Abstract
Background:
Low vitamin D levels have been associated with an increased risk of multiple sclerosis (MS), although it remains unknown whether this relationship varies by age.
Objective:
The objective of this paper is to investigate the association between vitamin D3 supplementation through cod liver oil at different postnatal ages and MS risk.
Methods:
In the Norwegian component of the multinational case-control study Environmental Factors In Multiple Sclerosis (EnvIMS), a total of 953 MS patients with maximum disease duration of 10 years and 1717 controls reported their cod liver oil use from childhood to adulthood.
Results:
Self-reported supplement use at ages 13–18 was associated with a reduced risk of MS (OR 0.67, 95% CI 0.52–0.86), whereas supplementation during childhood was not found to alter MS risk (OR 1.01, 95% CI 0.81–1.26), each compared to non-use during the respective period. An inverse association was found between MS risk and the dose of cod liver oil during adolescence, suggesting a dose-response relationship (p trend = 0.001) with the strongest effect for an estimated vitamin D3 intake of 600–800 IU/d (OR 0.46, 95% CI 0.31–0.70).
Conclusions:
These findings not only support the hypothesis relating to low vitamin D as a risk factor for MS, but further point to adolescence as an important susceptibility period for adult-onset MS.
Keywords: Multiple sclerosis, vitamin D, timing, environmental risk factors, susceptibility, age, supplementation, cod liver oil
Introduction
A low vitamin D level is one of the factors most consistently associated with multiple sclerosis (MS).1 Yet, it is not well understood at which age an adequate exposure might be especially important and an intervention optimally timed to modify MS risk.2
Observational studies investigating the timing of exposure reached different conclusions regarding the possibly most susceptible postnatal period: childhood,3 adolescence,4 childhood and adolescence,5 adulthood.6 Migration and space-time cluster studies also pointed to different postnatal susceptibility periods,7–10 though these findings could also reflect the effect of other environmental risk factors.2 Furthermore, lower vitamin D exposure and serum levels during the prenatal phase have been associated with increased risk of MS later in life and could mark an independent susceptibility period.11–13
Serum vitamin D levels are influenced by sun exposure and diet.1 Cod liver oil is an important dietary vitamin D source in high-latitude countries like Norway where there is no sun-induced vitamin D production during the winter.14 Norwegian Health Authorities have recommended 5 ml of cod liver oil daily (400 IU of vitamin D) for more than 60 years to prevent diseases like rickets, formerly more prevalent in areas with little access to vitamin D-rich fatty fish.15 A survey from 1997 estimated that about 35% of the Norwegian population and 50% of those aged 60–79 were still using the supplement on a daily basis.16
As the literature is inconsistent in delimiting a critical window in which vitamin D might act, we investigated the association between the postnatal timing of cod liver oil supplementation, an important oral vitamin D source in Norway, and the risk of developing MS.
Methods
The EnvIMS study
The multi-national multicenter case-control study of Environmental Factors In Multiple Sclerosis (EnvIMS) was launched to investigate environmental risk factors for MS and examine possible differences between distinct populations. A self-administered postal questionnaire (EnvIMS-Q) was designed including detailed questions about age-specific past exposures such as sun habits, diet, supplement use, past medical history, lifestyle, occupational and hormonal factors.17 The questionnaire has been assessed for acceptability, feasibility and reliability.17 The complete study design and methodology has been published elsewhere.17
The Norwegian component of the EnvIMS study was approved by the Regional Ethical Committee for Medical and Health Research for Western Norway (n. 11, 18.12.2008). All eligible study participants received an invitation to participate in the form of an information letter explaining the study objectives, relevance and instructions for participation. Completion and return of the questionnaire implied participants’ informed consent.
Study population
These analyses used the Norwegian EnvIMS data collected in 2008. The cases, diagnosed according to the McDonald criteria,18 were recruited from the Norwegian MS-registry and Biobank.19 Only patients with disease duration shorter than 10 years were eligible for participation. Of the 1368 invited cases, 953 (69.7%) returned the questionnaire. The response rate was 72% among women as compared to 64.6% in men.
Controls, frequency-matched on age and sex, were randomly selected in a 4:1-ratio from the population-based National Registry in Norway (Folkeregisteret). Of the 4728 invited controls, 1717 (36.3%) participated. The response rate in women was again higher than in men (39.4% vs. 29.4%).
