Abstract
Caffeine’s wakefulness-promoting and sleep-disrupting effects are well established, yet whether caffeine affects human circadian timing is unknown. Here we show that evening caffeine consumption delays the human circadian melatonin rhythm in vivo, and chronic application of caffeine lengthens the circadian period of molecular oscillations in vitro primarily via an adenosine receptor/cyclic AMP-dependent mechanism. In a double-blind, placebo controlled, ~49-day long within-subject study, we found the equivalent amount of caffeine as that in a double espresso 3 hours before habitual bedtime induced a phase delay of the circadian melatonin rhythm in humans by ~40 minutes. This magnitude of delay was nearly half of the magnitude of the phase-delaying response induced by exposure to 3-hours of evening bright-light (~3000 lux; ~7 Watts/m2) that began at habitual bedtime. Furthermore, using human osteosarcoma U2OS cells expressing clock gene luciferase reporters, we found a dose-dependent lengthening of circadian period by caffeine. By pharmacological dissection and siRNA knockdown we established that perturbation of adenosine receptor signaling, but not ryanodine receptor or phosphodiesterase activity, is sufficient to account for caffeine’s effects on cellular timekeeping. We also used a cyclic AMP biosensor to show that caffeine increased cyclic AMP levels, indicating that caffeine can influence a core component of the cellular circadian clock. Taken together, our findings demonstrate that caffeine influences human circadian timing and gives new insight into how the world’s most widely consumed psychoactive drug impacts upon human physiology.
Introduction
The circadian system is a key regulator of daily sleep-wakefulness timing, as well as other physiological and behavioral processes. The timing of endogenous circadian clocks can be shifted by environmental factors such as light, feeding, physical activity, and pharmacological agents. For example, bright light exposure around typical bedtime delays the master circadian clock and sleep timing in humans (1,2,3).
Caffeine is a methylxanthine that exerts its actions in part by opposing the effects of the neuromodulator adenosine via competitive binding to serpentine adenosine receptors (4,5) where it acts as an antagonist with inverse agonist activity (6,7). Caffeine binding to neuronal adenosine receptors causes release of excitatory neurotransmitters, and more generally results in changes to intracellular cAMP signaling and cellular metabolism across a wide range of cells and tissues (5,8). Caffeine also acts at several intracellular targets to modulate second messengers, including cyclic AMP (cAMP) signaling. First, caffeine competitively inhibits phosphodiesterases (PDEs), the enzymes that degrade cAMP; and thus at a cellular level, caffeine can stimulate cAMP-dependent signaling by more than one mechanism. Caffeine also binds to intracellular calcium-channel ryanodine receptors (RyR) leading to intracellular Ca2+ release (5). Whether caffeine influences circadian timing in humans is unknown.
Caffeine has been shown to lengthen the circadian period of conidiation rhythms in the red bread mold Neurospora crassa (9), the phototaxic rhythm of the green alga Chlamydomonas reinhardii (10), and the activity rhythm of the fruit fly Drosophila melanogaster (11). Caffeine can also phase shift the ocular compound action potential rhythm in the sea snail Bulla goudiana (12). Caffeine can acutely reduce levels of the pineal hormone melatonin in humans on the day of administration (13,14) and induce immediate-early gene expression (cFos) in the master circadian clock, the suprachiasmatic nucleus (SCN), in rodent models (15). Furthermore caffeine can advance and delay the phase of the electrical activity rhythm in SCN from isolated rat and hamster brain (16,17) and lengthen the period of the activity rhythms in mice, as well as the hPer2 rhythm in human osteosarcoma U2OS cells and the mPer2 rhythm in mouse NIH3T3 fibroblasts (18). These findings suggest that caffeine may also influence human circadian timing. Therefore, we first tested the hypothesis that evening caffeine consumption would phase delay the endogenous circadian melatonin rhythm. The onset of the melatonin rhythm, tested under constant conditions, is considered the most accurate and precise measure of circadian timing in humans. Melatonin is a hormone whose concentrations in bodily fluids are rhythmic and driven by the SCN. Melatonin is also the primary hormonal signal to the body of internal biological night and its onset initiates a physiological cascade that promotes sleep and associated physiological functions in humans. To understand caffeine’s activity at the cellular level, we also explored mechanisms by which caffeine affects circadian timing by examining its influence upon the circadian clock in human cells.
Results
Caffeine delays circadian melatonin phase in humans
We investigated how evening caffeine influences human circadian phase compared with evening exposure to broad spectrum bright-light, a potent environmental time cue for the human circadian clock (1,19). We also tested the combination of caffeine and bright-light to determine if together, they induced a greater phase shift than either alone. We conducted five approximately 49 day long, circadian phase-shifting trials using a sensitive within-subject design (Fig. S1). Circadian phase was examined under constant routine conditions (2,20; constant wakefulness, semi-recumbent posture, ambient temperature and dim light, with meals equally distributed across the circadian cycle in hourly snacks) on the day before and after exposure to four randomized, double-blind, placebo-controlled interventions: dim-light placebo (~1.9 lux; ~0.6 Watts/m2), dim-light caffeine (2.9 mg/kg body mass, equivalent to 200 mg caffeine in a 69 kg person), bright-light placebo (~3000 lux equivalent to approximately one-third of the maximal light exposure provided by light therapy devices; ~7 Watts/m2) and bright-light caffeine. Caffeine was administered 3 hours prior to participants’ habitual bedtime (Fig. 1), and the 3 hours of bright-light exposure began at habitual bedtime.
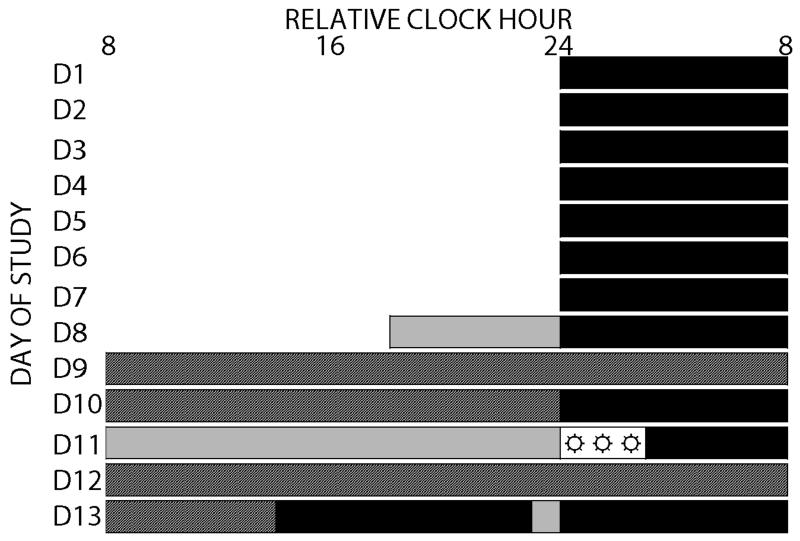
Fig 1. Protocol for human experiment.
