Highlights
-
•
We measure brain activation during anticipation of reward and loss in adolescents.
-
•
We compare incentive responses in six groups formed based on substance use patterns.
-
•
No activation differences emerge between the cannabis-only group and the other groups.
-
•
Tobacco-only users have less reward response than polysubstance and alcohol-only users.
-
•
Tobacco-only users show decreased reward activation compared to the control group.
Keywords: Adolescent, Cannabis, Tobacco, fMRI, Monetary incentive delay
Abstract
Numerous questions surround the nature of reward processing in the developing adolescent brain, particularly in regard to polysubstance use. We therefore sought to examine incentive-elicited brain activation in the context of three common substances of abuse (cannabis, tobacco, and alcohol). Due to the role of the nucleus accumbens (NAcc) in incentive processing, we compared activation in this region during anticipation of reward and loss using a monetary incentive delay (MID) task. Adolescents (ages 14–18; 66% male) were matched on age, gender, and frequency of use of any common substances within six distinct groups: cannabis-only (n = 14), tobacco-only (n = 34), alcohol-only (n = 12), cannabis + tobacco (n = 17), cannabis + tobacco + alcohol (n = 17), and non-using controls (n = 38). All groups showed comparable behavioral performance on the MID task. The tobacco-only group showed decreased bilateral nucleus accumbens (NAcc) activation during reward anticipation as compared to the alcohol-only group, the control group, and both polysubstance groups. Interestingly, no differences emerged between the cannabis-only group and any of the other groups. Results from this study suggest that youth who tend toward single-substance tobacco use may possess behavioral and/or neurobiological characteristics that differentiate them from both their substance-using and non-substance-using peers.
1. Introduction
Despite a legacy of being characterized by “storm and stress” (Hall, 1904), there continues to be great controversy regarding whether decision-making in adolescence is inherently adaptive or harmful (Mills et al., 2014). Existing empirical data have generally fallen toward the side of risk, as captured by several prevailing theories of adolescent neurodevelopment [e.g., “dual process” (Somerville et al., 2010); “triadic” (Ernst, 2014)]. Broadly, these theories suggest a developmental imbalance between neural reward and control systems that arises because subcortical reward circuitry reaches maturity earlier than frontally mediated executive control regions (Steinberg, 2010, Galvan, 2014). This developmental pattern may contribute to differences in adolescent decision-making, particularly if there is an opportunity for reward (Galvan et al., 2007, Chung et al., 2011).
While experimentation with substances is within the realm of typical adolescent behavior (Shedler and Block, 1990), there is some concern that substance use may exacerbate and/or enhance risks associated with the developing brain (e.g., Squeglia et al., 2009, Lubman et al., 2007). Importantly, despite the prevalence of substance use during adolescence, the relationship between substance use and reward processing in the adolescent brain is not fully understood (Geier, 2013). However, given the well-established link between substance use and altered reward circuitry in adults (Volkow et al., 2003), there is reason to believe that substance use during adolescence may interact with developing reward processing substrates.
One challenge in adolescent addiction research is that youth tend toward polysubstance use, using different substances together, often based on availability (Moss et al., 2014). American adolescents tend to abuse three substances: alcohol (75.6%), cannabis (48.6%), and tobacco (48.1%) (Kann et al., 2014). Despite the established use and clustering of these substances, most neuroimaging studies have examined only one, or at maximum two, substance per evaluation. This is problematic from a neural-circuitry perspective as prior studies revealed different patterns of brain response for single- vs. poly-substance using youth (i.e., alcohol-only, cannabis-only vs. alcohol + cannabis; e.g., Jacobus et al., 2013, Bava et al., 2009). Moreover, although research has begun to address co-occurring alcohol and cannabis use, few studies included tobacco. This is important given the possible impact of tobacco on the developing brain and on subsequent problem alcohol and cannabis use (Whelan et al., 2014). Thus, in a comprehensive model of adolescent reward processing, it is crucial to evaluate how these three substances interact with developing reward neurocircuitry; to our knowledge, this has not yet been done.
In line with adult research (e.g., Knutson et al., 2001a, Abler et al., 2006), examinations of reward processing in adolescent substance users have focused on the nucleus accumbens (NAcc). This structure receives dopaminergic input from the ventral tegmental area (VTA) in response to reinforcing stimuli (Spanagel and Weiss, 1999). During adolescence, the NAcc is highly responsive to rewards (Galvan et al., 2006, Ernst et al., 2005). This may stem from naturally occurring developmental changes in dopaminergic functioning, including increased dopamine receptor expression in the NAcc and greater striatal dopamine release during adolescence (Galvan, 2010, Laviola et al., 2003, Tarazi et al., 1998).
These normative changes in NAcc functioning and reward sensitivity may impact the development and maintenance of addiction (Volkow and Baler, 2014). Indeed, during adulthood, the ventral striatum (VS), which encompasses the NAcc, mediates the reinforcing effects of substances of abuse (Robinson and Berridge, 2000, Everitt and Robbins, 2005, Wise and Dopamine, 2004). Further, reward-related NAcc activation is associated with increased willingness to take risks (Knutson et al., 2008, Knutson and Greer, 2008). Individuals who experience greater VS/NAcc-mediated reward responses may thus be reinforced for engaging in risky and immediately rewarding behaviors such as substance use. Specifically, greater trait impulsivity has been observed in several studies of adolescent substance use (Stautz and Cooper, 2013, Fernández-Artamendi et al., 2015). Further, impulsivity is associated with greater reward sensitivity among substance users (Bjork et al., 2008). Thus, heightened reward sensitivity in the VS/NAcc may sustain problem substance use, potentially via amplifying the value of substance-related rewards in adolescents (and particularly among those with high impulsivity) (Karoly et al., 2013, Koob and Volkow, 2010).
In terms of substance-specific mechanisms of action, nicotine facilitates dopaminergic neurotransmission within brain reward pathways via activating nicotinic actylcholine (nAch) receptors, including those located on nerve terminals in VTA projections to the NAcc (D'souza and Markou, 2011). Alcohol influences dopaminergic transmission in the NAcc through activating GABAA receptors, which disinhibit GABAergic neurons in the VTA, causing dopamine release from the VTA to NAcc (Gilpin and Koob, 2008). In contrast, the mechanism of action of cannabis on these reward structures is less well understood. However, it seems to involve the binding of delta-9-tetrahydrocannabinol [THC] to CB1 receptors and selectively inducing dopamine release within the NAcc (French, 1997). It is currently unclear how these substances consumed in various combinations may differentially impact neural reward structures. Thus, across all three substances and different combinations thereof, the NAcc represents an important target for examining reward processing in adolescent substance users.
However, to date, few studies have investigated the relationship between these substances and reward neurocircuitry in adolescents. Of the existing studies, the monetary incentive delay (MID) task (see Balodis and Potenza, 2014) has gained support for its ability to quantify critical functional differences in reward processing across development (Bjork et al., 2004) and addiction (e.g., Filbey et al., 2013, van Hell et al., 2010, Wrase et al., 2007). Briefly, the MID evaluates changes in blood-oxygen level dependent (BOLD) response during the experience of anticipating receiving a reward (“positive incentives”) and avoiding a loss (“negative incentives”). The VS/NAcc has emerged as a particularly relevant structure mediating brain response to this task (Knutson et al., 2001b). In terms of substance use, Heitzeg and colleagues (2014) found that, in contrast to adults (Wrase et al., 2007), alcohol using youth showed positive correlations between reward-related NAcc activation and alcohol use. Cannabis-using young adults have shown increased VS activation during reward anticipation compared to non-users, with VS response correlated with greater cannabis use (Filbey et al., 2013, Nestor et al., 2010). In contrast, among tobacco users, studies have suggested lower VS responses during MID reward anticipation in adolescents (Peters et al., 2011) and adults (Rose et al., 2013).