Exposure, outcome and covariates
Cod liver oil is an important source of vitamin D in the Norwegian population.16 The recommended one teaspoon (two capsules) daily of cod liver oil (5 ml) of the most commonly used Norwegian brand (Möller’s, Axellus AS, Oslo) contains 10 μg (400 IU) of vitamin D3, 250 μg of vitamin A, 10 mg of vitamin E and 1.2 g of the omega-3 fatty acids EPA and DHA. A Norwegian survey estimated that the majority of consumers use one tablespoon (15 ml) as a serving size.20
Given the importance of cod liver oil as a source of vitamin D in Norway, the use of this supplement was explored in several questions in the EnvIMS-Q. Participants were asked to report whether they had used cod liver oil or capsules “never” or at ages “0–6,” “7–12,” “13–15,” “16–18,” “19–24” and “25–30.” The age-scale was adapted to the Norwegian school system. Additionally, the frequency of supplement use at ages 13–19 during the winter and the rest of the year was explored using two separate variables on a six-point scale including “never/seldom,” “1–3 times/month,” “1 time/week,” “2–3 times/week,” “4–6 times/week” and “7+ times/week.” Another question classified the usual quantity of cod liver oil consumed at each serving during the same period into “no use,” “half a teaspoon,” “one teaspoon,” “half a tablespoon” and “one tablespoon or more.” The special interest of the investigators in this period was based on previous findings indicating that adolescence might be especially important for MS risk modification.21
Further, data on important covariates were retrieved from the questionnaire. The level of sun exposure was estimated by summer outdoor activity at ages “0–6,” “7–12,” “13–15,” “16–18,” “19–24,” “25–30” and “in recent years,” and quantified as “not that often,” “reasonably often,” “quite often” and “virtually all the time.” The frequency of consumption of vitamin D-rich fatty fish at main meals at ages 13–19 was explored on a six-point scale with “never,” “1 time/month,” “2–3 times/month,” “1 time/week,” “2 times/week” and “3 and more times/week” for a) “herring,” b) “mackerel,” c) “halibut, flounder” and d) “salmon, trout,” respectively. From the information elicited about infectious mononucleosis, past occurrence of the disease was used as covariate (“yes,” “no” or “I don’t remember”). Participants also reported their body shape at five-year age intervals based on a nine-point scale derived from body sketches,22 a figure rating scale, which has been shown to reflect well individuals’ body mass index (BMI).23,24 Information on the smoking habits (smoking onset never or after versus before MS onset) and the participants’ level of education (elementary, middle, high school or university) was also included in the analyses.
Finally, participants reported whether they had a family history of MS (affected parent, sibling or child) and whether they had asked their parents or another person for help in recalling information.
Statistical analyses
Statistical analyses were performed using STATA 13.1 (StataCorp, College Station, TX, USA). The associations between exposure and outcome were estimated through logistic regression and reported as odds ratios (OR) with 95% confidence intervals (CIs). All estimates were adjusted for sex and year of birth (six-year categories to create balanced subgroups).
According to the distribution of age at disease onset in the cases, an index age with corresponding distribution was assigned to controls taking into account age at time of study. Participants were considered exposed only if the exposure of interest occurred before the index postnatal age or age at disease onset. Participants exposed only after this period were considered unexposed.
Based on reported cod liver oil use at different ages, three variables were created: 1) cod liver oil use during childhood (ages 0–12) regardless of use during the other periods compared to no use during childhood, 2) cod liver oil use during adolescence (ages 13–18) regardless of use during the other periods compared to no use during adolescence and 3) cod liver oil use during adulthood (ages 19–30) regardless of use during the other periods compared to no use during adulthood. The effect of these variables was estimated in i) separate models adjusted for age and sex, ii) simultaneously in the same model and finally iii) also adjusted for different covariates.
Additionally, MS risk was compared for cod liver oil use continuously from birth up to different ages to investigate whether the duration of exposure was important. Information about frequency and quantity of supplement use at ages 13–19 was analyzed both as a categorical and a continuous variable using those who reported never having taken cod liver oil or capsules during a certain season as reference.
Consumption frequency of fatty fish during adolescence was examined by assigning scores between 0 and 5 to each participant to account for how frequently on the six-point scale “herring,” “mackerel,” “halibut, flounder” and “salmon, trout,” respectively, were consumed. These scores were added up to an overall score ranging from 0 to 20 reflecting the fatty-fish consumption. To facilitate analyses of the overall score, a quintile-inspired five-point scale from 1 to 5 was created grouping overall scores of 0, 1–2, 3–4, 5–6, ⩾7 and analyzed as a continuous variable.
Further, reported sun exposure during the summer at ages 0–12, 13–18 and 19–30, history of infectious mononucleosis, smoking prior to MS onset, body size at age 15, frequency of consumption of oily fish at ages 13–19 and years of education were added to the model to adjust for possible confounding. In a second step interaction terms were created to test for effect modification by sex and age at disease onset.