After approximately seven days ambulatory monitoring, participants remained in an environment free of external time cues (days 8-13) under dim-light during scheduled wakefulness (~1.9 lux; ~0.6 Watts/m2) and darkness during scheduled sleep (black bars) in the laboratory. Example of in-laboratory procedures: Day eight included an 8-hour sleep opportunity. Days 9-10 consisted of a 40-hour constant routine (dark hashed grey bars). On day 11, participants received either caffeine or rice-powder filled placebo (pill symbol) 3-hour prior to habitual bedtime and 3-hour exposure (☼) to bright (~3000 lux; ~7 Watts/m2) or continued exposure to dim-light (light grey bars; ~1.9 lux; ~0.6 Watts/m2) beginning at habitual bedtime. Days 12-13 consisted of a 30-hour constant routine. Lab procedures were repeated four times across ~49 days (D1-49; Fig. S1). Relative clock hour shown with 2400h assigned to bedtime; actual times were dependent upon and relative to participant’s habitual bedtime.
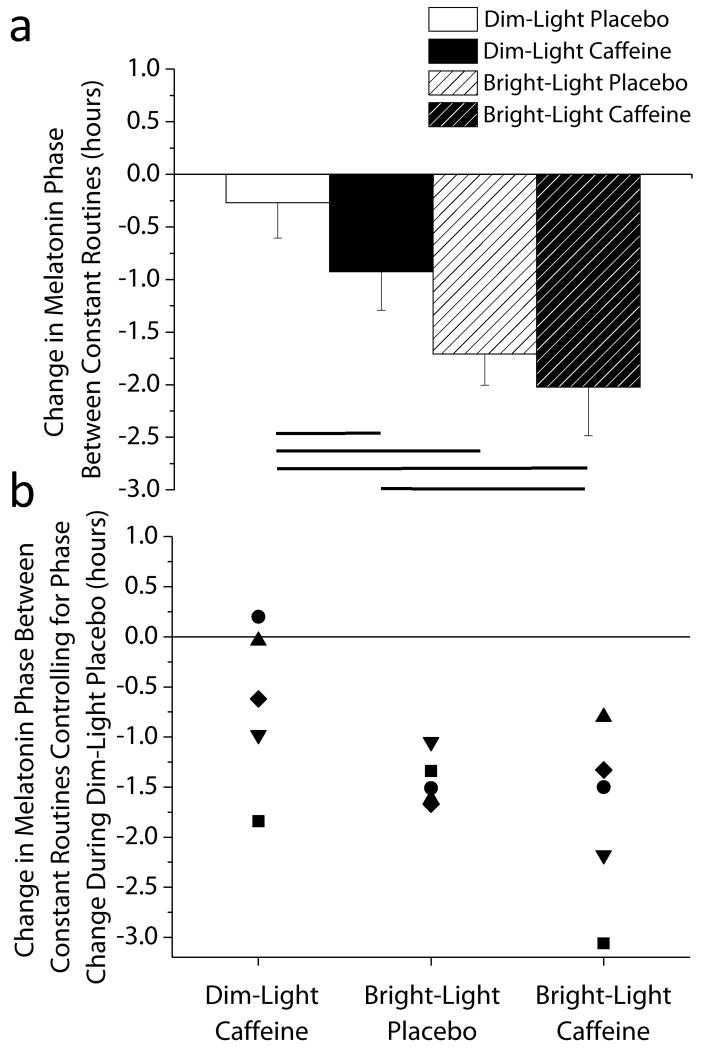
We found that the dim-light caffeine stimulus induced a significant phase delay that was ~40 minutes larger than for dim-light placebo—a large effect size (Cohen’s d= 0.93). Bright-light alone and bright-light caffeine induced phase delays of ~85 and ~105 minutes, respectively, more than dim-light placebo; these too were large effect sizes (d= 2.25 and 3.66) (Fig. 2A). Furthermore, bright-light caffeine significantly delayed circadian phase more than caffeine in dim light, also with a large effect size (d= 2.13) (Fig. 2A). No significant differences were observed between dim-light caffeine and bright-light placebo (p=0.07) or between bright-light placebo and bright-light caffeine (p=0.26), although effect sizes were large and medium (d= 1.17 and 0.79). Unexpectedly, bright-light caffeine did not induce a greater phase shift than bright-light placebo. It is possible that the light intensity used was saturating for the phase shifting response (21) and adding caffeine had no additional influence because of a ceiling effect. Thus, it will be important to test lower light intensities to determine whether caffeine potentiates phase shifts by light and also whether caffeine administered at the beginning of the light pulse has an influence on the induced phase shift.
Fig 2. Phase shifting response for each condition.
(A) Average phase shifts. Circadian phase delays are denoted as negative numbers and error bars represent standard error of mean. Lines represent significant differences between conditions at endpoints of the line (Dunnett’s test dim-light placebo versus dim-light caffeine p=0.011, versus bright-light placebo p=0.0007, versus bright-light caffeine p=0.0003). Data are mean ± SEM, n=5. (B) Individual differences in the phase shifting response controlling for phase change during the dim light-placebo control condition. Symbols represent individual subjects.
