Abstract
Background
Acute bronchiolitis is the commonest cause of hospitalisation in infancy. Currently management consists of supportive care and oxygen. A Cochrane review concluded that, “nebulised 3 % saline may significantly reduce the length of hospital stay”. We conducted a systematic review of controlled trials of nebulised hypertonic saline (HS) for infants hospitalised with primary acute bronchiolitis.
Methods
Searches to January 2015 involved: Cochrane Central Register of Controlled Trials; Ovid MEDLINE; Embase; Google Scholar; Web of Science; and, a variety of trials registers. We hand searched Chest, Paediatrics and Journal of Paediatrics on 14 January 2015. Reference lists of eligible trial publications were checked. Randomised or quasi-randomised trials which compared HS versus either normal saline (+/− adjunct treatment) or no treatment were included. Eligible studies involved children less than 2 years old hospitalised due to the first episode of acute bronchiolitis. Two reviewers extracted data to calculate mean differences (MD) and 95 % Confidence Intervals (CIs) for length of hospital stay (LoS—primary outcome), Clinical Severity Score (CSS) and Serious Adverse Events (SAEs). Meta-analysis was undertaken using a fixed effect model, supplemented with additional sensitivity analyses. We investigated statistical heterogeneity using I2. Risk of bias, within and between studies, was assessed using the Cochrane tool, an outcome reporting bias checklist and a funnel plot.
Results
Fifteen trials were included in the systematic review (n = 1922), HS reduced mean LoS by 0.36, (95 % CI 0.50 to 0.22) days, but with considerable heterogeneity (I2 = 78 %) and sensitivity to alternative analysis methods. A reduction in CSS was observed where assessed [n = 516; MD −1.36, CI −1.52, −1.20]. One trial reported one possible intervention related SAE, no other studies described intervention related SAEs.
Conclusions
There is disparity between the overall combined effect on LoS as compared with the negative results from the largest and most precise trials. Together with high levels of heterogeneity, this means that neither individual trials nor pooled estimates provide a firm evidence-base for routine use of HS in inpatient acute bronchiolitis.
Electronic supplementary material
The online version of this article (doi:10.1186/s12890-015-0140-x) contains supplementary material, which is available to authorized users.
Keywords: Bronchiolitis, Bronchodilator agents, Nebulizers and vaporizers, Randomized controlled trials as topic, Hypertonic saline, Systematic review, Meta-analysis
Background
Acute bronchiolitis is the most common cause for hospitalisation in infancy and childhood, with 1–3 % of all infants admitted to hospital during their first winter [1–9]. Obstruction of the airways results in severe breathing difficulties caused by common respiratory viruses infecting the lungs [1–8, 10–13]. The peak age of incidence is between 1 and 6 months for babies admitted with this condition [9]. Current management involves supportive care, minimal handling, supplemental oxygen and fluids [4, 14–16]. The median duration of admission is 3 days, considerably higher than for other acute paediatric admissions (median 1 day). The course of the illness or length of hospital stay has not been impacted by treatments including oral and inhaled steroids, antiviral agents and a variety of bronchodilators.
A number of small studies have suggested that nebulised hypertonic saline may influence the course of the illness resulting in a reduction in the duration of hospitalisation for infants admitted with “acute bronchiolitis” [17–20]. A Cochrane review, which last updated its searches in May 2013, included 11 trials involving 1090 infants with mild to moderate acute viral bronchiolitis (500 inpatients, six trials; 65 outpatients, one trial; and 525 emergency department patients, four trials) [21]. The review concluded that “current evidence suggests nebulized 3 % saline may significantly reduce the length of hospital stay and improve the clinical severity score in infants with acute viral bronchiolitis.” Our team felt that this conclusion is difficult to justify on two counts. Firstly, the findings are hampered by high levels of heterogeneity, and we believe these warrant further exploration. Secondly, a number of relevant published trials, published at the time appear to have been overlooked; this is still the case as of the 2013 update. Finally, since 2013, a number of large studies addressing this topic have been published.
As a result, we made a registration on the PROSPERO database and undertook a separate systematic review on the effect of hypertonic saline on the length of stay of infants admitted to hospital for acute bronchiolitis. The PROSPERO protocol is available (Additional file 1). This review does not investigate the use of hypertonic saline in infants with bronchiolitis in the emergency department.
Methods
This study is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance [22]. A checklist is available (Additional file 2). Since this study was a literature review of previously reported studies, ethical approval or additional consent from participants was not required.
Protocol and registration
The protocol was registered with the PROSPERO database (CRD42014007569) [23] on 03 March 14 and the registration was updated on 18 December 2014.
Eligibility criteria
We included published and unpublished randomised controlled trials involving children up to the age of 2 years, hospitalised as the result of a first episode of acute bronchiolitis. Trials were included if hypertonic saline versus either normal saline (+/− adjunct treatment) or no treatment were the interventions. Studies were grouped in pre-specified subgroups as follows: (1) Nebulised hypertonic saline alone vs normal saline; (2) Nebulised hypertonic saline plus a bronchodilator (e.g. salbutamol) vs. normal saline; (3) Nebulised hypertonic saline plus a bronchodilator vs. normal saline plus same bronchodilator; (4) Nebulised hypertonic saline alone or plus a bronchodilator vs. no intervention. We applied no restrictions based on the concentration, dose or administration of the intervention or control. We excluded studies not published in English.
Literature search and information sources
We searched Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (via Ovid), EMBASE from inception to January 2015, Web of Science from 2010 to January 2015 and Google Scholar. We used 2010 as a cut off, the date on which Zhang and colleagues ran their searches for the 2011 update [24]. We used the terms “bronchiolitis” and “hypertonic saline” to identify ongoing unpublished data in the following registries: Clinicaltrials.gov; UK Clinical Trials Gateway (UKCTG); CRD databases (DARE NHS EED, HTA); controlled-trials.com; centrewatch.com and National Research Register (NNR). On 14th January 2015 we also hand-searched Chest, Paediatrics and Journal of Paediatrics using the terms “hypertonic saline” and “bronchiolitis”. One reviewer (HC) checked the reference lists of all eligible trial publications for other relevant trials. The final search strategy is outlined in Additional file 3.
Study selection
Two reviewers (CM and HC) independently assessed eligibility, differences were resolved through discussion with third reviewers (DH and ME). Where the titles or abstracts suggested eligibility, we retrieved and screened the full paper. Unsuccessful attempts were made to contact trial investigators of three unpublished studies [25–27] to request additional unreported data.
Data extraction
CM and HC used a standardised data collection tool to include study characteristics, population characteristics and risk of bias [28]. Population and study data comprised of country where the trial was conducted, age, disease severity, intervention and comparator details (concentration, dose, delivery mechanism), and details of any adjunct treatments given (β2 agonist or epinephrine). We extracted baseline and follow-up outcome data for Length of hospital stay (LoS), final Clinical Severity Scores (CSS) using the scoring system described by Wang and colleagues [29], readmission rate and adverse events. Adverse event data was collected however reported but of particular interest were tachycardia, hypertension, pallor, tremor, nausea, vomiting and acute urinary retention. DH helped resolve any discrepancies with any of the data items.
Risk of bias
We separately assessed the potential for systematic error within individual studies using the Cochrane risk of bias tool [30] and the following dimensions of methodological quality: (1) generation of allocation sequence; (2) allocation concealment; (3) blinding (participant and researchers); (4) blinding of outcome assessors; (5) completeness of outcome data; (6) selective outcome reporting. Studies were grade as being at, “low”, “high” or “unclear” risk of bias. Any discrepancies were discussed between the data extractors until both reached a unanimous decision. Where an unclear grading was given we contacted trial authors to obtain further information and searched for the study protocol to identify sources of reporting bias. We used standard methods (based on the Cochrane Handbook) to assess funnel plot symmetry as we had greater than 10 trials in the meta-analysis [30]. We employed methods suggested by Dwan and colleagues [31] to assess the risk of outcome reporting bias for each of the outcomes using the Outcome Reporting Bias (ORB) classification whereby trials are scored as “high risk”, “low risk” or “partial risk”. The full classification table is available in Additional file 4.
Synthesis
The primary outcome was LoS. Secondary outcomes were adverse events, final CSS scores and rate of readmission. Data on LoS and final mean CSS was used to calculate mean differences (MD) with 95 % Confidence Intervals (CIs) for each outcome. Where trials included arms using different concentrations of hypertonic saline in addition to a control arm we treated them as two separate trials, dividing the control arm numbers by two in order not to double-count control participants. Meta-analyses were undertaken in RevMan Version 5.2 and Stata version12 using both fixed (Inverse-Variance) and random effect (Der Simonian & Laird) models [32]; additional sensitivity analyses used the metasens package as implemented within the R software version 3.1.3[33]. The four pre-specified intervention subgroups were separated in the main forest plot to give subgroup estimates of treatment effects. Statistical heterogeneity—a measure of within-trial variation—was investigated using the I2 statistic [30]: 0–40 % indicating unimportant levels; 30–60 % showing moderate heterogeneity; 50–100 % as demonstrable heterogeneity within trials. Sensitivity analyses were undertaken as proposed by Deschartres et al. [34] along with meta-regression to assess whether heterogeneity could be attributed to measurable sources. An assessment for the potential of publication bias was made via assessment of the funnel plot generated for all trials included in the meta-analysis. We produced a narrative description of adverse events and readmission rates.