Results
Mean study age and sex distribution were similar in both groups (Table 1). Cases were significantly more likely than controls to have smoked before MS onset, experienced infectious mononucleosis, reported a lower educational level, a large body size at age 15 and infrequent summer sun exposure during adolescence.
Table 1.
| Cases (n = 953) | Controls (n = 1717) | |
|---|---|---|
| Age at study, mean (SD) | 44.8 (10.5) | 46.0 (10.8) |
| Male, n (%) | 286 (30.0) | 461 (26.9) |
| Age at disease onset, mean (SD) | 37.6 (10.2)g | n.a. |
| Disease duration, mean (SD) | 7.2 (2.7)g | n.a. |
| Smoking before MS onsetc, n (%) | 545 (58.9) | 853 (50.7) |
| Infectious mononucleosis, n (%) | ||
| “Yes” | 160 (17.3) | 155 (9.3) |
| “No” | 729 (78.7) | 1486 (88.8) |
| “I don’t remember” | 37 (4.0) | 33 (2.0) |
| Educational level, n (%) | ||
| High school or lower | 538 (57.1) | 802 (47.3) |
| University career | 402 (42.7) | 890 (52.5) |
| Body size at age 15, n (%)d | ||
| Normal (silhouette 1–4)e | 794 (87.0) | 1486 (89.5) |
| Large (silhouette 5–9)f | 119 (13.0) | 174 (10.5) |
| Summer sun exposure, n (%) | ||
| Lower vs. higher at age 13–15 | 302 (32.7)/623 (67.4) | 456 (27.4)/1210 (72.6) |
| Lower vs. higher at age 16–18 | 487 (52.4)/443 (47.6) | 779 (46.8)/885 (53.2) |
EnvIMS: Environmental Factors In Multiple Sclerosis; SD: standard deviation; n: count; n.a.: not applicable.
Missing data for covariates ranging from 0% to 3.6%.
Cases asked significantly more often for help than controls to recall information when filling out the questionnaire (mother: 34.5 vs. 17.2%, father: 6.9 vs. 2.5%, other person: 8.3% vs. 4.2%).
Only 0.9 % in this group smoked less than three years.
Based on Stunkard’s figure rating scale.
Body mass index (BMI) ranges from 18.9 to 23.5 kg/m2.
BMI ranges from 26.1 to 43.3 kg/m2 including overweight and obesity.
Based on data from the Norwegian MS-registry and Biobank.
Timing of cod liver oil use
Supplementation habits did not vary with sex, but with age at the time of study. Supplementation during childhood, adolescence and adulthood was more common among participants born before 1962.
Cod liver oil use was reported by 54.4% of cases and 55.9% of controls during at least one of the age ranges of interest between birth and age 30. Information on the age-specific supplementation was missing for 11.6% of participants. The association between the risk of developing MS and cod liver oil use varied considerably depending on the timing of the supplementation (Table 2). A marked inverse association was observed for intake at ages 13–18 after adjusting for age, sex and supplementation during the other periods. Neither cod liver oil use during childhood nor adult life was associated with reduced disease risk compared to non-use during those periods.
Table 2.
Association between cod liver oil use at different ages and the risk of MS.
| Use compared to no use of cod liver oil during |
|||
|---|---|---|---|
| Childhood (0–12 y)a | Adolescence (13–18 y)a | Adulthood (19–30 y)a | |
| Cases n (%) | 301 (31.8) | 196 (20.6) | 165 (17.4) |
| Controls n (%) | 631 (36.9) | 473 (27.6) | 309 (18.0) |
| OR (95% CI)b | OR (95% CI)b | OR (95% CI)b | |
| Model 1c | 0.82 (0.69–0.97)f | 0.70 (0.58–0.85)g | 1.00 (0.81–1.24) |
| Model 2d | 1.01 (0.81–1.26) | 0.67 (0.52–0.86)h | 1.17 (0.93–1.47) |
| Model 3e | 1.01 (0.79–1.29) | 0.72 (0.55–0.96)f | 1.18 (0.92–1.51) |
MS: multiple sclerosis; y: years; n: count; OR: odds ratio; CI: confidence interval.
Continuous or periodical use regardless of prior and subsequent use.
OR of MS for age at disease onset after the exposure period of interest by cod liver oil use during specific age periods compared to no use during the same period.
Model 1: Separate model for each age period. Adjusted for age and sex.
Model 2: All three age periods included in the same model. Adjusted for age and sex.
