Abstract
Although Yoga has the potential to be an alternative physical activity to enhance bone health, there is a lack of high quality evidence for this type of intervention. The purpose of this randomized controlled trial was to examine the effects of a progressive 8-month Ashtanga-based Yoga program on bone turnover markers (BTM), areal bone mineral density (aBMD) and volumetric bone characteristics in premenopausal women. Thirty-four premenopausal women (35-50 years) were randomly assigned either to a Yoga group (YE, n = 16) or a control group (CON, n = 18). Participants in YE group performed 60 minutes of an Ashtanga-based Yoga series 2 times/week with one day between sessions for 8 months, and the session intensity was progressively increased by adding the number of sun salutations (SS). Participants in CON were encouraged to maintain their normal daily lifestyles monitored by the bone specific physical activity questionnaire (BPAQ) at 2 month intervals for 8 months. Body composition was measured by dual energy x-ray absorptiometry (DXA). Bone formation (bone alkaline phosphatase, Bone ALP) and bone resorption (Tartrate-Resistant Acid Phosphatase-5b, TRAP5b) markers were assessed at baseline and after 8 months. aBMD of total body, lumbar spine and dual proximal femur and tibia bone characteristics were measured using DXA and peripheral Quantitative Computed Tomography (pQCT), respectively. We found that the serum Bone ALP concentrations were maintained in YE, but significantly (p = 0.005) decreased in CON after the 8 month intervention, and there were significant (p = 0.002) group differences in Bone ALP percent changes (YE 9.1 ± 4.0% vs. CON -7.1 ± 2.3%). No changes in TRAP5b were found in either group. The 8-month Yoga program did not increase aBMD or tibia bone strength variables. Body composition results showed no changes in weight, fat mass, or % fat, but small significant increases in bone free lean body mass occurred in both groups. The findings of this study suggest that regular long-term Ashtanga Yoga had a small positive effect on bone formation but did not alter aBMD or tibia bone characteristics in premenopausal women.
Key points.
Regular long-term Ashtanga-based Yoga program had a small positive effect on bone formation, but no effects were found on bone resorption.
None of the bone density or geometry variables were changed by the 8-month Ashtanga-based Yoga intervention.
Future Yoga interventions should focus on longer duration and greater frequency to elicit improvements in bone mineral density.
Key words: Yoga, Sun Salutations, bone formation, bone resorption, bone mineral density, bone strength
Introduction
Physical activity is a key component in maintaining healthy bone mass during adulthood (Kohrt et al., 2004). Significant improvements in bone mineral density (BMD) have been documented in premenopausal women for exercise interventions employing either high impact exercises (Bailey and Brook-Wavell, 2010; Winters-Stone and Snow, 2006) or resistance exercises that produce high joint reaction forces (Bemben et al., 2011; Lohman et al., 1995). In addition to such high intensity exercises, alternative activities may provide more choice and increase motivation in women who cannot engage in traditionally recommended exercises. Among non-traditional forms of exercise, Tai Chi (a traditional Chinese martial art) has been increasingly recognized as a safe and alternative exercise for maintaining healthy bone in older adults (U.S., 2004). Results of cross-sectional studies indicated that participants who did regular Tai Chi had higher BMD in the lumbar spine and the proximal femur (Qin et al., 2002; 2005) and lower rates of bone loss (Qin et al., 2002). A 24-week feasibility study in the elderly (79.1± 1.8 years) showed that both Tai Chi and resistance training groups had higher levels of serum bone alkaline phosphatase (Bone ALP), while the Tai Chi group exhibited a greater increase in serum Bone ALP than the resistance group after 6 weeks of training (Shen et al., 2007).
Both Yoga and Tai Chi are similar in that they involve coordinated movements with deep breathing and weight bearing postures that require strong upper and lower body muscular strength and balance. Due to its purported physical and mental benefits, Yoga is becoming a popular physical activity in modern western society (Yoga, 2008). Numerous cross-sectional and intervention studies have shown the health benefits of Yoga on blood glucose levels in individuals with diabetes (Gokal et al., 2007; Khatri et al., 2007), depression (Krishnamurthy and Telles, 2007; Shapiro et al., 2007; Woolery et al., 2004), blood pressure (Madanmohan et al., 2004; Ray et al., 2001), and muscle strength (Bhutkar et al., 2011; Kim et al., 2012; Tran et al., 2001). A recent meta-analysis of yoga randomized controlled trials in older adults (63.5-77.5 years) by Patel et al. (2012) reported medium effect sizes for self-rated health status (0.66) and maximal oxygen uptake (0.54). However, there is a lack of randomized controlled trials with Yoga as the primary intervention and bone variables as the primary outcomes. There were only two randomized controlled studies included in this meta-analysis that reported BMD as an outcome variable, with both studies using light yoga as a control condition (Ades et al., 2005; Blumenthal et al., 1991). The findings were mixed as BMD did not increase after either a progressive, 6-month resistance training or the low-intensity yoga in older women (68-77 years) with coronary heart disease (Ades et al., 2005); but BMD did increase in men (60-83 years) after 14 months of both aerobic exercise and light yoga programs (Blumenthal et al., 1991).
Presently, it is not clear whether the intensity of Yoga interventions is high enough to induce positive effects on BMD in a younger healthy population. For example, Hagins et al. (2007) reported that the metabolic costs of Hatha Yoga, averaged across the entire session, represent low levels of physical activity. Also, a recent study showed that a sequence of 28 Hatha Yoga postures produced low peak ground reaction forces (GRF) to upper and lower extremities that ranged from 0.22 to 1.47 times body weight (Wilcox et al., 2012). On the other hand, Ashtanga Yoga, or power Yoga, is performed at a higher intensity than Hatha Yoga and has been shown to elicit significantly higher heart rates than either Hatha or gentle Yoga (Cowen and Adams, 2007). It consists of asanas (postures), vinyasa (movement), and ujjayi (victorious breath). The Ashtanga Yoga sequence uses upper and lower body strength with coordinated movements, which may strengthen muscle and increase flexibility. Sun Salutations (SS) are an example of an Ashtanga Yoga sequence, where yoga postures are performed dynamically with combinations of forward and backward bending poses (Omkar et al., 2011). Based on the mathematical model developed by Omkar et al. (2011) using biomechanical principles, the postures of the Sun Salutation sequence theoretically produce dynamic joint strains and moments similar to other daily activities and exercises.
There is a paucity of studies examining the effects of Yoga, particularly the Ashtanga style, on bone turnover markers (BTM). In a small pilot study (n = 8), Balk et al. (2009) examined BTM responses of sedentary osteopenic postmenopausal women to 12 weeks of one hour per week Hatha Yoga classes in addition to home Yoga practice. They reported a trend for a moderate positive correlation between total minutes of Yoga practice and percent change in Bone ALP. A 12-week weight-bearing Yoga training program performed three days per week significantly decreased the bone resorption marker in the Yoga group (−26.9%) compared to the control group (−0.8%) in postmenopausal women (Phoosuwan et al., 2009). Neither of these studies were randomized controlled trials and it is not clear that sources of biological variability in BTM (e.g., diurnal variation, food intake) were adequately controlled.
Ashtanga Yoga has potential to be an alternative physical activity to prevent osteoporosis; however, there is a lack of randomized controlled trials with bone outcomes, especially in premenopausal women. Therefore, the purpose of this randomized controlled study was to examine the effects of a progressive 8-month Ashtanga Yoga exercise program on bone metabolism in middle-aged premenopausal women. The primary variables of interest were the bone turnover markers (BTM) given the duration and moderate intensity of the yoga intervention. We also assessed bone characteristics (density, geometry, strength) by DXA and pQCT, although these variables were of secondary interest because of their well-documented small effect sizes (Kelley et al., 2013). We chose an 8 month duration based on previous randomized controlled exercise intervention studies related to bone metabolism that ranged from 6 months to 2 years (Kelley et al., 2013; Polidoulis et al., 2012). We hypothesized that the Yoga intervention would elicit increases in the bone formation marker and decreases in the bone resorption marker. Because the program included a progressive jumping component, we expected positive BMD and bone strength adaptations to the Yoga training.