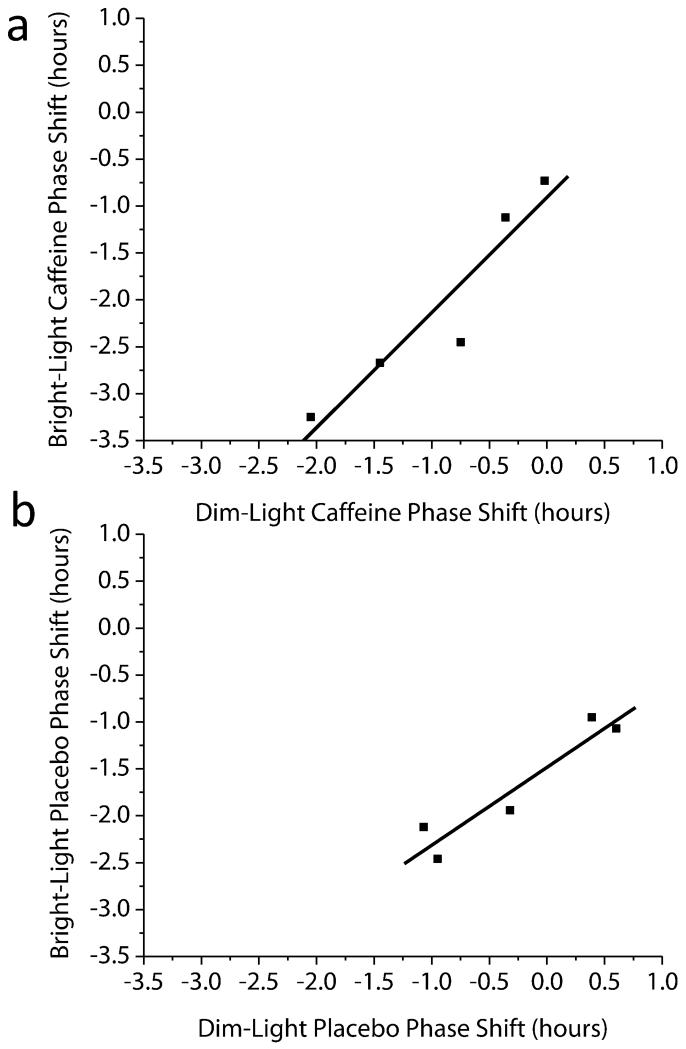
In human circadian research, there have been few studies in which all subjects studied have been exposed to multiple phase shifting stimuli and thus we compared the consistency of the phase shift response to the stimuli tested. Because the period of the circadian clock is on average longer than 24-hours (22,23,24), under experimental dim-light placebo conditions, circadian phase in most individuals delays gradually from one day to the next. We observed a significant positive correlation between this gradual phase change under dim-light placebo and the magnitude of phase delay in response to bright-light placebo (Fig. 3b; r=0.95, p=0.015). We also observed a significant positive correlation between the phase shifts induced by dim-light caffeine and bright-light caffeine (Fig. 3a; r=0.94, p=0.016) such that the greater the phase delay in response to dim-light caffeine, the greater the phase delay in response to bright-light caffeine. No correlation was observed between phase shifts induced by bright-light placebo and dim-light caffeine (r=0.17, p=0.78), and between bright-light placebo and bright-light caffeine (r=0.33, p=0.58). Furthermore, we quantified whether there was systematic inter-individual variability in the phase shifting response to the stimuli tested using the intraclass correlation coefficient (ICC). The ICC was used to test how consistent were the magnitude of the phase shifts for the caffeine and light stimuli within individuals. We found ICCs in the moderate range (ICC=0.46) for the phase shift response between the two placebo conditions and in the substantial range (ICC=0.66) between the two caffeine conditions. The latter shows stable individual differences in the response to placebo and to caffeine stimuli, as 46% to 66% of the variance in the phase shift response was explained by stable shifts among individuals. Such findings suggest that there are robust and stable individual differences in the phase shifting response to caffeine, and moderate stable individual differences in the phase resetting shifting response to bright light, with the change in phase observed during the dim-light placebo control condition. Weaker ICC’s between light and caffeine stimuli (slight ICC=0.11; for bright-light placebo and bright light-caffeine, fair ICC = 0.23; for dim-light placebo and bright light-caffeine, fair ICC = 0.27; for dim-light placebo and dim-light caffeine, fair ICC = 0.35) show that the consistency of individual differences in response to these stimuli is less and suggests that these phase resetting stimuli may work via different mechanisms on the human circadian clock. Thus, the slight to fair ICC’s between phase shifts for dim-light caffeine and bright-light placebo indicate that the proportion of variance in the data explained by systematic inter-individual variability is less when comparing light and caffeine stimuli. Because participants were exposed to all interventions, our protocol permitted control for the influence of individual differences in circadian period (23) on the phase shifting response (2,24). This procedure increased our sensitivity to detect phase shifts when using a relatively small number of subjects. Four of five subjects in the dim-light caffeine condition, and five of five in the bright-light placebo and bright-light caffeine conditions showed delays compared to the dim-light placebo control condition. When controlling for responses to the dim-light placebo control condition, individual differences in phase shifting to caffeine, to bright-light and to their combination were observed (Fig. 2b). Caffeine alone and in combination with bright-light appears to show larger individual differences in the phase shifting response when compared to bright light only (Pitman-Morgan Test; dim-light caffeine p=0.06, bright-light caffeine p< 0.05). It thus appears that caffeine increases individual differences in the phase shifting response to light, perhaps indicating an influence of caffeine on light-induced phase shifts or an influence of the integration of photic and non-photic stimuli by the circadian clock. Why larger individual differences in the phase shifting response to caffeine occur is unclear, however, they may be related to genetic or epigenetic variations in caffeine pharmacokinetics and sensitivity to caffeine. For example, polymorphism in the adenosine A2A receptor gene (ADORA2A) is linked with individual differences in sleep disruption effects of caffeine and habitual caffeine consumption (25,26,27).
Fig 3. Association between phase shifts induced by different conditions.
(A) Dim-Light Caffeine with Bright-Light Caffeine (B) Dim-Light Placebo and Bright-Light Placebo. Symbols represent individual subjects, and solid line represents the best linear fit to the data.
Mechanisms by which caffeine affects circadian timekeeping in human cells
The variation in the phase shifting response in humans could be more readily explained if the mechanism by which caffeine affects the circadian clock were better understood. To address this question directly, we employed an in vitro assay based upon bioluminescent reporters of circadian transcriptional rhythms in the well-characterized human U2OS cell line. Period-lengthening in response to chronic caffeine has been reported both at the behavioral level in rodents and flies (11,18,28), as well as at the cellular level in isolated rodent SCN ex vivo and human U2OS cells in vitro (16,18). Human U2OS cells recapitulate the key observation under investigation (18) and similar to the SCN, primarily express the higher affinity, ubiquitous and abundant A1 adenosine receptor (A1R, encoded by the ADORA1 gene) (29,30) as well as PDEs and ryanodine receptors. U2OS cells thus seemed an ideal platform with which to delineate the cellular target of caffeine in the context of its action upon circadian rhythms in humans.