Taken together, the MID literature (e.g., Balodis and Potenza, 2014) points to differences in VS activation during reward anticipation among users of different substances. Critically, these relationships may be complicated by poly- vs. single-substance use. For example, among adults, tobacco use is associated with decreased striatal reward responses (Rose et al., 2013), but interestingly, this is not the case among smokers who also use cannabis (van Hell et al., 2010, Nestor et al., 2010). Similarly, among adults, alcohol is associated with decreased reward-related striatal responses (Wrase et al., 2007, Beck et al., 2009), but not among drinkers who smoke tobacco (Bjork et al., 2012). Finally, cannabis is associated with increased striatal reward responding, even among individuals who also use tobacco (van Hell et al., 2010, Nestor et al., 2010).
Thus, in this first preliminary study of this kind, we aimed to deconstruct these relationships in adolescents, by comparing reward and loss anticipation (i.e., positive and negative incentive processing) in the NAcc among single- versus poly-substance users of the three most prevalent substances in this age group (alcohol, cannabis, and tobacco; Kann et al., 2014). Based on prior studies (e.g., Filbey et al., 2013, Nestor et al., 2010), we hypothesized that cannabis-only users would show greater incentive responses compared to the tobacco-only and non-using control groups, and that the cannabis + tobacco group would show greater incentive responses compared to the tobacco-only group. We further expected that the alcohol-only group would show greater incentive response compared to the tobacco-only and non-using groups (see Heitzeg et al., 2014) and that the tobacco-only group would also show decreased incentive responses compared to the non-using controls (e.g., Peters et al., 2011). Given the absence of prior MID studies comparing neural incentive responses in single-substance versus polysubstance-using adolescents, we did not have a priori hypotheses regarding the relationships between the cannabis + tobacco and the cannabis + tobacco + alcohol groups. Therefore, we used an exploratory approach to guide those comparisons.
2. Material and methods
2.1. Participants
All study procedures were approved by the participating university Institutional Review Board. We also obtained a federal Certificate of Confidentiality as an additional level of protection for participating youth. For this evaluation, we recruited 239 high-risk youth (155 male, mean age = 15.91 years [SD = 1.2]) as part of an ongoing study (blinded for review) examining neural moderators of two STI/HIV behavioral risk interventions. All data included herein were collected prior to randomization to, and receipt of, the behavioral interventions. Participants were recruited by trained research staff who introduced the project at local community diversion and alternative to incarceration programs, informing youth about the voluntary nature of participation. Informed assent (written) and parent/guardian consent (audiorecorded) was required for participation.
To be included in the study youth had to be between the ages of 14–18 years, a participant within our community partner program, and fluent in English. Note that youth were not recruited for participation in this study based on their current substance use, but rather, the parent study was focused on HIV/STI risk reduction. Thus, adolescents in the larger pool included those with no current substance use in addition to more frequent substance users. To facilitate generalizability of the parent study outcomes to the widest community of youth, exclusionary criteria were kept purposefully broad. Further, youth were neither screened for, included on, or excluded on the basis of potential co-occurring neuropsychiatric disorders, somatic conditions, or co-occurring substance use. Youth were excluded only on the basis of taking antipsychotic medication, endorsing MRI contraindications (e.g., current pregnancy, non-removable metal in the body), and loss of consciousness (>5 min during past 6 months).
Eligible youth completed behavioral measures assessing substance use and other health risk behaviors and an MRI scan. Adolescents were not allowed to participate if intoxicated, and were prohibited from using any substances (including tobacco) for at least 3 hours prior to the scan. As compensation for participating in this component of the study, adolescents could earn between $20 and $70, depending on their task performance during the scan.
2.2. Substance use groups
Because the aim of the present analysis was to examine incentive-based processes using the MID task for single- vs. poly-substance users across the three predominant substances of abuse, we created six groups (total N = 132) matched for age, gender, and substance use frequency. Groups were based on past month alcohol, cannabis, and tobacco use, as assessed in an interviewer-administered calendar recall (e.g., timeline follow-back; see Section 2.3). To capture adolescents who were within the top tier of this sample in terms of severity for each substance use category, we defined alcohol use as ≥2 or more alcohol use days in the past month, cannabis use as ≥10 cannabis use days in the past month, and tobacco use as ≥27 tobacco use days in the past month. These thresholds represented the top third of users within each respective substance. Using these thresholds, n = 14 youth used only cannabis, n = 34 used only tobacco, n = 12 used only alcohol, n = 17 used cannabis and tobacco, n = 17 used cannabis, tobacco and alcohol, and n = 38 reported 0 days of alcohol, cannabis, and tobacco use (and thus comprised the non-using control group). There were too few people in the sample who reported using alcohol and tobacco only or alcohol and cannabis only to create these comparison groups. To ensure that greater relative levels of substance use did not drive the observed effects, groups were matched on frequency of use of common substances (i.e., the tobacco-only group and the cannabis + tobacco group were matched on frequency of tobacco use, the cannabis-only group and the cannabis + tobacco group were matched on frequency of cannabis use, etc.). Group characteristics are listed in Table 1.
Table 1.
Characteristics of participating sample (N = 132).