Methods
Participants
Healthy premenopausal women volunteers between the ages of 35 and 50 years were recruited from the surrounding Oklahoma City metro area. The inclusion criteria were as follows: healthy premenopausal women volunteers between the ages of 35 and 50 years; free of chronic back/joint problems and cardiovascular disease; non-smokers; not pregnant; not on hormonal birth control; not taking antihypertensive drugs or any medication that affects bone density; those who had not been engaged in a weight training program and/or a Yoga program for at least 12 months prior to the study; and women who were medically stable, ambulatory, and capable of undergoing physical strength testing and training. The exclusion criteria were as follows: women outside of the 35-50 years age range; those who exceeded the weight limit (300 pounds) of the bone densitometer; those who were taking medications known to affect BMD such as steroid hormones, calcitonin, or corticosteroids; women who did not have regular menstrual cycles; those with thyroid problems or who were diabetic; and any women with physical and mental disabilities preventing them from being strength tested and trained, including orthopedic or arthritic problems.
Research design
This randomized control 8 month intervention study (ClinicalTrials.gov NCT02163668. Registered 13 June 2014) utilized a mixed factorial research design, with group (Yoga, Control) as the between subjects factor and time (Pre, Post intervention) as the within subjects factor. Qualified participants completed the written informed consent, a series of questionnaires for health/menstrual history and lifestyle factors, bone scans and blood draw during the first visit to the laboratory at baseline. A second visit was used to assess muscular strength (data previously reported by Kim et al., 2012). After the intervention, menstrual history, calcium intake and physical activity questionnaires were administered; and bone scans, a blood draw and strength testing were conducted. The study design for the 8-month Ashtanga-based Yoga intervention is shown in Figure 1.
Figure 1.
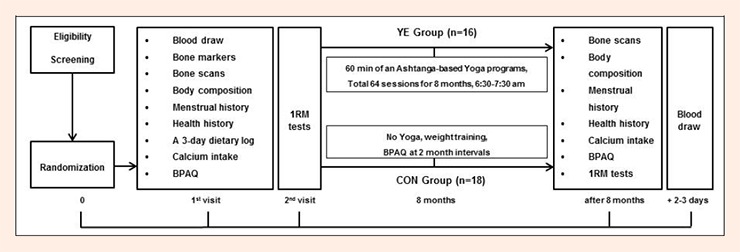
Study design for the 8-month Ashtanga –based Yoga intervention. YE: Yoga Exercise, CON: Control, BPAQ: bone-specific physical activity, 1RM: 1 repetition maximum
We recruited the participants from August to September in 2009 and Yoga intervention started in October 2009 and ended in June 2010. A total of 91 women initially volunteered for the study; however, 44 potential participants were excluded as they did not meet the inclusion/exclusion criteria (Figure 2). After screening, 47 participants were enrolled and randomized to either the Yoga (YE, n = 27) or control (CON, n = 20) groups. Participants in the YE group were instructed to maintain their normal daily activities, and not to begin any new exercise programs, especially resistance exercise, during the study. Participants in CON group were instructed to simply maintain their usual level of physical activity for the same time period that should not include any Yoga exercise.
Figure 2.
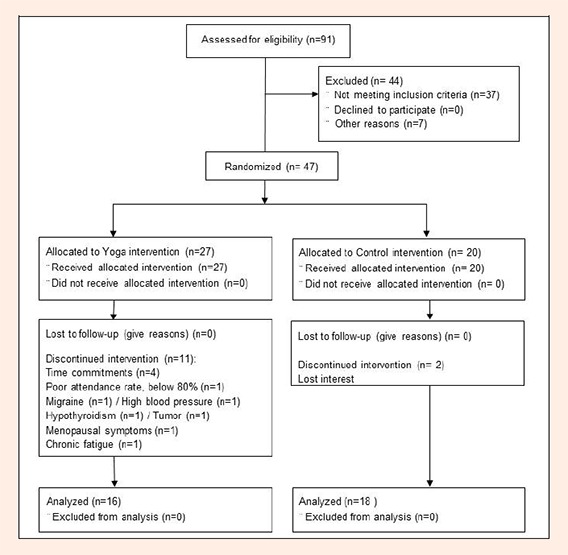
Flow chart of the recruitment process and research design (CONSORT 2010).
The available data on the effects of yoga on bone, or even the effects of randomized controlled exercise interventions on BTM, particularly in middle-aged premenopausal women, are sparse. We estimated effect sizes for BTM responses from several short duration (6-8 week) intervention studies. Small effect sizes for Bone ALP were calculated for a 6 week study in overweight men and women undergoing weight loss combined with weight bearing exercise (d = 0.25, Hinton et al., 2006), and for an 8 week randomized controlled study in young college age women performing combined aerobic and resistance training (d = 0.37, Lester et al., 2009). A medium effect size (d = 0.59) was determined for a 1 year walking and jumping intervention in premenopausal women (36 ± 7 years) conducted by Shibata et al. (2003). We also performed a power analysis using the bone resorption marker data in a 12 week yoga intervention in postmenopausal women published by Phoosuwan et al. (2009) and found a large effect size (d = 0.92) for the yoga treatment. Based on these studies, detecting a significant BTM response in the treatment group requires sample sizes ranging from 9 to 101 for 80% power. We used an effect size of 0.55 to estimate a sample size of 22 for 80% power for the BTM responses. Based on a meta-analysis of randomized controlled trials in premenopausal women, Kelley et al. (2013) reported a small effect size (g = 0.342) for the effects of exercise on the femoral neck aBMD site, which would require a sample size of 107 per group for 80% power. We recognize that the effect sizes for aBMD and vBMD response are small, thus, we used the BTM responses as the primary variables of interest in this study. We used a computerized random number generator for allocation. A researcher that was not blinded conducted the random allocation sequence, enrolled participants, and assigned participants to interventions. All methods and procedures were approved by the University of Oklahoma Institutional Review Board.
Questionnaires
All participants visited the laboratory to complete the following questionnaires at baseline: menstrual history, the bone-specific physical activity (BPAQ) (Weeks and Beck, 2008), health status, calcium intake, and 3-day dietary log. Menstrual cycle characteristics were assessed by self-report using our standard laboratory menstrual history questionnaire. Participants reported the number of menstrual periods in the previous 12 months, current and past oral contraceptive use, and whether they experienced menopausal symptoms. Women who reported irregular menstrual cycles, current oral contraceptive use, or symptoms of menopause (e.g., hot flashes, irregular menstrual cycle length) were excluded from the study. The menstrual history questionnaire was administered again at the end of the intervention to confirm menstrual characteristics of the participants had not changed during the intervention. One woman was excluded from the data analyses because she reported the onset of menopausal symptoms during the study.
Daily calcium intake, including supplements, was assessed by a food frequency questionnaire (Musgrave et al., 1989) at baseline and at the end of the intervention. Participants whose calcium intake was below 1000 mg/day were advised to increase their calcium intakes to meet the 1000 mg/day recommendation. They were given an information sheet that listed food items with high calcium contents, but they were not prescribed calcium supplements. Participants also completed a 3-day dietary log to estimate nutrient and caloric intakes at baseline. Participants were asked to record everything that they ate for two days during the week and one day during the weekend, including the food/drink item with brand names, the amount ingested, and method of preparation as specific as possible. There were missing dietary intake data for 2 participants (1 YE and 1 CON). The Diet Analysis Plus 9 data base (Cengage Learning, Inc., KY) was used for dietary analysis.
Participants completed the BPAQ at baseline, during (CON group only) and at the end of the intervention to estimate levels of physical activities that impose mechanical loads on the skeleton. The BPAQ consists of independent sections for past (from one year of age) and current (previous 12 months) physical activity participation. We used the total score to measure the physical activity levels at baseline and after 8 months. The current physical activity score was used to monitor physical activity levels for the CON group at 2 month intervals for the 8 months.
