced secretion. *P < .05 (n = 3) from control levels obtained in the absence of YM201636, apilimod, or baflomycin A1.
Phosphatidylinositol 3-phosphate 5-kinase, PIKfyve (Fab1 in yeast), acts on PI(3)P to produce phosphatidylinositol-3,5-bisphosphate [PI(3,5)P2], a signaling molecule that represents <0.01% of total phosphoinositides.9 PIKfyve localizes to EE by binding to PI(3)P via a zinc-finger fyve-domain (Fab1, YOTB, Vac1 and Early endosomal antigen 1).9 PIKfyve also phosphorylates PI to produce phosphatidylinositol 5-phosphate [PI(5)P] in vitro, but studies attribute the cellular effects of PIKfyve to PI(3,5)P2 as PI(5)P is produced from PI(3,5)P2 dephosphorylation.10 PIKfyve controls two endosomal pathways: 1) retrograde trafficking from the EE to the trans-Golgi network (TGN) and 2) maturation of the EE to the late endosome (LE)/lysosome.9 Although genetic ablation of PIKfyve is embryonic lethal, knockdown by small-interfering RNA, expression of a dominant negative kinase-dead PIKfyve mutant, or use of the PIKfyve-specific inhibitor YM201636 (6-amino-N-[3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin-2-yl)phenyl]pyridine-3-carboxamide), causes enlargement of the EE and retention of material from LE/lysosomes, and TGN.9
In addition to the classic zymogen granule (ZG) secretory pathway, acinar cells also have a parallel secretory pathway that arises from immature secretory granules and TGN and uses anterograde endosomal trafficking through EE and recycling endosome (RE) to deliver secretory zymogens to the apical plasma membrane (see Figure 1A). Under basal conditions, this is termed the constitutive-like pathway (CLP); when it is stimulated by low-level CCK-8 or muscarinic agonists, it is termed the minor-regulated pathway (MRP).11 In addition to mediating anterograde secretion, the EE compartment serves as a sorting compartment that gives rise to LE. We recently demonstrated that anterograde endosomal trafficking through EE and RE in pancreatic acini plays an important role in mediating zymogen secretion.12 Here we manipulated acinar EE to LE/lysosome trafficking, in part, by targeting PIKfyve enzyme activity and examined the effects on secretory function and the intracellular activation of trypsinogen during acute pancreatitis in isolated acinar cells from rodents and humans. The results supported that PIKfyve activity mediates trafficking from the EE to LE resulting in secretory inhibition, intracellular trypsin accumulation, and cellular damage.
Materials and Methods
Antibodies
Anti-hemagglutinin (HA) mouse monoclonal antibody was purchased from Cell Signaling Technology (Beverly, MA). Anti–lysosomal-associated membrane protein 1 (LAMP1) used for immunofluorescence was purchased from Assay Designs (Ann Arbor, MI). Anti-Rab11a, anti-Rab5, and anti-LAMP1 (used for Western blotting) were purchased from AbCam (Cambridge, MA). Anti-Rab5 was purchase from BD Transduction Laboratories (Lexington, KY). The PIP(3,5) antibody (cat. no. ZP035) was from Eschelon Biosciences (Salt Lake City, UT). Alexa-conjugated rabbit and mouse secondary antibodies were purchased from Invitrogen/Life Technologies (Carlsbad, CA). Anti-PIKfyve was a kind gift from Lois Weisman (University of Michigan). Peroxidase conjugated secondary antibodies were purchased from GE Healthcare Bio-Sciences (Pittsburgh, PA). All antibodies were characterized before western blotting and immunofluorescence labeling studies by serial dilutions to determine optimal conditions and negative controls (usually secondary antibody alone) to ensure specificity (data not shown).
Other Reagents
Phadebas Amylase Assay kit was purchased from Magle Life Sciences (Lund, Sweden). Cholecystokinin (CCK-8) was purchased from Research Plus (Barnegat, NJ). Dulbecco’s minimal essential medium (DMEM), essential amino acids, fetal bovine serum (FBS), TrypLE Express, penicillin and streptomycin, Alexa-conjugated phalloidin, Image-iT FX Signal Enhancer, and Prolong gold antifade reagent with 4′,6-diamidino-2-phenylindole were purchased from Invitrogen. Bovine serum albumin, 12-O-tetradecanoylphorbol-13-acetate, and a protease inhibitor cocktail containing AEBSF, aprotinin, EDTA, leupeptin, and E64 were purchased from Calbiochem (San Diego, CA). Protein determination reagent and nonfat dry milk were purchased from Bio-Rad Laboratories (Hercules, CA). DNA Maxi, Mini-prep kits, polymerase chain reaction reagents, and CytoTox 96 were purchased from Promega (Madison, WI). The QuikChange XL Site-Directed Mutagenesis Kit, restriction enzymes were purchased from Fermentas (Vilnius, Lithuania). Trypsin substrate Boc-Glu-Ala-Arg-MCA, and cathepsin B substrate Arg-Arg-MCA were purchased from Peptides International (Louisville, KY). Formaldehyde and Tissue Tek were purchased from Electron Microscopy Sciences (Hatfield, PA). SuperSignal West Femto Chemiluminescent Substrate was purchased from Thermo Scientific (Waltham, MA). YM201636 was purchased from Chemdea (Ridgewood, NJ). Apilimod was purchased LGM Pharma (Boca Raton, FL).
Adenovirus Production
PIKfyve wild-type (WT) was a kind gift from Dr. Louis Weisman (University of Michigan). Point mutations of PIKfyve WT were generated by substituting lysine residue 1877 with glutamate using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and were confirmed by DNA sequencing. The HA-tagged PIKfyve WT and K1877E adenoviruses were produced and purified by Vector BioLabs (Malvern, PA). Canine HA-Rab5 WT, S34N, and Q79L were a kind gift from Brian P. Ceresa (University of Louisville). Production of adenovirus was performed using Adeno-X Adenoviral System 3 (Clonetech, Mountain View, CA). Enhanced green fluorescent protein (EGFP)-Rab11a WT, Q70L, and S25N adenovirus were a kind gift from Rejji Kuruvilla (Johns Hopkins University). Harvested adenovirus from AD-293 was purified and concentrated by cesium chloride centrifugation. Adenovirus titer was determined by plaque assay using agarose overlay on AD-293 cells. The effects of adenoviruses on acinar damage was assessed by lactate dehydrogenase (LDH) leakage (Table 1)
Table 1.