As an antagonist of broadly expressed adenosine receptors, caffeine can attenuate the regulation of adenylyl cyclase activity by extracellular adenosine. In the case of the A1R, caffeine blocks the activation of Gi/o by adenosine: effectively increasing cAMP production (31,32). As noted, caffeine also has activity against intracellular targets: as an inhibitor of PDE, acute caffeine can reduce the rate of cAMP degradation, and as an activator of RyR acute caffeine increases calcium-release from intracellular stores. Dynamic cAMP signaling is required for normal circadian timekeeping, and chronic modulation of cAMP turnover lengthens the period of cellular circadian rhythms (33). Furthermore period lengthening has been reported previously in response to drugs known to be more selective than caffeine (a weak ligand) for either adenosine receptors (CGS-15943, a potent non-xanthine adenosine antagonist/inverse agonist) or PDEs (3-isobutyl-1-methylxanthine, IBMX) (33,34,35). Thus caffeine, CGS-15943 and IBMX all lengthen circadian period in cultured human cells; in contrast we observed that chronic administration of ryanodine had no effect on cellular timekeeping (Fig. S2), and so we excluded the ryanodine receptor from further consideration as the caffeine target mediating effects on circadian rhythms.
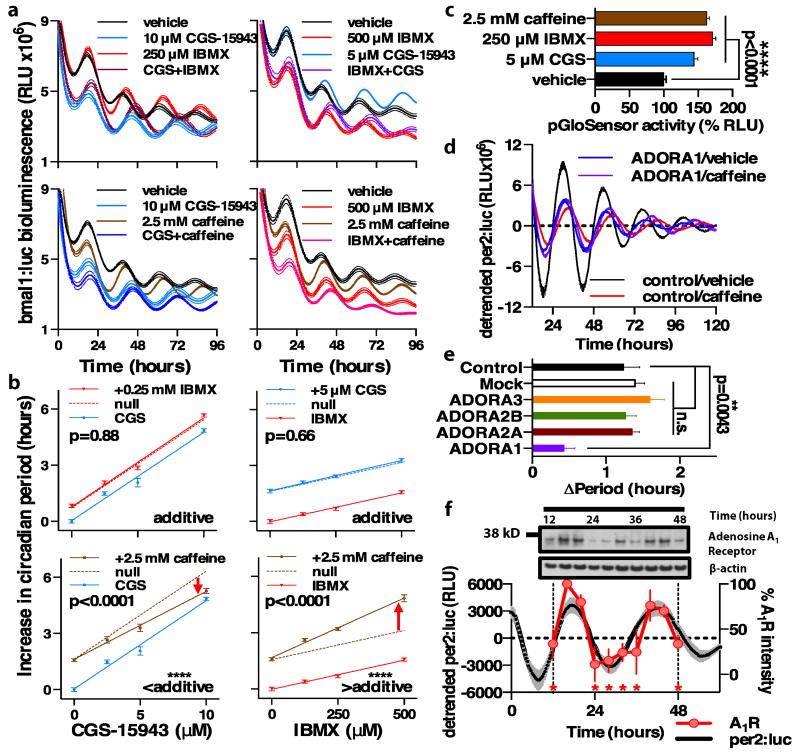
To determine whether caffeine’s effect upon the cellular clockwork is primarily through PDEs or adenosine receptors, we exploited the differing pharmacodynamics and selectivities of CGS-15943 and IBMX during functional assays of cellular timekeeping to compare the relative caffeine-sensitive contributions of the two potential mechanisms. Using the dose-dependent effects of the two drugs upon cellular circadian period detected by bioluminescence, we analyzed their interactions at several concentrations to pharmacologically dissect the mechanism by which caffeine interacts with the cellular clockwork. We also used a non-competitive inhibitor of adenylyl cyclase (THFA, which slows the rate of Gsα–stimulated cAMP synthesis) (33) and has previously been demonstrated to lengthen circadian period in vitro and in vivo across a wide range of model systems (33,36,37), as a control to confirm that caffeine’s effect is cAMP-mediated. We hypothesized that either CGS-15943 or IBMX lengthen circadian period by acting through the same cellular target as caffeine. When two drugs act at the same site, the target saturates at a lower effective concentration, therefore when applied in combination with caffeine whichever drug exhibits a less than additive period-lengthening must act through the same cellular target as caffeine. If the postulates underlying our hypothesis were correct we predicted that, because they target different mechanisms within the same signaling pathway, the slope of the dose response for the combined action of CGS-15943 and IBMX upon circadian period should be equal to the sum of their effects when applied separately. We observed this to be the case (Fig. 4a,b upper panels, Fig. S3, S4). As anticipated, we found that caffeine lengthened circadian period reported by bmal1:luc and per2:luc in a dose-dependent manner (Fig. S3a,b, S4a,b – left hand panels), consistent with prior findings using the Period2 promoter (18). We found that lengthening of circadian period by caffeine is significantly less than additive with increasing doses of CGS-15943, strongly implying that they act at the same target. Moreover, caffeine acts more than additively, or synergistically, with increasing doses of IBMX, indicating that caffeine and IBMX cannot be competing at the same cellular targets in this context. Finally as anticipated, THFA the inhibitor of adenylyl cyclase, which acts upstream of PDEs but downstream of adenosine receptors in cAMP signal transduction, modulated the effect of caffeine, IBMX and CGS (Fig. S3, S4), supporting the role of cAMP as the second messenger system stimulated by caffeine in this context (33,38). We confirmed this by using a well-characterized post-translational cAMP intramolecular complementation biosensor (pGloSensorTM). Caffeine, CGS-15943, and IBMX all increased average cAMP levels over 6 days by ~50%, at concentrations that evoked intermediate increases in period (Fig. 4c).
Fig 4. Caffeine increases circadian period in cultured human cells in vitro in an adenosine receptor/cAMP-dependent fashion.