Yoga intervention
The Ashtanga-based Yoga training program consisted of 64 Yoga sessions on Mondays and Wednesdays from 6:30 to 7:30 am for 8 months. Participants performed 60 minutes of a Yoga series including 15 minutes of warm-up exercises, 35 minutes of Yoga postures and 10 minutes of cool-down. Dynamic and static stretching were introduced during the warm-up at the beginning with either sitting, supine, or standing postures. A certified Yoga instructor led all Yoga sessions and precisely taught Yoga postures with consistent instructions. Modified postures were taught to participants who were not able to perform the standard postures. For example, when participants performed a downward-facing dog pose (posture 8), participants who had tight hamstrings and inflexible ankles were instructed to bend knees, slightly raise heels, or put a rolled mat under their heels. We provided the Yoga blocks and straps for participants who were willing to use them.
Thirty five minutes of postures consisted of SS (Figure 3), standing, balancing, sitting, and supine postures. During the first 4 months, SS I with a triangle pose and warrior series were instructed and jumping (backward between postures 3 and 5 and forward between postures 18 and 20) was progressively included so that 12 jumps were performed in each session by the end of month 4. In the second 4 months, SS II with jumping for the same postures was performed with a total of 14 jumps by month 8. In postures 2 and 21(upward hand pose), the participant slowly raises the arms alongside the ears with inhalation. In postures 3 and 20 (standing forward bend pose), the participant bends the torso forward from the hip joints, not from the waist. In this posture, the participant was encouraged to lift and lengthen the front torso and to release a little more fully into the forward bend with each exhalation. In postures 4 and 19 (half-stand forward bend pose), from the standing forward bend, the participant gently presses the palms or fingertips into the floor and lifts the top of sternum up away from the floor and looks forward. The participant was asked to be careful not to compress the back of the neck. From posture 4 to 5 (plank pose), the participants slightly bends their knees and jumps back. If the participant had back pain or fear of jumping back, she was asked to move legs back one by one. The arms are perpendicular to the floor and the shoulders directly over the wrist. This plank posture strengthens the arms, wrists, and spine as well as the abdomen. In postures 6 and 16 (four-limbed staff pose), the participant slowly lowers torso and legs to a few inches above the floor. If the participant felt pain with their shoulders or back, she was asked to bend the knees on the floor. In postures 7 and 17 (upward-facing dog pose), the participant firmly presses the inner hands into the floor and gently lifts torso up and legs a few inches off the floor. From posture 7 to 8 (down-ward facing dog pose), the participant slowly lifts the pelvis toward the ceiling and straightens spine naturally. The head should be kept between the upper arms. The benefits of this posture are to stretch the shoulders, hamstrings, calves, and hands and strengthen the arms and legs. In posture 10 (warrior I), from the down-ward facing dog pose, the participant slowly lifts the right leg back (posture 9) and moves the right leg forward. The right knee over the right ankle is perpendicular to the floor and gently raises both arms above the head. In posture 11(warrior II), the participant stretches the arms between the shoulder blades. In posture 12 (reverse warrior), the participant gently lowers left arm and raises right arm to the ceiling and stretches right side. In posture 13(side angle pose), the participant gently lowers right arm on the right knee and lifts left arm to the ceiling. In this posture, body weight moves to the right knee. After the side angle posture, the participant slowly moves back to the warrior II pose, plank pose, lower-limbed pose, upward-facing dog pose, and down-ward facing dog pose. Finally, the participant did the same sequence on their left leg (postures 9-18).
Figure 3.
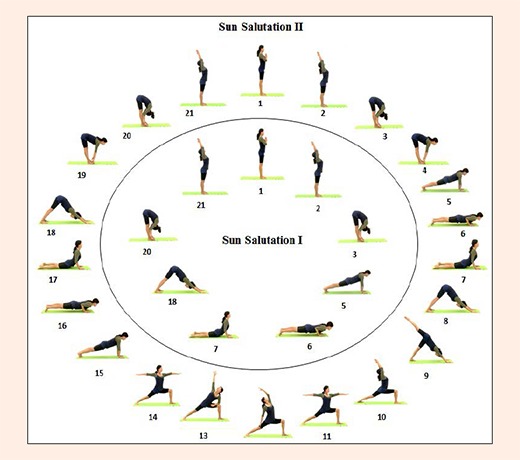
Yoga postures for Sun Saluation I and II sequences.
We progressively increased the exercise intensity by increasing the number of SS and jumps during the 8 month intervention (Table 1). The postures were static and held for approximately five to ten breaths each. On the first day of the Yoga intervention, participants were informed about proper Yoga attire and safety consideration for performing Yoga postures. Participants were encouraged to inform the Yoga instructor prior to class if they had any medical or other conditions such as muscle soreness that could affect their performance. Participants self-monitored their exercise intensity by measuring their heart rate using the palpation method (radial artery) and by recording their Ratings of Perceived Exertion (RPE) after the SS exercises. Each participant recorded heart rate and RPE after SS every Wednesday. During the training program, the average RPE ranged from 12 (light exertion) to 14 (somewhat hard) and about half of the women had exercise HR between 60-80% of their estimated maximum HR (HRmax= 220 – age) (data not shown). The room was maintained at a temperature of 21-23 °C.
Table 1.
Ashtanga-based Yoga postures and Sun Salutations (SS) monthly progression.
| Time | Months (Mondays / Wednesdays) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 15 mins | Sitting warm-up | Sitting/Standing warm-up | Sitting / Standing warm-up | Sitting / Standing warm-up |
| 35 mins | *SS I Triangle Warrior II Reverse warrior Side angle Wide legged forward bend Single-leg balance Tree Side plank Bridge Double legs |
SS I Triangle Warrior II Reverse warrior Side angle Wide legged forward bend Tree Standing forward bend Cat & Cow Boat / Side plank Rolling like a ball Double legs lift Dynamic bridge |
SSI Triangle Warrior I, II Reverse warrior Side angle Extended side angle Revolved extended side angle Wide legged forward bend Tree Chair Side plank Dolphin Pose Dolphin plank Rolling like a ball Double legs lift Dynamic bridge |
SSI Triangle Warrior I, II Reverse warrior Side angle Extended side angle Revolved extended side angle Wide legged forward bend Variation of Tree / Gate Forward bending / Low lunge Seated forward bend / Plow Boat / Rolling like a ball Side plank / Sphinx Sphinx / Dolphin plank Dolphin / Bow Roll-up Crisscross crunches Dynamic bridge |
| 10 mins | Cool down | Cool down | Cool down | Cool down |
| SSI #(jumps) | 3(6) | 4(8) | 5(10) | 6(12) |
| Time | Months (Mondays / Wednesdays) | ||||
|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | ||
| 15 mins | Supine / Sitting warm-up | Supine / Sitting & Standing warm-up |
Supine / Sitting & Standing warm-up |
Supine / Standing warm-up | |
| 35 mins | *SS II Triangle Warrior II Variation of tree / Revolved side angle Chair / Standing forward bend Mermaid / Wide angle Side plank / Rolling like a ball Side reclining leg lift / Plow Dolphin plank / Side plank Bow / Dolphin plank Roll-up Crisscross crunches Dynamic bridge |
SS II Triangle Revolved triangle Intense side stretch Eagle Standing forward bend / Rolling like a ball Camel / Fish Side plank / Plow Bow / Shoulder stand Roll-up Crisscross crunches Dynamic bridge |
SSII Triangle Revolved triangle Half moon Warrior III Tree / Eagle Standing forward bend / Cow face Camel / Fish Side plank / Plow Bow / Shoulder stand Roll-up Crisscross crunches Dynamic bridge |
SSII Triangle Revolved triangle Half moon Warrior III Eagle Standing forward bend / Gate Camel / Low lunge Side plank / Fish Dolphin plank / Plow Bow / Shoulder stand Roll-up Crisscross crunches Dynamic bridge |
|
| 10 mins | Cool down | Cool down | Cool down | Cool down | |
| SSII #(jumps) | 4(8) | 5(10) | 6(12) | 7(14) | |
# number of SS performed; (number of jumps performed) in each session
Body composition
We used dual energy x-ray absorptiometry (DXA, GE Lunar Prodigy, GE Medical Systems, encore 2002 Software, version 10.50.086) to measure body composition of the whole body. Quality Assurance (QA) scans were performed daily and qualified technicians conducted all measures following standard manufacturer’s procedures (GE Lunar Prodigy). Participants were asked to remove all metal and attenuating materials prior to being scanned. We measured standing height to the nearest 0.1 cm and weight to the nearest 0.1 kg using a wall stadiometer and a Tanita BWB-800 digital scale (Tanita Corporation of America, Inc., Arlington Heights, IL). Scan modes were determined by the software based on the truncal thickness: thick > 25cm, standard 13-25 cm, think < 13 cm. We obtained measures of total fat mass (Total FM) (kg), bone free lean body mass (BFLBM) (kg) and % body fat (% Fat) from the total body DXA scan. Short term precision (root mean square coefficient of variation (CV%)) for body composition variables in this laboratory for Total FM, BFLBM, and % Fat are 2.74%, 1.39% and 2.5%, respectively.