Active PIKfyve Enhances Acinar Injury Assayed by LDH Release, and Inactive PIKfyve or Active Rab5 Reduce Injury
| Time and Treatment | LDH Release (% of Total) Mean ± SE |
|---|---|
| Fresh | |
| Basal | 0.82 ± 0.18 |
| 10 nM CCK | 6.63 ± 0.70a |
| 6 h | |
| GFP | 1.11 ± 0.41 |
| PIKfyve WT | 2.33 ± 0.72 |
| PIKfyve K1877E | 1.69 ± 0.34 |
| 16 h | |
| Control | 0.85 ± 0.31 |
| GFP | 1.17 ± 0.25 |
| PIKfyve WT | 8.81 ± 1.34a |
| PIKfyve K1877E | 0.83 ± 0.11 |
| Rab5 WT | 0.56 ± 0.19 |
| Rab5 Q79L | 1.31 ± 0.33 |
| Rab11a WT | 1.42 ± 0.30 |
| Rab11a Q70L | 0.69 ± 0.11 |
| Rab11a S25N | 1.03 ± 0.16 |
| 24-h collagen | |
| GFP | 1.13 ± 0.13 |
| PIKfyve WT | 2.46 ± 0.47 |
| PIKfyve K1877E | 1.31 ± 0.12 |
Note: Activity present in media after 3 hours of incubation of acini and expressed as percentage of total cellular LDH activity. As a positive control, acini were stimulated with 10 nM CCK for 3 hours to induce cell damage, resulting in a sixfold increase in LDH leakage. CCK, cholecystokinin; GFP, green fluorescent protein; LDH, lactate dehydrogenase; PIKfyve, phosphatidylinositol 3-phosphate 5-kinase; SE, standard error; WT, wild type.
P < .05, n = 3 independent experiments each performed in duplicate.
Short-Term Culture of Pancreatic Acini
The University of Wisconsin Committee on Use and Care of Animals approved all studies involving animals. Pancreatic acini were isolated from male Harlan Sprague-Dawley rats and C57BL/6 mice by collagenase digestion as previously described.13 Culturing of acini was performed similarly to the method described.14 In brief, isolated acini were cultured in 8–10, 100 × 15 mm plastic petri dishes in 20 mL DMEM supplemented with 0.5% FBS, 0.02% soybean trypsin inhibitor, and penicillin and streptomycin for 16 hours, unless otherwise noted, at 37°C, 5% CO2 in a humidified atmosphere. Adenoviruses were added to acini at specified titers in the culture medium for 6 or 16 hours of incubation. At 16 hours, greater than 99% of acini expressed green fluorescent protein (GFP; data not shown). For the 24-hour culture on collagen, the mouse acini were cultured as previously described elsewhere.15
Immunofluoresecence Analysis of Phosphatidylinositol-3,5-Bisphosphate Levels
Immunofluorescence microscopy was conducted on cryostat sections of rat pancreatic lobules that were treated 30 minutes with YM201636 ± CCK-8 (30 minutes). The PIP(3,5) antibody was from Eschelon (1:10; cat. no. ZP035). Secondary was anti-mouse Alexa 488 (1:100). Actin was stained with phalloidin-Alexa 647. We used 4′,6-diamidino-2-phenylindole to stain nuclei. Quantification was performed previously reported elsewhere.12 All experiments were conducted on at least three separate tissue preparations.
Cell Surface Labeling of LAMP1
For external cell-surface labeling, fresh acini were treated ±1 μM YM201636 for 30 minutes followed by CCK for 5 minutes at 37°C. LAMP1 labeling was performed and quantified as previously described elsewhere.14
Electron Microscopy
Rat pancreatic lodules were fixed in 2% formaldehyde/2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at 4°C. Following rinsing in phosphate buffer, lobules were incubated in 2% osmium in 0.1M phosphate buffer overnight at room temp with gentle agitation. Lobules were exposed to a series of ethanol dehydrations done in a microwave at 45°C, followed by polyprolylene dehydration at room temp and placed in a 50:50 mix of EPON and polypropylene overnight at room temp with gentle agitation. Epon was exchanged three times at 45°C followed by a final exchange at 60°C in a microwave (30 minutes each) before final embedding. Sections (80 nm) were evaluated with a Philips CM 120 electron microscope. Captured images were converted to TIFF files and edited for publication in Adobe PhotoShop (Adobe Systems, Mountain View, CA).
Assays
For YM201636 and apilomod studies, acini were preincubated in HEPES buffer containing agents for 30 minutes in a 37°C water bath with gentle agitation before the acinar secretory assays were performed in the presence of inhibitor or vehicle (0.1% dimethyl sulfoxide [DMSO]). For the protease activity assays, acini were treated as control or hyperstimulated with 10 nM CCK-8 for 30 minutes. Trypsin and cathepsin B activities were measured fluorometrically using a method described previously elsewhere.16, 17 The acinar lysates, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting analyses were conducted as described elsewhere.18, 19
To measure LDH release, acini were pretreated with 0.1% DMSO or 10 μM YM201636 for 15 minutes then left untreated or stimulated with 10 nM CCK-8 for 3 hours. The supernatant was collected from the final 3 hours of culture and was analyzed for LDH activity using a procedure previously described elsewhere.20
Human Acini
Human acinar cells were obtained from cadaveric donors as a byproduct of pancreatic islet isolation conducted at the University of Wisconsin Department of Surgery, Islet Research Laboratory. Human pancreata were obtained through the University of Wisconsin Hospital and Clinics Organ Procurement Organization with consent obtained for research from next of kin and authorization by the Health Sciences institutional review board. Islets were isolated using a modification of the automated Ricordi method.21 Collagenase NB1, neutral protease (Serva Electrophoresis, Heidelberg, Germany), and DNase (Roche Applied Science, Indianapolis, IN) were infused into the main pancreatic duct. Islets were separated from acinar tissue by centrifugation on a continuous Biocoll gradient (Biochrom, Berlin, Germany) in a COBE 2991 cell processor (Lakewood, CO). Acinar cells were washed in DMEM and further purified by allowing to pellet by gravity though DMEM containing 4% bovine serum albumin. Cells were treated as described for rodent acini.