Results
Searches and selection
After removal of duplicates, the searches yielded 1489 citations, from which 1443 were excluded at the title/abstract stage (Fig. 1). We retrieved 46 full papers for further review of which 28 were excluded because they were not a controlled trial (n = 4) [35–38], were in the wrong setting (n = 7) [39–45] or wrong population or intervention (n = 15) [17, 46–59], or were not available in English (n = 2) [60, 61]. All included studies were parallel group, participant-randomised trials. 3 were multi-centre; 9 were single centre and the number of centres was unknown for 6. We identified 18 trials (2225 participants, excluding one trial as the number of participants is unknown [26]) eligible for inclusion at the full paper review stage (Fig. 1). Details of all excluded trials at the full stage can be found in Additional file 5.
Fig. 1.
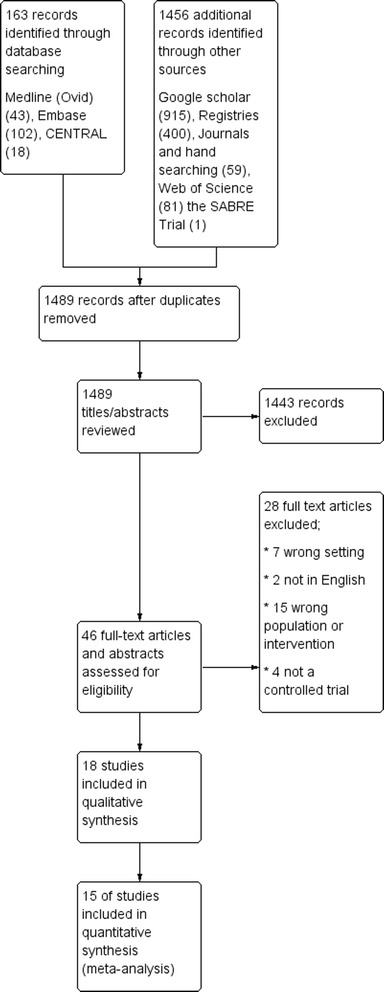
Study flow diagram
Study characteristics
The remaining 18 trials (total number of children included in the analysis) were conducted in: Italy (n = 106) [62]; United Arab Emirates and Canada (n = 91) [20]; China (n = 205) [63, 64]; Israel (n = 93) [18, 19]; Argentina (n = 82) [25]; India (n = 388) [65–67]; Qatar (n = 171) [68]; Georgia (n = 42) [69]; The Netherlands (n = 247) [70]; USA (n = 190) [71]; Turkey (n = 69) [27]; Mexico (n = unknown) [26]; Nepal (n = 59) [72] and UK (n = 290) [73] (Additional file 6). The sample size ranged from 40 [67] to 317 [73] participants. Eligibility criteria varied significantly in terms of patient characteristics and disease severity (Additional file 7). The upper age limit for subjects ranged from 12 to 24 months. Amongst the range of clinical characteristics required for inclusion were ‘bronchiolitis with temperature >38 °C’ , ‘first episode of wheezing with evidence of viral infection’ , ‘wheezing’ , ‘wheeze and/or crackles’ , ‘crackles’ , and ‘first episode of bronchiolitis’. In terms of severity, oxygen saturation in air was specified in 4 studies and ranged from <97 to <92 %. Numbers of participants allocated to each group was clearly defined in all but three trials [26, 27, 69] whose data was not usable in the meta-analysis as attempts to contact the author were fruitless.
Population characteristics
The average age ranged from 2.6 months [19] to 8.6 months (median = 4.5 months). Five studies including children under 12 months age only [18, 19, 65, 70, 72]. The heterogeneity of study design and patient populations was emphasised by the indicators of disease severity at entry to the study. Baseline oxygen saturations were reported in nine studies [18–20, 62, 65, 66, 68, 70, 72]; these ranged from very mild (SaO2 of 90.5 % [64]) to significant respiratory compromise (SaO2 of 97.4 % [68]). Clinical scoring systems were used to classify disease severity in 15 of 18 studies [18–20, 26, 27, 62–68, 70–72]. Based on the scores at entry, the disease severity varied from mild/moderate [n = 5] [27, 63, 67, 69, 70], moderate [n = 5] [18–20, 25, 65],moderate to severe [n = 4] [26, 64, 66, 68] and severe [n = 2] [62, 73] confirming the heterogeneity of disease severity across the studies. A severity classification was not provided for Ojha et al. and Silver et al. [71, 72], but based on their characteristics were subjectively classified “severe” and “mild/moderate” respectively. Disease severity was assessed in fifteen of the 18 trials using a variety of scoring systems to characterise participants as “mild”, “moderate” or “severe” or a combination of each; Wang et al. CSS scoring system [29] (n = 8) [18, 19, 62–65, 67, 70], Respiratory Distress Assessment Instrument (RDAI) score (n = 3) [20, 66, 71], Bronchiolitis Severity Score (n = 1) [68], Bronchiolitis Clinical Score (n = 1) [69], Respiratory assessment score (n = 1) [27], Respiratory Distress Scale Sant Joan de Deu Hospital (n = 1) [26] and Clinical scoring of respiratory distress (n = 1) [72] (Table 1).
Table 1.
Population characteristics
| Study | Age—mean (SD) | Gender | Disease severity | Length of hospital stay mean (SD) (days) | Final CSS score |
|---|---|---|---|---|---|
| Al-Ansari 2010 et al. [68] | Intervention (3 % HS): 3.84 (2.84) | Intervention (3 % HS): F19 M39 | Moderate to severe | Intervention (3 % HS): 1.4 (1.41) | NR |
| Intervention (5 % HS): 4.02 (2.56) | Intervention (5 % HS): F26 M31 | Intervention (5 % HS): 1.56 (1.38) | |||
| Control: 3.30 (2.43) | Control: F26 M31 | Control: 1.88 (1.76) | |||
| Espelt et al. 2012 [25] | NR | Intervention: F24 M26 | Moderate | Intervention: 5.8 (2.7) | NR |
| Control: F26 M24 | Control: 5.47 (2.1) | ||||
| Everard et al. 2014 [73] | Intervention: 3.3 (2.6) | Intervention: F69 M73 | Severe | Intervention: 4.19 (3.20) | NR |
| Control: 3.4 (2.8) | Control: F64 M85 | Control: 4.22 (3.52) | |||
| Giudice et al. 2012 [62] | Intervention: 4.8 (2.3) | Intervention: F18 M34 | Severe | Intervention: 4.9 (1.3) | Intervention: 6.5 (1.6) |
| Control: 4.2 (1.6) | Control:F19 M35 | Control: 5.6 (1.6) | Control: 7.7 (1.6) | ||
| Kuzik et al. 2007 [20] | Intervention: 4.4 (3.7) | Intervention: F20 M27 | Moderate | Intervention: 2.6 (1.9) | NR |
| Control: 4.6 (4.7) | Control: F19 M30 | Control: 3.5 (2.9) | |||
| Luo et al. 2010 [63] | Intervention: 6.0 (4.3) | NR | Mild to | Intervention: 6 (1.2) | Intervention: 1.5 (0.5) |
| Control: 5.6 (4.5) | moderate | Control: 7.4 (1.5) | Control: 2.9 (0.7) | ||
| Luo et al. 2011 [64] | Intervention: 5.9 (4.1) | NR | Moderate to severe | Intervention: 4.8 (1.2) | Intervention: 1.7 (0.6) |
| Control: 5.8 (4.3) | Control: 6.4 (1.4) | Control: 3.1 (0.7) | |||
| Maheshkumar et al. 2013 [67] | NR | NR | Mild to moderate | Intervention: 2.25 (0.89) | NR |
| Control: 2.88 (1.76) | |||||
| Mandelberg et al. 2003 [18] | Intervention: 3 (1.2) | Intervention: F12 M15 | Moderate | Intervention: 3 (1.2) | NR |
| Control: 2.6 (1.9) | Control: F9 M15 | Control: 4 (1.9) | |||