| Cannabis-only n or M (SD) | Tobacco-only n or M (SD) | Cannabis + tobacco n or M (SD) | Alcohol-only n or M (SD) | Cannabis + Tobacco + Alcohol n or M (SD) | Control (no substance use) n or M (SD) | Test statistic | p-value | |
|---|---|---|---|---|---|---|---|---|
| n = 14 | n = 34 | n = 17 | n = 12 | n = 17 | n = 38 | |||
| Age (range 14–18 years) | 15.79 (1.4) | 16.29 (1.2) | 15.76 (1.15) | 16.00 (1.23) | 15.94 (.97) | 15.76 (1.2) | F(5, 126) = .858 | .51 |
| Gender | χ2(1, N = 132) = 2.614 | .76 | ||||||
| Male | 11 | 21 | 13 | 8 | 10 | 24 | ||
| Female | 3 | 13 | 4 | 4 | 7 | 14 | ||
| Race | χ2(4, N = 132) = 23.596 | .26 | ||||||
| Caucasian | 1 | 1 | 2 | 1 | 4 | 4 | ||
| African American | 1 | 2 | 0 | 2 | 1 | |||
| Hispanic American | 10 | 19 | 8 | 7 | 8 | 26 | ||
| American Indian/Alaskan Native | 1 | 1 | 1 | 2 | 0 | 0 | ||
| Multi-racial | 1 | 11 | 6 | 2 | 3 | 7 | ||
| MID Performancea | ||||||||
| Accuracy—LOSS trialsb | .66 (.05) | .67 (.04) | .66 (05) | .66 (.06) | .67 (.05) | .67 (.04) | F(5, 126) = .337 | .89 |
| Reaction time—LOSS trialsb | 192.11 (18.7) | 200.93 (13.5) | 202.88 (33.9) | 197.46 (15.4) | 193.03 (17.0) | 199.67 (14.7) | F(5, 126) = .955 | .45 |
| Accuracy—REWARD trialsc | .65 (.05) | .68 (.04) | .67 (.05) | .67 (.05) | .67 (.04) | .67 (.05) | F(5, 126) = 1.063 | .38 |
| Reaction time—REWARD Trialsc | 189.82 (17.1) | 192.23 (15.8) | 198.50 (33.8) | 192.75 (14.6) | 192.74 (15.7) | 197.70 (23.5) | F(5, 126) = .528 | .76 |
| Past month substance use (TLFB)a | ||||||||
| No. of cannabis use days | 20.4 (8.9) | n/a | 24.4 (6.5) | n/a | 24.8 (6.9) | n/a | F(2, 45) = 1.640 | .21 |
| Cannabis hits per using day | 14.3 (11.4) | n/a | 19.4 (25.2) | n/a | 18.1 (16.5) | n/a | F(2, 45) = .290 | .75 |
| No. of cigarette smoking days | n/a | 29.59 (.8) | 29.71 (.6) | n/a | 29.65 (.9) | n/a | F(2, 65) = .133 | .88 |
| Cigarettes per smoking day | n/a | 5.99 (7.2) | 6.92 (6.8) | n/a | 9.26 (10.0) | n/a | F(2, 65) = .985 | .38 |
| No. of drinking days | n/a | n/a | n/a | 3.5 (2.4) | 5.82 (4.2) | n/a | t(27) = 1.723 | .10 |
| Drinks per drinking day | n/a | n/a | n/a | 6.97 (3.5) | 8.00 (2.7) | n/a | t(27) = .909 | .37 |
| Lifetime substance use | ||||||||
| Age at first cannabis use | 12.93 (1.9) | 11.30 (2.2) | 11.35 (2.1) | 12.58 (1.9) | 10.53 (2.6) | 11.87 (2.6) | F(5, 116) = 2.256 | .05 |
| Age at first alcohol use | 13.42 (2.3) | 12.47 (2.1) | 11.88 (2.2) | 13.00 (2.3) | 12.06 (1.3) | 12.63 (2.8) | F(5, 118) = .893 | .49 |
| Alc dependence (AUDIT) | 2.83 (3.0) | 2.97 (4.3) | 5.21 (3.9) | 9.58 (5.1) | 12.71 (5.8) | 1.17 (2.0) | F(5, 118) = 24.87 | <.001* |
| Cannabis dependence (MDS) | 3.08 (2.3) | 2.56 (3.3) | 3.35 (2.5) | 2.5 (2.4) | 4.38 (2.8) | .42 (.8) | F(5, 124) = 7.929 | <.001* |
| Impulsivity (ImpSS) | 10.23 (3.6) | 10.71 (4.2) | 10.25 (3.1) | 12.00 (4.7) | 12.82 (4.1) | 10.16 (4.2) | F(5, 126) = 1.351 | .25 |
MID = monetary incentive delay; TLFB = time-line follow-back; AUDIT = alcohol use disorders identification test; MDS = marijuana dependence scale; ImpSS = impulsivity and sensation seeking scale.
Group comparisons conducted using 1-way ANOVA.
Collapsing across all incentive magnitudes for LOSS trials
Collapsing across all incentive magnitudes for REWARD trials
Significant differences.
2.3. Measures
2.3.1. Demographics questionnaire
This measure collected general demographic information including age, gender, and racial/ethnic background.
2.3.2. 30-day time-line follow-back (TLFB; Sobell and Sobell, 1992)
The TLFB is an interviewer-administered measure that uses a calendar format to facilitate recall and estimates of daily alcohol, tobacco, and cannabis use. The TLFB measures substance consumption over the past month, including quantity of use (amount of substance use per day) and frequency of use (total number of substance use days) for alcohol, cannabis, and tobacco. This measure has been successfully used across many studies to assess adolescent substance use (e.g., Chung et al., 2004) with good reliability and validity (Donohue et al., 2004).
2.3.3. Alcohol use disorders identification test (AUDIT; Saunders et al., 1993)
This 10-item measure was developed by the World Health Organization to assess alcohol consumption, drinking behavior, adverse psychological reactions to alcohol, and alcohol-related problems, and to provide an overall estimate of hazardous drinking symptoms. The maximum score on this measure is 40. In our sample, the AUDIT demonstrated good internal consistency (α = .838).
2.3.4. Marijuana dependence scale (MDS; Stephens et al., 2000)
The MDS is a dichotomous measure of endorsed DSM IV criteria, which generates a summed estimate of cannabis dependence (e.g., “When I smoked marijuana, I often smoked more or for longer periods of time than I intended”). In our sample, the MDS demonstrated good internal consistency (α = .862).
2.3.5. The impulsivity and sensation seeking scale (ImpSS; Zuckerman, 2002)
The ImpSS consists of 19 items measuring impulsivity and sensation seeking, and can be evaluated as subscale (e.g., impulsivity, sensation seeking) or a full summed scale. Individuals high in impulsivity have been found to have the tendency to take risks for novel, varied, and intense experiences (e.g., Zuckerman, 1994). In our sample, the ImpSS demonstrated good internal consistency (α = .807). For the present analyses, we used the full summed scale.
2.4. Functional magnetic resonance imaging task
Numerous versions of the monetary incentive delay (MID) task have been used in neuroimaging research over the last decade. For the present study, we utilized a version of the task that has gained empirical support through research on young adult cannabis users (see Filbey et al., 2013, for procedures and parameters). Specifically, receiving actual earnings has been shown to improve youth performance in fMRI tasks (Chung et al., 2011). Thus, consistent with administration procedures for cannabis-using young adults, immediately prior to entering the scanner, all participants were clearly informed that they would receive the amount of money they earned during the task as soon as they exited the scanner. Throughout the task, participants were provided information about their cumulative earnings (presented as a screenshot of their cumulative sums). As promised, all youth received their earnings in cash once they exited the scanner.