(A) Representative examples of grouped raw bioluminescence data (mean ± SEM, n=6) showing the effect of different concentrations and combinations of IBMX, CGS-15943 and caffeine on human U2OS cells stably expressing bmal1:luciferase. (B) Grouped quantification of circadian period (mean period ± SEM, n=6) showing dose-dependent lengthening of circadian period in response to CGS-15943 (blue, left panels) or IBMX (red, right panels) ± a fixed concentration of another period-lengthening drug (either 0.25 mM IBMX, red; 5 μM CGS-15943, blue; 2.5 mM caffeine, brown). Solid line depicts the linear regression in each case (R2≥0.98), with broken lines representing the null hypothesis (null, simple additive drug action i.e. no change in the slope). In each sub-panel sum-of-squares F-test p-values are reported, where p<0.05 indicates rejection of the null hypothesis (same slope for both groups). The significance and drug additivity are summarized below. Red arrows indicate that caffeine acts synergistically with IBMX, but less than additively with CGS-15943. (C) All 3 drug treatments significantly increase cAMP signaling reported by pGloSensor activity over 6 days, plotted as mean ± SEM (n=4) relative to vehicle control. p<0.0001 by one-way ANOVA, and by Bonferroni’s multiple comparisons test for each drug vs. vehicle. (D) ADORA1 knockdown attenuates the period lengthening effect of 2.5 mM caffeine in U2OS cells, representative detrended group mean ± SEM is shown (n=4) (E) Grouped quantification of period lengthening by caffeine following siRNA knockdown of each adenosine receptor isoform (n=7 or n=8); one-way ANOVA, p=0.0002. By Bonferroni’s multiple comparisons test, p=0.0043 for control vs. ADORA1, with no significant difference vs. any other group (n.s., p>0.67). (F) Adenosine A1 receptor is rhythmically expressed in U2OS cells in phase with per2:luc. Upper panel, representative A1R immunoblot; lower panel, normalized grouped A1R abundance (mean ± SEM, n=3, p<0.0001 by one-way ANOVA, * indicates p<0.0001 for each time point vs. 16 hours by Bonferroni’s multiple comparisons test). Per2:luc rhythms, recorded in parallel (n=4), are shown for reference.
To further substantiate that the effect of caffeine upon the cellular clock is attributable to its action at adenosine receptors, we predicted that incubating human cells with an alternative selective adenosine receptor antagonist would again result in a dose-dependent lengthening of circadian period. We chose the well-characterized xanthine derivative 8-(p-sulfophenyl) theophylline (8-SPT) (32), because it is membrane-impermeant and therefore cannot act at intracellular sites (PDE, RyRs) and because it is structurally unrelated to CGS-15943. We further predicted that, as with CGS-15943, co-application with 2.5 mM caffeine would reduce the gradient of the dose-dependent period-lengthening elicited by 8-SPT. We expected caffeine and 8-SPT to compete for the same target, adenosine receptors, which can be saturated. Both predictions were confirmed to be the case (Fig. S5).
We next asked whether a specific adenosine receptor is responsible for the effect of caffeine on cellular rhythms. To address this, we assessed the effect of caffeine upon cells subject to siRNA knockdown for each of the human adenosine receptors, using cocktails of previously validated target-specific siRNAs at two different siRNA/cell ratios. Only with knockdown of ADORA1 expression did we observe a significant attenuation of the dose-dependent lengthening of cellular circadian period (Fig. 4d, e, Fig. S6). Moreover by immunoblotting for A1R over a U2OS time course, with bioluminescence recorded from parallel cultures, we observed that A1R protein levels oscillate in phase with per2:luc activity (Fig. 4f). It is thus plausible that the major action of caffeine upon the cellular clockwork is to attenuate an endogenous circadian regulation of cAMP-mediated inputs to the transcriptional clockwork e.g. via functional cAMP/Ca2+-response elements in the Period 1 & 2 promoters (33). If daily cycles of A1R expression occur in human cells and tissues in vivo, then clearly it would play a very similar role to that proposed for RGS16 and CRYPTOCHROME1/2 in mouse SCN and liver, respectively (36,39).
Taken together therefore, whilst we cannot exclude the possibility that additional targets for caffeine might be of relevance in vivo, our in vitro findings indicate that caffeine influences circadian timing by acting primarily via A1 adenosine receptors, thereby modulating endogenous regulation of cAMP signaling, a core component of the cellular circadian clock (33).
Discussion
Our findings demonstrate that caffeine affects the phase of the human circadian clock, as measured by the melatonin rhythm driven by the human SCN and that caffeine primarily affects human cellular circadian clocks by an A1R/cAMP-dependent mechanism. Administration of evening caffeine induced a phase delay that was nearly half the size of the phase-shift induced by evening exposure to bright light. Continuous exposure of human cells in vitro to caffeine also lengthened the period of the cellular circadian clock, in a manner dependent on adenosine receptor/cAMP signaling. Whether caffeine also influences circadian timing in peripheral tissues (40) in vivo (e.g., liver and extra-SCN neuronal clocks) remains to be determined. Nevertheless, our observation that A1R levels oscillate cell-autonomously in human U2OS cells reported here is complemented by the observation that ADORA1 transcript levels are also circadian regulated in mouse in vivo, e.g. in heart, liver and adipose tissues (41).
Although our experiments show that caffeine can delay the human circadian clock in vivo and lengthen the period of the human circadian clock in vitro, further research is needed using similar sensitive within-subject designs and additional circadian phase markers to explore whether caffeine can also phase advance circadian timing and whether our dose-dependent responses in vitro translate to dose-dependent responses in vivo. These studies were performed in a transformed cell line and thus it will be important to confirm whether these observations hold true in non-transformed cells. Future studies should also test the influence of caffeine on circadian timing across a range of cell and tissue types and whether the A1 adenosine receptor remains primarily responsible in each case. Furthermore, we cannot discount a direct interaction between caffeine and photic inputs to the SCN master clock in vivo. For example, adenosine is reported to influence retinal function and transmission of photic information via the retinohypothalamic tract (RHT). Moreover, adenosine administration has been reported to attenuate phase shifts induced by light exposure (42) and caffeine has been shown to enhance the period lengthening effects of light in mice (28). Furthermore, adenosine receptor mRNA is expressed in retinal ganglion cells (43), however whether these include the melanopsin retinal ganglion cells that project to the SCN is unknown. Adenosine may reduce photic input to the SCN by altering RHT transmission and glutamate release onto the SCN (31).
The finding that caffeine influences human circadian physiology may have implications for the pathophysiology and perhaps treatment of some circadian sleep-wake disorders. For example, beyond increasing daytime exposure to sunlight and reducing evening exposure to electrical light (19), avoiding evening caffeine may help to treat problematic delayed sleep timing through circadian as well as established wakefulness-sleep mechanisms (5). Our phase delay results with caffeine may also contribute to the reported association between higher caffeine intake and being an evening chronotype (44). Properly timed caffeine use may also be of benefit with respect to shifting circadian timing, potentially assisting with circadian adaptation to large phase delays required when flying across many time zones westward, as well as sustaining wakefulness until bedtime in the new time zone. Trials are needed to test the latter, and it will be important to monitor for caffeine-induced sleep disruption under such conditions, which of course could worsen jet lag.