| Nemsadze et al. 2013 [69] | NR | NR | Mild to moderate | Intervention: 4.4 (1.1) | NR |
| Control: 4.9 (1.2) | |||||
| Ojha et al. 2014 [72] | Intervention: 8.61 (5.74) | NR | NR | Intervention: 1.87 (0.96) | NR |
| Control: 8.51 (4.24) | Control: 1.82 (1.18) | ||||
| Ozdogan et al. 2014 [27] | Overall: 7.1 (5.48) | NR | Mild to moderate | NR | NR |
| Pandit et al. 2013 [66] | NR | NR | Moderate to severe | Intervention: 3.92 (1.72) | NR |
| Control: 4.08 (1.90) | |||||
| Sharma et al. 2013 [65] | Intervention: 4.93 (4.31) | Intervention: F28 M97 | Moderate | Intervention: 2.64 (0.88) | NR |
| Control: 4.18 (4.24) | Control: F31 M92 | Control: 2.66 (0.93) | |||
| Silver et al. 2014 [71] | Intervention: 3.86 (3.01) | Intervention (3%HS): F31 M62 | NR | Intervention: 2.49 (1.64) | NR |
| Control: 4.39 (2.95) | Control: F37 M60 | Control: 2.47 (1.76) | |||
| Sosa-Bustamante et al. 2014 [26] | NR | NR | Moderate to severe | NR | NR |
| Tal et al. 2006 [19] | Intervention: 2.8 (1.2) | Intervention: F11 M10 | Moderate | Intervention: 2.6 (1.4) | Intervention: 5.35 (1.3) |
| Control:2.3 (0.7) | Control: F7 M13 | Control: 3.5 (1.7) | Control: 6.45 (1) | ||
| Teunissen et al. 2014 [70] | Intervention (3 % HS): 3.6 (5.2) | Intervention (3 % HS): F40 M44 | Mild to moderate | Intervention (3 % HS): 3.43 (2.24) | Intervention (3 % HS): 3.87 (3.15) |
| Intervention (6 % HS): 3.4 (3.8) | Intervention (6 % HS): F35 M48 | Intervention (6 % HS): 3.74 (2.99) | Intervention (6 % HS): 5.16 (4.20) | ||
| Control: 3.6 (5.0) | Control: F31 M49 | Control: 2.82 (2.25) | Control: 4.61(5.38) |
F Female, M male, NR Not reported, HS Hypertonic saline
Intervention characteristics
All trials administered 3 % hypertonic saline via a nebuliser as the active intervention; three trials were designed with an additional arm using higher concentrations of hypertonic saline at 5 % [27, 68] or 6 % [70]. Fifteen of the eighteen trials stated the amount of hypertonic saline administered which ranged from 3 ml [25, 67] to 4 ml [18–20, 26, 63–66, 70–73] or in one trial 5 ml [68]. Four trials compared hypertonic saline versus normal saline alone [20, 64, 71, 72] or versus standard care [73] with no additional treatments. Where the flow rate was stated (n = 10) it ranged between 5 and 10 L/min [18, 19, 62, 65–68, 70, 71, 73]. Investigators in the other trials administered different adjunct treatments alongside hypertonic saline: five trials administered epinephrine/adrenaline [18, 19, 62, 66, 68]; seven administered a β2 agonist (salbutamol, albuterol) [25–27, 63, 65, 67, 70]; one trial did not specify any additional medication [69] and five trials did not administer different adjunct treatments alongside hypertonic saline [20, 64, 71–73].
Comparator characteristics
The majority of trials stated that they administered 0.9 % of normal saline as the control group except one where “oxygen therapy plus best supportive care” was the control [73] or where authors did not state the concentration of normal saline [67, 69]. Eight of the trials gave oxygen therapy in both the intervention and control arms [20, 62, 64, 65, 67, 68, 70, 73], nine trials did not specify whether oxygen therapy was provided [18, 19, 25–27, 63, 69, 71, 72] and one trial stated oxygen therapy was provided in the intervention arm but did not state whether this was provided in the control arm [66]. The flow rate of the nebulisers in the control groups matched those in the intervention arm of each trial.
Risk of bias
The risk of bias was variable across studies (Additional file 8 and Fig. 2). Eleven trials had clearly adequate allocation concealment, using pharmacy to prepare and administer solutions [20, 62], sequential identical containers [19, 65, 70], using both these methods [72], a web-based randomisation system [71, 73] or sealed, opaque envelopes [64, 66, 68]. Randomisation was adequately described in eleven trials based on lists generated by computer systems or computer generated random number tables [19, 20, 62, 64–68, 71–73]. Participants and investigators were blinded to the treatment given again in fourteen of the 18 studies; three trials were “open label” [25, 66, 73] and one gave no description of blinding [65]. Blinding of the outcome assessor for the primary outcome was adequately described with the attending physician who made the decision to discharge blinded in five studies [18, 19, 63, 64, 71] and all medical staff, participants and staff blinded in one trial [70]. Participant withdrawal and the use of intention to treat analysis were clearly explained in all but five trials [25–27, 68, 69].
Fig. 2.
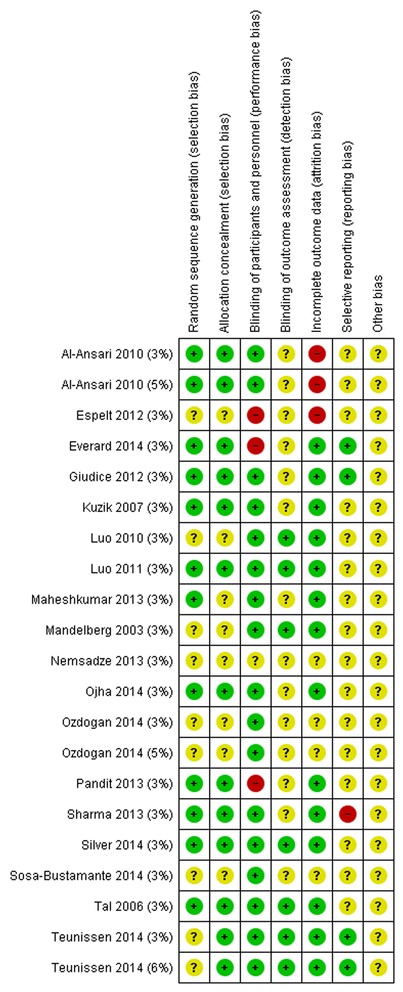
Risk of bias
We graded two of these as being at high risk of bias: one provided no explanation regarding the uneven distribution of withdrawals [25] and one did not state which arms the 16 patients were excluded from [68]. The median loss to-follow-up at the time of the primary outcome assessment (LoS) was 8 % (range 0 % [20, 63, 66, 67] to 18 % [25, 72]; twelve studies did not complete an intention to treat analysis, presenting instead an ‘available case analysis’ for only those participants for whom a LoS could be identified [18–20, 25, 62, 64, 65, 68, 70–73]. Three studies completed a full analysis on all participants randomised [63, 66, 67].
Results and synthesis
Data from three studies [26, 27, 69] could not be included in the analysis of the primary outcome (LoS) because it was unclear how many participants were randomised to each study arm or the mean and SD LoS was unavailable; attempts to contact the authors failed. Twelve trials presented available case analyses for the primary outcome, LoS, and the denominators for these trials express the number analysed, rather than the number randomised (Figs. 3 and 4) [18–20, 25, 62, 64, 65, 68, 70–73].
Fig. 3.
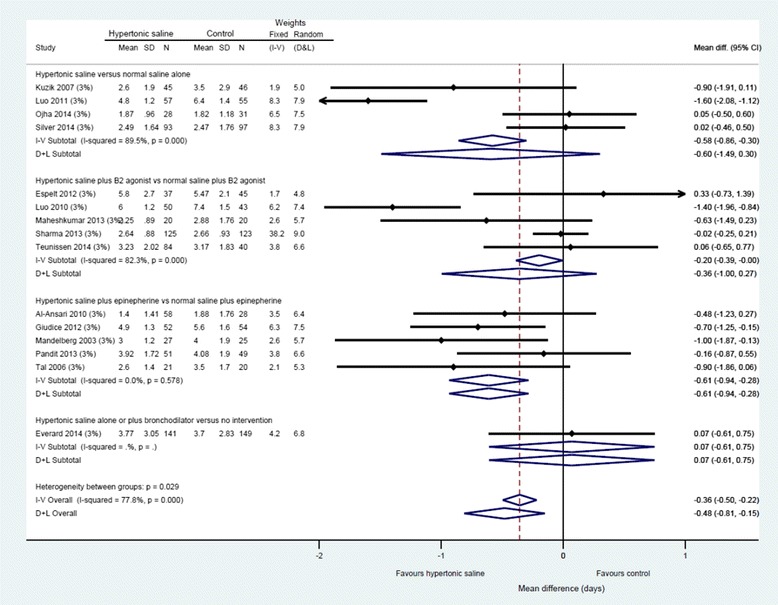
Difference in length of hospital stay by intervention subgroup, 3 % hypertonic saline
Fig. 4.