Bone turnover markers
Resting blood samples (approximately 6 ml) were obtained by venipuncture from an antecubital vein between 8:00 am and 10:00 am at baseline and 2-3 days after the last Yoga session. Participants fasted for at least 8 hours prior to blood collection. Participants were instructed to abstain from vigorous exercise prior to the blood draws. A centrifuge was used to separate the serum from the red blood cells. The serum was aliquoted into microtubes, and frozen at -848C until the bone marker assays were performed. The bone turnover markers were assayed in duplicate and all time points for a participant were measured in the same assay. The bone formation marker, Bone ALP, was measured with the MicroVue™BAP EIA Kit (Quidel Corporation, San Diego, CA, U.S.A.). The range of intra-assay CVs was 0.6-2.2%. The bone resorption marker, TRAP5b, was measured in duplicate using the MicroVue™TRAP5b Enzyme Immunoassay Kit (Quidel Corporation, San Diego, CA, U.S.A.). The range of intra-assay CV was 0.4-6.1%. The bone marker ratio, Bone ALP/TRAP5b, was also calculated.
Areal bone mineral density
We used DXA (GE Lunar Prodigy, GE Medical Systems, encore 2002 Software, version 10.50.086) to assess aBMD of total body, lumbar spine (L1-L4), and dual proximal femur (total hip, femoral neck, trochanter) at baseline and the end of the 8 month study. The CV% for the aBMD of total body, total hip (Right, Left), femoral neck (Right, Left), trochanter (Right, Left), and spine (L1-L4) are ranged from 0.68% to 1.39%.
Volumetric bone mineral density and bone geometry
Peripheral Quantitative Computed Tomography (pQCT) (XCT 3000, Software version 6.00, Stratec Medizintechnik GmbH, Pforzheim, Germany) was used to acquire tibia bone characteristic variables at baseline and the end of the 8 month study. A pQCT technician ran the cone phantom daily to monitor QA. Tibial length of the non-dominant leg was measured from the medial malleolus to the tibial plateau. Participants were seated in the scanning chair with the non-dominant leg supported by holder centered in gantry. Tibia scans were obtained at 4%, 38% and 66% of the bone length proximal to reference line, respectively. One qualified technician, who was not blinded to participant allocation, performed all scans and analyses. A voxel size of 0.4 mm was used for all sites at the scout view speed of 40 mm/sec and CT speed of 20 mm/sec, respectively. At the distal tibia (4%), contour mode 3 at 169 mg/cm3 and peel mode 4 at 650 mg/cm3 with a 10% peel were used to determine total volumetric BMD (ToD, mg/cm3), total bone area (ToA, mm2), trabecular volumetric BMD (TraD, mg/cm3), and trabecular area (TraA, mm2). For 38% and 66% analyses, cort mode 2 at 710 mg/cm3 was used to define cortical results and cort mode 2 at 480 mg/cm3 was used to obtain torsional strength for strength-strain index (SSI). We measured ToD (mg/cm3), ToA (mm2), CoD (mg/cm3), cortical area (CoA, mm2), cortical thickness (CTh, mm), and strength strain index (SSI, mm3). The CV% for the pQCT bone measurements ranged from 0.57% to 0.83% at the 4% tibia; from 0.31% to 1.21 % at the 38%; and from 0.50% to 0.95% at the 66% tibia.
Statistical analyses
Data are reported as means D standard error (SE). All data were determined to be normally distributed by the Kolmogorov-Smirnov test. We used the per protocol approach to analyze all data. We compared baseline physical characteristics between groups using independent t- tests. For calcium intake, body composition, BTM, aBMD and tibia bone characteristic variables, two-way mixed factorial analysis of variance (ANOVA) [Group (YE vs. CON) w Time (pre vs. post)] with repeated measures was used to analyze group responses to the intervention. If a significant group f time interaction occurred, paired samples t-tests with Bonferroni adjustment were used as post-hoc tests to determine significant time differences within each group.
Percent changes in dependent variables were calculated (%Δ = [(post – pre) / pre] / 100) and group differences in percent change variables were determined by independent t-tests. Pearson correlation coefficients were computed to determine the relationships between BTM responses and percent changes in calcium intake and body composition variables. Analysis of covariance (ANCOVA) was used to compare group differences in percent change BTM variables using percent changes in calcium intake, and body composition variables as covariates.
All statistical procedures were performed using SPSS for Windows 21.0 version (Chicago, IL). Effect sizes (ES) (d) and power analyses were calculated using G*Power 3.192.2 software (Faul et al., 2007). ES classifications of trivial (d < 0.20), small (d >0.20 to <0.50), medium (d >0.50 to <0.80) and large (d >0.80) were used (Kelley et al., 2013). The level of significance was set at p ≤ 0.05.
Results
Thirteen participants did not complete the intervention: 4 because of time commitments; 6 because of recent diagnoses of serious migraine, high blood pressure, hypothyroidism, tumor, menopausal symptoms, or chronic fatigue; 1 participant was excluded from the analyses due to poor attendance (below 80%); 1 could not be contacted, and 1 did not want to participate in post testing due to personal reasons. None of the reasons for dropping out were related to Yoga intervention. Thirty-four participants (YE, n = 16; CON, n = 18) completed the entire 32 weeks of the study (Figure 2). The average attendance rate of YE participants was 92.6% for the 8 months.
Participant characteristics
Table 2 shows the baseline physical characteristics, BPAQ, calcium intake, and dietary intake estimates for each group. The CON group had significantly (p < 0.05) higher total caloric intake, carbohydrate intake, and magnesium intake; however, total caloric intake expressed relative to body weight (g/kg) was not significantly different between groups. The remaining variables were not significantly different between YE and CON groups at baseline (p > 0.05). As previously mentioned, participants whose baseline calcium intake were less than 1000 mg/day (n = 9 in YE and n = 8 in CON groups) were given an instruction sheet with food choices for increasing their intake to at least 1000 mg/day during the intervention. Both groups significantly increased (p < 0.001) their mean calcium intakes after 8 months; and there was no significant difference (p > 0.05) between groups (YE, 1438 ± 192 mg/day; CON, 1594 ± 181mg/day). However, at the post test, 4 YE and 3 CON participants still had calcium intakes below 1000 mg/day. Current BPAQ scores assessed at 2 month intervals for CON participants documented that they did not significantly alter their physical activity levels during the study (2 months, 1.51 ± 0.59; 4 months, 2.70 ± 1.08; 6 months, 1.02 ± 0.35; 8 months, 0.88 ± 0.40; p > 0.05).
Table 2.
Baseline descriptive characteristicsof YE and CON Groups. Data are means (±SE).
| Variable | YE (n=16) | CON (n=18) | |
|---|---|---|---|
| Age (years) | 45.7 (1.0) | 43.2 (1.0) | |
| Height (m) | 1.63 (.01) | 1.61 (.01) | |
| Weight (kg) | 69.7 (3.3) | 70.0 (2.2) | |
| BMI (kg/m2) | 26.0 (1.0) | 27.0 (1.0) | |
| Tibia length (mm) | 364(6) | 366(4) | |
| BPAQ | 22.7 (6.5) | 25.7 (5.7) | |
| Calcium Intake (mg/day) | 1025(137) | 1162(176) | |
| Total CIa | Kcal | 1732(108) | 2184(163) * |
| Kcal/kg | 26.5 (2.5) | 31.2 (2.6) | |
| Protein a | Total g | 71.6 (6.0) | 88.3 (8.4) |
| g/kg | 1.1 (.1) | 1.2 (.1) | |
| CHO (g) a | 207.4 (16.1) | 272.8 (25.8) * | |
| Fat (g) a | 71.0 (5.6) | 84.9 (7.5) | |
| Vitamin D (µg) a | 4.4 (2.1) | 5.9 (1.6) | |
| Magnesium (mg) a | 211.1 (31.7) | 326.3 (43.8) | |
YE: Yoga Exercise, CON: Control, BPAQ: Bone-Specific Physical Activity Questionnaire Total Score, Total CI: Total Caloric Intake, CHO: Carbohydrate.
a YE n=15, CON n=17
* p < 0.05 significant group effect
Body composition variables
Table 3 shows the baseline and post test body composition changes for each group. There were no significant group differences for any of the baseline body composition variables or for percent changes in any of these variables from baseline to post testing. However, a significant time effect was found for BFLBM (p < 0.01), which showed a small but significant increase for both groups.