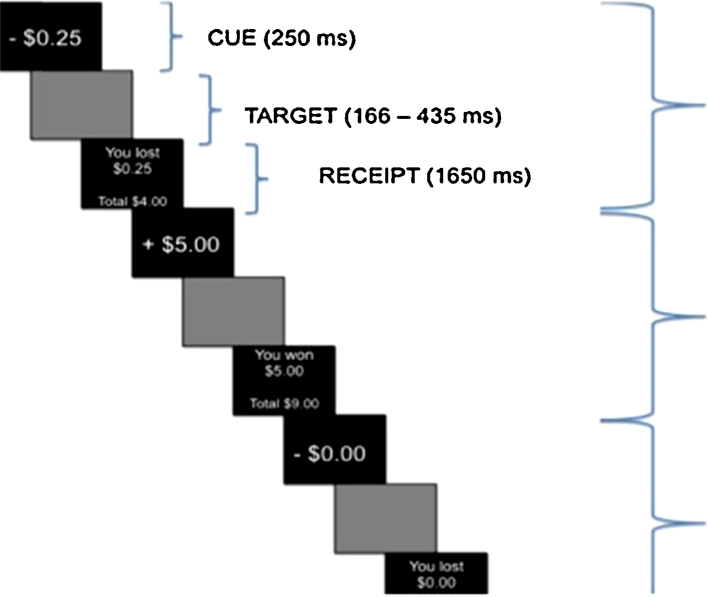
Project staff informed subjects that to register their responses on this task, they were required to press a button when they saw a target square appear on the screen. Staff explained that during REWARD trials, correct responding (hitting the button) would result in earning money, whereas during LOSS trials, responding would prevent losing money. Finally, during NEUTRAL trials, responding would not impact earnings, but reaction times would be measured and recorded. Prior to entering the scanner, all youth completed a 7-min practice session to ensure adequate understanding of the task. Each trial began with a 250 ms cue screen that alerted youth to the trial type (REWARD; LOSS; NEUTRAL). All cue screens also informed youth about the amount of money that could be won or lost on that trial (either $.20, $1.00 or $5.00). This task included 54 REWARD trials, 54 LOSS trials, and 36 NEUTRAL trials. Across the 108 total REWARD and LOSS trials, there were 36 total trials for each level of incentive magnitude ($.20, $1.00 and $5.00). Each target was presented for 166–436 ms, during which time participants had the opportunity to respond. The target was followed by a delay period (1165–1934 ms), and a feedback screen (1650 ms), which let youth know whether or not they had been successful within the trial, the sum they had just gained/lost, and their running monetary total (see Fig. 1). The task was programmed using an adaptive algorithm implemented to allow for a 66% success rate. Briefly, the algorithm simply adjusts the buffer period between the cue and the target square until the 66% accuracy rate is achieved. If the participant has achieved more than 66% correct, this buffer is shortened to try to decrease their hit rate; if the participant is below 66% correct, the buffer period is increased. For the present analyses, we focused on the anticipation phase of the task for comparison with existing literature (Balodis and Potenza, 2014, Silverman et al., 2014).
Fig. 1.
MID task design. Illustration of task conditions and regressors. The anticipation regressor is composed of the cue + target time periods.
2.5. Image acquisition
MRI images were acquired using a 3.0 Tesla Siemens Trio MRI whole-body scanner (Erlangen, Germany) with a 12-channel head coil. High resolution T1-weighted anatomical images were acquired with a 5-echo magnetization-prepared rapid gradient-echo (MP-RAGE) sequence with the following parameters: [TR (repetition time) = 2.53 s, 7○ flip angle, number of excitations (NEX) = 1, slice thickness = 1 mm, FOV (field of view) = 256 mm, in-plane resolution = 256 × 256]. During the task, functional images were acquired using a single-shot, gradient-echo echo-planar pulse sequence (TR = 2000 ms; TE = 29 ms; flip angle = 75○; FOV = 240 mm; matrix size = 64 × 64). Structural images were collected with a sagittal orientation and functional images were acquired parallel to the anterior commissure (AC)–posterior commissure (PC) line + 5° to 10°, to diminish susceptibility artifacts.
2.6. Image processing and statistical analyses
Analysis of functional images was conducted using AFNI (version = AFNI_2011_12_21_1014 [November 4, 2014] [64-bit]). The first image of each run along with two dummy scans were eliminated to account for T1 equilibrium effects, resulting in a total of 524 images for the final analyses. Anomalous values were first identified using a despiking algorithm in AFNI (Cox, 1996), and then replaced based on temporally neighboring values. All time-series data were then spatially registered in two- and three-dimensional space to the second EPI image of the first run to reduce the effects of head motion, and were temporally interpolated to the first slice to account for differences in slice acquisition. Framewise displacement (FD) was calculated on the first derivatives of the head motion data following transformation of rotations to a 100 mm diameter sphere (Power et al., 2012). Data were then spatially blurred using an 8 mm Gaussian full-width half-maximum filter and converted to standard stereotaxic coordinate space (Talairach and Tournoux, 1988). A voxel-wise general linear model analysis was used to estimate data fit by convolving a double-gamma variate function with the study design matrix. A total of 13 regressors were used to model the anticipation phase (3 regressors for the different magnitudes of REWARD trials, 3 for the different magnitudes of LOSS trials and 1 for NEUTRAL trials) and the feedback phase (3 regressors for hits and 3 regressors for misses during REWARD, LOSS, and NEUTRAL trials). In addition, 12 nuisance motion regressors were included in the model (6 motion parameters and their derivatives). Error trials were modeled separately for each trial-type to eliminate error variance (Mayer et al., 2012). Percent signal change for correct trials (“HITS”) was calculated by dividing the beta coefficients for each condition by the average model intercept (β0) and used in all second level analyses. Prior to selection of subjects to include in the present analyses, adolescents who demonstrated excessive motion during the scan were removed from the larger pool of subjects. Average translational and rotational motion and mean FD were calculated, and individuals were considered outliers and removed if they were more than two standard deviations above the group average on any motion parameter. No significant differences in motion were observed between the six groups included in the present analyses.
To validate that the task was functioning as expected in this group of adolescents, we examined main effects of the task by conducting 1-sample t-tests across the entire sample (n = 132) for REWARD and LOSS trials across the whole brain. To measure the effect of the task independent of level of incentive magnitude, we collapsed across all 3 levels of REWARD and across all 3 levels of LOSS to form two contrasts of interest: REWARDCombined-NEUTRAL and LOSSCombined-NEUTRAL. All voxel-wise whole brain results were corrected for false positives at p < .05 based on 10,000 Monte-Carlo simulations implemented in AFNI (statistical threshold of p < .005 in conjunction with a minimum cluster size = 2432 μL).
Next, to focus specifically on the brain areas most directly related to our hypotheses, we tested two regions of interest (ROIs)—the right NAcc and left NAcc. These ROIs were chosen based on previous research on the role of these regions in reward response and substance use (e.g., Bjork et al., 2008, Yau et al., 2012, Weiland et al., 2013). Bilateral NAcc masks were created using 5 mm diameter spheres centered at the following Talairach coordinates: left NAcc (−10, 12, −3) and right NAcc (8, 12, −2). The left and right NAcc were examined separately because the left and right hemispheres have demonstrated differential patterns of activation during the MID task (e.g., Knutson et al., 2001a, Balodis et al., 2012). In line with our focus on incentive processing, effect sizes were extracted for left and right NAcc during the anticipatory period of each trial. Anticipatory activation during the NEUTRAL trials was subtracted from activation during anticipation across all 3 levels of REWARD ($.20, $1.00 $5.00) and LOSS ($.20, $1.00 $5.00) separately, as well as collapsed across all three levels of reward and loss, to create 8 contrasts of interest (REWARD$5-NEUTRAL, REWARD$1-NEUTRAL, REWARD$.20-NEUTRAL, REWARDCombined-NEUTRAL, LOSS$5-NEUTRAL, LOSS$1-NEUTRAL, and LOSS$.20-NEUTRAL, LOSSCombined-NEUTRAL) for both right and left NAcc.
To examine group differences in left and right NAcc activation during anticipation of REWARD and LOSS, we conducted a series of one-way analyses of variance (ANOVA) tests using SPSS (Version 21), with group as the independent variable (IV), and incentive condition (collapsed across all three incentive levels) as the dependent variable (DV). Thus, a total of four ANOVA tests were performed, examining group differences in left NAcc REWARDCombined-NEUTRAL, left NAcc LOSSCombined-NEUTRAL, right NAcc REWARDCombined-NEUTRAL and right NAcc LOSSCombined-NEUTRAL. Significant group differences in the left and right NAcc for the REWARD trials were followed up by pairwise comparisons in SPSS, comparing the six groups separately for each of the eight contrasts of interest (i.e., Left REWARD$5-NEUTRAL, Left REWARD$1-NEUTRAL, Left REWARD$.20-NEUTRAL, Left REWARDCombined-NETURAL, Right REWARD$5-NEUTRAL, Right REWARD$1-NEUTRAL, Right REWARD$.20-NEUTRAL and Right REWARDCombined-NEUTRAL). As we did not have sufficient evidence to support directional a priori hypotheses between all groups, we did not apply corrections for multiple testing to the results of these t-tests, given that the goal of exploratory formative research is to reduce type II error (Jaeger and Halliday, 1998).