Model 3: All three age periods included in the same model. Adjusted for age, sex, smoking before disease onset, history of infectious mononucleosis, sun exposure, body shape at age 15, education, and consumption of fatty fish.
P value < 0.05.
P value < 0.0001.
P value < 0.005.
The association between cod liver oil supplementation during adolescence and MS risk was not meaningfully altered after adjusting for sun exposure, infectious mononucleosis, smoking, body size, oily fish consumption and education. No significant differences were seen in the effect between men and women and according to age at disease onset (data not shown).
We observed that 404 cases (42.4%) and 368 controls (21.4%) asked for help in completing the questionnaire. Of these, 34.1% vs. 42.2% used cod liver oil during childhood, 19.8% vs. 30.2% during adolescence and 15.2% vs. 19.4% during adulthood, comparing cases and controls, respectively. When restricting the analysis of the fully adjusted model 3 (Table 2) to those asking for help, the pattern of association remained similar. The respective OR (95% CI) were 0.99 (0.66–1.49) for childhood, 0.67 (0.42–1.06) for adolescence and 0.99 (0.64–1.54) for adulthood use.
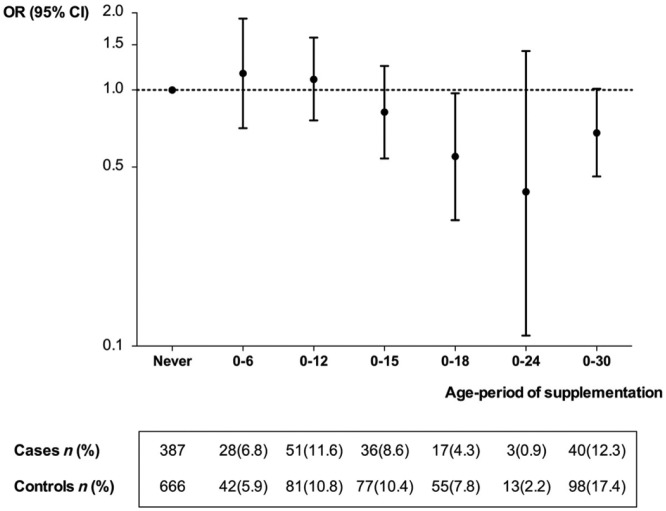
Continuous supplementation from birth up to a certain age was increasingly more strongly associated with a reduced MS risk the longer the supplementation lasted, except for the longest period from birth to age 30 (Figure 1).
Figure 1.
Association between cod liver oil use during increasingly longer age-periods and MS risk.
MS: multiple sclerosis; OR: odds ratio; CI: confidence interval. n: count.
OR of MS in groups with cod liver oil use continuously from birth up to a specific age compared to those who reported no use in the past and number of cases and controls.
Supplementation during adolescence
Supplementation during adolescence was further analyzed for seasonality, frequency and habitual serving size.
The habit of using (liquid) cod liver oil during adolescence was more common during the winter (33.7% and 43.6%) than the rest of the year (19.7% and 23.9%), both in cases and controls. Information was missing for 15.9% of participants for intake during winter and 29.3% during the rest of the year. The frequency of intake most often reported during adolescence in the winter, if any, was “7+ times a week” both for cases (41.8%) and controls (39.3%). The serving size most commonly reported was “1 tablespoon” for cases (58.7%) and controls (64.3%).
Table 3 shows the association between MS risk and increasing doses of vitamin D3 as estimated from the frequency and quantity of supplementation in the winter during adolescence, suggesting a dose-response relationship (p trend = 0.001) and the strongest protective effect for 600–800 IU/d. The estimates did not substantially change after adjusting for a selection of confounders (Table 3), nor after also adding family history of MS into the model.
Table 3.
Association between average daily intake of vitamin D3 through monthly supplemented cod liver oil in the winter during adolescence (ages 13–19) and MS risk for age at disease onset >19.
| Cod liver oil(ts/month) | Vitamin D3(IU/day) | Cases n (%) | Controls n (%) | ORa | 95% CI |
|---|---|---|---|---|---|
| Noneb | – | 525 (66.0) | 784 (56.1) | 1.00 | – |
| 1–15 | ⩽ 200 | 79 (9.9) | 160 (11.5) | 0.74 | 0.55–0.99c |
| 16–30 | 201–400 | 55 (6.9) | 125 (9.0) | 0.68 | 0.48–0.95c |
| 31–45 | 401–600 | 14 (1.8) | 38 (2.7) | 0.58 | 0.31–1.08 |
| 46–60 | 601–800 | 32 (4.0) | 104 (7.4) | 0.46 | 0.31–0.70d |
| >60 | >800 | 90 (11.3) | 186 (13.3) | 0.77 | 0.58–1.02 |
MS: multiple sclerosis; n: count; ts: teaspoons; IU: international units; OR: odds ratio; CI: confidence interval.