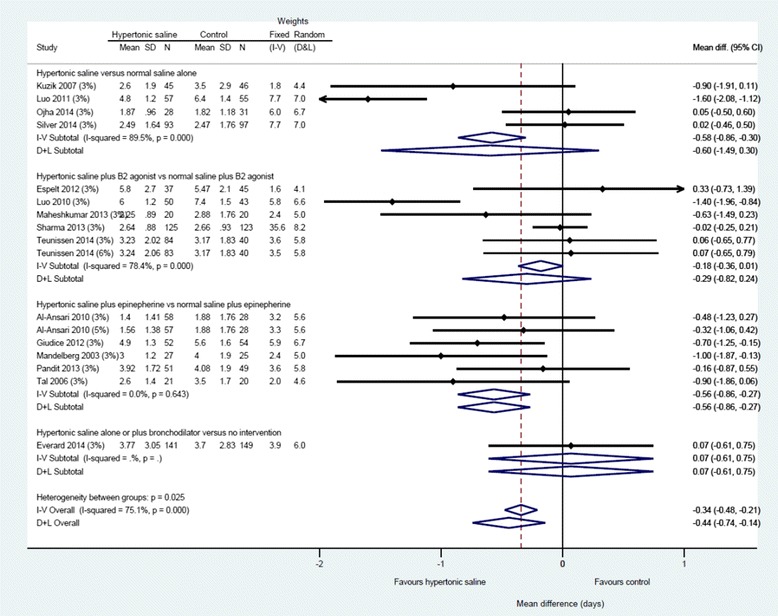
Difference in length of stay by intervention subgroup, all concentrations of hypertonic saline
Across fifteen studies (n = 1922 participants), hypertonic saline, compared with any comparator, reduced mean LoS by approximately a third of a day using a fixed effect approach [mean difference = −0.36, 95 % CI −0.50 to −0.22 days; Fig. 3]. Reflecting the high levels of statistical heterogeneity (I2 = 78 %) a random-effects analysis was undertaken: this gave a pooled effect size of −0.44 (−0.14 to −0.74) The between-trials variance (τ2 = 0.27) can be used to derive a prediction interval for the of effect size in a future: this ranged from −1.59 to +0.71. [74] The results are presented in (pre-specified) subgroups based on standard care, but considerable heterogeneity remains even within these. The funnel plot for LoS was not classically symmetrical, but neither was it suggestive of publication bias or small study effects (see Fig. 5).
Fig. 5.
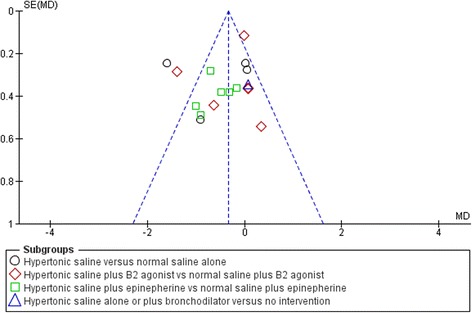
Funnel plot, difference in length of hospital stay (whole group)
To further explore the heterogeneity we performed sensitivity analyses. Firstly, the contribution of each study to the overall effect size and heterogeneity were assessed graphically by constructing a Baujat plot (Fig. 6) [75]. The figure shows, for each trial, the impact of excluding it from the final analysis (vertical axis), plotted against its contribution to the heterogeneity statistic (horizontal axis). As a general guide, with homogeneity the contribution to heterogeneity (horizontal axis) should range between approx. 0–5 for each study. Two studies are clear outliers, contributing excessively to the heterogeneity [63, 64]; a third contributes to both heterogeneity and effect size, which is to be expected given it contains 38 % of the overall weight [65].
Fig. 6.
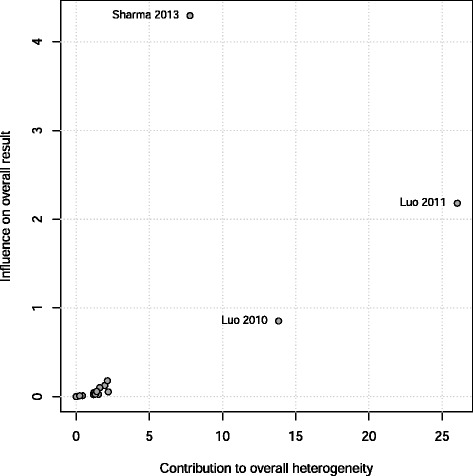
Baujat plot comparing weight to overall heterogeneity
Secondly, we reproduce the approach taken by Dechartres [34] in which the original analyses are compared to those derived from i) the single most precise trial (the trial with the narrowest confidence interval [65]); ii) the pooled average of largest 25 % of trials; iii) the beta0 limit-analysis proposed by Rücker et al. (2011) to control for small-study effects [76]; and iv) restricted to trials at low overall risk of bias. For the latter, we undertook two analyses, the first allowing unblinded trials which were otherwise considered low RoB, and the second limiting to blinded low RoB trials. These secondary analyses are depicted in Fig. 7. Lastly, we repeated the analyses excluding the two outlying trials identified above [63, 64]. The resulting analyses exhibit considerable disparities. The overall results are contradicted by the largest and most precise trials, both of which estimate virtually no difference between the groups. Excluding the two Luo trials greatly reduces the heterogeneity with the overall I2 falling from 75 to 23 % and the overall effect size reduced to a more modest (albeit statistically significant) difference of 3.8 h (I-V analysis; 95 % CI 0.2 to 7.2 h) or 5.0 h (D&L analysis; 95 % CI 0.2 to 9.6 h).
Fig. 7.
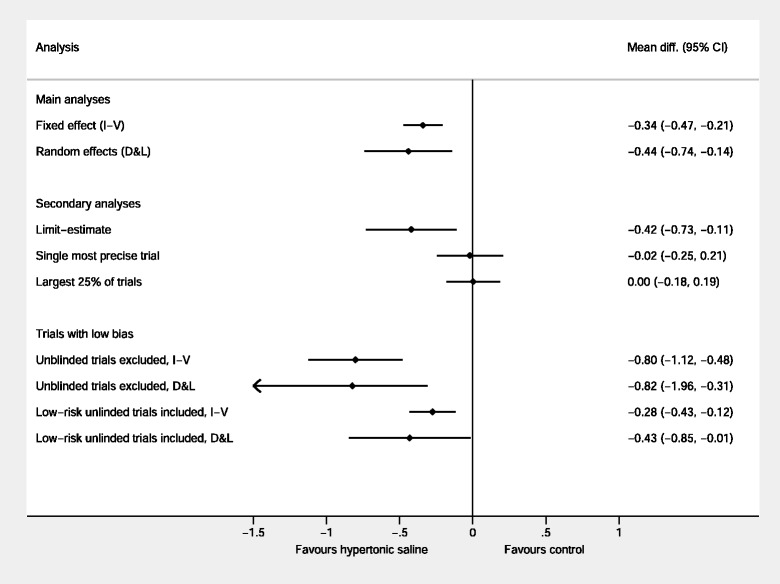
Sensitivity analyses based on precision, size, small study effects and risk of bias
Lastly we used meta-regression to explore whether any of the sources of heterogeneity could explain the inconsistency (Fig. 8). Specifically, we assessed the relationship between the effect sizes observed within each trial against its i) mean age; ii) mean baseline oxygen saturation; and iii) severity classification. None of the associations were statistically significant and, more importantly, the residual heterogeneity statistics (ie variation remaining after adjustment) was virtually unchanged, indicating the between-trial variation was not well explained by any of the three characteristics explored.
Fig. 8.
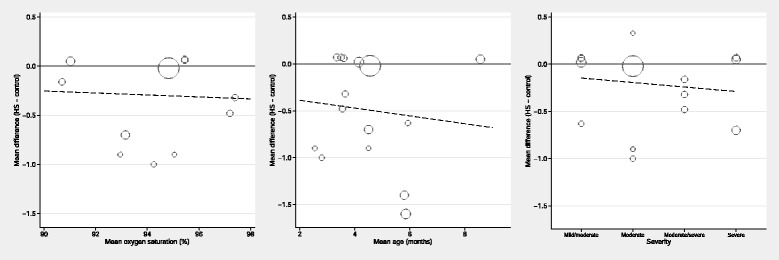
Meta-regression investigating age, baseline oxygen saturation, and severity classification as sources of heterogeneity
Five trials contributed to the mean difference in final CSS scores (n = 516, MD −1.36 (95 % CI −1.52 to −1.20); we observed no statistical heterogeneity [19, 62–64, 70]. One trial reported one SAE possibly related to the intervention [73], no other studies described intervention related SAE’s.
In an analysis of three studies that reported readmission rate [68, 71, 73] (651 participants), no difference was observed between hypertonic saline and control [RR 0.90, 95 % CI 0.52 to 1.55]; there was no statistical heterogeneity (I2 = 0 %). A decision was made not to attempt a meta-analysis of adverse event data which, where reported, was mainly narrative and not consistently captured across trials. We present a narrative summary in Additional file 9. One study described one SAE, of bradycardia and desaturation during administration of the nebuliser, which had resolved by the following day [73], no other studies described any serious adverse events related to the intervention of hypertonic saline.
