Abstract
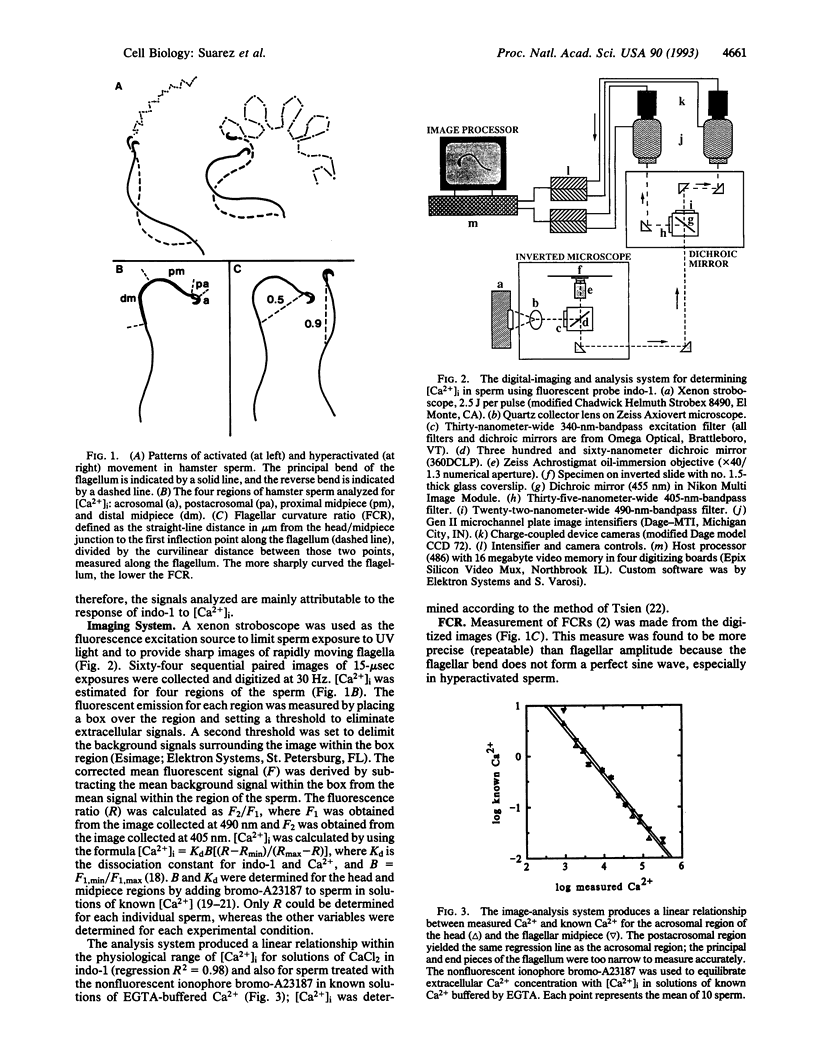
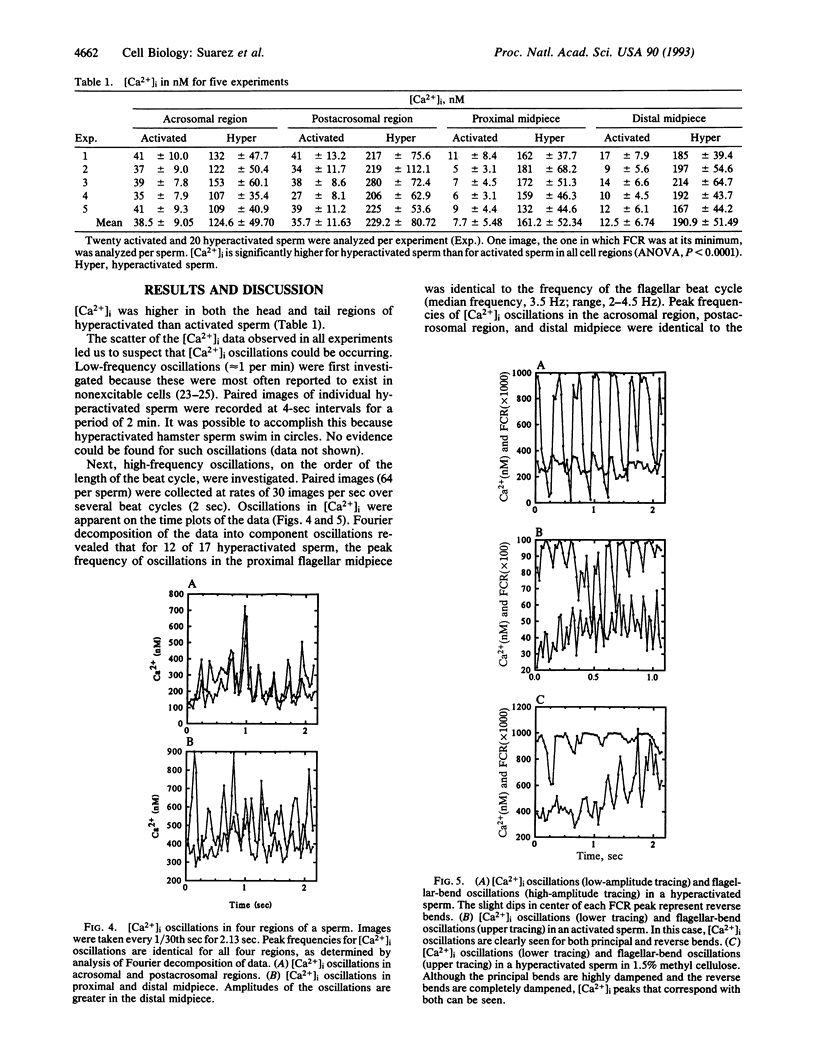
At some time before fertilization, mammalian sperm undergo a change in movement pattern, termed hyperactivation. There is evidence that hyperactivation offers an advantage to sperm for detaching from the oviductal mucosa, for penetrating viscoelastic substances in the oviduct, and for penetrating the zona pellucida. Hyperactivation is known to require extracellular calcium, but little else is known about the mechanisms by which calcium affects sperm movement. The calcium-sensitive fluorescent dye indo-1 was used to follow intracellular calcium levels ([Ca2+]i) in individual moving sperm. Sperm were loaded with 10 microM of the acetoxymethyl ester form of the dye and then rinsed. The dye was excited at 340 nm by using a filtered xenon stroboscope, and images at the 405-nm and 490-nm excitation maxima were simultaneously digitized at 30 per sec for 2.1 sec. [Ca2+]i was significantly higher in the acrosomal and postacrosomal regions of the head and in the flagellar midpiece (the principal piece could not be measured) in hyperactivated than in nonhyperactivated sperm (P < 0.0001). [Ca2+]i oscillations were detected in the proximal half of the midpiece that were identical in frequency to the flagellar-beat-cycle frequency in 12 of 17 hyperactivated sperm (median, 3.5 Hz). Rapid [Ca2+]i oscillations were also detected in the acrosomal and postacrosomal regions, as well as in the distal midpiece. Oscillations were not eliminated by dampening the flagellar bending with methyl cellulose. The [Ca2+]i oscillations detected in sperm are significantly more rapid than oscillations detected in other cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. F., First N. L., Lardy H. A. Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. J Biol Chem. 1976 Jul 10;251(13):3881–3886. [PubMed] [Google Scholar]
- Bancel F., Salmon J. M., Vigo J., Viallet P. Microspectrofluorometry as a tool for investigation of non-calcium interactions of Indo-1. Cell Calcium. 1992 Jan;13(1):59–68. doi: 10.1016/0143-4160(92)90030-v. [DOI] [PubMed] [Google Scholar]
- Bavister B. D. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989 Jun;23(2):139–158. doi: 10.1002/mrd.1120230202. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berruti G. Calpactin-like proteins in human spermatozoa. Exp Cell Res. 1988 Dec;179(2):374–384. doi: 10.1016/0014-4827(88)90276-5. [DOI] [PubMed] [Google Scholar]
- Berruti G., Porzio S. Evidence for a calsequestrin-like calcium-binding protein in human spermatozoa. Eur J Cell Biol. 1990 Jun;52(1):117–122. [PubMed] [Google Scholar]
- Bradley M. P., Forrester I. T. A sodium-calcium exchange mechanism in plasma membrane vesicles isolated from ram sperm flagella. FEBS Lett. 1980 Nov 17;121(1):15–18. doi: 10.1016/0014-5793(80)81255-5. [DOI] [PubMed] [Google Scholar]