Materials and Methods
Human Participant Protocol
Twenty phase shifting protocols were conducted with five healthy participants [(three females) aged 24.0±2.8 years, BMI 23.9±0.9; weight 70.6±11.9 kg (mean±SD)] who maintained regular approximately eight hour sleep-wakefulness schedules based on habitual sleep and wake times for approximately seven days prior to laboratory visits (range 4-10 days; Fig. 1) verified by sleep logs, voicemail time-stamped sleep and wake times, and wrist actigraphy (Actiwatch-L, Minimitter-Respironics,). Participants abstained from over-the-counter medications, supplements, and caffeine for two-weeks, naps for one-week, exercise for three-days and alcohol for two-days prior to and throughout the experiment [Exclusion criteria: medical, psychiatric, or sleep disorders as determined by history, physical and psychiatric exams, abnormal blood chemistries (comprehensive metabolic panel and complete blood cell counts) or clinical electrocardiogram; drug, nicotine or medication use; habitual sleep duration <7 hours or >9 hours; BMI <18.5 or >27; pregnancy; shiftwork or living below the local 1600 m altitude in the year prior; travel >1 time zone three-weeks prior]. Urine toxicology and alcohol breath testing performed each visit. Participants gave written informed consent and procedures were approved by the University of Colorado Boulder Institutional Review Board and the Colorado Clinical and Translational Sciences Institute Scientific Advisory and Review Committee.
Participants arrived approximately six hours prior to habitual bedtime each laboratory visit. Double-blind conditions were performed by the Clinical and Translational Research Center pharmacist who provided pills identical in appearance. The allocation sequence was concealed until interventions were assigned and data prepared for statistical analysis. Pills were five capsules, containing rice flour placebo or caffeine (2.9mg/kg total dose; Gallipot, Inc). Ceiling mounted fluorescent lamps (Sylvania Octron 32W T8 bulbs) provided broad spectrum white light exposure with a color temperature similar to sunlight at midday (6500K). During the 3-hour light exposure session, subjects were under the direct supervision of research assistants who remained in the suite to ensure that the intended intensity of illumination was achieved. Subjects maintained constant posture while alternating between fixing their gaze on a target for six minutes or free gaze for six minutes. Average light intensities during the fixed gaze were 2985±388 lux (mean±SD) for bright light and 1.9±0.4 lux for dim light interventions. Light exposure was timed to induce a maximal phase delay based on published phase and illuminance response curves (3,21). Saliva for melatonin assessment was collected every 30-60 minutes and frozen at −80°C until assayed by ELISA (IBL International).
Human Phase Shift Data Analysis
Phase shifts were determined as change in timing of dim-light melatonin onset (DLMO) between constant routines. The salivary DLMO was defined as the linearly interpolated time point when melatonin levels exceeded and remained two standard deviations above the stable baseline mean (20). Data were analyzed with repeated measures ANOVA and using planned comparisons with Bonferroni correction for multiple comparisons. One-tailed Dunnett’s test was used for hypothesis driven comparisons versus dim-light placebo control and dependent t-tests for caffeine and bright-light comparisons. Associations for phase shifts between conditions were analyzed with Pearson’s r and intraclass correlations. A two-way mixed model ANOVA was used to derive intraclass correlation coefficients (ICC) with subject as a random factor and phase shifting stimuli as a fixed factor. The ICC model (45) used the following formula:
where MSS = mean square subject, MSE = mean square error, k = number of sleep transitions, n = number of subjects, and MSC = mean square condition. The following arbitrary benchmarks from Landis and Koch (46) were used to describe the strength of agreement of ICC scores: <0.00=poor, 0.00-0.20=slight, 0.21-0.40=fair, 0.41-0.60=moderate, 0.61-0.80=substantial, 0.81-1.00=almost perfect. Statistical analyses were performed using Statistica (version 10.0; Statsoft Inc.). Effect sizes (Cohen’s d) were calculated to determine the size of phase shifting effects. Standard interpretations of Cohen’s d effect size were used: small, d=0.2; moderate, d=0.5; large, d=0.8. Pitman-Morgan Tests were used for post-hoc comparisons of the difference in variance between paired conditions. Circadian DLMO phase relative to bedtime prior to interventions was similar (p=0.41): dim-light placebo −1.7±0.9h (mean ± SD; 95%CI: −2.8 to −0.6h); dim-light caffeine −1.5±0.6h (95%CI: −2.2 to −0.7h); bright-light placebo −2.3±1.2h (95%CI: −3.8 to −0.8h); bright-light caffeine −2.1±0.9h (95%CI: −3.2 to −0.9h). Phase shift data were normally distributed.
Cell Culture Protocol
Human U2OS cells stably expressing per2:luc or bmal1:luc were generated as described (47). One day prior to bioluminescence imaging, cells were trypsinised, resuspended in culture medium and seeded into 6x96-well white plates (LUMITRAC, greiner bio-one) at a density of 104 cells/well, and incubated in a humidified incubator for 24 hours (37°C, 5% CO2). After 24 hours, when the cell monolayer was observed to be 100% confluent in clear duplicate plates, the cell medium was replaced with 100 μl Air Medium containing various drugs, as described (48). Stock solutions of IBMX and CGS-15943 were dissolved in DMSO at 100 mM and 20 mM, respectively, prior to being diluted to working concentrations in Air Medium. 100 mM stocks of THFA, 8-SPT and caffeine were made up in Air Medium directly. Final DMSO content was controlled for within each 96-well plate, and never exceeded 0.5%, a concentration observed to have no significant effect on the circadian period of U2OS bioluminescence rhythms under these conditions (Fig. S3,S4). Plates were sealed with adhesive film (Thermo Scientific) and immediately transferred to the recording incubator. Bioluminescence imaging was performed at 37°C over six days in an Alligator (Cairn Research) with n=6 replicates for each experimental condition. Drug concentrations were employed that have previously been shown to lie within the quasi-linear portion of the dose-response curve (around the IC50 value), with respect to effects on cellular circadian period (18,33,34). For comparison of cellular cAMP signaling activity, U2OS cells stably expressing the pGloSensor bioluminescent cAMP reporter (Promega) were generated, with the recording taking place over six days under conditions similar to the circadian bioluminescence assays, except that in order to facilitate reporter activity 1 mM luciferin was included in the recording media and cells were incubated continuously at 30°C. Total bioluminescence collected over six days were normalized to vehicle controls.
All siRNA reagents were purchased from Santa Cruz Biotechnology. Pools of target-specific siRNAs against each human adenosine receptor (sc-39848, sc-39850, sc-29642, sc-39854) or a control siRNA (sc-37007) were transfected into per2:luc U2OS cells in 96 well plates using siRNA Reagent System (sc-45064) following the manufacturer’s recommended protocol, using either 1.25 pmol or 3.75 pmol siRNA per well. Mock, or untransfected, cells were also assayed to control for any non-specific effect of siRNA transfection upon cellular rhythms. Bioluminescence assays, or lysis of parallel cultures for immunoblotting, began 72 hours later, when cells were fully confluent.