Discussion
Summary of findings
Overall hypertonic saline was associated with a reduced length of stay, with a pooled difference of −0.36, [95 % CI −0.50 to −0.22]. The evidence for this result is of moderate varies quality (GRADE summary of findings in Table 2). However, our confidence in this estimate is undermined by the excessive heterogeneity of the results (I2 = 78 %) and inconsistency between the main and sensitivity analysis in general. Of particular note is that the overall pooled estimate is contradicted by the four largest component trials [65, 70, 71, 73], all of which found no benefit of hypertonic saline. The true impact of hypertonic saline therefore appears to depend greatly on the patient population within which each trial was conducted, and in particular the level of standard care.
Table 2.
Summary of findings table
| Assumed risk | Corresponding risk | Relative effect (95 % CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Outcomes | Normal saline (+/− adjunct treatment) or oxygen therapy plus best supportive care | Hypertonic saline (+/− adjunct treatment) | |||
| Hypertonic saline versus normal saline alone (days) | The mean length of hospital stay ranged across control groups from 1.82 to 6.4 days | The mean length of hospital stay ranged across hypertonic saline groups from 1.87 to 4.8 days | 0.58 (95 % CI −0.86 to −0.30) days | 452 (4 inpatient trials) | ⊕ ⊕ ⊕ ⊕ higha |
| Hypertonic saline plus B2 agonist vs normal saline plus B2 agonist (days) | The mean length of hospital stay ranged across control groups from 2.66 to 7.4 days | The mean length of hospital stay ranged across hypertonic saline groups from 2.25 to 6 days | 0.18 (95 % CI −0.36 to 0.01 days) | 710 (5 inpatient trials) | ⊕ ⊕ ⊕⊖ moderateb |
| Hypertonic saline plus epinepherine vs normal saline plus epinephrine (days) | The mean length of hospital stay ranged across control groups from 1.88 to 5.6 days | The mean length of hospital stay ranged across hypertonic saline groups from 1.4 to 4.9 days | 0.56 (95 % CI −0.86 to −0.27 days) | 470 (5 inpatient trials) | ⊕ ⊕ ⊕ ⊕ highc |
| Hypertonic saline alone or plus bronchodilator versus no intervention (days) | The mean length of hospital stay for control groups was 3.7 days | The mean length of hospital stay in the hypertonic saline group was 3.7 days | 0.07 (95 % CI −0.61 to 0.27 days) | 290 (1 inpatient trial) | ⊕ ⊕ ⊕⊖ moderated |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95 % confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95 % CI)
CI Confidence interval; RR Risk Ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
aSubstantial heterogeneity; all studies double blinded and generally low risk of bias
bSubstantial heterogeneity; one study had incomplete outcome data and was un-blinded
cNo heterogeneity; one study had incomplete outcome data and one was un-blinded
dSingle study, no blinding
Strengths and limitations of the findings
High levels of statistical heterogeneity associated with the meta-analysis dominate the results making their interpretation challenging. The addition of concomitant medications may explain some of this heterogeneity across intervention subgroups, with as the largest effect sizes found in trials where hypertonic saline was given alone. Studies were conducted across a number of different healthcare settings with diverse local services, usual care, guidelines (e.g. definition of “fit to discharge”) and disease severity at entry, all of which must contribute to the extensive intra-trial variation observed. That many of the largest trials contributed such little weight to the analysis undermines our confidence in the results. Furthermore, the absence of re-admission rate in the majority of trials may suggest the intervention is not as economically beneficial as the results suggest.
A key strength is the inclusion of 15 of the 18 trials in the meta-analysis of the primary outcome. This facilitated both investigation of publication bias and allowed for subgroup analyses and aggregate data meta-regression, although there was incomplete data in relation to secondary outcomes and trial design features.
One can argue the potential of publication bias based on the uncharacteristic funnel plot shape. Systematic error is not thought to always be caused by application of language restrictions to meta-analyses despite the potential reduction of the precision of pooled estimates [77]. Nonetheless, restriction of trials to only English articles may have altered the precision, effect size, heterogeneity and overall risk of bias.
Summary of heterogeneity
In undertaking this systematic review it has become apparent that there are a number of semantic, methodological and cultural differences across the studies, all of which impacts on the results obtained and the generalisability of an individual trial’s findings. We propose some of these factors below, and offer an explanation for how these may impact on the interpretation of the review’s findings.
-
i)
The definition of ‘acute bronchiolitis’ differs between countries, and indeed across clinicians in the same institution. Inevitably this diversity was reflected in the description of infants included which variously specified wheeze and or crackles (n = 4) [20, 68, 70, 73]; a first episode of wheezing (n = 5) [62–66]; “bronchiolitis” (n = 4) [19, 67, 71, 72]; or bronchiolitis with a temperature >38C (n = 1) [18]; while information was absent in four others [25–27, 69]. The term “wheeze” is itself open to interpretation (and sometimes misinterpretation) within the medical profession [78–82], and may be taken to include children presenting with their first exacerbation of asthma, and manifesting as bronchospasm. The occurrence of this is less common among younger patients, and as a consequence we may have expected the effect size to vary according to the mean age of the study population. Nevertheless, our meta-regression to investigate this was equivocal.
A more immediate explanation is that the impact of HS varied with severity. All patients included in this study met the definition of acute bronchiolitis as used in the UK, Australia and parts of Europe which in summary involves apparent viral infection, signs of lower respiratory tract disease with airflow obstruction manifest by increased work of breathing, hyperinflation of the chest and widespread crackles, with or without intermittent wheeze. Clearly there are considerable differences in setting and in the types of patients included in different studies.
-
ii)
Variation among discharge criteria
The consistency of the outcomes—specifically ‘length of stay’ and ‘fit for discharge’—is self-evidently defined and assessed in very different ways across the studies. Moustgaard et al. suggest that definition of outcomes in trials is a widespread problem[83]. The studies set (sometimes arbitrary) criteria regarding when the patient stay started, including “from study entry, which was within 12 h of admission” (n = 2) [20, 62]; from hospital admission (n = 3) [65, 68, 73]; or from first dose of study medication (n = 2)[70, 71]; information was absent for the remaining 11 studies [18, 19, 25–27, 63, 64, 66, 67, 69, 72]. The reported time to entry into study varied from 3 to 24 h, and generally did not specify whether “entry” corresponded to consent or first treatment. The latter criterion in particular represents a huge proportion of an admission in units with mean stays of 72 h or less. Similarly, discharge was defined and assessed differently across studies. In one study the discharge assessment used a continuous discharge criteria [73],but in at least five others the decision to discharge was made only once a day [18, 19, 63, 65, 66], meaning the time of discharge is effectively a discrete outcome which occurs at intervals of 24 h. Although this inevitably overestimates the real time taken to be fit for discharge, it does so equally for both groups and would be expected to underestimate rather than overestimate the difference between the groups. With this in mind we have no explanation for why the positive studies are based on a once daily clinical assessment.
In the remaining studies the frequency of assessment for discharge was unclear. We present a summary of the discharge criteria in Additional file 10.
The criteria for discharge range from saturating 92 % or greater in air & oral feeding >75 % of usual intake [73] to no respiratory signs or symptoms for the previous 12 h [63, 64]. As may be expected, stricter criteria leads to longer LoS. The criteria that patients should be free of any signs or symptoms is curious as it has been well documented that the symptoms associated with acute bronchiolitis persists for many days or even weeks [73, 84]. Behrendt et al. previously noted a marked variation in length of stay of patients admitted with RSV bronchiolitis with very short admissions [median approx. 72 h] in USA, UK and Northern Europe as compared with significantly longer admissions in Germany and Southern Europe [85] a finding corroborated by more recent trials that have been included herein. These longer admissions were associated with increased co-morbidities such as diarrhoea which may be as a result of nosocomial infection resulting from longer admission times. This cultural difference is again noted with none of the Italian subjects in the study of Giudice being discharged before 72 h, a period beyond the mean ‘length of stay’ in the Dutch, UK and USA study [70, 73, 85]. Finally, the subjects in the Luo studies with mild to moderate [63] bronchiolitis remained in hospital longer than those with more severe disease [64], a finding which is somewhat difficult to explain.
-
iii)
Publication, generalisability and other biases
This difference in practice may also, in large part, explain the differences in observed treatment effects in the large UK, Dutch and USA studies which found no benefit as compared with the apparently large effect observed in other studies [70, 71, 73]. While early indications of a potential benefit may have been attributable to publication bias [86, 87] the positive effects of later large studies may be attributable to study design and cultural effects. It is of note that all the recent studies of hypertonic saline have failed to demonstrate any benefit yet the ‘meta-analysis still appears to favour the treatment. This effect is largely driven by the relatively large studies of Luo et al. and it is likely that this is explicable when considering discharge criteria in more detail (see above).