All estimates adjusted for age and sex; p-trend for association = 0.001, OR (95% CI) = 0.91 (0.87–0.96). Adjusting in addition for smoking before disease onset, history of infectious mononucleosis, sun exposure, body shape at age 15, education, and consumption of fatty fish: p trend = 0.025, OR (95% CI) = 0.94 (0.89–0.99). The effect estimate did not materially change when adjusting in addition for supplementation during adolescence in the summer.
Reference group consists of those who reported neither an intake of cod liver oil liquid nor capsules.
P value < 0.05.
P value < 0.0001.
The association was significant for supplementation only during the winter. For the supplement use in other seasons we found no evidence of an association between doses of cod liver oil during adolescence and MS (OR 1.03, 95% CI 0.84–1.26, p trend = 0.773, adjusted for supplementation during adolescence in the winter and the covariates listed in the methods).
Fish consumption
Consumption of fatty fish during adolescence was associated with a significant reduction in risk for later MS development (OR 0.92, 95% CI 0.86–0.99, p trend = 0.047, adjusted for all covariates mentioned in the methods). This OR was related to one step on the five-point scale and is equivalent to an OR of 0.72 comparing most frequent with no fish consumption. When further adjusting for cod liver oil use during adolescence, the result did not meaningfully change, but was no longer significant (OR 0.93, 95% CI 0.86–1.01, p trend = 0.085).
Discussion
We found an inverse association between MS risk and cod liver oil supplementation during adolescence and a dose-response protective effect suggested for higher dosages of vitamin D consumed through cod liver oil. However, no association was observed for supplementation during childhood or adulthood. These findings suggest that adolescence might be an especially susceptible period for disease risk modification through dietary vitamin D and are in line with previous observational4,21 and experimental25 studies. Other environmental risk factors like infectious mononucleosis,26 high BMI24 and other lifestyle factors27 have also been suggested to act mainly during adolescence.
Previous studies in the same area found that sun exposure through outdoor activity during adolescence was associated with a decreased disease risk only when exposure occurred during the summer,4,28 while the association between MS and cod liver oil use during adolescence in the present study was only significant for intake during the winter when sun-induced vitamin D-production ceases. These seasonal differences suggest that the risk-modifying effect is vitamin D mediated.
A low vitamin D level is one of the risk factors most consistently associated with an increased risk of MS. A prospective study reported a decreased MS risk among adult nurses comparing supplemental intake of ⩾400 IU/d of vitamin D to no intake.6 However, it is unclear how vitamin D levels in adulthood correlate with those during adolescence.
Our findings indicate an especially sensitive period during adolescence for MS risk modification but probably not the only one.21 Higher doses of vitamin D may be needed during childhood and adulthood to reach the same degree of risk modification as during adolescence.21,25 Even though we did not find an association between overall cod liver oil use during childhood or adulthood and MS risk, we could not evaluate whether a protective effect was restricted to high-dose users at these ages, as this information was not collected.
A prospective study in the United States (US) did not find an association between total recalled dietary vitamin D intake during adolescence and MS risk.29 Intake of ⩾400 IU/d of vitamin D from multivitamins during adolescence showed, however, a nonsignificant reduced MS risk of an order of magnitude similar to the findings in our study under the rare-disease-assumption.29 Power might not have been optimal to yield significant results particularly since diet contributes only to a small extent to the vitamin D status compared to sun exposure in the studied area.29 In the area of the present study there is virtually no contribution of sun exposure during winter.
There are alternative explanations to our findings. Adolescence might be the most sensitive period in which vitamin D unfolds its observed immunomodulatory effects30 or is of importance in the terminal phase of brain development. Another explanation could be that vitamin D supplementation during different periods in life might not lead to comparable serum levels. No age-dependent difference was, however, experimentally found in how dietary vitamin amounts translate into serum levels.25
Our results might also be due to chance, but this is unlikely considering the strong association and conformity with previous findings. We adjusted the estimates for other known environmental risk factors, but we cannot exclude the possibility of residual confounding.
Alternatively, the inverse association between cod liver oil use during adolescence and MS risk might be due to a longer period of exposure rather than the right timing. Adolescent cod liver oil users may be more likely to have additionally consumed the supplement during the other periods resulting in a longer exposure. However, when analyzing continuous supplementation from birth over increasingly longer periods, the strength of association did not steadily increase. The longest supplementation period was less strongly associated with a reduced MS risk than the shorter ones.