Western Blotting
Cells washed in ice cold PBS buffer then lysed directly in 2XLDS sample buffer (Life Technologies) supplemented with 10 mM TCEP, then heated at 70°C for 10 minutes. Western blotting was performed using the Novex NuPAGE system (Life Technologies) under reducing conditions with 4-12% Bis-Tris gradient gels using MES buffer following the recommended protocol. Protein transfer to nitrocellulose for blotting was performed using the iBlot system (Life Technologies). Blocking and all antibody incubations were performed in 0.25%/0.25% w/w BSA/non-fat dried milk (Marvel) in Tris buffered saline/0.05% Tween-20 (TBST). After 1 hour in blocking buffer, after three brief washes in TBST, primary antibody incubations were performed overnight at 4°C using 1:5000 anti-actin (SCBT, sc-47778), anti-A1R (Proteintech, 55026-1-AP), and antibodies against human adenosine A2A, A2B and A3 receptors (Abcam ab3461, ab40002 and ab136051, respectively,). Membranes were washed in TBST three times for 10 minutes and then incubated with the appropriate 1:10,000 HRP-conjugated secondary antibody (Sigma-Aldrich) for 60 min. Four 10-min TBST washes were then performed before performing chemiluminescence detection using Immobilon ECL reagent (Merck Millipore).
Bioluminescence Data Analysis
Analysis of bioluminescence data was done using a modified version of the CellulaRhythm algorithm (49). Raw luminescence data were detrended by fitting a 4th order polynomial function using linear regression. The detrended traces were then fitted to a damped cosine curve using nonlinear least squares to the following equation:
where m = Slope, k = Damping rate, and t = Time. Due to transient luminescence changes after medium changes, the first 20 hours of luminescence data were excluded from the analysis. Graphs were plotted, and statistical analyses performed, in Graphpad Prism version 6.0 for Mac (GraphPad Software).
Supplementary Material
Acknowledgments
We thank the Clinical Translational Research Center physicians, nurses, pharmacist, dieticians, and research assistants; N. Gerhart, S. M. Nguyen, P. P. Serapio, S. Henriques da Costa, C. Frezza, K. Feeney and G. Rey for assistance with this study. We thank R. Kram for comments on an earlier version of the manuscript.
Funding: Supported by National Institutes of Health (NIH) R01 HL081761, by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082, and the Howard Hughes Medical Institute in collaboration with the Biological Sciences Initiative and Undergraduate Research Opportunities Program at the University of Colorado Boulder. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. J.S.O. is supported by the Medical Research Council (MC_UP_1201/4) and the Wellcome Trust (093734/Z/10/Z).
Author contributions
T.M.B.: study design, data collection, data analysis, and manuscript preparation; R.R.M. data collection, data analysis, and manuscript preparation; A.W.M.: data analysis, and manuscript preparation; E.D.C.: study design, data collection, data analysis, and manuscript preparation; J.A.S.: data collection, data analysis, and manuscript preparation; S.C.B.: data collection, data analysis, and manuscript preparation; C.M.J.: data collection, data analysis, and manuscript preparation; J.S.O.: study design, data collection, data analysis, and manuscript preparation; K.P.W.: study design, data collection, data analysis, and manuscript preparation.
Footnotes
Fig. S1. 49 day protocol for human experiment.
Fig S2. Ryanodine does not lengthen circadian period reported by per2:luc in cultured human cells in vitro.
Fig. S3. Caffeine increases circadian period reported by bmal1:luc in cultured human cells in vitro, in an adenosine receptor/cAMP-dependent fashion.
Fig. S4. Caffeine increases circadian period reported by per2:luc in cultured human cells in vitro, in an adenosine receptor/cAMP-dependent fashion.
Fig S5. Caffeine acts at the same site as 8-(p-sulfophenyl)theophylline (8-SPT) to increase circadian period in cultured human cells in vitro.
Fig S6. Quantification of adenosine receptor siRNA knockdown efficacy.
Competing interests: J.A.S: Financial relationships: consulting fees for Zeo, Inc.; J.S.O: Funding: Medical Research Council and the Wellcome Trust; K.P.W.: Funding: NIH, NASA, National Space Biomedical Research Institute, Henry Ford Hospital, Philips Inc., Takeda Pharmaceuticals America, University of Colorado Boulder; Financial relationships: consulting fees from or served as a paid member of scientific advisory boards for NIH, Northwestern American Waterways Project, Takeda Pharmaceuticals, Torvec Inc., Zeo, Inc.; Speaker honorarium fees from Associated Professional Sleep Societies, Potomac Center for Medical Education, American College of Chest Physicians, American Academy of Sleep Medicine, and the National Institutes of Health.