Table 3.
Body composition variables. Data are means (±SE).
| Variable | YE (n=16) | CON (n=18) | ||||
|---|---|---|---|---|---|---|
| Baseline | 8-month | %Δ | Baseline | 8-month | %Δ | |
| Weight (kg) | 69.7 (3.3) | 70.9 (3.5) | 1.8 (1.1) | 70.0 (2.2) | 70.1 (1.9) | .5 (1.4) |
| Total FM (kg) | 27.8 (2.2) | 28.5 (2.6) | 1.6 (2.8) | 28.2 (2.0) | 27.7 (1.8) | .3 (3.5) |
| BFLBM (kg)** | 38.8 (1.3) | 39.5 (1.2) | 2.0 (.6) | 38.6 (.8) | 39.3 (.8) | 1.9 (.8) |
| % Fat | 39.4 (1.6) | 39.3 (1.9) | -.6 (1.7) | 39.8 (1.9) | 39.2 (1.8) | -.7 (1.9) |
YE: Yoga Exercise, CON: Control, %Δ : percent change, Total FM: total fat mass, BFLBM: Bone Free Lean Body Mass.
**p<0.01 significant time effect.
Bone marker responses
The results for bone marker responses are presented in Table 4. The Bone ALP reference range for premenopausal women 25-44 years of age is 11.6 – 29.6 (U/L) (MicroVue™BAP EIA Kit). The TRAP5b reference range for premenopausal women 30-44 years of age is 1.5 – 4.3 (U/L) (MicroVue™TRAP5b Enzyme Immunoassay Kit). All participants had values within the references at baseline and after 8 months. There were no significant differences between groups for Bone ALP, TRAP5b, or the Bone ALP:TRAP5b ratio at baseline. There was no significant group or time effect (p > 0.05) for Bone ALP; however, a significant group × time interaction (p = 0.002) was detected. Paired samples t-tests with Bonferroni adjustment indicated that Bone ALP significantly decreased (p = 0.005) for the CON group, whereas YE had similar Bone ALP concentrations before and after the intervention (p = 0.08). Also, there was a significant group difference (p = 0.002, d = 1.23) in percent changes for Bone ALP as the YE group showed an increase (9.1 ± 4.0%), and the CON group had a decrease (−7.1 ± 2.3%) after the intervention (Figure 4). For TRAP5b concentrations, no significant group, time, or group × time interaction effects were detected (Table 4), and there were no significant group differences in percent changes in TRAP5b (Figure 4).
Table 4.
Bone marker responses before and after training. Data are means (±SE).
| Variable | YE (n=16) | CON (n=18) | 8-month | |
|---|---|---|---|---|
| Baseline | 8-month | Baseline | 8-month | |
| Bone ALP (U/L)** | 25.0 (1.8) | 26.8 (1.8) | 29.1 (2.3) | 27.0 (2.4)†† |
| TRAP5b (U/L) | 2.5 (.2) | 2.4 (.2) | 2.6 (.2) | 2.3 (.2) |
| Ratio | 10.8 (.8) | 11.6 (.7) | 12.0 (1.1) | 12.9 (1.5) |
YE: Yoga Exercise, CON: Control, Bone ALP: bone Alkaline Phosphatase, TRAP5b: Tartrate-Resistant Acid Phosphatase 5b, Ratio: Ratio of Bone ALP to TRAP5b.
**p<0.01 significant group
× time interaction
†† p<0.01 baseline vs. 8-month for CON.
Figure 4.
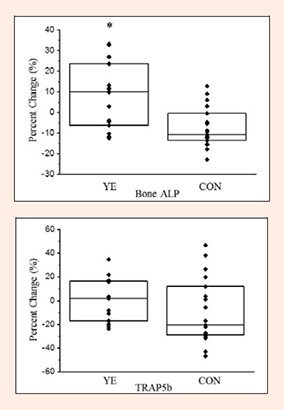
Individual percent changes in bone markers after 8 months of training. Bars represent the median (quartile), YE: Yoga Exercise, CON: Control, Bone ALP: Bone Alkaline Phosphatase, TRAP5b: Tartrate-Resistant Acid Phosphatase 5b. *p<0.05 Significant group difference
Correlational analysis for the entire sample determined that percent changes in calcium intake were not significantly correlated to percent changes in either BTM. Percent change in Bone ALP was not significantly correlated to body composition percent changes; however, percent change in TRAP5b was negatively related to percent change in body weight (r = −0.44, p = 0.009) and to percent change in total fat mass (r = − 0.47, p = 0.005). The BTM marker findings described above were not altered when percent changes in calcium intake or body composition variables (weight, percent fat, BFLBM) were used as covariates in ANCOVA procedures.
Areal bone mineral density and pQCT variables
Table 5 shows the baseline, 8 month and percent changes for aBMD at the total body, right and left hip (total hip, femoral neck and trochanter), and the lumbar spine (L1-L4) measured by DXA. At baseline, there were no significant differences for any aBMD variables between groups (p > 0.05). Baseline T-scores were in the osteopenia range (−1.1 to −2.4) for 4 YE and 3 CON participants at the hip sites, and 2 YE and 2 CON participants at the spine (L1-L4). No significant group, time or group × time interaction effects for aBMD were detected (p > 0.05). Also, there were no group differences in percent changes for aBMD values (p > 0.05). Baseline, 8 month, and percent change for pQCT outcomes are presented in Table 6.
Table 5.
Areal BMD (aBMD) before and after training. Data are means (±SE).
| aBMD | YE (n=16) | CON (n=18) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8-month | %Δ | Baseline | 8-month | %Δ | ||
| Total Body | 1.169 (.018) | 1.163 (.018) | -.5 (.3) | 1.184 (.017) | 1.184 (.017) | .0 (.3) | |
| Right | Total Hip | .990 (.028) | .989 (.028) | .2 (.3) | 1.037 (.026) | 1.040 (.026) | .1 (.3) |
| Femoral Neck | .963 (.026) | .956 (.025) | -.9 (.5) | 1.011 (.025) | 1.011 (.024) | .1 (.8) | |
| Trochanter | .789 (.028) | .789 (.028) | -.1 (.5) | .798 (.026) | .807 (.027) | 1.1 (.6) | |
| Left | Total Hip | .999 (.026) | .996 (.027) | -.4 (.4) | 1.030 (.024) | 1.031 (.025) | .0 (.3) |
| Femoral Neck | .971 (.024) | .963 (.025) | -1.0 (.7) | .995 (.023) | .999 (.024) | .3 (.5) | |
| Trochanter | .793 (.026) | .788 (.026) | -.8 (.4) | .805 (.024) | .808 (.025) | .3 (.5) | |
| Spine (L1-L4) | 1.210 (.033) | 1.210 (.033) | .0 (.6) | 1.233 (.031) | 1.231 (.031) | -.1 (.5) | |
All values expressed in g/cm2. YE: Yoga Exercise, CON: Control, %Δ : percent change. No significant group, time, or group × time interaction for all aBMD variables (p>0.05).
Table 6.
