Abstract
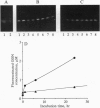
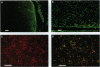
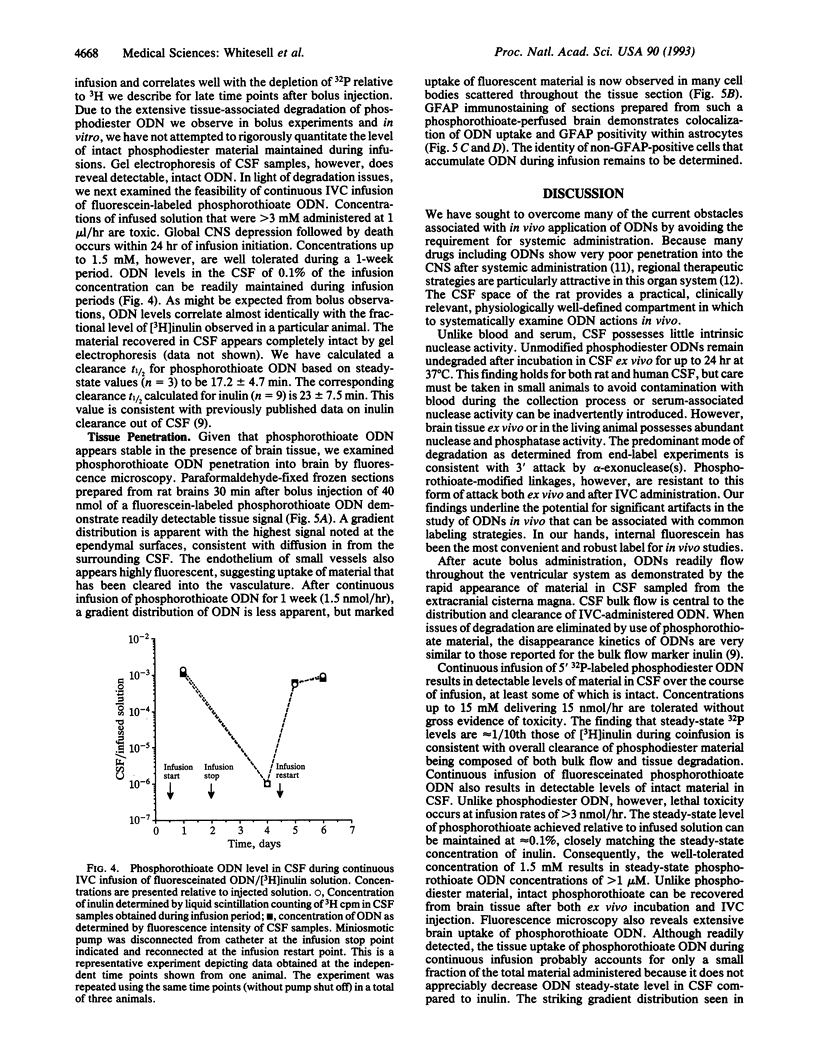
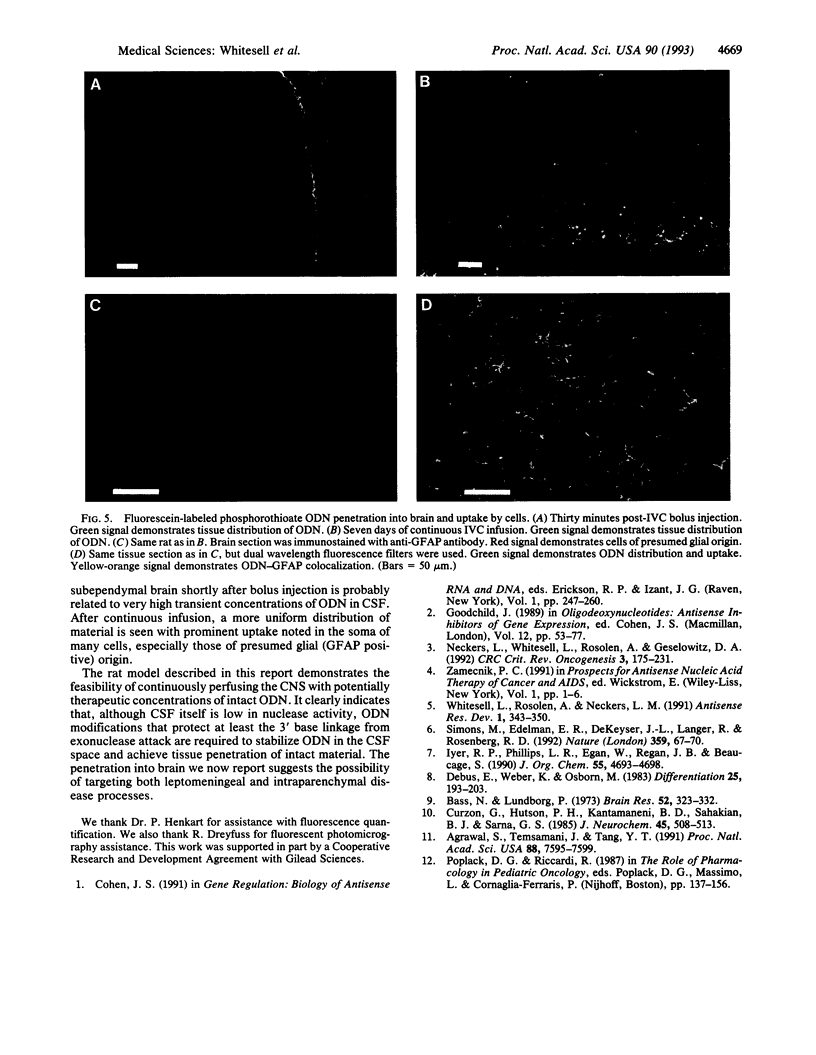
We report experiments in the rat demonstrating the feasibility of intraventricular administration of oligodeoxynucleotides (ODNs) as a regional treatment approach to disorders within the central nervous system (CNS). Although we find little intrinsic nuclease activity in cerebrospinal fluid (CSF), phosphodiester ODNs are rapidly degraded by brain-associated alpha-exonuclease activity. Phosphorothioate ODNs, however, appear resistant to degradation in the CNS and, after intraventricular administration, we find they are cleared in a manner consistent with CSF bulk flow. Continuous infusion of ODN at 1.5 nmol/hr by miniosmotic pump can maintain micromolar concentrations of intact phosphorothioate ODN in CSF for at least 1 week without obvious neurologic or systemic toxicity. After infusion, extensive brain penetration and marked cellular uptake, especially by astrocytic cells, is demonstrated.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Temsamani J., Tang J. Y. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass N. H., Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-( 14 C)inulin after intrathecal infusion. Brain Res. 1973 Mar 30;52:323–332. doi: 10.1016/0006-8993(73)90668-9. [DOI] [PubMed] [Google Scholar]
- Curzon G., Hutson P. H., Kantamaneni B. D., Sahakian B. J., Sarna G. S. 3,4-Dihydroxyphenylethylamine and 5-hydroxytryptamine metabolism in the rat: acidic metabolites in cisternal cerebrospinal fluid before and after giving probenecid. J Neurochem. 1985 Aug;45(2):508–513. doi: 10.1111/j.1471-4159.1985.tb04017.x. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Neckers L., Whitesell L., Rosolen A., Geselowitz D. A. Antisense inhibition of oncogene expression. Crit Rev Oncog. 1992;3(1-2):175–231. [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Rosolen A., Neckers L. M. In vivo modulation of N-myc expression by continuous perfusion with an antisense oligonucleotide. Antisense Res Dev. 1991 Winter;1(4):343–350. doi: 10.1089/ard.1991.1.343. [DOI] [PubMed] [Google Scholar]