3. Results
3.1. MID behavioral performance
Across the six groups, participants averaged 67% HITS for all REWARD trials (median reaction time = 195 ms), as well as 67% hits on the loss trials (median reaction time = 199 ms). These results are consistent with the performance algorithm built into the task. We also conducted six 1-way ANOVAs with group as the IV and task performance (accuracy for REWARD, LOSS, and NEUTRAL trials and median reaction time for REWARD, LOSS, and NEUTRAL trials) as the DVs. No significant group differences emerged in reaction time for REWARD, LOSS, or NEUTRAL trials, or on percentage of hits for REWARD, LOSS, or NEUTRAL trials.
3.2. Task validation: main effects of anticipation of reward and loss
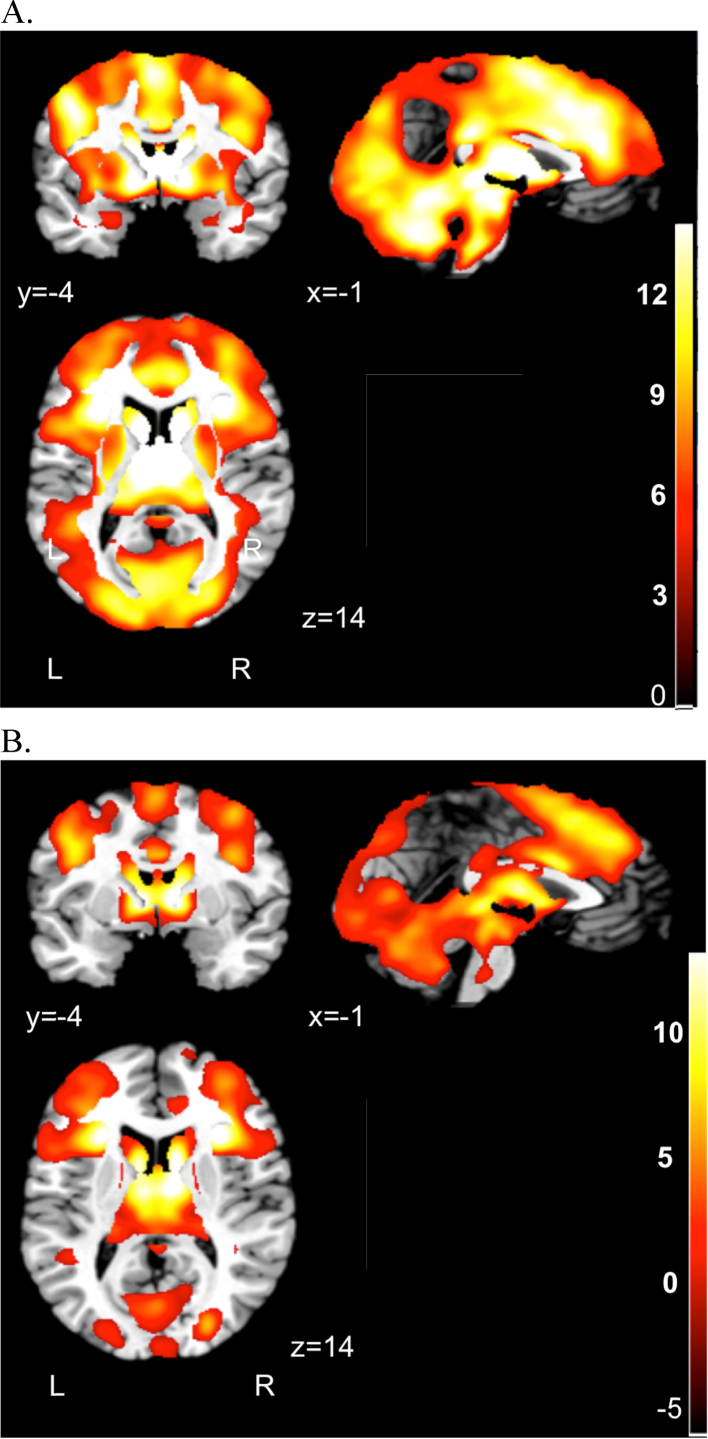
As expected, 1-sample t-tests across the entire sample (N = 132) demonstrated that REWARD relative to NEUTRAL trials activated a large network that included the bilateral caudate, thalamus, cingulate, and left insula (see Supplementary Table 1 for local extrema of activation). In addition, the LOSS relative to NEUTRAL contrast showed activation in a large network that included the bilateral insula, caudate, thalamus, and anterior cingulate (see Supplementary Table 2 for local extrema of activation). This is consistent with task effects observed in prior adolescent and adult MID studies (e.g., Knutson et al., 2001a, Bjork et al., 2004, Bjork et al., 2010). Brain areas showing significant activation during the REWARDCombined-NEUTRAL contrast are in Fig. 2a, and activation for the LOSSCombined-NEUTRAL contrast is shown in Fig. 2b.
Fig. 2.
Task main effects across entire sample (N = 132). (A) Whole brain activation during the REWARD > NEUTRAL anticipation contrast. (B) Whole brain activation during the LOSS > NEUTRAL anticipation contrast. Statistical maps thresholded at p < .005. The color bar indicates t range. Left (L) and right (R) hemispheres are marked in the bottom of the figure.
3.3. Group differences in REWARD and LOSS responding
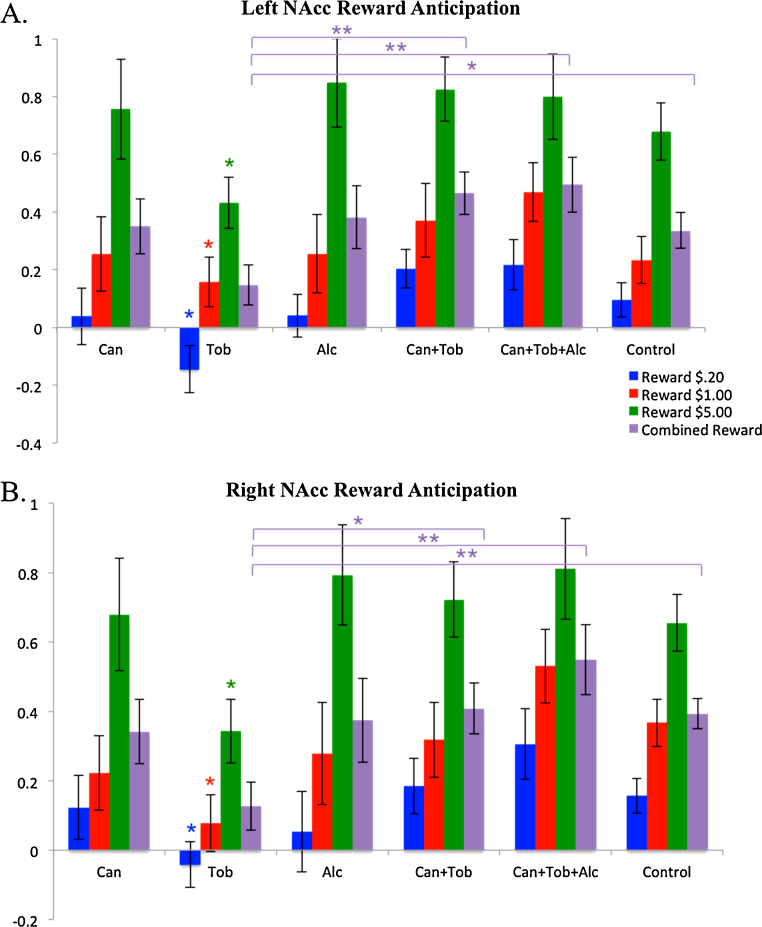
Results of ANOVAs for NAcc activation demonstrated a significant effect of group on left NAcc activation for the REWARDCombined-NEUTRAL contrast, F = (5, 126) = 2.722, p = .023, and on right NAcc activation for the REWARDCombined-NEUTRAL contrast, F = (5, 126) = 3.985, p = .002. No significant main effects of group were observed for right or left NAcc activation for LOSSCombined-NEUTRAL contrasts.