Data Materials and Availability: n/a
References
- 1.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE. Bright Light Resets the Human Circadian Pacemaker Independent of the Timing of the Sleep-Wake Cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 2.Wright KP, Jr., Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. [DOI] [PubMed] [Google Scholar]
- 3.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 5.Nehlig A, Daval J, Debry G. Caffeine and the Central Nervous System: Mechanisms of Action, Biochemical, Metabolic and Psychostimulant Effects. Brain Res. Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 6.Shryock JC, Ozeck MJ, Belardinelli L. Inverse agonists and neutral antagonists of recombinant human A1 adenosine receptors stably expressed in Chinese hamster ovary cells. Mol. Pharmacol. 1998;53:886–893. [PubMed] [Google Scholar]
- 7.Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, Tate CG, Weir M, Marshall FH. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 2011;19:1283–1293. doi: 10.1016/j.str.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman JF. Circadian Periodicity in Neurospora - Alteration by Inhibitors of Cyclic-Amp Phosphodiesterase. Science. 1975;190:789–790. doi: 10.1126/science.173018. [DOI] [PubMed] [Google Scholar]
- 10.Goodenough JE, Bruce VG. The Effects of Caffeine and Theophylline on The Phototactic Rhythm of Chlamydomonas Reinhardii. Biol. Bull. 1980;159:649–655. [Google Scholar]
- 11.Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J. Neurosci. 2009;29:11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralph MR, Khalsa SBS, Block GD. Cyclic Nucleotides and Circadian Rhythm Generation in Bulla Gouldiana. Comp. Biochem. Physiol. A. 1992;101:813–817. [Google Scholar]
- 13.Wright KP, Jr., Badia P, Myers BL, Plenzler SC, Hakel M. Caffeine and light effects on nighttime melatonin and temperature levels in sleep-deprived humans. Brain Res. 1997;747:78–84. doi: 10.1016/s0006-8993(96)01268-1. [DOI] [PubMed] [Google Scholar]
- 14.Wright KP, Jr., Myers BL, Plenzler SC, Drake CL, Badia P. Acute effects of bright light and caffeine on nighttime melatonin and temperature levels in women taking and not taking oral contraceptives. Brain Res. 2000;873:310–317. doi: 10.1016/s0006-8993(00)02557-9. [DOI] [PubMed] [Google Scholar]
- 15.Shearman LP, Weaver DR. Distinct pharmacological mechanisms leading to c-fos gene expression in the fetal suprachiasmatic nucleus. J. Biol. Rhythms. 2001;16:531–540. doi: 10.1177/074873001129002222. [DOI] [PubMed] [Google Scholar]
- 16.Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Muñoz M, Dent MA, Granados-Fuentes D, Hall AC, Hernández-Cruz A, Harrington ME, Aguilar-Roblero R. Circadian modulation of the ryanodine receptor type 2 in the SCN of rodents. Neuroreport. 1999;10:481–486. doi: 10.1097/00001756-199902250-00007. [DOI] [PubMed] [Google Scholar]
- 18.Oike H, Kobori M, Suzuki T, Ishida N. Caffeine lengthens circadian rhythms in mice. Biochem. Biophys. Res. Commun. 2011;410:654–658. doi: 10.1016/j.bbrc.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 19.Wright KP, Jr., McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, Wright KP., Jr. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36:1617–1624. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright KP, Jr., Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-hour pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc. Natl. Acad. Sci. USA. 98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy JF, Cain SW, Chang AM, Phillips AJ, Münch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Jr., Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS One. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rétey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin. Pharmacol. Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 26.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am. J. Clin. Nutr. 2007;86:240–244. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- 27.Byrne EM, Johnson J, McRae AF, Nyholt DR, Medland SE, Gehrman PR, Heath AC, Madden PA, Montgomery GW, Chenevix-Trench G, Martin NG. A genome-wide association study of caffeine-related sleep disturbance: confirmation of a role for a common variant in the adenosine receptor. Sleep. 2012;35:967–975. doi: 10.5665/sleep.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Diepen HC, Lucassen EA, Yasenkov R, Groenen I, Ijzerman AP, Meijer JH, Deboer T. Caffeine increases light responsiveness of the mouse circadian pacemaker. Eur. J. Neurosci. 2014;40:3504–3511. doi: 10.1111/ejn.12715. [DOI] [PubMed] [Google Scholar]
- 29.Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol. Endocrinol. 2005;19:1555–1568. doi: 10.1210/me.2004-0381. [DOI] [PubMed] [Google Scholar]
- 30.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, van den Pol AN. Adenosine modulation of calcium currents and presynaptic inhibition of GABA release in suprachiasmatic and arcuate nucleus neurons. J. Neurophysiol. 1997;77:3035–3047. doi: 10.1152/jn.1997.77.6.3035. [DOI] [PubMed] [Google Scholar]
- 32.Conlay LA, Conant JA, deBros F, Wurtman R. Caffeine alters plasma adenosine levels. Nature. 1997;389:136. doi: 10.1038/38160. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto KH, Kiuchi R, Ishida M, Ukai-Tadenuma M, Minami Y, Kito R, Nakao K, Kishimoto W, Yoo SH, Shimomura K, Takao T, Takano A, Kojima T, Nagai K, Sakaki Y, Takahashi JS, Ueda HR. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. USA. 2009;106:15744–15749. doi: 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu PH, Phillis JW, Nye MJ. Alkylxanthines as adenosine receptor antagonists and membrane phosphodiesterase inhibitors in central nervous tissue: evaluation of structure-activity relationships. Life Sci. 1982;31:2857–2867. doi: 10.1016/0024-3205(82)90676-2. [DOI] [PubMed] [Google Scholar]
- 36.Doi M, Ishida A, Miyake A, Sato M, Komatsu R, Yamazaki F, Kimura I, Tsuchiya S, Kori H, Seo K, Yamaguchi Y, Matsuo M, Fustin JM, Tanaka R, Santo Y, Yamada H, Takahashi Y, Araki M, Nakao K, Aizawa S, Kobayashi M, Obrietan K, Tsujimoto G, Okamura H. Circadian regulation of intracellular G-protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat. Commun. 2011;2:327. doi: 10.1038/ncomms1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cell Mol. Life Sci. 2013;70:2985–2998. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narishige S, Kuwahara M, Shinozaki A, Okada S, Ikeda Y, Kamagata M, Tahara Y, Shibata S. Effects of caffeine on circadian phase, amplitude and period evaluated in cells in vitro and peripheral organs in vivo in PER2::LUCIFERASE mice. Br. J. Pharmacol. 2014;171:5858–69. doi: 10.1111/bph.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe A, Moriya T, Nisikawa Y, Araki T, Hamada T, Shibata S, Watanabe S. Adenosine A1-receptor agonist attenuates the light-induced phase shifts and fos expression in vivo and optic nerve stimulation-evoked field potentials in the suprachiasmatic nucleus in vitro. Brain Res. 1996;740:329–336. doi: 10.1016/s0006-8993(96)00881-5. [DOI] [PubMed] [Google Scholar]
- 43.Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB. Localization of adenosine receptor messenger RNAs in the rat eye. Exp. Eye Res. 1997;65:595–602. doi: 10.1006/exer.1996.0352. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara K, Miyasita A, Inugami M, Fukuda K, Yamazaki K, Miyata Y. Differences in the time or frequency of meals, alcohol and caffeine ingestion, and smoking found between ‘morning’ and ‘evening’ types. Psychol. Rep. 1985;57:391–396. doi: 10.2466/pr0.1985.57.2.391. [DOI] [PubMed] [Google Scholar]
- 45.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol. Methods. 1996;1:30–46. [Google Scholar]
- 46.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 47.Valekunja UK, Edgar RS, Oklejewicz M, van der Horst GT, O’Neill JS, Tamanini F, Turner DJ, Reddy AB. Histone methyltransferase MLL3 contributes to genome-scale circadian transcription. Proc. Natl. Acad. Sci. USA. 2013;110:1554–1559. doi: 10.1073/pnas.1214168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill JS, Hastings MH. Increased coherence of circadian rhythms in mature fibroblast cultures. J. Biol. Rhythms. 2008;23:483–488. doi: 10.1177/0748730408326682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc. Natl. Acad. Sci. USA. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.