Statistical Analysis
All data are the mean and standard error (SEM) of at least three separate tissue preparations performed in triplicate. P values were calculated using analysis of variance (ANOVA) and post-hoc paired Student t test. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Inhibition of PIKfyve Enhances Acinar Secretion
In contrast the classic ZG pathway, the CLP uses anterograde endosomal trafficking through EE and RE to mediate low-level secretion (see Figure 1A). The EE serves as a sorting compartment that also gives rise to the LE. Maturation of the EE to LEs requires the formation PI(3,5)P2. Of the three classes of PI3-kinase enzymes, class I, which generates PI(3,4,5)P3 from PI(4,5)P2, and class III (Vps34), which generates PI(3)P from PI, are both inhibited by wortmannin and LY294002 (see Figure 1B). PI(3,5)P2 is generated from PI(3)P in the EE by the single enzyme PIKfyve.
Inhibition of PIKfyve can be achieved using the inhibitors YM201636 and apilimod; these have a greater than 100-fold and 1000-fold higher selectivity for PIKfyve than other PI kinases, respectively.22, 23 Because LEs and lysosomes have been implicated as the site of zymogen activation during acute pancreatitis, we investigated how altering EE to LE trafficking by inhibition of PIKfyve would affect acinar cell function.
PIKfyve is absent from purified ZGs enriched in vesicle-associated membrane protein 8 (VAMP8) but is present in microsomal fractions positive for lysosome-associated protein LAMP1 (see Figure 1C). Inhibition of PIKfyve with YM201636 has a biphasic effect on maximal (100 pM) and supramaximal (10 nM) CCK-8–stimulated secretion, identical to its effects on neuroendocrine cells.24 Concentrations up to 1 μM augment both basal (see Figure 1D) and stimulated (Total − Basal) secretion (see Figure 1E), whereas 10 uM augments only basal secretion.
PIKfyve inhibition enhanced secretion over a full range of CCK-8 concentrations and in response to thapsigargin, phorbol ester, CPT-cAMP, or their simultaneous additions, indicating it does not acutely effect CCK receptor activation (not shown). Quantification of PI(3,5)P2 levels was estimated using immunofluorescence microscopy of cryostat sections of pancreatic lobules treated with YM201636 ± CCK-8 (see Figure 1F). PI(3,5)P2 was detected throughout the cytoplasm, and as anticipated was also observed in nonacinar cell types, presumably nerve, duct, stellate, and islet cells. Stimulation with low (30 pM) or supramaximal (10 nM) CCK-8 increased PI(3,5)P2 levels by approximately 40% whereas YM201636 strongly reduced basal and stimulated levels, as has been reported in skeletal muscle.25
Finally, inhibition of PIKfyve in rat acini with apilimod (150 nM) significantly augmented basal, maximal, and supramaximal CCK-8–stimulated secretion (see Figure 1G) whereas inhibition of PI3-kinases with wortmannin or LY294002 had no effect, as reported elsewhere.6, 7, 8 Likewise, blocking EE to LE trafficking using the vacuolar H+-ATPase inhibitor bafilomycin A1 similarly elevated basal, maximal, and supramaximal CCK-8–stimulated secretion as reported using monensin or chloroquine to disrupt lysosomal pH.26
Early Endosome Trafficking Regulates Amylase Secretion
To confirm the secretory effects of these pharmacologic agents, acinar PI(3,5)P2 levels were acutely modulated using adenoviral expression of human HA-PIKfyve WT or the kinase-dead mutant K1877E, which has a potent dominant negative effect on endogenous enzyme activity.27, 28 Based on the inhibitor studies, we anticipated expression of WT PIKfyve would increase and the dominant negative K1877 mutant decrease secretion (Figure 2A). Enhanced expression of HA-PIKfyve WT in acinar suspension cultures was lethal at periods >8–12 hours (see Table 1); however, 6-hour expression did not significantly elevate LDH release from cells and resulted in a 30% reduction in maximal and supramaximal CCK-stimulated secretion (see Figure 2B). At 6 hours, dominant negative HA-PIKfyve K1877E tended toward increasing secretion. However, robust expression at 16 hours resulted in a threefold increase in basal secretion but had no effect on stimulated secretion if basal was subtracted from total values (see Figure 2C), as seen with high levels of YM201636 (see Figure 1D and E). These results demonstrate that increasing EE to LE trafficking by activation of PIKfyve inhibits secretion whereas PIKfyve inhibition increases secretion.
Figure 2.
Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve)-mediated endosomal trafficking regulates amylase secretion. (A) Diagram depicting that increased expression of wild-type (WT) PIKfyve enhances early endosome (EE) to late endosome (LE) trafficking and reduces anterograde trafficking from the EE, whereas dominant negative PIKfyve K1778 has the opposite effect. (B) Total amylase secretion (Basal + Stimulated) from rat acini cultured 6 hours with 106 pfu/mL adenovirus coding for green fluorescent protein (GFP), PIKfyve WT, or dominant-negative PIKFyve K1877E and treated as basal or with maximal (100 pM) and supramaximal (10 nM) CCK-8. WT PIKfyve expression significantly reduced secretion. (C) Rat acini cultured as in B but for 16 hours. Dominant negative PIKfyve K1877E significantly enhanced basal secretion. HA-PIKfyve K177E expression was confirmed by immunoblotting (top gels).
We alternatively manipulated EE to LE progression by targeting Rab5, which when activated by guanosine triphosphate (GTP) binding promotes formation of the EE compartment by controlling EE homotypic fusion. Rab5-GTP hydrolysis is also essential for the transition from EE to LE, and introduction of a hydrolysis-resistant GTP-trapped Q79L mutant can be used to inhibit EE to LE maturation (Figure 3A).29 In acini, Rab5-GTP Q79L significantly enhanced basal but not maximal CCK-stimulated secretion calculated by subtracting basal from total secretion (see Figure 3B). In comparison, expansion of the EE compartment by expression of Rab5 WT (expressed at slightly higher levels than the Q79L) enhanced both basal and CCK-8-stimulated secretion. Note that endogenous Rab5 expression is lost during 16-hour acinar culture. We recently demonstrated that rescue of Rab5 expression during acinar culture restores the acinar endosomal system, which is necessary for a robust secretory response.14 Importantly, although Rab5 WT, Q79L, and PIKfyve K1877E expression all elevated basal secretion, they had no effects on LDH leakage from cells, indicating that the increased amylase release is not secondary to cell damage (see Table 1).