In summary therefore, there remains considerable heterogeneity which are not germane to being captured and quantified by standard meta-regression tools. Clearly, a large amount of the heterogeneity is driven by two trials from the same team, led by Luo [63, 64], with outlying results, relatively small sample sizes but narrow confidence intervals (around a day, compared with a day-and-a-half in SABRE [73] and the other large northern European study—Teunissen 2014 [70]). The removal of these two studies from the main analysis considerably reduces the effect sizes and statistical significance in the analyses to a more modest (and minimal) impact. Nevertheless, this does not eliminate heterogeneity completely.
Finally, there choice whether to favour a fixed- or random-effects analysis remains open to debate, with strong and apparently compelling proponents on both sides [32, 88–91]. The presence of unexplained heterogeneity goes against the assumption of a single underlying (fixed) effect, and this is commonly taken to justify the random effect model. When the heterogeneity is excessive however, the random effect model has the unfortunate operational characteristic of allocating similar weights to all trials, irrespective of their size and precision. Our decision to pre-specify a fixed-effect as the primary analysis was taken to counter this limitation. That said, we are unable to offer a clinically sensible reason why the largest trial should be allocated only 4 % of the weight in this analysis. Given this, together with the large and unexplained heterogeneity in general, our recommendation is that no single overall summary measure—fixed, random or otherwise—is an adequate reflection of the identified trials. Although we investigated response in relation to dose (3, 5 or 6 %), the studies did not provide data on frequency or duration of HS, which may also have varied across studies.
Strengths and limitations compared to other reviews
Building on the review conducted by Zhang and colleagues which contained 11 RCTs (n = 1090), our review included 15 trials (n = 1922) which included three much larger trials which unanimously showed null results [65, 70, 73]. We limited our inclusion criteria to trials of inpatient infants, whereas Zhang et al. also included outpatient and emergency department trials. Al-Ansari has been included in our review despite being included in the emergency department group by Zhang and colleagues, as the length of stay infers that the patients were admitted [68]. Despite this, our meta-analysis included a further 8 trials [25, 65–67, 70–73] which altogether unearthed significantly higher levels of heterogeneity than that stated in the previous Cochrane review. A potential explanation is that we applied no restrictions in terms of dose or way the intervention was administered, and in addition we included data from one unpublished study: Zhang et al. made no statement in regards to these.
Duplication is not without merit—it enables the replicability of methods to be demonstrated, as well as adding weight to or disputing the current evidence base [92–94]. Even when faced with identical data, approaches taken and interpretations made can differ between researchers [95]. A well-defined rationale for any such duplicate review, as required by the PRISMA checklist (though not explicitly) [94], provides transparency regarding overlaps and subsequently, allows for informed debate about its value to the evidence base [95].
Implications for policy and practice
The disparities between the results of the largest, most precise trials and all the included trials, together with high levels of heterogeneity, mean that neither individual trials nor pooled estimates provide a firm evidence-base for the use of hypertonic saline in inpatient acute bronchiolitis [34]. The refutation of initially large treatment effects in small trials by larger trials stronger is a phenomenon which is observed more widely and should not surprise the reader [96]. For instance, in the treatment of acute bronchiolitis, initial evidence supporting the use of β2-agonists has also been overturned as successive trials have been published [97].
Further research
Our aggregate level data analysis was unable to identify specific settings and characteristics which influence the effect of HS on LoS. Systematic sensitivity analyses, ideally based on individual patient data and regression analyses are warranted to better understand why hypertonic saline showed substantial benefit in some trials yet none in others [34]. In the absence of this, there is no robust evidence to support the use of hypertonic saline.
Conclusions
Claims that hypertonic saline achieves small reductions in LoS must be treated with scepticism based on the 15 known trials of HS. The findings appear at best highly dependent on trial design and local policies. We cannot rule out the possibility that inhaled HS offers symptomatic relief but have no data to support or deny this possibility.
Acknowledgements
No specific funding supported the initial work on this review, however revisions to the work were undertaken during the write-up of the Hypertonic Saline in Acute Bronchiolitis: Randomised Controlled Trial and Economic Evaluation (SABRE). This project was funded by the Health Technology Assessment programme (HTA 09/91/22) and will be published in full in the Health Technology Assessment journal series. Further information available at: http://www.nets.nihr.ac.uk/projects/hta/099122. This report presents independent research commissioned by the NIHR. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, CCF, NETSCC, the Health Technology Assessment programme or the Department of Health. The Authors gratefully acknowledge the contribution of unpublished data by Bettina Loza of the VieCuri Medisch Centrum voor Noord Limburg, Venlo, The Netherlands and Alyssa Silver of The Children’s Hospital of Montefiore, Bronx, New York.
Additional files
PROSPERO registration. (PDF 109 kb)
PRISMA statement. (DOCX 24 kb)
Search strategies. (DOCX 20 kb)
ORBIT classification outcome matrix. (DOCX 16 kb)
Excluded studies at full paper review stage. (DOCX 20 kb)
Study characteristics. (DOCX 43 kb)
Clinical issues. (DOCX 34 kb)
Risk of bias. (DOCX 21 kb)
Adverse events narrative. (DOCX 22 kb)
Discharge criteria. (DOCX 18 kb)
Footnotes
Competing interests
The authors declare that they have no completing interests.
Authors’ contributions
CM participated in the design and conception of the systematic review, designed data collection tools, coordinated and conducted the data collection, contributed to the analysis, interpretation of the results and drafted the manuscript. HC coordinated and conducted the data collection, contributed to the analysis and drafted the manuscript. DH participated in the design and conception of the systematic review, coordinated and conducted the data collection, contributed to the analysis, interpretation of the results and drafted the manuscript. MB participated in statistical analyses, interpretation of the results and drafted the manuscript. MLE contributed to the analysis, interpretation of the results and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Chin Maguire, Email: c.maguire@sheffield.ac.uk.
Hannah Cantrill, Email: h.cantrill@sheffield.ac.uk.
Daniel Hind, Email: d.hind@sheffield.ac.uk.
Mike Bradburn, Email: m.bradburn@sheffield.ac.uk.
Mark L. Everard, Email: mark.everard@uwa.edu.au
References
- 1.Elliot S, Ray C. Viral infections of the lower respiratory tract. In: Taussig L, Landau L, editors. Pediatric respiratory medicine. 2nd edition. Philadelphia: MosbyElsevier; 2009. pp. 481–490. [Google Scholar]
- 2.Everard M. Respiratory syncytial virus bronchiolitis and pneumonia. In: Taussig L, Landau L, editors. Paediatric respiratory medicine. 2nd edition. St Louis: Mosby; 2009. [Google Scholar]
- 3.Smyth RL, Openshaw PJM. Bronchiolitis. Lancet. 2006;368:312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 4.Scottish Intercollegiate Guidelines Network SIGN. Bronchiolitis in children - a national clinical guideline [http://www.sign.ac.uk/pdf/sign91.pdf]
- 5.Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande SA, Northern V. The clinical and health economic burden of respiratory syncytial virus disease among children under 2 years of age in a defined geographical area. Arch Dis Child. 2003;88:1065–9. doi: 10.1136/adc.88.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin AJ, Gardner PS, Mcquillin J. Epidemiology of respiratory viral infection among paediatric inpatients over a six-year period in North-East England. Lancet. 1978;312:1035–1038. doi: 10.1016/S0140-6736(78)92351-6. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, et al. Risk factors for hospital admission with RSV bronchiolitis in England: A population-based birth cohort study. PLoS One. 2014;9:e89186. doi: 10.1371/journal.pone.0089186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Respiratory Syncytial Virus Activity - United States, July 2008-December 2009 [http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5908a4.htm] [PubMed]
- 11.Fleming DM, Elliot AJ, Cross KW. Morbidity profiles of patients consulting during influenza and respiratory syncytial virus active periods. Epidemiol Infect. 2007;135:1099–108. doi: 10.1017/S0950268807007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot AJ, Fleming DM. Influenza and respiratory syncytial virus in the elderly. Expert Rev Vaccines. 2008;7:249–58. doi: 10.1586/14760584.7.2.249. [DOI] [PubMed] [Google Scholar]
- 13.Falsey A, Hennessey P, Formica M, Cox C, Walsh E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–5. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds E, Cook C. The treatment of bronchiolitis. J Pediatr. 1963;63:1205–7. doi: 10.1016/S0022-3476(63)80215-2. [DOI] [PubMed] [Google Scholar]
- 15.Panickar JR, Dodd SR, Smyth RL, Couriel JM. Trends in deaths from respiratory illness in children in England and Wales from 1968 to 2000. Thorax. 2005;60:1035–8. doi: 10.1136/thx.2005.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming DM, Pannell RS, Cross KW. Mortality in children from influenza and respiratory syncytial virus. J Epidemiol Community Health. 2005;59:586–90. doi: 10.1136/jech.2004.026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarrell EM, Tal G, Witzling M, Someck E, Houri S, Cohen HA, et al. Nebulized 3 % hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest. 2002;122:2015–2020. doi: 10.1378/chest.122.6.2015. [DOI] [PubMed] [Google Scholar]
- 18.Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3 % hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest. 2003;123:481–487. doi: 10.1378/chest.123.2.481. [DOI] [PubMed] [Google Scholar]
- 19.Tal G, Cesar K, Oron A, Houri S, Ballin A, Mandelberg A. Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. ISR Med Assoc J Imaj. 2006;8:169–173. [PubMed] [Google Scholar]
- 20.Kuzik BA, Al-Qadhi SA, Kent S, Flavin MP, Hopman W, Hotte S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr. 2007;151:266–270. doi: 10.1016/j.jpeds.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2013;7:CD006458. doi: 10.1002/14651858.CD006458.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin Maguire, Hannah Cantrill. Nebulised hypertonic saline solution for acute bronchiolitis in infants: a systematic review and meta-analysis. PROSPERO 2014:CRD42014007569 Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014007569.