3.3.1. Cannabis group differences
Pairwise comparisons using t-tests revealed no significant group differences in left or right NAcc activation between the cannabis group and any of the other groups.
3.3.2. Tobacco group differences
We observed significant NAcc activation differences for the tobacco-only group compared to both polysubstance groups, the control group and the alcohol-only group during REWARD trials. Specifically, the tobacco-only group had lower left NAcc activation compared to the control group during REWARD$.20-NEUTRAL (t(70) = 2.421, p = .018) and REWARDCombined-NEUTRAL trials (t(70) = 2.026, p = .047). We found a similar pattern in the right NAcc, whereby the tobacco-only group showed less activation than the control group across all three levels of reward (REWARD$.20-NEUTRAL, t(70) = 2.412, p = .018; REWARD$1-NEUTRAL, t(70) = 2.734, p = .008; REWARD$5-NEUTRAL, t(70) = 2.539, p = .013) and collapsed across reward levels, REWARDCombined-NEUTRAL, t(56.236) = 3.258, p = .002.
The tobacco-only group also demonstrated less activation in the left NAcc compared to the cannabis + tobacco group for low and high rewards (REWARD$.20-NEUTRAL, t(49) = 2.799, p = .007; REWARD$5-NEUTRAL, t(49) = 2.659, p = .011) and collapsed across reward levels (REWARDCombined-NEUTRAL, t(49) = 2.843, p = .006). The same pattern was observed for the right NAcc (REWARD$.20-NEUTRAL, t(49) = 2.063, p = .044; REWARD$5-NEUTRAL, t(49) = 2.513, p = .015; REWARDCombined-NEUTRAL, t(49) = 2.540, p = .014).
In addition, the tobacco-only group demonstrated less activation in the left NAcc compared to the cannabis + tobacco + alcohol group across all 3 levels of reward (REWARD$.20-NEUTRAL, t(49) = 2.780, p = .008; REWARD$1-NEUTRAL, t(49) = 2.189, p = .033; REWARD$5-NEUTRAL, t(49) = 2.254, p = .029) and collapsed across reward levels (REWARDCombined-NEUTRAL, t(49) = 2.905, p = .005). The same pattern of results also emerged in the right NAcc (REWARD$.20-NEUTRAL, t(49) = 2.964, p = .005; REWARD$1-NEUTRAL, t(49) = 3.279, p = .002; REWARD$5-NEUTRAL, t(49) = 2.821, p = .007; REWARDCombined-NEUTRAL, t(49) = 3.480, p = .001).
Finally, the tobacco-only group showed decreased activation compared to the alcohol-only group for the highest reward condition for both the left NAcc (REWARD$5-NEUTRAL, t(44) = 2.373, p = .022) and the right NAcc (REWARD$5-NEUTRAL, t(44) = 2.546, p = .014).
3.3.3. Polysubstance group differences
No pairwise differences in NAcc activation emerged between either of the two polysubstance groups (cannabis + tobacco; cannabis + tobacco + alcohol), or between either polysubstance group and the control group on any of the REWARD-NEUTRAL contrasts. Left and right NAcc activation for REWARD-NEUTRAL contrasts across all six groups are shown in Fig. 3.
Fig. 3.
Group differences in mean percent signal change in left and right NAcc for anticipation of Reward relative to Neutral trials. (A) Left NAcc activation for REWARD > NEUTRAL contrasts at each level of Reward. (B) Right NAcc activation for REWARD > NEUTRAL contrasts at each level of Reward. Note that y-axis range −.4 to 1 in (A) and y-axis range −.2 to 1 in (B). Purple bars indicate significant differences in REWARDCombined-NEUTRAL trials. Blue, red and green asterisks indicate significant differences at each individual level of reward (specific group differences at each level of reward described in text). Effect sizes were extracted from masks of the NAcc using a 5 mm sphere centered at x = −10, y = 12, z = −3 (Talairach space). Error bars represent ± 1 standard error, *p < .05, **p < .01. Can = Cannabis, Tob = Tobacco, Alc = Alcohol. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
This study took a first step at evaluating the comparative neural responses during incentive anticipation among adolescents across the three most prevalent substances of abuse (alcohol, tobacco, and cannabis). We focused on the NAcc, an area within the VS that has been strongly implicated in reward processing in both the developmental and addiction literatures (e.g., Knutson et al., 2001a; Ernst et al., 2005; Heitzeg et al., 2014). In this preliminary examination, we observed whole brain effects supporting the validity of the MID task among 14–18 year olds. Our ROI analysis indicated significant differences in NAcc activation during reward anticipation by substance use group, with a unique response pattern emerging among the tobacco-only group. Of note, we observed nearly identical patterns of reward responding in the left and right NAcc, which strengthens our confidence in these results. We hypothesized decreased reward responding for youth in the tobacco-only group compared to the alcohol-only, cannabis-only, cannabis + tobacco and control groups (Nestor et al., 2010, Peters et al., 2011). Given the lack of published research on polysubstance users of alcohol, tobacco and cannabis, we did not have clear directional hypotheses about this group, and thus treated this portion of the analysis as exploratory.
Contrary to our a priori hypotheses, no differences emerged between the tobacco-only and cannabis-only groups. However, as predicted, we observed differences in reward responding in bilateral NAcc in the tobacco-only group compared to the non-using control group. Specifically, we found decreased activation in the tobacco-only compared to the control group, which is consistent with previous findings suggesting that tobacco is associated with blunted responses to non-substance-based rewards (such as financial incentives) in the VS (e.g., Peters et al., 2011, Rose et al., 2013, Wilson et al., 2014). We also observed greater responses in the alcohol-only compared to the tobacco-only group for the highest level of reward, which is consistent with some prior research showing increased reward responding in adolescent drinkers (e.g., Heitzeg et al., 2014).