Figure 3.
Altering Rab5- or Rab11-mediated endosomal trafficking controls acinar secretion. (A) Diagram demonstrating that overexpression of wild-type (WT) or GTP-locked Rab5 Q79L inhibits early endosome (EE) to late endosome (LE) maturation and enhances anterograde endosomal trafficking. (B) Acini were cultured 16 hours with 106 pfu/mL adenovirus coding for green fluorescent protein (GFP), Rab5 WT, or active Rab5 Q79L. Endogenous (end) and exogenous canine Rab5 levels were determined by immunoblotting (top). Both Rab5 constructs enhanced basal and total secretion (Basal + Stimulated) compared with GFP control. (C) Diagram demonstrating that GTP-locked Rab11a Q70L enhances and GDP-locked Rab11a S24N decreases anterograde endosomal trafficking. Acini were cultured 16 hours with 105 pfu/mL adenovirus expressing GFP, EGFP-Rab11a WT, active GTP-locked Q70L, or GDP-locked inactive S25N. Expression was determined by immunoblotting (top). Rab11a Q70L enhanced and Rab11a S24N decreased the basal and total CCK-8 stimulated secretion. (E) Data from 16-hour cultures in Figures 2C, 3B, and 3D plotted to show basal and stimulated (Total – Basal) secretion. Agents that increase EE to LE trafficking enhance only basal secretion, whereas agents that alter EE to RE trafficking modulate basal and stimulated secretion. *P < .05 (n = 3) compared with control GFP levels.
Anterograde trafficking from the EE to RE is dependent on Rab11a, which is localized to the apical domain of the acinar cell and has an important role in epithelial polarity.14, 30 Inhibition of EE to LE trafficking by Rab5-GTP expression does not impact Rab11a-dependent plasma membrane recycling via the RE.31 To promote EE to RE trafficking, a constitutively active GTP-trapped Rab11a mutant, Q70L, was used and conversely a dominant negative guanosine diphosphate (GDP)-trapped S25N mutant was used to block EE to RE trafficking (see Figure 3C).32 Endogenous Rab11a expression remains relatively stable during 16 hours of acinar culture (see Figure 3D). Expression of EGFP-Rab11a WT or Rab11a-GTP Q70L does not impact endogenous Rab11a levels, but Rab11a-GDP S25N down-regulates it. Strikingly, Rab11a-GTP Q70L enhanced both basal and maximal-CCK-8 stimulated secretion, and conversely Rab11a-GDP S25N reduced both basal and maximally stimulated secretion. Collectively, these results demonstrate that molecular approaches that inhibit EE to LE maturation (PIKfyve K1877E and Rab5 Q79L) mainly promote basal secretion, whereas approaches that influence anterograde endosomal trafficking (Rab5 WT, Rab11a Q70L, and Rab11a S24N) impact both basal and stimulated secretion (see Figure 3E).
Inhibition of PIKfyve Enhances LAMP1 Externalization
We recently reported that cell-surface labeling of LAMP1, which is absent from ZG, may be used as a marker of endolysosomal exocytosis in acinar cells.14, 33 As a significant portion of post-Golgi LAMP1 traffics from EE to LE and lysosomes, EE to LE progression was blocked by PIKfyve inhibition, resulting in a significant increase in basal LAMP1 surface labeling of apical membrane (Figure 4). LAMP1 labeling is greatly enhanced after 5 minutes maximal CCK-8 (100 pM) stimulation and is almost fully inhibited by supramaximal (100 nM) stimulation. PIKfyve inhibition did not impact LAMP1 exocytosis by maximal CCK-8, but completely reversed the inhibitory effect of supramaximal CCK-8. These data suggest that early during CCK-8 hyperstimulation, apical endolysosomal exocytosis is inhibited and by default increases trafficking from the EE to LE and lysosome.
Figure 4.
Inhibition of phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) augments endolysosomal exocytosis. Lysosome-associated membrane protein 1 (LAMP1) surface labeling was measured in nonpermeabilized rat acini preincubated 30 minutes with 0.1% dimethyl sulfoxide as control or 10 μM YM201636. Cells were treated as basal or stimulated for 5 minutes with maximal (100 pM) or supramaximal (100 nM) CCK-8 (cholecystokinin-1). Lower panel is a quantification of pixel intensity under each condition. *P < .05 (n = 3).
Inhibition of PIKfyve Decreases Early Markers of Acinar Pancreatitis in Vitro
PIKfyve inhibition with YM201636 caused a concentration-dependent decrease in both basal and supramaximal CCK-stimulated intracellular trypsin levels by >95% at 10 μM YM201636 (Figure 5A). PIKfyve inhibition also strongly reduced intracellular trypsin accumulation in response to the bile salt, taurolithocholic acid 3-sulfate (TLC), and the nicotine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a major toxin in cigarette smoke (see Figure 5B). Similar results were also achieved with apilimod and the H+-ATPase inhibitor bafilomycin A1 (see Figure 5C). Consistent with reduced zymogen activation, PIKfyve inhibition significantly attenuated acinar necrosis in response to supramaximal CCK-8, as determined by LDH leakage from cells in response to these models of acinar pancreatitis (see Figure 5D).
Figure 5.
Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) inhibition blocks intracellular trypsin accumulation during acinar pancreatitis. (A) Rat acini were pretreated for 30 minutes with 0.1% dimethyl sulfoxide (DMSO) as control, or indicated concentrations of YM201636 before we measured the intracellular trypsin activity under basal or supramaximal (10 nM) cholecystokinin (CCK-8)-stimulated conditions (30 minutes). (B) Rat acini were pretreated 30 minutes with 0.1% DMSO as control or with 10 μM YM201636 then stimulated with supramaximal CCK-8 (10 nM), 500 μM taurolithocholic acid 3-sulfate (TLC), or 100 nM 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) for 30 minutes before the intracellular trypsin activity was measured. (C) Rat acini were pretreated 30 minutes with 0.1% DMSO as control or with the PIKfyve inhibitors YM201636 (10 μM) and apilimod (Apil) (150 nM), or the H+-ATPase inhibitor bafilomycin A (BafA1) (1 μM) followed by supramaximal CCK-8 (10 nM) for 30 minutes before the intracellular trypsin activity was measured. (D) Rat acini were treated as in B, and the leakage of lactate dehydrogenase activity after 3 hours of stimulation was quantified as a measure of acinar necrosis. (E) Mouse acini were cultured for 24 hours on collagen-coated plates with 105 pfu/mL adenovirus expressing green fluorescent protein (GFP), PIKfyve wild type (WT), or dominant negative K1877E and either were left untreated (basal) or were stimulated with supramaximal (10 nM) CCK-8 for 30 minutes. HA-PIKfyve levels were determined by immunoblotting (top). A–D: *P < .05 compared with DMSO-treated control cells; E: *P < .05 compared with GFP control.
Unlike suspension cultures of rat acini, mouse acini cultured for 24 hours on collagen-coated plates in the presence of FBS and isobutyl methyl xanthine maintain secretion and supramaximal CCK-induced intracellular trypsinogen activation.15 Increasing cellular PI(3,5)P2 levels by enhanced expression of PIKfyve WT elevated basal and greatly potentiated trypsin accumulation in response to supramaximal stimulation (see Figure 5). Accordingly, dominant negative PIKfyve K1877E fully blocked trypsinogen activation in response to supramaximal CCK-8. Together these data demonstrate that enhanced EE to LE trafficking is required for intracellular trypsin accumulation.
Investigation of the EE to LE pathway in human pancreatic acini was conducted after isolation of cells from cadaveric donors. Human acini showed a biphasic amylase secretory response to maximal (10 μM) and supramaximal (1 mM) concentrations of the muscarinic agonist carbachol (Figure 6A). Similar to rodent cells, PIKfyve inhibition elevated only basal secretion and fully prevented the secretory inhibition caused by a supramaximal carbachol. Importantly, inhibition of PIKfyve in human acinar cells likewise blocked premature intracellular trypsinogen activation induced by supramaximal carbachol (see Figure 6B).
Figure 6.
Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) inhibition prevents supramaximal carbachol-induced inhibition of amylase secretion and intracellular trypsin accumulation in human acini. (A) Isolated human acini were pretreated for 30 minutes with 10 μM YM201636 or 0.1% dimethyl sulfoxide (DMSO) as a control and stimulated with maximal (10 μM) or supramaximal (1 mM) carbachol (CCh) for 30 minutes before the total amylase secretion was measured. PIKfyve inhibition strongly enhanced basal secretion. (B) Human acini were treated as in A, and the intracellular trypsin activity was determined. PIKfyve inhibition significantly reduced trypsin activity to basal levels in response to supramaximal CCh. *P < .05 (n = 3), comparing control with YM201636 treatment under each condition.
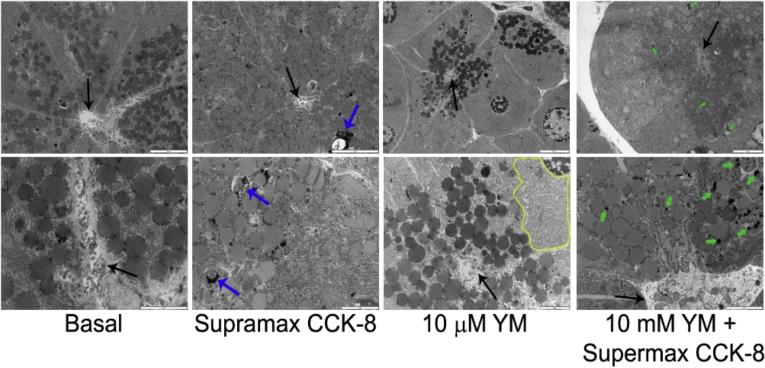
Analysis of acinar morphology by electron microscopy of thin sections of rat pancreatic lobules treated with supramaximal CCK-8 (100 nM) for 30 minutes revealed an enhanced appearance of autophagic vacuoles compared with basal conditions (Figure 7). After 30 minutes preincubation with YM201636, some minor dilation of the endoplasmic reticulum was noted (in figure, outlined with yellow line). Preincubation with YM201636 followed by supramaximal CCK-8 appeared to reduce the presence of autophagic vacuoles. Additionally, the appearance of numerous electron-dense lysosomes in YM-treated cells (in figure, green arrows) suggested that acute inhibition of EE to LE trafficking did not prevent lysosome formation.
Figure 7.
Ultrastructural analysis of acinar cells after phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) inhibition. Rat pancreatic lobules were treated with 10 μM YM201636 or 0.1% dimethyl sulfoxide (DMSO) as a control for 30 minutes followed by stimulation with supramaximal (100 nM) cholecystokinin (CCK-8) for 30 minutes. Electron micrographs were obtained from 80-nm thick osmium-stained sections. Black arrows indicate acinar lumen; blue arrows indicate autophagic vacuoles and/or fusion of a multivesicular endosome with a lysosome; the yellow line outlines the slightly dilated endoplasmic reticulum; green arrows indicate lysosomes. Top row, scale line = 5 μm; bottom row, scale line = 2 μm.
PIKfyve Inhibition Does Not Prevent Cathepsin B Activation
The absence of intracellular trypsin accumulation after PIKfyve inhibition suggests that 1) trypsinogen activation did not occur, 2) it was activated and secreted,20 or 3) it was activated and rapidly degraded by cathepsin L.15, 34 We found no evidence of trypsin activity or activated carboxypeptidase immunoreactivity in the extracellular medium after PIKfyve inhibition and high-dose CCK-8 stimulation (data not shown), supporting that activated zymogens were not secreted from cells.
As intracellular trypsinogen activation is thought to be mediated by lysosomal cathepsin B,1, 35 we investigated cathepsin B maturation and activity. Supramaximal caerulein stimulation of acini is known to result in a twofold increase in cathepsin B activity but a greater than 50% reduction in the level of cathepsin B protein.36 We likewise detected a twofold increase in intracellular cathepsin B activity at 30 minutes in response to supramaximal CCK-8 (Figure 8A). Unexpectedly, PIKfyve inhibition alone elevated basal cathepsin B activity independent of CCK-8. Accordingly, cathpsin B maturation at 60 minutes under basal conditions was greatly increased by PIKfyve inhibition; moreover, the reduction in enzyme seen in response to high-dose CCK-8 was blocked (see Figure 8B and C). Together these data suggest acutely blocking EE to LE trafficking by PIKfyve inhibition might enhance zymogen trafficking to the plasma membrane and prevent mixing with activated lysosomal hydrolases rather than inhibiting cathpesin B-catalyzed trypsinogen activation. However, we cannot rule out a coincident induction of cathepsin L and rapid degradation of trypsin.15
Figure 8.