- Breitbart H., Rubinstein S. Calcium transport by bull spermatozoa plasma membranes. Biochim Biophys Acta. 1983 Jul 27;732(2):464–468. doi: 10.1016/0005-2736(83)90063-9. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Calcium sensors in sea urchin sperm flagella. Cell Motil Cytoskeleton. 1991;18(2):123–130. doi: 10.1002/cm.970180207. [DOI] [PubMed] [Google Scholar]
- Demott R. P., Suarez S. S. Hyperactivated sperm progress in the mouse oviduct. Biol Reprod. 1992 May;46(5):779–785. doi: 10.1095/biolreprod46.5.779. [DOI] [PubMed] [Google Scholar]
- Devor D. C., Ahmed Z., Duffey M. E. Cholinergic stimulation produces oscillations of cytosolic Ca2+ in a secretory epithelial cell line, T84. Am J Physiol. 1991 Mar;260(3 Pt 1):C598–C608. doi: 10.1152/ajpcell.1991.260.3.C598. [DOI] [PubMed] [Google Scholar]
- Drobnis E. Z., Yudin A. I., Cherr G. N., Katz D. F. Hamster sperm penetration of the zona pellucida: kinematic analysis and mechanical implications. Dev Biol. 1988 Nov;130(1):311–323. doi: 10.1016/0012-1606(88)90437-x. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Corron M. E., Kim T. D., Babcock D. F. Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol. 1992 Aug;152(2):304–314. doi: 10.1016/0012-1606(92)90137-6. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Tombes R. M., First N. L., Babcock D. F. An adhesion-associated agonist from the zona pellucida activates G protein-promoted elevations of internal Ca2+ and pH that mediate mammalian sperm acrosomal exocytosis. Dev Biol. 1989 Sep;135(1):133–146. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Gnessi L., Ruff M. R., Fraioli F., Pert C. B. Demonstration of receptor-mediated chemotaxis by human spermatozoa. A novel quantitative bioassay. Exp Cell Res. 1985 Nov;161(1):219–230. doi: 10.1016/0014-4827(85)90506-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kann M. L., Feinberg J., Rainteau D., Dadoune J. P., Weinman S., Fouquet J. P. Localization of calmodulin in perinuclear structures of spermatids and spermatozoa: a comparison of six mammalian species. Anat Rec. 1991 Aug;230(4):481–488. doi: 10.1002/ar.1092300407. [DOI] [PubMed] [Google Scholar]
- Lindemann C. B., Goltz J. S. Calcium regulation of flagellar curvature and swimming pattern in triton X-100--extracted rat sperm. Cell Motil Cytoskeleton. 1988;10(3):420–431. doi: 10.1002/cm.970100309. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Macek M. B., Shur B. D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature. 1992 Jun 18;357(6379):589–593. doi: 10.1038/357589a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Yuzaki M., Nakada K., Shirakawa H., Nakanishi S., Nakade S., Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992 Jul 10;257(5067):251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Michikawa Y., Baba T., Okinaga S., Arai K. Calreticulin is present in the acrosome of spermatids of rat testis. Biochem Biophys Res Commun. 1992 Jul 31;186(2):668–673. doi: 10.1016/0006-291x(92)90798-p. [DOI] [PubMed] [Google Scholar]