Lastly, our findings might be due to the protective effect of other cod liver oil ingredients. Vitamin A and E as well as omega-3 fatty acids have become of interest as possible disease-modifying candidates of MS.31–33 Fewer studies focused on a risk-modulating potential and results are not consistent. A U-shaped pattern of association between serum vitamin A levels and MS risk was reported in a registry-based smaller cohort study.34 However, intake of carotenoids, vitamin E and omega-3 fatty acids assessed by food frequency questionnaires was unrelated to disease risk in two large cohorts of women.35,36 We cannot exclude the possibility that vitamin A in cod liver oil contributed to our findings. The dose-response relationship observed for use during adolescence might be attributed to a protective effect of vitamin A, which increases along with vitamin D in higher doses of cod liver oil. It is unknown whether this nutrient acts age-dependently and could thus explain the findings on the timing of exposure to cod liver oil. Salzer and colleagues reported similar associations for younger participants (16–26 years) as in the entire cohort.34 Even if age at exposure was not addressed in detail, this observation contradicts somewhat the presence of a strong age-dependent effect for vitamin A. Moreover, evidence for an age-varying action of vitamin D exists beyond studies focusing on dietary sources, and thus independent of vitamin A.4
The MS prevalence is lower in populations with consumption of fish,37 and earlier studies suggested an inverse association between fish consumption and MS.4,38 Focusing on vitamin D-rich fatty fish, we found that consuming it during adolescence might be protective against the disease. The association was similar but no longer significant after adjusting for cod liver oil use. Our study might be underpowered to show subtle associations.
Previous studies investigating and comparing associations in different age periods in humans were smaller and could not account for all of the known risk factors of MS in the analyses.4,5 In this present large case-control study we analyzed cod liver oil supplementation, widely used in the Norwegian population, as a dietary proxy for vitamin D intake and serum levels. Investigating and comparing various age periods we found evidence of clear age differences in how vitamin D might affect MS risk even after adjusting for possible confounding.
Case-control studies, although efficient to conduct and suited to study past exposures in detail, are subject to methodological limitations. Despite the population-based sampling of both cases and controls, selection bias could, for instance, be an issue considering the different response rates between both groups. Compared to those included in the study, individuals not responding could have a different distribution of some of their main characteristics affecting the correlation between exposure and disease. We do not have any information on such a possible relation, but we found a higher proportion of controls with the highest level of education compared to the cases, indicating a higher socioeconomic status in controls. We accounted for this by adjusting for confounding by education.
Recall bias is another potential threat to the validity of findings in case-control studies. Nevertheless, the overall proportion of participants reporting cod liver oil use did not differ among cases and controls, and it is unlikely that participants were biased in recalling the timing of this supplementation. Knowledge about the period in life most susceptible to MS risk modification is not yet established.
In order to reduce the risk for misclassified responses, non-differential due to memory issues in general and differential due to deteriorating cognitive function in MS patients, only cases with a maximum disease duration of 10 years were eligible for the study. Furthermore, the age-period scale used to explore supplementation habits was adapted to the Norwegian school system to facilitate recall. In addition, participants were encouraged to ask their parents for help to correctly reconstruct past exposures if needed.
In conclusion, our findings suggest that adolescence might be an important postnatal age-period for an MS risk-reduction. Commonly used doses of vitamin D contained in cod liver oil might contribute to modify MS risk when supplemented throughout adolescence. Further studies are needed to confirm our findings and to investigate whether higher doses might potentially be as protective during childhood or adulthood.
Acknowledgments
We thank Prof Alberto Ascherio from the Department of Nutrition, Harvard T.H. Chan School of Public Health, USA, for his valuable scientific advice and discussion. Further, the authors wish to acknowledge Bettina Galanti, Department of Clinical and Experimental Medicine, University of Sassari, Italy (European substudy administration and logistics), Sally Killborn, Research Institute of the McGill University Health Centre, Montreal Canada (EnvIMS-Q format, dissemination, graphics), Erin Lundy, Department of Mathematics and Statistics, McGill University (data quality assessment), Azadeh Shohoudi, Department of Mathematics and Statistics, McGill University (data quality assessment), and Bin Zhu, Research Institute of the McGill University Health Centre, Montreal Canada (statistical assistance).
Footnotes
Conflicts of interest: M. Cortese has nothing to declare.
T. Riise has nothing to declare.
K. Bjørnevik has nothing to declare.
T. Holmøy has received speaker honoraria, travel support and unrestricted research grants from Sanofi Aventis, Biogen Idec, Bayer Schering, Novartis, and Merck Serono.