Inhibition of phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) enhances intracellular cathepsin B activity and maturation. (A) Isolated rat acini were pretreated 30 minutes with 10 μM YM201636 or 0.1% dimethyl sulfoxide (DMSO) as a control before treatment as basal or with supramaximal (10 nM) CCK-8 stimulation. Intracellular cathepsin B activity was measured using a fluorogenic substrate. (B, C) Isolated rat acini were treated as in A but stimulated for 60 minutes, and cathepsin B maturation was determined by immunoblotting and densitometry. *P < .05 (n = 3) compared with control in panel C.
Discussion
The CLP and MRP were previously identified in acini by their release of newly synthesized enzymes during pulse-chase labeling studies11, 37 (Figure 9A). The CLP and MRP are proposed to originate from vesicles that bud off the TGN and immature secretory granules, and traffic to an EE compartment. The CLP was proposed to enter a RE intermediate before secretion, whereas the MRP directly undergoes secretion in response to low-level stimulation.14 However, whether the CLP and MRP use distinct trafficking routes is unclear; we therefore refer here mainly to the CLP when describing anterograde endosomal trafficking. We previously measured endolysosomal exocytosis in acini by cell-surface labeling of externalized LAMP1, an EE, LE, and lysosomal protein that is absent from purified ZGs.14, 33 Based in part on the sensitivity of this pathway to the Golgi-disrupting agent brefeldin A (BFA), it was proposed to represent the CLP and MRP.
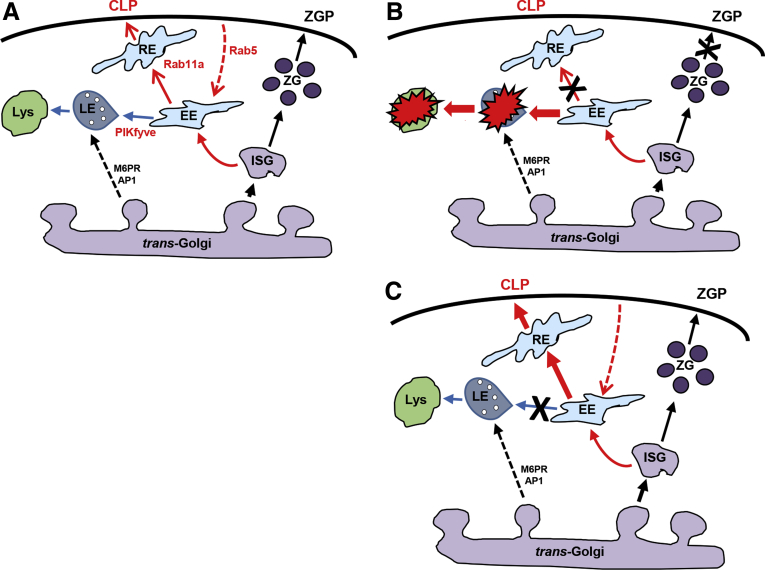
Figure 9.
Anterograde endosomal trafficking in acinar cells. (A) In the classic zymogen granule pathway (ZGP), immature secretory granules (ISG) bud from the trans-Golgi and mature by selective removal of membrane proteins and small amounts of zymogens carried in bulk that enter the early endosomal (EE) compartment. This gives rise to the constitutive-like pathway (CLP), which under basal conditions further traffics via recycling endosomes (RE) to the apical plasma membrane. The EE also give rise to the late endosome (LE), and this step is dependent on phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) activity. Lysosomal proteins containing mannose-6-phosphate (M6P) are also delivered directly to the LE and lysosome from the trans-Golgi by adaptor protein 1 (AP1)-dependent clathrin-coated vesicle formation. Rab5 controls formation of the EE, and Rab11a mediates trafficking from the EE to RE. (B) Early during pancreatitis, secretory inhibition occurs (indicated by X), and zymogens trafficking from EE to LE compartments is enhanced, presumably leading to enhanced zymogen activation. (C) Inhibition of EE to LE trafficking enhances CLP secretion and reduces zymogen activation, potentially by preventing zymogen mixing with lysosomal hydrolases in LE and lysosomes. Note that the minor-regulated pathway (MRP), which is proposed to traffic from EE to plasma membrane, is not shown.
We recently reported that CLP trafficking is dependent on Rab5 and its associated binding proteins D52 and EEA1.12 Moreover, trafficking within the CLP was shown to be essential to maintain VAMP8-mediated ZG secretion, which comprises 50% of the overall secretory response. Our current study expands these findings by demonstrating that inhibition of EE to LE and EE to TGN trafficking by PIKfyve inhibition likewise promotes acinar secretion. Whether this enhanced secretion is a consequence of endolysosomal vesicle exocytosis or represents fusion of these vesicles with VAMP8 containing zymogen granules before exocytosis is currently under investigation.12
Evidence that pharmacologic and molecular inhibition of PIKfyve or expression of Rab5-GTP Q79L to inhibit EE to LE/lysosome trafficking enhanced secretion, and that altering Rab11a activity to modulate anterograde endosomal trafficking likewise controlled secretion, clearly underscores the importance of endosomal trafficking in shaping the acinar secretory response. Taken together with the LAMP1 surface-labeling studies, these findings suggest that during supramaximal acinar stimulation, the combination of apical secretory inhibition and activation of PIKfyve results in enhanced zymogen trafficking from EE to LE/lysosomes, thereby promoting trypsinogen cleavage by cathepsin B and the retention of activated trypsin (see Figure 9B). Conversely, inhibition of PIKfyve enhances anterograde trafficking and zymogen secretion in turn, preventing intracellular trypsinogen activation (Figure 9C).