- Owen C. S., Sykes N. L., Shuler R. L., Ost D. Non-calcium environmental sensitivity of intracellular Indo-1. Anal Biochem. 1991 Jan;192(1):142–148. doi: 10.1016/0003-2697(91)90199-4. [DOI] [PubMed] [Google Scholar]
- Ralt D., Goldenberg M., Fetterolf P., Thompson D., Dor J., Mashiach S., Garbers D. L., Eisenbach M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2840–2844. doi: 10.1073/pnas.88.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S. S., Dai X. B., DeMott R. P., Redfern K., Mirando M. A. Movement characteristics of boar sperm obtained from the oviduct or hyperactivated in vitro. J Androl. 1992 Jan-Feb;13(1):75–80. [PubMed] [Google Scholar]
- Suarez S. S., Dai X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol Reprod. 1992 Apr;46(4):686–691. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- Suarez S. S. Hamster sperm motility transformation during development of hyperactivation in vitro and epididymal maturation. Gamete Res. 1988 Jan;19(1):51–65. doi: 10.1002/mrd.1120190106. [DOI] [PubMed] [Google Scholar]
- Suarez S. S., Katz D. F., Overstreet J. W. Movement characteristics and acrosomal status of rabbit spermatozoa recovered at the site and time of fertilization. Biol Reprod. 1983 Dec;29(5):1277–1287. doi: 10.1095/biolreprod29.5.1277. [DOI] [PubMed] [Google Scholar]
- Suarez S. S., Katz D. F., Owen D. H., Andrew J. B., Powell R. L. Evidence for the function of hyperactivated motility in sperm. Biol Reprod. 1991 Feb;44(2):375–381. doi: 10.1095/biolreprod44.2.375. [DOI] [PubMed] [Google Scholar]
- Suarez S. S. Sperm transport and motility in the mouse oviduct: observations in situ. Biol Reprod. 1987 Feb;36(1):203–210. doi: 10.1095/biolreprod36.1.203. [DOI] [PubMed] [Google Scholar]
- Suarez S. S., Vincenti L., Ceglia M. W. Hyperactivated motility induced in mouse sperm by calcium ionophore A23187 is reversible. J Exp Zool. 1987 Nov;244(2):331–336. doi: 10.1002/jez.1402440218. [DOI] [PubMed] [Google Scholar]
- Tash J. S., Krinks M., Patel J., Means R. L., Klee C. B., Means A. R. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol. 1988 May;106(5):1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Wahl M., Lucherini M. J., Gruenstein E. Intracellular Ca2+ measurement with Indo-1 in substrate-attached cells: advantages and special considerations. Cell Calcium. 1990 Aug;11(7):487–500. doi: 10.1016/0143-4160(90)90081-5. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Weigensberg B. I., Lough J., More R. H., Katz E., Pugash E., Peniston C. Effects of estradiol on myointimal thickenings from catheter injury and on organizing white mural non-occlusive thrombi. Atherosclerosis. 1984 Sep;52(3):253–265. doi: 10.1016/0021-9150(84)90055-8. [DOI] [PubMed] [Google Scholar]
- Wilde M. W., Ward C. R., Kopf G. S. Activation of a G protein in mouse sperm by the zona pellucida, an egg-associated extracellular matrix. Mol Reprod Dev. 1992 Apr;31(4):297–306. doi: 10.1002/mrd.1080310411. [DOI] [PubMed] [Google Scholar]