Given parallels with the animal literature in this area, it is possible that acute withdrawal from nicotine may explain the reduced striatal activation by reward cues observed among adolescent smokers in this study (Epping-Jordan et al., 1998). Using animal models, nicotine withdrawal has been linked to reduced dopamine release in the NAcc (Hildebrand et al., 1998), blunting both tonic and phasic dopaminergic activity in the striatum (Zhang et al., 2012). Evidence suggests that upregulated dopamine transporter activity contributes to the decrease in extracellular striatal dopamine observed during early nicotine withdrawal (Hadjiconstantinou et al., 2011). As subjects in this study were required to abstain from smoking cigarettes for 3 hours before the scanning session, it is possible that the experience of acute nicotine withdrawal may have influenced NAcc activation during the scan. Future studies may consequently consider measuring withdrawal symptoms before the MID task.
Note that the tobacco-only group also showed decreased reward responding compared to the polysubstance groups (cannabis + tobacco; cannabis + tobacco + alcohol), who may have also been experiencing nicotine withdrawal. Importantly, this finding was expected based on prior work demonstrating that tobacco + cannabis users have greater striatal reward responses than tobacco-only users (van Hell et al., 2010, Nestor et al., 2010). Yet, comparison with existing studies, including the Nestor study (Nestor et al., 2010), should be done with caution, as subjects in the present study reported heavier smoking; thus, the case could be made that this set of adolescents might have a greater propensity to experience acute nicotine withdrawal. Notably, our results cannot be attributed to greater tobacco use in the tobacco-only versus polysubstance groups, as the design of the study purposefully ensured that all three groups were matched for equal levels of tobacco use. Additionally, as we found no evidence of differences in impulsivity across our groups, it is unlikely that our activation findings may have been driven by personality traits associated with increased reward responsiveness.
Instead, we cautiously speculate that our results may be attributable to the fact that the impact of tobacco alone on adolescent neural responses may differ from the impact of tobacco in combination with other substances. In line with prior work in this area, the use of multiple substances in synergy may exert a neuroprotective effect (Jacobus et al., 2009), potentially due to the opposing or interactive nature of other substances (i.e., cannabis), when used in tandem with alcohol and/or tobacco (Nestor et al., 2010, Bachtell and Ryabinin, 2001, Penetar et al., 2005). Extending the conclusions of Jacobus and colleagues (Jacobus et al., 2009) that cannabis may protect against alcohol-related neural damage, we suggest that our results may be due to the influence of cannabis upon what would otherwise be deleterious neural alterations associated with tobacco use in our polysubstance groups.
Alternatively, our pattern of findings may suggest that adolescent tobacco-only users represent a behaviorally or neurocognitively unique group in terms of reward processing. Indeed, it is possible that given greater restrictions on both tobacco use and tobacco advertising in recent years, regular tobacco-only use (i.e., daily smoking) is less prevalent for adolescents than in decades past (Wakefield et al., 2008). Moreover, without implying causation, tobacco use has also been associated with higher levels of psychopathology (Talati et al., 2013). Given that numerous psychiatric disorders emerge during adolescence (Paus et al., 2008), one could argue that the tobacco-only group may comprise a subset of youth who might be transitioning into psychiatric illness or symptoms (potentially including addiction), and for whom the unique pattern of neural activation during the MID task may be a biomarker (e.g., Whelan et al., 2014, Pizzagalli et al., 2009, Guyer et al., 2012). However, the absence of diagnostic data on this sample precludes definitive conclusions on this possibility. Rather, this is a crucial direction for further work in this area.
It is also unclear whether decreased reward responding among tobacco users develops over time as a result of tobacco exposure or represents a neurobiological vulnerability factor for initiating and sustaining smoking (Dagher et al., 2001). Because the participants in this sample are adolescents, and thus relatively new smokers, these results are consistent with the idea that lower reward sensitivity (at least among youth who only use tobacco) may represent a risk factor, at the very least, for initiation and maintenance of regular smoking.
Regarding the potential impact of personality factors on reward responding during the MID, we did not observe differences in impulsivity across any of the groups. One interpretation of this finding may be that as a group, high-risk adolescents such as those included herein, demonstrate high levels of impulsivity (White et al., 1994) and substance use (Chassin, 2008), so differences in impulsivity are not apparent across substance use groups due to ceiling effects. Here, all adolescents within each group scored high on impulsivity, suggesting that impulsivity may not be the temperamental feature that differentiates reward processing by substance subgroup for this population.
Among polysubstance users, incentive-elicited NAcc responses in the cannabis + tobacco and cannabis + tobacco + alcohol groups showed a stronger parallel to non-substance-using youth than to single-substance users (namely those who only use tobacco). These data are in line with prior studies showing that brain structure among youth who exhibited recent binge drinking and cannabis use were more in line with non-using youth, as compared to alcohol-only users (Jacobus et al., 2009). Although that was not the precise pattern observed here, one interpretation of our findings is that experimentation with multiple substances might be more developmentally typical. Consequently, it may be the case that youth who move toward selecting only one substance, which may be more unusual in this age group, may use for reasons of pre-existing neural risk (e.g., family history; genetic risk factors) rather than due to the social reasons that support or drive more normative adolescent explorations of substance use in this age range. In the present study, we did not explore any potential underlying vulnerability factors (e.g., family history, genetic risk), however, this is an important next step needed to address causation in future studies.
Finally, we expected that the cannabis-only group would show increased reward responding in the NAcc compared to the tobacco-only and control groups. However, no differences emerged between the cannabis-only group and any of the other groups. Importantly, cannabis use is becoming increasingly normative in adolescent populations, particularly among socioeconomically disadvantaged youth like the high-risk youth in this sample (Redonnet et al., 2012). Thus, examining the relationship between cannabis use and developing brain structure and function is especially timely. This is an area of great current debate (e.g., Batalla et al., 2013, Vaidya et al., 2012, Weiland et al., 2015, Filbey et al., 2014, Gilman et al., 2014), and it is not clear whether and/or to what extent cannabis use is associated with MID response in adolescents.
4.1. Limitations and future directions
A notable strength of the present study is the matched-group design, which allowed us to observe brain activation differences that are not simply due to differences in the degree of use of the same substance between relevant user groups. However, although this study suggests that tobacco-only users may possess behavioral and neurobiological characteristics that differentiate them from their single-substance (i.e., alcohol-only) and polysubstance using peers, there are a number of factors that limit the interpretation of our results. Given the relatively small sample sizes and the cross-sectional nature of this study, these results do not provide evidence suggesting that particular patterns of incentive-elicited responses in the NAcc lead to substance use in adolescence, or that adolescent substance use causes abnormalities in neural reward processes. Larger longitudinal studies among adolescents are needed to explicate the temporal nature of the relationship between striatal reward responding and alcohol, cannabis, tobacco and the various combinations thereof. For example, recent cross-institutes initiatives within the National Institutes of Health will help determine whether adolescents who use a single substance (e.g., the tobacco-only users in the present study) are likely to remain users of only this substance or to transition to using multiple substances over time. Relatedly, this type of cross-site longitudinal study will help elucidate the relationship between emerging psychiatric illness and substance use trajectories during adolescence.
This sample was comprised of high-risk youth involved in alternatives to justice programming; future work would benefit from replication with community-based youth. Relatedly, to be as inclusive as possible within the parent study, and to yield results with the maximum potential for generalizability, we did not collect data on psychiatric comorbidity. Although we excluded individuals taking antipsychotic or anticonvulsant medications, it is possible that some subjects included in the present analyses would have endorsed symptoms of psychiatric disorders. Because psychiatric illness is often associated with substance use (Kandel et al., 1997) and also appears to be related to neural reward responding during the MID (e.g., Pizzagalli et al., 2009, Guyer et al., 2012), future studies should control for psychiatric disorders. In addition, we did not collect detailed histories regarding lifetime substance use and dependence. Future research should include measures of lifetime substance use (including tobacco), as this could provide useful information for interpreting differences in brain activation across substance use groups.