The initial site of trypsinogen activation measured by trypsin-activated peptide immunoreactivity on electron micrographs was shown to occur in small vesicles adjacent to ZGs.38 Others using biochemical fractionation and immunoelectron microscopy demonstrated activated trypsin and trypsin-associated peptide localization occurs in large cytoplasmic vacuoles after supramaximal stimulation.39 Further, active trypsin is detected in a compartment containing lysosomal enzymes including cathepsin B,1 and colocalizes with LAMP1.1, 40 Organelle acidification by the vacuolar ATPase is required for cathpesin B maturation/activation.41 Blocking cathepsin B activation by inhibition of vacuolar ATPases in various cell types also blocks EE to LE/lysosome trafficking and redirects cargo to the RE before its exocytosis.42 Accordingly, we show that, just as with PIKfyve inhibition, treatment of acini with baflomycin enhanced acinar secretion. These findings indicate that the protective effects of vacuolar ATPase inhibitors against zymogen activation are due, at least in part, to an inhibition of EE to LE/lysosome trafficking as well as the inhibition of lysosome acidification.
Acute inhibition of PIKfyve enhanced intracellular cathepsin B maturation and activity, consistent with a similar study reporting that PIKfyve inhibition does not block lysosome hydrolase maturation.43 A recent study reported that PI(3,5)P2 promotes assembly of the vacuolar ATPase in yeast, although the extent to which PIKfyve inhibition prevents organelle acidification is uncertain.44 Short-term PIKfyve inhibition did not prevent but enhanced cathepsin B activation/maturation, which likely reflects that the enzyme is sorted from the TGN directly to the LE/lysosome via a mannose-6-phosphate receptor and adaptor protein 1 (AP1)-dependent clathrin coat assembly pathway (Figure 9). In comparison, lysosomal membrane proteins are controlled by AP3 and traffic through EE and LE intermediates.45 This suggests that zymogens including trypsinogen may be trafficked by the AP3 pathway during formation of the CLP and MRP. Interestingly, BFA, which inhibits both AP1- and AP3-dependent trafficking, blocks colocalization of zymogens with lysosomal hydrolases and trypsinogen activation. Moreover, BFA did not acutely reduce total cellular cathepsin B activity or protein levels, further supporting that blocking zymogen trafficking to the lysosome effectively inhibits intracellular activation.36
PIKfyve has a differential sensitivity to inhibition with respect to EE to TGN versus EE to LE trafficking. PIKfyve small-interfering RNA knockdown by 70% inhibits trafficking from the EE to TGN but leaves LE maturation unaffected.9 In comparison, dominant negative PIKfyve or a maximal concentration of YM201636 blocks both EE to TGN as well as LE trafficking.9 High-dose YM201636 or expression of dominant negative PIKfyve K1877E elevated basal but not stimulated secretion, whereas lower concentrations elevated both basal and stimulated secretion. The most effective inhibition of intracellular trypsin accumulation occurred with higher doses of YM201636, further implicating EE to LE trafficking. Likewise, constitutively active Rab5-GTP blocks LE maturation46 and in acinar cells enhanced basal but not stimulated secretion, suggesting that basal secretion of zymogens provide the protective effects of these agents.
A final consideration is that although a large body of evidence supports zymogen and lysosomal hydrolase colocalization as the trigger for trypsinogen activation, the identity of this “activation compartment” remains uncertain. Recent studies propose zymogen activation occurs as a consequence of enhanced autophagy during acute pancreatitis.47 Further, autophagy is ultimately impaired during acute pancreatitis due to lysosomal dysfunction involving cathepsin and LAMP1 and LAMP2 degradation. One possibility is that inhibition of anterograde endosomal secretion and consequent accumulation of cargo including zymogens in EE, LE, and lysosomes may contribute to lysosomal dysfunction. Clearly, a more comprehensive understanding of the extremely dynamic nature and functional overlap of membrane trafficking events shared by the endosomal and autophagic pathways should provide key insight into the pathobiology of acute pancreatitis.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by National Institutes of Health grants RO1DK07088, PO1DK098108, and USDA/HATCH WIS01583 (to G.E.G.); National Institutes of Health grants RO1DK54021, NIAAAR21 (AA020847), PO1DK098108, and a Veterans Administration Merit and Career Development Award (to F.S.G.); a City of Hope subcontract, and National Institutes of Health grant NIDDK 1UC4DK098085-01 (to L.A.F.); and National Institutes of Health grant T32DK007665 (to S.W.M. and E.K.J.).
References
- 1.Saluja A., Hashimoto S., Saluja M. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987;253:G508–G516. doi: 10.1152/ajpgi.1987.253.4.G508. [DOI] [PubMed] [Google Scholar]
- 2.Gukovsky I., Gukovskaya A.S., Blinman T.A. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 3.Saluja A.K., Lerch M.M., Phillips P.A. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 4.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 5.Toker A., Cantley L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 6.Gukovsky I., Cheng J.H., Nam K.J. Phosphatidylinositide 3-kinase gamma regulates key pathologic responses to cholecystokinin in pancreatic acinar cells. Gastroenterology. 2004;126:554–566. doi: 10.1053/j.gastro.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Singh V.P., Saluja A.K., Bhagat L. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387–1395. doi: 10.1172/JCI12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer L., Gukovskaya A.S., Young S.H. Phosphatidylinositol 3-kinase regulates Ca2+ signaling in pancreatic acinar cells through inhibition of sarco(endo)plasmic reticulum Ca2+-ATPase. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1200–G1212. doi: 10.1152/ajpgi.00212.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ho C.Y., Alghamdi T.A., Botelho R.J. Phosphatidylinositol-3,5-bisphosphate: no longer the poor PIP2. Traffic. 2012;13:1–8. doi: 10.1111/j.1600-0854.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- 10.Zolov S.N., Bridges D., Zhang Y. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci USA. 2012;109:17472–17477. doi: 10.1073/pnas.1203106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castle J.D., Castle A.M. Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci. 1996;109:2591–2599. doi: 10.1242/jcs.109.10.2591. [DOI] [PubMed] [Google Scholar]