M.T. Kampman has nothing to declare.
S. Magalhaes has nothing to declare.
M. Pugliatti has nothing to declare.
C. Wolfson has nothing to declare.
K-M. Myhr has received speaker honoraria, travel support and/or unrestricted research grants from Bayer Schering, Biogen Idec, Genzyme, Sanofi Aventis, Novartis, and Merck Serono.
Funding: This work was supported by the Italian MS Society/Foundation FISM (grant number 2007/R/14, 2008/R/19); The Western Norway Regional Health Authority/ Helse Vest (grant number 911421/2008, 911474/2009); The University of Bergen, Norway (2007); The Norwegian MS Society (2011) and The Multiple Sclerosis Society of Canada (2011–2013).
Contributor Information
Marianna Cortese, The KG Jebsen Centre for MS-Research, Department of Clinical Medicine, University of Bergen, Norway/Department of Global Public Health and Primary Care, University of Bergen, Norway/Department of Nutrition, Harvard T.H. Chan School of Public Health, USA.
Trond Riise, Department of Global Public Health and Primary Care, University of Bergen, Norway/The Norwegian Multiple Sclerosis Competence Center, Department of Neurology, Haukeland University Hospital, Norway.
Kjetil Bjørnevik, Department of Global Public Health and Primary Care, University of Bergen, Norway/Department of Nutrition, Harvard T.H. Chan School of Public Health, USA/The Norwegian Multiple Sclerosis Competence Center, Department of Neurology, Haukeland University Hospital, Norway.
Trygve Holmøy, Institute of Clinical Medicine, University of Oslo, Norway/Department of Neurology, Akershus University Hospital, Norway.
Margitta T Kampman, Department of Neurology, University Hospital of North Norway, Norway.
Sandra Magalhaes, Department of Epidemiology and Biostatistics and Occupational Health, McGill University, Canada.
Maura Pugliatti, Department of Global Public Health and Primary Care, University of Bergen, Norway/Department of Clinical and Experimental Medicine, University of Sassari, Italy/Division of Medicine, McGill University, Canada.
Christina Wolfson, Department of Epidemiology and Biostatistics and Occupational Health, McGill University, Canada/Research Institute of the McGill University Health Centre, Canada.
Kjell-Morten Myhr, The KG Jebsen Centre for MS-Research, Department of Clinical Medicine, University of Bergen, Norway/The Norwegian Multiple Sclerosis Competence Center, Department of Neurology, Haukeland University Hospital, Norway.
References
- 1. Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012; 8: 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Handel AE, Giovannoni G, Ebers GC, et al. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol 2010; 6: 156–166. [DOI] [PubMed] [Google Scholar]
- 3. Islam T, Gauderman WJ, Cozen W, et al. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007; 69: 381–388. [DOI] [PubMed] [Google Scholar]
- 4. Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 2007; 254: 471–477. [DOI] [PubMed] [Google Scholar]
- 5. van der Mei IA, Ponsonby AL, Dwyer T, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: Case-control study. BMJ 2003; 327: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004; 62: 60–65. [DOI] [PubMed] [Google Scholar]
- 7. Pugliatti M, Riise T, Sotgiu MA, et al. Evidence of early childhood as the susceptibility period in multiple sclerosis: Space-time cluster analysis in a Sardinian population. Am J Epidemiol 2006; 164: 326–333. [DOI] [PubMed] [Google Scholar]
- 8. Riise T, Gronning M, Klauber MR, et al. Clustering of residence of multiple sclerosis patients at age 13 to 20 years in Hordaland, Norway. Am J Epidemiol 1991; 133: 932–939. [DOI] [PubMed] [Google Scholar]
- 9. Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis: Evidence from a migrant population in Australia. Brain 2000; 123 (Pt 5): 968–974. [DOI] [PubMed] [Google Scholar]
- 10. Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol 1995; 47: 425–448. [PubMed] [Google Scholar]
- 11. Staples J, Ponsonby AL, Lim L. Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: Longitudinal analysis. BMJ 2010; 340: c1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirzaei F, Michels KB, Munger K, et al. Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann Neurol 2011; 70: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munger KL, Soilu-Hänninen M, Åivo J, et al. In utero 25-hydroxyvitamin D and risk of multiple sclerosis among offspring in the Finnish Maternity Cohort. 2014 Joint ACTRIMS-ECTRIMS Meeting Poster Session 1, Boston, USA Mult Scler 2014; 20: 67–284, Poster no. P330.25205048 [Google Scholar]