- 24.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2009;2011:CD006458. doi: 10.1002/14651858.CD006458.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Efficacy of Nebulized Hypertonic Saline in the Treatment of Acute Bronchiolitis (NCT01238848) [http://www.clinicaltrials.gov/ct2/show/NCT01238848?term=espelt+bronchiolitis&rank=1]
- 26.Nebulized 3 % Hypertonic Saline Solution Treatment of Bronchiolitis in Infants (NCT02233985) [https://clinicaltrials.gov/ct2/show/study/NCT02233985?term=“Hypertonic+saline”+AND+“bronchiolitis”&lup_s=01/03/2014&rank=8]
- 27.Ozdogan S, Koker O, Kose G, Yildirmak Y. The Efficacy Of Nebulized Hypertonic Saline In AcuteBronchiolitis In Hospital Setting: A Randomized And Double Blind Trial. Am J Respir Crit Care Med 2014;189(11): A2740. URL: http://www.atsjournals.org/doi/abs/10.1164/ajrccmconference. 2014.189.1_MeetingAbstracts.A2740.
- 28.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145:106–9. doi: 10.1164/ajrccm/145.1.106. [DOI] [PubMed] [Google Scholar]
- 30.Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] [www.cochrane-handbook.org]
- 31.Dwan K, Gamble C, Kolamunnage-Dona R, Mohammed S, Powell C, Williamson PR. Assessing the potential for outcome reporting bias in a review: A tutorial. Trials. 2010;11:52. doi: 10.1186/1745-6215-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Altman DG. Systematic reviews in health care: Meta-analysis in context, second edition - Wiley online library. London: BMJ; 2001. [Google Scholar]
- 33.Advanced Statistical Methods to Model and Adjust for Bias in Meta-Analysis [http://cran.r-project.org/web/packages/metasens/metasens.pdf]
- 34.Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA. 2014;312:623. doi: 10.1001/jama.2014.8166. [DOI] [PubMed] [Google Scholar]
- 35.Mandelberg A. Hypertonic saline in the treatment of acute bronchiolitis in the emergency department. Arch Pediatr Adolesc Med. 2010;164:395–397. doi: 10.1001/archpediatrics.2010.37. [DOI] [PubMed] [Google Scholar]
- 36.Principi T, Komar L. A critical review of “a randomized trial of nebulized 3 % hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department.”. J Popul Ther Clin Pharmacol. 2011;18:e273–e274. [PubMed] [Google Scholar]
- 37.Hom J, Fernandes RM. When should nebulized hypertonic saline solution be used in the treatment of bronchiolitis? Paediatr Child Health. 2011;16:157–8. doi: 10.1093/pch/16.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauvaget E, David M, Bresson V, Retornaz K, Bosdure E, Dubus JC. Nebulized hypertonic saline and acute viral bronchiolitis in infants: Current aspects]. [Review] [French. Arch Pediatr. 2012;19:635–641. doi: 10.1016/j.arcped.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Ipek IO, Yalcin EU, Sezer RG, Bozaykut A. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in moderate bronchiolitis. Pulm Pharmacol Ther. 2011;24:633–7. doi: 10.1016/j.pupt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol-normal saline, epinephrine-normal saline, and 3 % saline in mild bronchiolitis. Pediatr Pulmonol. 2010;45:41–7. doi: 10.1002/ppul.21108. [DOI] [PubMed] [Google Scholar]
- 41.Grewal S, Ali S, McConnell DW, Vandermeer B, Klassen TP. A randomized trial of nebulized 3 % hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department. Arch Pediatr Adolesc Med. 2009;163:1007–1012. doi: 10.1001/archpediatrics.2009.196. [DOI] [PubMed] [Google Scholar]
- 42.Kuzik BA, Flavin MP, Kent S, Zielinski D, Kwan CW, Adeleye A, et al. Effect of inhaled hypertonic saline on hospital admission rate in children with viral bronchiolitis: A randomized trial. CJEM Can Emerg Med care. 2010;12:477–484. doi: 10.1017/s1481803500012690. [DOI] [PubMed] [Google Scholar]
- 43.Sezer GR, Bozaykut A, Ipek IO, Uyur E, Seren PL, Paketci C. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol / hypertonic saline combination in first bronchiolitis attack. Acta Paediatr. 2010;99:153. [Google Scholar]
- 44.Jacobs JD, Foster M, Wan J, Pershad J. 7 % Hypertonic saline in acute bronchiolitis: A randomized controlled trial. Pediatrics. 2014;133:e8–13. doi: 10.1542/peds.2013-1646. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Baker C, Lang ME, Schrager SM, Liley FF, Papa C, Mira V, Balkian A, Mason WH. Nebulized Hypertonic Saline for Bronchiolitis: A Randomized Clinical Trial. JAMA Pediatr. 2014 Jul;168(7):657-63. doi: 10.1001/jamapediatrics.2014.301. Erratum in: JAMA Pediatr. 2014 Oct;168 (10):971. [DOI] [PubMed]
- 46.Gupta N, Puliyel A, Manchanda A, Puliyel J. Nebulized hypertonic-saline vs epinephrine for bronchiolitis: Proof of concept study of cumulative sum (CUSUM) analysis. Indian Pediatr. 2012;49:543–7. doi: 10.1007/s13312-012-0122-5. [DOI] [PubMed] [Google Scholar]
- 47.Khashabi J, Salari LS, Karamiyar M, Mussavi H. Comparison of the efficacy of nebulized L-epinephrine, salbutamol and normal saline in acute bronchiolitis: A randomized clinical trial. Med J Islam Repub Iran. 2005;19:2005. [Google Scholar]
- 48.Lines DR, Bates ML, Rechtman AR, Sammartino LP. Efficacy of nebulised ipratropium bromide in acute bronchiolitis. Pediatr Rev Commun. 1992;6:1992. [Google Scholar]
- 49.Milner A. The role of anticholinergic in acute bronchiolitis in infancy. [French] Arch Pediatr. 1995;2:159S–162S. doi: 10.1016/0929-693X(96)89885-X. [DOI] [PubMed] [Google Scholar]
- 50.Patel H, Platt RW, Pekeles GS, Ducharme FM. A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824. doi: 10.1067/mpd.2002.129844. [DOI] [PubMed] [Google Scholar]
- 51.Tinsa F, Rhouma AB, Ghaffari H, Boussetta K, Zouari B, Brini I, et al. A randomized, controlled trial of nebulized terbutaline in the first acute bronchiolitis in infant less than 12 months old. Tunis Med. 2009;87:200–3. [PubMed] [Google Scholar]
- 52.Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D, et al. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27–35. doi: 10.1056/NEJMoa022226. [DOI] [PubMed] [Google Scholar]
- 53.Postiaux G, Louis J, Labasse HC, Gerroldt J, Kotik AC, Lemuhot A, et al. Evaluation of an alternative chest physiotherapy method in infants with respiratory syncytial virus bronchiolitis. Respir Care. 2011;56:989–994. doi: 10.4187/respcare.00721. [DOI] [PubMed] [Google Scholar]
- 54.Hariprakash S, Alexander J, Carroll W, Ramesh P, Randell T, Turnbull F, et al. Randomized controlled trial of nebulized adrenaline in acute bronchiolitis. Pediatr Allergy Immunol. 2003;14:134–139. doi: 10.1034/j.1399-3038.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 55.Chowdhury D, Al HM, Khalil M, Al-Frayh AS, Chowdhury S, Ramia S. The role of bronchodilators in the management of bronchiolitis: A clinical trial. Ann Trop Paediatr. 1995;15:77–84. doi: 10.1080/02724936.1995.11747752. [DOI] [PubMed] [Google Scholar]
- 56.Nenna R, Tromba V, Berardi R, De AD, Papoff P, Sabbatino G, Moretti C, Midulla F: Recombinant human deoxyribonuclease treatment in hospital management of infants with moderate-severe bronchiolitis. Eur J Inflamm 2009;7:169–74.