Tibia bone characteristics before and after training. Data are means (±SE).
| Variable | YE (n=16) | CON (n=18) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8-month | %Δ | Baseline | 8-month | %Δ | ||
| Tibia 4% | ToD (mg/cm3) | 284.1 (9.5) | 284.5 (8.9) | .3 (.7) | 300.5 (8.9) | 301.9 (8.4) | .6 (1.1) |
| ToA (mm2) | 958.5 (29.4) | 952.4 (28.8) | -.3 (1.3) | 879.4 (27.7) | 873.9 (27.2) | -.5 (1.5) | |
| TraD(mg/cm3) | 232.5 (6.9) | 232.8 (6.8) | .1 (.2) | 236.7 (6.5) | 236.3 (6.4) | -.1 (.4) | |
| TraA (mm2) | 813.7 (29.0) | 807.7 (27.8) | -.3 (1.6) | 733.3 (27.3) | 726.2 (26.2) | -.7 (1.9) | |
| Tibia 38% | ToD (mg/cm3) | 938.2 (14.4) | 936.0 (14.2) | -.2 (.1) | 919.3 (13.5) | 919.8 (13.4) | .1 (.1) |
| ToA (mm2) | 353.2 (8.9) | 353.5 (8.7) | .1 (.2) | 351.2 (8.3) | 351.7 (8.2) | .2 (.2) | |
| CoD(mg/cm3) | 1189.6 (6.7) | 1187.1 (6.9) | -.2 (.1) | 1182.6 (6.3) | 1182.9 (6.5) | .0 (.1) | |
| CoA(mm2) | 265.1 (7.1) | 265.3 (6.9) | .1 (.3) | 261.1 (6.7) | 261.6 (6.5) | .2 (.3) | |
| CTh (mm) | 5.3 (.1) | 5.3 (.1) | .0 (.3) | 5.2 (.1) | 5.3 (.1) | .1 (.3) | |
| SSI (mm3) | 1450.3 (50.6) | 1447.3 (49.0) | -.2 (.4) | 1442.4 (47.7) | 1436.8 (46.2) | -.2 (.7) | |
| Tibia 66% | ToD (mg/cm3) | 656.2 (15.5) | 655.3 (15.4) | -.1 (.3) | 659.1 (14.6) | 660.3 (14.5) | .2 (.5) |
| ToA (mm2) | 533.0 (15.6) | 533.1 (14.9) | .1 (.2) | 522.5 (14.7) | 521.3 (14.1) | -.2 (.4) | |
| CoD(mg/cm3) | 1141.5 (7.2) | 1137.6 (7.4) | -.3 (.1) | 1131.9 (6.8) | 1131.0 (7.0) | -.1 (.1) | |
| CoA(mm2) | 270.6 (6.8) | 272.1 (6.9) | .5 (.2) | 270.4 (6.4) | 270.6 (6.5) | .0 (.3) | |
| CTh (mm) | 3.9 (.1) | 3.9 (.1) | .6 (.3) | 4.0 (.1) | 4.0 (.1) | .2 (.5) | |
| SSI (mm3) | 2226.5 (80.9) | 2219.6 (77.3) | -.2 (.3) | 2158.1 (76.3) | 2154.1 (72.9) | -.1 (.4) | |
YE: Yoga Exercise, CON: Control, %Δ : percent change, ToD : total volumetric BMD, ToA: total bone area, TraD: trabecular volumetric BMD, TraA: trabecular area, SSI: strength strain index, CoD: cortical volumetric BMD, CoA: cortical area, CTh: cortical thickness. *p=0.005 significant time effect
At baseline, there were no significant group differences for volumetric BMD, geometry and strength variables at the tibia 4%, 38% and 66%. After 8 months, there was an only significant time effect for CoD at the tibia 66% site as both groups significantly (p = 0.005) decreased. There were no significant group, time or group × time interaction effects for the rest of pQCT outcomes. No significant group differences inpercent changes for pQCT outcomes were detected after the 8 month intervention (p > 0.05)
Discussion
Our study is the first randomized controlled trial examining the effects of a progressive Ashtanga Yoga training program on bone turnover markers and bone characteristics in premenopausal women. The primary unique finding was that the bone formation marker, serum Bone ALP, showed different responses for the two groups as it was maintained in YE, but significantly decreased in CON during the 8 month intervention. Also, the percent changes in Bone ALP were in opposite directions for the two groups; YE had a small increase, whereas CON had a decrease, yielding a net benefit of 16.2% for the Yoga treatment group. The bone resorption marker did not change for either group. These patterns of BTM responses are similar to those reported in short duration exercise interventions in young college age women (Lester et al., 2009) and in overweight men and women (Hinton et al., 2006). Lester et al. (2009) found that Bone ALP significantly increased in the resistance training (16.8%) and combined resistance and aerobic training (15.8%) groups, while it decreased in the control group. There was no significant training group effect for TRAP5b, although it significantly decreased at the end of the 8 week intervention for all three groups. Hinton et al. (2006) examined BTM responses to weight-bearing aerobic exercise in overweight and obese men and women who underwent a 5% reduction in body weight. They reported a significant increase in Bone ALP (5.7%) at the end of the 6 week intervention with no concomitant change in bone resorption markers, thus, the addition of weight-bearing exercise to the weight loss treatment ameliorated the negative effects of energy restriction on bone turnover rates in overweight men and women.
It is interesting to note that the Ashtanga-based Yoga program was able to maintain Bone ALP concentrations in these premenopausal women. There are seasonal variations in bone turnover markers, which tend to be lowest in the summer months and highest in the winter months, with amplitudes reported as high as 17.7% (Woitge et al., 1998). Blumsohn et al. (2003) reported TRAP5b had a significant time of year difference with an amplitude of 3.5% of the individual annual mean, whereas Bone ALP had a non-significant amplitude of 2.0%. Also, circannual rhythm accounted for a small percentage of the within-subject variance for both markers (Bone ALP, 1.5%; TRAP5b, 0.8%). Since we collected the baseline blood samples in October and the post-training blood samples in June, we would expect a decrease in the bone markers for the control group, which was documented for both Bone ALP (-7.1%) and TRAP5b (-8.3%). However, the YE group did not show this seasonal pattern for Bone ALP, as it increased +9.1%, suggesting the yoga intervention was associated with a small but significant increase in bone formation. The YE group TRAP5b response was close to the within-subject variance accounted for by seasonal rhythm for this resorption marker reported by Blumsohn et al. (2003).
There are limited studies that have examined the effects of alternative forms of exercise on bone turnover markers in women. The few available studies on yoga training were not randomized controlled trials and it is not clear that sources of biological variability (e.g., diurnal variation, food intake, exercise) in BTM were adequately controlled. Phoosuwan et al. (2009) investigated the effects of a weight-bearing Yoga training program on bone markers in postmenopausal women (50-60 years). They found that 3 times a week weight-bearing Yoga training for 12 weeks had a positive effect by decreasing the bone resorption marker, a response not observed in the control group. However, they found no effects on the bone formation marker for either group. On the other hand, a 12-week Yoga training (once a week) study in osteopenic postmenopausal women showed a trend for a positive moderate correlation between the amount of Yoga practice and Bone ALP levels, but not with urinary type I collagen cross-linked N-telopeptide levels, the bone resorption marker (Balk et al., 2009). Similar to our results, a 6-month Tai Chi training (3 times per week 40 min exercise sessions) was effective in increasing Bone ALP in the elderly (ages) after 6 weeks of training (Shen et al., 2007). In contrast to previous studies, the strengths of our study are the randomization of the treatment and control for diurnal variation and acute food intake on BTM concentrations as all blood samples were obtained at the same time in the morning in a fasted state.