- 14. Engelsen O, Brustad M, Aksnes L, et al. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol 2005; 81: 1287–1290. [DOI] [PubMed] [Google Scholar]
- 15. Birgisdottir BE, Brantsaeter AL, Kvalem HE, et al. Fish liver and seagull eggs, vitamin D-rich foods with a shadow: Results from the Norwegian Fish and Game Study. Mol Nutr Food Res 2012; 56: 388–398. [DOI] [PubMed] [Google Scholar]
- 16. Alexander J, Frøyland L, Hemre G-I, et al. A comprehensive assessment of fish and other seafood in the Norwegian diet. The Norwegian Scientific Committee for Food Safety (VKM), http://www.vkm.no/dav/d94dff429b.pdf (2006, accessed 20 April 2015). [Google Scholar]
- 17. Pugliatti M, Casetta I, Drulovic J, et al. A questionnaire for multinational case-control studies of environmental risk factors in multiple sclerosis (EnvIMS-Q). Acta Neurol Scand Suppl 2012: 43–50. [DOI] [PubMed] [Google Scholar]
- 18. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 19. Myhr KM, Grytten N, Aarseth JH. The Norwegian Multiple Sclerosis Registry and Biobank. Acta Neurol Scand Suppl 2012: 20–23. [DOI] [PubMed] [Google Scholar]
- 20. Johansson LR, Solvoll K, Bjørneboe GE, et al. Intake of very-long-chain n-3 fatty acids related to social status and lifestyle. Eur J Clin Nutr 1998; 52: 716–721. [DOI] [PubMed] [Google Scholar]
- 21. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006; 296: 2832–2838. [DOI] [PubMed] [Google Scholar]
- 22. Stunkard AJ, Sørensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983; 60: 115–120. [PubMed] [Google Scholar]
- 23. Stunkard A. Old and new scales for the assessment of body image. Percept Mot Skills 2000; 90: 930. [DOI] [PubMed] [Google Scholar]
- 24. Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009; 73: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adzemovic MZ, Zeitelhofer M, Hochmeister S, et al. Efficacy of vitamin D in treating multiple sclerosis-like neuroinflammation depends on developmental stage. Exp Neurol 2013; 249: 39–48. [DOI] [PubMed] [Google Scholar]
- 26. Hernan MA, Zhang SM, Lipworth L, et al. Multiple sclerosis and age at infection with common viruses. Epidemiology 2001; 12: 301–306. [DOI] [PubMed] [Google Scholar]
- 27. Hedström AK, Åkerstedt T, Hillert J, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol 2011; 70: 733–741. [DOI] [PubMed] [Google Scholar]
- 28. Bjørnevik K, Riise T, Casetta I, et al. Sun exposure and multiple sclerosis risk in Norway and Italy: The EnvIMS study. Mult Scler 2014; 20: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 29. Munger KL, Chitnis T, Frazier AL, et al. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol 2011; 258: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain 2009; 132: 1146–1160. [DOI] [PubMed] [Google Scholar]
- 31. Torkildsen O, Wergeland S, Bakke S, et al. Omega-3 fatty acid treatment in multiple sclerosis (OFAMS Study): A randomized, double-blind, placebo-controlled trial. Arch Neurol 2012; 69: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 32. Løken-Amsrud KI, Myhr KM, Bakke SJ, et al. Alpha-tocopherol and MRI outcomes in multiple sclerosis—association and prediction. PLoS One 2013; 8: e54417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Løken-Amsrud KI, Myhr KM, Bakke SJ, et al. Retinol levels are associated with magnetic resonance imaging outcomes in multiple sclerosis. Mult Scler 2013; 19: 451–457. [DOI] [PubMed] [Google Scholar]
- 34. Salzer J, Hallmans G, Nyström M, et al. Vitamin A and systemic inflammation as protective factors in multiple sclerosis. Mult Scler 2013; 19: 1046–1051. [DOI] [PubMed] [Google Scholar]
- 35. Zhang SM, Willett WC, Hernan MA, et al. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am J Epidemiol 2000; 152: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 36. Zhang SM, Hernan MA, Olek MJ, et al. Intakes of carotenoids, vitamin C, and vitamin E and MS risk among two large cohorts of women. Neurology 2001; 57: 75–80. [DOI] [PubMed] [Google Scholar]
- 37. Swank RL, Lerstad O, Strøm A, et al. Multiple sclerosis in rural Norway—its geographic and occupational incidence in relation to nutrition. N Engl J Med 1952; 246: 722–728. [DOI] [PubMed] [Google Scholar]
- 38. Bäärnhielm M, Olsson T, Alfredsson L. Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult Scler 2014; 20: 726–732. [DOI] [PubMed] [Google Scholar]