- 57.Bertrand P, Aranibar H, Castro E, Sanchez I. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Pediatr Pulmonol. 2001;31:2001. doi: 10.1002/ppul.1040. [DOI] [PubMed] [Google Scholar]
- 58.Nenna R, Papoff P, Moretti C, De Angelis D, Battaglia M, Papasso S, Bernabucci M, Cangiano G, Petrarca L, Salvadei S, Nicolai A, Ferrara M, Bonci E, Midulla F: Seven percent hypertonic saline-0.1 % hyaluronic acid in infants with mild-to-moderate bronchiolitis. Pediatr Pulmonol. 2014;49:919–25. [DOI] [PubMed]
- 59.Bueno Campaña M, Olivares Ortiz J, Notario Muñoz C, Rupérez Lucas M, Fernández Rincón A, Patiño Hernández O, Calvo Rey C: High flow therapy versus hypertonic saline in bronchiolitis: randomised controlled trial. Arch Dis Child 2014;99(6):511-5. doi: 10.1136/archdischild-2013-305443. [DOI] [PubMed]
- 60.Park JY, Jeong YM, Jeong SJ, Seo SS. The efficacy of nebulized 3 percent hypertonic saline solution and fenoterol in infants with bronchiolitis. Korean J Pediatr. 2005;48:518–522. [Google Scholar]
- 61.Zheng W, Li L, Chengfung H, Yunmei H, Wei L. The effects of inhalation of the 3 % hypertonic saline solution with ambroxol hydrochloride in the treatment of 43 bronchiolitis patients. J Pediatr Pharm. 2012;18.
- 62.Giudice M, Saitta F, Leonardi S, Capasso M, Niglio B, Chinellato I, et al. Effectiveness of nebulized hypertonic saline and epinephrine in hospitalized infants with bronchiolitis. Int J Immunopathol Pharmacol. 2012;25:485–491. doi: 10.1177/039463201202500218. [DOI] [PubMed] [Google Scholar]
- 63.Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatr Int. 2010;52:199–202. doi: 10.1111/j.1442-200X.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 64.Luo Z, Fu Z, Liu E, Xu X, Fu X, Peng D, et al. Nebulized hypertonic saline treatment in hospitalized children with moderate to severe viral bronchiolitis. Clin Microbiol Infect Off Eur Soc Clin Microbiol Infect. 2011;17:1829–1833. doi: 10.1111/j.1469-0691.2010.03304.x. [DOI] [PubMed] [Google Scholar]
- 65.Sharma BS, Gupta MK, Rafik SP. Hypertonic (3 %) saline vs 0.9 % saline nebulization for acute viral bronchiolitis: A randomized controlled trial. Indian Pediatr. 2013;50:743–7. doi: 10.1007/s13312-013-0216-8. [DOI] [PubMed] [Google Scholar]
- 66.Pandit S, Dhawan N, Thakur D. Utility of hypertonic saline in the management of acute bronchiolitis in infants: A randomised controlled study. Int J Clin Pediatr. 2013;2:24–29. [Google Scholar]
- 67.Maheshkumar KB, Karunakara BP, Nagalli MM, Mallikarjuna HB: Aerosolised hypertonic saline in hospitalized young children with acute bronchiolitis: a randomized controlled clinical trial. Journal of Pediatric Sciences (ISSN:1309–1247) 2013.
- 68.Al-Ansari K, Sakran M, Davidson BL, El SR, Mahjoub H, Ibrahim K. Nebulized 5 % or 3 % hypertonic or 0.9 % saline for treating acute bronchiolitis in infants. J Pediatr. 2010;157:630–634. doi: 10.1016/j.jpeds.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 69.Nebulized 3 % hypertonic saline in the treatment of acute bronchiolitis in infants. [http://www.ersnet.org/learning_resources_player/abstract_print_13/files/Abstract_book_2013.pdf]
- 70.Teunissen J, Hochs AHJ, Vaessen-Verberne A, Boehmer ALM, Smeets CCJM, Brackel H, et al. The effect of 3% and 6% hypertonic saline in viral bronchiolitis: A randomised controlled trial. Eur Respir J. 2014;44(4):913–21. doi: 10.1183/09031936.00159613. [DOI] [PubMed] [Google Scholar]
- 71.A Study of Hypertonic Saline for Infants Hospitalized With Bronchiolitis (NCT01488448) [https://clinicaltrials.gov/ct2/show/NCT01488448]
- 72.Ojha A, Mathema S, Sah S, Aryal U. A comparative study on use of 3 % saline versus 0.9 % saline nebulisation in children with bronchiolitis. J Nepal Heal Res Counc. 2014;12:39–43. [PubMed] [Google Scholar]
- 73.Everard ML, Hind D, Ugonna K, Freeman J, Bradburn M, Cooper CL, et al. SABRE: A multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis. Thorax. 2014;69:1105–1112. doi: 10.1136/thoraxjnl-2014-205953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baujat B, Mahé C, Pignon J, Hill C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat Med. 2002;30:2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 76.Rucker G, Carpenter J, Schwarzer G. Detecting and adjusting for small-study effects in meta-analysis. Biom J. 2011;53:351–368. doi: 10.1002/bimj.201000151. [DOI] [PubMed] [Google Scholar]
- 77.Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–44. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 78.Elphick H, Everard M. Noisy breathing in children. In: David T, editor. Recent advances in paediatrics. London: The Royal Society of Medicine; 2002. [Google Scholar]
- 79.Elphick H, Ritson S, Rodgers H, Everard M. When a “Wheeze” is not a wheeze: Acoustic analysis of breath sounds in infants. Eur Respir J. 2000;16:593–7. doi: 10.1034/j.1399-3003.2000.16d04.x. [DOI] [PubMed] [Google Scholar]
- 80.Elphick HE, Lancaster GA, Solis A, Majumdar A, Gupta R, Smyth RL. Validity and reliability of acoustic analysis of respiratory sounds in infants. Arch Dis Child. 2004;89:1059–63. doi: 10.1136/adc.2003.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasterkamp H. Nomenclature used by health care professionals to describe breath sounds in asthma. CHEST J. 1987;92:346. doi: 10.1378/chest.92.2.346. [DOI] [PubMed] [Google Scholar]
- 82.Spiteri MA, Cook DG, Clarke SW. Reliability of eliciting physical signs in examination of the chest. Lancet. 1988;1:873–5. doi: 10.1016/S0140-6736(88)91613-3. [DOI] [PubMed] [Google Scholar]
- 83.Moustgaard H, Bello S, Miller FG, Hróbjartsson A. Subjective and objective outcomes in randomized clinical trials: Definitions differed in methods publications and were often absent from trial reports. J Clin Epidemiol. 2014;67:1327–1334. doi: 10.1016/j.jclinepi.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 84.Bisgaard H, Flores-Nunez A, Goh A, Azimi P, Halkas A, Malice M-P, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med. 2008;178:854–60. doi: 10.1164/rccm.200706-910OC. [DOI] [PubMed] [Google Scholar]
- 85.Behrendt CE, Decker MD, Burch DJ, Watson PH. International variation in the management of infants hospitalized with respiratory syncytial virus. Int RSV Study Group Eur J Pediatr. 1998;157:215–20. doi: 10.1007/s004310050798. [DOI] [PubMed] [Google Scholar]
- 86.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- 87.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990;263:1385–9. doi: 10.1001/jama.1990.03440100097014. [DOI] [PubMed] [Google Scholar]
- 88.Hunter JE, Schmidt FL. Fixed effects vs. random effects meta-analysis models: Implications for cumulative research knowledge. Int J Sel Assess. 2000;8:275–292. doi: 10.1111/1468-2389.00156. [DOI] [Google Scholar]
- 89.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 90.Peto R. Why do we need systematic overviews of randomized trials? Stat Med. 1987;6:233–240. doi: 10.1002/sim.4780060306. [DOI] [PubMed] [Google Scholar]
- 91.Thompson SG, Pocock SJ. Can meta-analyses be trusted? Lancet. 1991;338:1127–30. doi: 10.1016/0140-6736(91)91975-Z. [DOI] [PubMed] [Google Scholar]
- 92.Siontis KC, Hernandez-Boussard T, Ioannidis JPA. Overlapping meta-analyses on the same topic: Survey of published studies. BMJ. 2013;347:f4501. doi: 10.1136/bmj.f4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garritty C, Tsertsvadze A, Tricco AC, Sampson M, Moher D. Updating systematic reviews: An international survey. PLoS One. 2010;5:e9914. doi: 10.1371/journal.pone.0009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moher D. The problem of duplicate systematic reviews. BMJ. 2013;347:f5040. doi: 10.1136/bmj.f5040. [DOI] [PubMed] [Google Scholar]
- 95.Krumholz H. The case for duplication of meta-analyses and systematic reviews. BMJ. 2013;347:f5506. doi: 10.1136/bmj.f5506. [DOI] [PubMed] [Google Scholar]
- 96.Ioannidis JPA. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–28. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 97.Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26:328–33. doi: 10.1097/MOP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]