We did not find any changes in aBMD of total body, lumbar spine (L1-L4), and dual proximal femur (total hip, femoral neck, trochanter) as measured by DXA. A cross-sectional study (Mukherjee et al., 2010) reported 14 premenopausal women who did Bikram (hot) Yoga at least 3 times a week for 3 or more years had generally higher BMD at the lumbar spine, hip and the whole body compared with the age, sex and race-matched controls. Similarly, results of Tai Chi studies reported that long-term postmenopausal Tai Chi participants have higher BMD and slower rates of bone loss than age-matched sedentary controls (Qin et al., 2002; 2005; Xu et al., 2004). In addition, 12 months of regular Tai Chi training significantly slowed bone loss in both trabecular and cortical compartments of the distal tibia in Tai Chi group as compared with the sedentary control group (Chan et al., 2004). We found no significant changes in the tibia pQCT variables (ToD, ToA, CTh, SSI) for total, cortical and trabecular bone, with the exception of cortical vBMD at the tibia 66%, which significantly decreased for both groups after the intervention. However, the CV% for cortical vBMD is 0.5% so these results are within the precision of this measurement. In contrast, Qin et al. (2002) found that postmenopausal women who did regular Tai Chi over 4 years had greater BMD in the trabecular compartment of the distal tibia compared with age-matched controls. In our study, the 8 month Yoga program was designed to improve BMD by progressively increasing the intensity by increasing the number of SS, and thus the number of jumps, performed each month. Hagins et al. (2007) suggested that increasing the number of SS may be a way to increase the intensity high enough to improve cardiorespiratory fitness. Although we increased the session intensity each month, it still may not have been sufficient intensity or duration to elicit meaningful improvements in aBMD or in vBMD. We previously reported that leg press strength was significantly increased in the YE group (Kim et al., 2012), suggesting that the force produced by muscle contractions during the yoga postures was sufficient to elicit adaptations in muscle but not in bone.
There are several imitations to our study. The attrition rate was 27.6%, which was mostly due to time commitments for 8 months and personal medical issues. Although not directly related to the Yoga intervention itself, the majority of the participants lost to follow up were from the Yoga group. In a meta-analysis of randomized controlled exercise interventions for BMD, Kelley et al. (2013) reported that dropout rates were higher in premenopausal (28-53%) vs. postmenopausal (14-23%) women and higher in exercise groups (17-26%) vs. control groups (12-21%). Our dropout rates are in line with previous randomized controlled trials. It is plausible that other factors contributed to changes in bone turnover markers. We did not measure serum Vitamin D concentrations to assess Vitamin D status, which plays a key role in general bone health (Rosen and Mayne, 2012). The menstrual cycle status of our participants before and after the intervention was determined by self-report and not verified by serum hormone measurements. Crandall et al. (2013) used a longitudinal design to examine the contribution of changes in estradiol and follicle-stimulating hormone (FSH) concentrations to BMD changes that occurred during three phases of the menopausal transition. They reported lumbar spine and femoral neck BMD losses of 0.28%/year and 0.27%/year during the pretransmenopause phase (baseline to 1 year before the final menstrual period). Also, in this phase FSH and estradiol concentrations were associated with rates of lumbar spine BMD bone loss but not femoral neck BMD loss. In our study, one woman was excluded from the analysis due to the onset of menopausal symptoms; however, the remaining participants reported regular menstrual cycles and no menopausal symptoms. Although it is possible that some of the women in our study could have experienced hormonal changes, we postulate that they would be in the pretransmenopause phase given their regular menstrual cycles.
The body composition characteristics of our participants is another possible limitation to this study. The women, on average, were overweight based on BMI, and both groups were encouraged to maintain their body weight during the intervention. There were no significant changes in body weight, Total FM, and %fat after the study; however, both groups showed small increases in BFLBM after the 8 months. As previously discussed, overweight subjects undergoing weight loss still showed significant improvements in bone formation in response to weight-bearing exercise (Hinton et al., 2006), therefore, we expect that the BTM responses in our study were minimally affected by the body composition of the participants. Finally, although each Yoga technique was precisely instructed in the same manner, the variations of Yoga postures, sequences, and duration of each movement were dependent on each participant’s physical abilities.
Conclusion
The findings of this study suggest that 8 months of Ashtanga-based Yoga had a small positive effect on bone formation but did not improve bone density or geometry in premenopausal women. Most previous studies investigating the possible beneficial effects of Yoga have focused on the potential uses as a therapeutic tool for reducing symptoms of depression and anxiety. The present study is the first report of a randomized controlled trial of an intervention for Ashtanga Yoga exercise to determine its effects on bone metabolism and BMD responses. Future studies are needed to quantify the intensity of weight-bearing Yoga postures, including Sun Salutations, and these can be measured by a force plate to determine ground reaction forces. Also, the utilization of longer duration and greater frequency weight-bearing Ashtanga Yoga programs are recommended to detect its effects on BMD in premenopausal women.
Acknowledgements
We gratefully acknowledge the women for their participation in our 8-month Yoga training study. Without their dedication, this study would not have been possible. We also thank to Harshvardhan, Jessica Smith, Carmen Chrisman for the data collection and the staff of the Huston Huffman Physical Fitness Center at OU for their support during the intervention. This study was funded in part by the University of Oklahoma College of Arts & Sciences and Graduate College
Biographies
SoJung KIM
Employment
Instructor in Sports Medicine Department at the Kyunghee University Global Campus
Degree
PhD
Research interests
Bone health and osteoporosis prevention
E-mail: bone1342@gmail.com
Michael G. BEMBEN
Employment
Professor and Chair in Health and Exercise Science Department at the University of Oklahoma
Degree
PhD
Research interests
Aging and neuromuscular function, Effects of resistance training interventions on muscle strength and size
E-mail: mgbemben@ou.edu
Allen W. KNEHANS
Employment
Professor and Chair, Department of Nutritional Sciences, University of Oklahoma Health Sciences Center
Degree
PhD
Research interests
Nutrition, Obesity
E-mail: allen-knehans@ouhsc.edu
Debra A. BEMBEN
Employment
Professor in Health and Exercise Science Department at the University of Oklahoma
Degree
PhD
Research interests
Effects of exercise interventions on bone density and bone metabolism in postmenopausal women
E-mail: dbemben@ou.edu
References
- Ades P.A., Savage P.D., Brochu M., Tischler M.D., Lee N.M., Poehlman E.T. (2005) Resistance training increases total daily energy expenditure in disabled older women with coronary heart disease. Journal of Applied Physiology 98, 1280-1285. [DOI] [PubMed] [Google Scholar]
- Bailey C.A., Brooke-Wavell K. (2010) Optimum frequency of exercise for bone health: a randomized controlled trial of a high-impact unilateral intervention. Bone 46, 1043-1049. [DOI] [PubMed] [Google Scholar]
- Balk J., Gluck M., Bernardo L., Catov J. (2009) The effect of yoga on markers of bone turnover in osteopenic women: a pilot study. International Journal of Yoga Therapy 19, 63-68. [Google Scholar]
- Bemben D.A., Bemben M.G. (2011) Dose-response effect of 40 weeks of resistance training on bone mineral density in older adults. Osteoporosis International 22, 179-186. [DOI] [PubMed] [Google Scholar]
- Bhutkar M.V., Bhutkar P.M., Taware G.B., Surdi A.D. (2011) How effective is sun salutation in improving muscle strength, general body endurance and body composition? Asian Journal of Sports Medicine 2, 259-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J.A., Emery C.F., Madden D.J., Schniebolk S., Riddle M.W., Cobb F.R., Higginbotham M., Coleman R.E. (1991) Effects of exercise training on bone density in older men and women. Journal of the American Geriatric Society 39, 1065-1070. [DOI] [PubMed] [Google Scholar]