- 12.Messenger S.W., Falkowski M.A., Thomas D.D.H. Vesicle associated membrane protein 8 (VAMP8)-mediated zymogen granule exocytosis is dependent on endosomal trafficking via the constitutive-like secretory pathway. J Biol Chem. 2014;289:28040–28053. doi: 10.1074/jbc.M114.593913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas D.D., Taft W.B., Kaspar K.M. CRHSP-28 regulates Ca(2+)-stimulated secretion in permeabilized acinar cells. J Biol Chem. 2001;276:28866–28872. doi: 10.1074/jbc.M102214200. [DOI] [PubMed] [Google Scholar]
- 14.Messenger S.W., Thomas D.D.H., Falkowski M.A. Tumor protein D52 controls trafficking of an apical endolysosomal secretory pathway in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2013;305:G439–G452. doi: 10.1152/ajpgi.00143.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mareninova O.A., Hermann K., French S.W. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawabata S., Miura T., Morita T. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem FEBS. 1988;172:17–25. doi: 10.1111/j.1432-1033.1988.tb13849.x. [DOI] [PubMed] [Google Scholar]
- 17.Mach L., Mort J.S., Glössl J. Maturation of human procathepsin B. Proenzyme activation and proteolytic processing of the precursor to the mature proteinase, in vitro, are primarily unimolecular processes. J Biol Chem. 1994;269:13030–13035. [PubMed] [Google Scholar]
- 18.Thomas D.D.H., Kaspar K.M., Taft W.B. Identification of annexin VI as a Ca2+-sensitive CRHSP-28-binding protein in pancreatic acinar cells. J Biol Chem. 2002;277:35496–35502. doi: 10.1074/jbc.M110917200. [DOI] [PubMed] [Google Scholar]
- 19.Kaspar K.M., Thomas D.D.H., Taft W.B. CaM kinase II regulation of CRHSP-28 phosphorylation in cultured mucosal T84 cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1300–G1309. doi: 10.1152/ajpgi.00534.2002. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri A., Kolodecik T.R., Gorelick F.S. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol. 2005;288:G235–G243. doi: 10.1152/ajpgi.00334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricordi C., Lacy P.E., Finke E.H. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 22.Jefferies H.B.J., Cooke F.T., Jat P. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9:164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai X., Xu Y., Cheung A.K. PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem Biol. 2013;20:912–921. doi: 10.1016/j.chembiol.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborne S.L., Wen P.J., Boucheron C. PIKfyve negatively regulates exocytosis in neurosecretory cells. J Biol Chem. 2008;283:2804–2813. doi: 10.1074/jbc.M704856200. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Lai Y.-C., Hill E.V. Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction-stimulated glucose uptake in skeletal muscle. Biochem J. 2013;455:195–206. doi: 10.1042/BJ20130644. [DOI] [PubMed] [Google Scholar]
- 26.De Lisle R.C., Williams J.A. Zymogen granule acidity is not required for stimulated pancreatic protein secretion. Am J Physiol. 1987;253:G711–719. doi: 10.1152/ajpgi.1987.253.6.G711. [DOI] [PubMed] [Google Scholar]
- 27.Sbrissa D., Ikonomov O.C., Deeb R. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem. 2002;277:47276–47284. doi: 10.1074/jbc.M207576200. [DOI] [PubMed] [Google Scholar]
- 28.Cabezas A., Pattni K., Stenmark H. Cloning and subcellular localization of a human phosphatidylinositol 3-phosphate 5-kinase, PIKfyve/Fab1. Gene. 2006;371:34–41. doi: 10.1016/j.gene.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Rink J., Ghigo E., Kalaidzidis Y. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Jing J., Prekeris R. Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity. Histol Histopathol. 2009;24:1171–1180. doi: 10.14670/hh-24.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceresa B.P., Lotscher M., Schmid S.L. Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(q79l)-induced changes in endosome geometry. J Biol Chem. 2001;276:9649–9654. doi: 10.1074/jbc.M010387200. [DOI] [PubMed] [Google Scholar]
- 32.Ren M., Xu G., Zeng J. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas D.D.H., Martin C.L., Weng N. Tumor protein D52 expression and Ca2+-dependent phosphorylation modulates lysosomal membrane protein trafficking to the plasma membrane. Am J Physiol Cell Physiol. 2010;298:C725–739. doi: 10.1152/ajpcell.00455.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wartmann T., Mayerle J., Kähne T. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology. 2010;138:726–737. doi: 10.1053/j.gastro.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halangk W., Lerch M.M., Brandt-Nedelev B. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlichenko L., Stolz D.B., Noel P. ADP-ribosylation factor 1 protein regulates trypsinogen activation via organellar trafficking of procathepsin B protein and autophagic maturation in acute pancreatitis. J Biol Chem. 2012;287:24284–24293. doi: 10.1074/jbc.M111.328815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arvan P., Castle J.D. Phasic release of newly synthesized secretory proteins in the unstimulated rat exocrine pancreas. J Cell Biol. 1987;104:243–252. doi: 10.1083/jcb.104.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otani T., Chepilko S.M., Grendell J.H. Codistribution of TAP and the granule membrane protein GRAMP-92 in rat caerulein-induced pancreatitis. Am J Physiol. 1998;275:G999–G1009. doi: 10.1152/ajpgi.1998.275.5.G999. [DOI] [PubMed] [Google Scholar]
- 39.Hofbauer B., Saluja A.K., Lerch M.M. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352–G362. doi: 10.1152/ajpgi.1998.275.2.G352. [DOI] [PubMed] [Google Scholar]
- 40.De Lisle R.C. Altered posttranslational processing of glycoproteins in cerulein-induced pancreatitis. Exp Cell Res. 2005;308:101–113. doi: 10.1016/j.yexcr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Waterford S.D., Kolodecik T.R., Thrower E.C. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem. 2005;280:5430–5434. doi: 10.1074/jbc.M413513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurtado-Lorenzo A., Skinner M., El Annan J. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- 43.Ikonomov O.C., Sbrissa D., Foti M. PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell. 2003;14:4581–4591. doi: 10.1091/mbc.E03-04-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S.C., Diakov T.T., Xu T. The signaling lipid PI(3,5)P2 stabilizes V1–V(o) sector interactions and activates the V-ATPase. Mol Biol Cell. 2014;25:1251–1262. doi: 10.1091/mbc.E13-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braulke T., Bonifacino J.S. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Rojas R., van Vlijmen T., Mardones G.A. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gukovskaya A.S., Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993–G1003. doi: 10.1152/ajpgi.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]