- Blumsohn A., Naylor K.E., Timm W., Eagleton A.C., Hannon R.A., Eastell R. (2003) Absence of marked seasonal change in bone turnover: a longitudinal and multicenter cross-sectional study. Journal of Bone and Mineral Research 18, 1274-1281. [DOI] [PubMed] [Google Scholar]
- Chan K., Qin L., Lau M., Woo J., Au S., Choy W., Lee K., Lee S. (2004) A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Archives of Physical Medicine and Rehabilitation 85, 717-722. [DOI] [PubMed] [Google Scholar]
- Cowen V.S., Adams T.B. (2007) Heart rate in yoga asana practice: A comparison of styles. Journal of Bodyworks and Movement Therapies 11, 91-95. [Google Scholar]
- Crandall C.J., Tseng C.H., Karlamangla A.S., Finkelstein J.S., Randolph J.F., Thurston R.C., Huang M.H., Zheng H., Greendale G.A. (2013) Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. Journal of Clinical Endocrinology and Metabolism 98, E654-E663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175-191. [DOI] [PubMed] [Google Scholar]
- Gokal R., Shillito L., Maharaj S. R. (2007) Positive impact of yoga and pranayam on obesity, hypertension, blood sugar, and cholesterol: a pilot assessment. Journal of Alternative and Complementary Medicine 13, 1056-1057. [DOI] [PubMed] [Google Scholar]
- Hagins M., Moore W., Rundle A. (2007) Does practicing hatha yoga satisfy recommendations for intensity of physical activity which improves and maintains health and cardiovascular fitness? BMC Complementary and Alternative Medicine 7, 40-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton P.S., Rector R.S., Thomas T.R. (2006) Weight-bearing, aerobic exercise increases markers of bone formation during short-term weight loss in overweight and obese men and women. Metabolism Clinical and Experimental 55, 1616-1618. [DOI] [PubMed] [Google Scholar]
- Kelley G.A., Kelley K.S., Kohrt W.M. (2013) Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. International Journal of Endocrinology 2013, 741639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley G.A, Kelley K.S. (2013) Dropouts and compliance in exercise interventions targeting bone mineral density in adults: a meta-analysis of randomized controlled trials. Journal of Osteoporosis 2013, 250423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri D., Mathur K.C., Gahlot S., Jain S., Agrawal R.P. (2007) Effects of yoga and meditation on clinical and biochemical parameters of metabolic syndrome. Diabetes Research and Clinical Practice 78, e9-10. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Bemben M.G., Bemben D.A. (2012) Effects of an 8-month yoga intervention on arterial compliance and muscle strength in premenopausal women. Journal of Sports Science and Medicine 11, 322-330. [PMC free article] [PubMed] [Google Scholar]
- Kohrt W.M., Bloomfield S.A., Little K.D., Nelson M.E., Yingling V.R. (2004) Physical activity and bone health. Medicine and Science in Sports and Exercise 36, 1985-1996. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy M.N., Telles S. (2007) Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. Journal of Gerontological Nursing 33, 17-23. [DOI] [PubMed] [Google Scholar]
- Lester M.E., Urso M.L., Evans R.K., Pierce J.R., Spiering B.A., Maresh C.M., Hatfield D.L., Kraemer W.J., Nindl B.C. (2009) Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training. Bone 45, 768-776. [DOI] [PubMed] [Google Scholar]
- Lohman T., Going S., Pamenter R., Hall M., Boyden T., Houtkooper L., Ritenbaugh C., Bare L., Hill A., Aickin M. (1995) Effects of resistance training on regional and total bone mineral density in premenopausal women: a randomized prospective study. Journal of Bone and Mineral Research 10, 1015-1024. [DOI] [PubMed] [Google Scholar]
- Madanmohan Udupa K., Bhavanani A.B., Shatapathy C.C., Sahai A. (2004) Modulation of cardiovascular response to exercise by yoga training. Indian Journal of Physiology and Pharmacology 48, 461-465. [PubMed] [Google Scholar]
- Mukherjee A., Mukherjee P., Rude R.R. (2010) Bikram Yoga as a countermeasure of bone loss in women. Chinese Medicine 1, 1-4. [Google Scholar]
- Musgrave K.O., Giambalvo L., Leclerc H.L., Cook R.A., Rosen C.J. (1989) Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. Journal of the American Dietetic Association 89, 1484-1488. [PubMed] [Google Scholar]
- Omkar S.N., Mour M., Das D. (2011) A mathematical model of effects of specific joints during practice of the Sun Salutation - a sequence of yoga postures. Journal of Bodywork & Movement Therapies 15, 201-208. [DOI] [PubMed] [Google Scholar]
- Patel N.K., Newstead A.H., Ferrer R.L. (2012) The effects of yoga on physical functioning and health related quality of life in older adults: a systematic review and meta-analysis. Journal of Alternative and Complementary Medicine 18, 902-917. [DOI] [PubMed] [Google Scholar]
- Phoosuwan M., Kritpet T., Yuktanandana P. (2009) The effects of weight bearing yoga training on the bone resorption markers of the postmenopausal women. Journal of the Medical Association of Thailand 92, 102-108. [PubMed] [Google Scholar]
- Polidoulis I., Beyene J., Cheung A.M. (2012) The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women--a systematic review and meta-analysis of randomized controlled trials. Osteoporosis International, 23, 39-51. [DOI] [PubMed] [Google Scholar]
- Qin L., Au S., Choy W., Leung P., Neff M., Lee K., Lau M., Woo J., Chan K. (2002) Regular Tai Chi Chuan exercise may retard bone loss in postmenopausal women: A case-control study. Archives of Physical Medicine and Rehabilitation 83, 1355-1359. [DOI] [PubMed] [Google Scholar]
- Qin L., Choy W., Leung K., Leung P.C., Au S., Hung W., Dambacher M., Chan K. (2005) Beneficial effects of regular Tai Chi exercise on musculoskeletal system. Journal of Bone and Mineral Metabolism 23, 186-190. [DOI] [PubMed] [Google Scholar]
- Ray U.S., Mukhopadhyaya S., Purkayastha S.S., Asnani V., Tomer O.S., Prashad R., Thakur L., Selvamurthy W. (2001) Effect of yogic exercises on physical and mental health of young fellowship course trainees. Indian Journal of Physiology and Pharmacology 45, 37-53. [PubMed] [Google Scholar]
- Rosen C.J., Mayne S.T. (2012) Vitamin D dose requirements for fracture prevention. The New England Journal of Medicine, 367, 1368; author reply 1369-1370. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Cook I.A., Davydov D.M., Ottaviani C., Leuchter A.F., Abrams M. (2007) Yoga as a Complementary Treatment of Depression: Effects of Traits and Moods on Treatment Outcome. Evidence-Based Complementary and Alternative Medicine 4, 493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.L., Williams J.S., Chyu M.C., Paige R.L., Stephens A.L., Chauncey K.B., Prabhu F. R., Ferris L.T., Yeh J.K. (2007) Comparison of the effects of Tai Chi and resistance training on bone metabolism in the elderly: a feasibility study. The American Journal of Chinese Medicine 35, 369-381. [DOI] [PubMed] [Google Scholar]
- Shibata Y., Ohsawa I., Watanabe T., Miura T., Sato Y. (2003) Effects of physical training on bone mineral density and bone metabolism. Journal of Physiological Anthropology and Applied Human Science 22, 203-208. [DOI] [PubMed] [Google Scholar]
- Tran M.D., Holly R.G., Lashbrook J., Amsterdam E.A. (2001) Effects of Hatha yoga practice on the health-related aspects of physical fitness. Preventive Cardiology 4, 165-170. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2004) Bone health and osteoporosis. A report of the Surgeon General. Rockville: Department of health and Human Services, Office of the Surgeon General. [Google Scholar]
- Weeks B.K., Beck B.R. (2008) The BPAQ: a bone-specific physical activity assessment instrument. Osteoporosis International 19, 1567-1577. [DOI] [PubMed] [Google Scholar]
- Wilcox S.J., Hager R., Lockhart B., Seeley M.K. (2012) Ground reaction forces generated by twenty-eight Hatha Yoga postures. International Journal of Exercise Science 5, 114-126. [PMC free article] [PubMed] [Google Scholar]
- Winters-Stone K.M., Snow C.M. (2006) Site-specific response of bone to exercise in premenopausal women. Bone 39, 1203-1209. [DOI] [PubMed] [Google Scholar]
- Woitge H.W., Scheidt-Nave C., Kissling C., Leidig-Bruckner G., Meyer K., Grauer A., Scharla S.H., Ziegler R., Seibel M.J. (1998) Seasonal variation of biochemical indexes of bone turnover: results of a population-based study. Journal of Clinical Endocrinology and Metabolism 83, 68-75. [DOI] [PubMed] [Google Scholar]
- Woolery A., Myers H., Sternlieb B., Zeltzer L. (2004) A yoga intervention for young adults with elevated symptoms of depression. Alternative Therapies in Health and Medicine 10, 60-63. [PubMed] [Google Scholar]
- Xu H., Lawson D., Kras A. (2004) A study on Tai Ji exercise and traditional chinese medical modalities in relation to bone structure, bone function and menopausal symptoms. Journal of Chinese Medicine-Hove 74, 3-7. [Google Scholar]
- Yoga S. (2008) 2008 Yoga in America Market Study. Yoga Journal. Available form URL: http://www.yogajournal.com/advertise/press_releases/10 [Google Scholar]