A minor limitation is the task design; a different version of the MID might yield a slightly different pattern of results. Further, a different analytical approach that might help disentangle the effects observed here would be to examine the BOLD response based on a continuous measure of substance use. However, in our effort to maintain group comparisons to differentiate between single versus poly-substance users, we opted to instead match level of use across relevant groups. Important next steps could involve comparing MID response to a continuous measure of quantity of substances ingested during the past month or examining incentive processing across adolescents reporting varying degrees of substance dependence. Relatedly, the nature of our analytical questions necessitated conducting a series of independent voxel-wise t-tests, and due to our uneven group sizes, using an alternative statistical approached such as a Tukey's test would not be ideal (Smith, 1971). Thus, our results may have been influenced by the particular statistical approach we selected. In addition, we did not observe any gender effects in the present investigation. This may be a consequence of our matched samples, and we encourage future evaluations of gender effects. Finally, it is important to note that although youth in the present study were not intoxicated at the time of the scan, we would expect state vs. trait differences in MID responding (Rose et al., 2013). Thus, future studies examining differences in MID responses during active intoxication across various substances may provide useful information.
The results from this investigation support the notion that individual propensity to use single or multiple substances during adolescence may be associated with underlying differences in neural incentive processing, and these differences are likely to interact with substance exposure to influence the trajectory of substance use across the stages of development.
Conflict of interest
The authors have not been involved in financial relationships that pose a conflict of interest for this work. The authors are in agreement regarding the content of this manuscript. The corresponding author will continue to take responsibility for informing the other authors of any editorial issues.
Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE 1144083 awarded to Hollis Karoly. This research was also supported by 1R01NR013332-01 (MPIs: Feldstein Ewing and Bryan). The authors acknowledge Dr. Francesca Filbey for providing the version of the MID task used in this project, as well as for descriptions of task procedures/parameters, and to Dr. James Bjork for his consultation on task design.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.05.005.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Hall G.S. vols. I and II. D. Appleton & Co.; New York: 1904. (Adolescence: Its Psychology and Relations to Physiology, Anthropology, Sociology, Sex, Crime, Religion, and Education). [Google Scholar]
- Jaeger R.G., Halliday T.R. On confirmatory versus exploratory research. Herpetologica. 1998:S64–S66. [Google Scholar]
- Mills K.L., Goddings A.L., Clasen L.S., Giedd J.N., Blakemore S.J. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci. 2014;36(3–4):147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014 doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Galvan A. Insights about adolescent behavior, plasticity, and policy from neuroscience research. Neuron. 2014;83(2):262–265. doi: 10.1016/j.neuron.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Chung T., Geier C., Luna B., Pajtek S., Terwilliger R., Thatcher D. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug Alcohol Depend. 2011;115(1–2):43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedler J., Block J. Adolescent drug use and psychological health: a longitudinal inquiry. Am. Psychol. 1990;45(5):612. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Squeglia L.M., Jacobus J., Tapert S.F. The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 2009;40(1):31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman D.I., Yücel M., Hall W.D. Substance use and the adolescent brain: a toxic combination? J. Psychopharmacol. 2007;21(8):792–794. doi: 10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- Geier C.F. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm. Behav. 2013;64(2):333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.J. The addicted human brain: insights from imaging studies. J. Clin. Invest. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H.B., Chen C.M., Yi H.Y. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Kann L., Kinchen S., Shanklin S.L., Flint K.H., Hawkins J., Harris W.A. Youth risk behavior surveillance—United States, 2013. MMWR Surveill. Summ. 2014;63(Suppl. 4):1–168. [PubMed] [Google Scholar]
- Jacobus J., Squeglia L.M., Bava S., Tapert S.F. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Frank L.R., McQueeny T., Schweinsburg B.C., Schweinsburg A.D., Tapert S.F. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R., Watts R., Orr C.A., Althoff R.R., Artiges E., Banaschewski T. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B., Walter H., Erk S., Kammerer H., Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31(2):790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22(11):521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G., Macri S., Morley-Fletcher S., Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Tarazi F.I., Tomasini E.C., Baldessarini R.J. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci. Lett. 1998;254(1):21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Baler R.D. Addiction science: uncovering neurobiological complexity. Neuropharmacology. 2014;76(Pt B):235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The psychology and neurobiology of addiction: an incentive–sensitization view. Addiction. 2000;95(8s2):91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Knutson B., Wimmer G.E., Kuhnen C.M., Winkielman P. Nucleus accumbens activation mediates the influence of reward cues on financial risk taking. Neuroreport. 2008;19(5):509–513. doi: 10.1097/WNR.0b013e3282f85c01. [DOI] [PubMed] [Google Scholar]
- Knutson B., Greer S.M. Anticipatory affect: neural correlates and consequences for choice. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stautz K., Cooper A. Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clin. Psychol. Rev. 2013;33(4):574–592. doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Fernández-Artamendi S., Martínez-Loredo V., Secades-Villa R., Garcia-Rodriguez O., Fernández-Hermida J. Impulsivity and early onset of alcohol and cigarette use in adolescents. Drug Alcohol Depend. 2015;146:e276. [Google Scholar]
- Bjork J.M., Smith A.R., Hommer D.W. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42(4):1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly H.C., Harlaar N., Hutchison K.E. Substance use disorders: a theory-driven approach to the integration of genetics and neuroimaging. Ann. N. Y. Acad. Sci. 2013;1282:71–91. doi: 10.1111/nyas.12074. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'souza M.S., Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict. Sci. Clin. Pract. 2011;6(1):4. [PMC free article] [PubMed] [Google Scholar]
- Gilpin N.W., Koob G.F. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res. Health. 2008;31(3):185. [PMC free article] [PubMed] [Google Scholar]
- French E.D. Δ9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci. Lett. 1997;226(3):159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- Balodis I.M., Potenza M.N. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task anticipatory reward processing in addiction. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., Dunlop J., Myers U.S. Neural effects of positive and negative incentives during marijuana withdrawal. PLOS ONE. 2013;8(5):e61470. doi: 10.1371/journal.pone.0061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell H.H., Vink M., Ossewaarde L., Jager G., Kahn R.S., Ramsey N.F. Chronic effects of cannabis use on the human reward system: an fMRI study. Eur. Neuropsychopharmacol. 2010;20(3):153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Wrase J., Schlagenhauf F., Kienast T., Wustenberg T., Bermpohl F., Kahnt T. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Nestor L., Hester R., Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49(1):1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Bromberg U., Schneider S., Brassen S., Menz M., Banaschewski T. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168(5):540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Rose E.J., Ross T.J., Salmeron B.J., Lee M., Shakleya D.M., Huestis M.A. Acute nicotine differentially impacts anticipatory valence-and magnitude-related striatal activity. Biol. Psychiatry. 2013;73(3):280–288. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A., Schlagenhauf F., Wüstenberg T., Hein J., Kienast T., Kahnt T. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66(8):734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum. Brain Mapp. 2012;33(9):2174–2188. doi: 10.1002/hbm.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M.M., Villafuerte S., Weiland B.J., Enoch M.-A., Burmeister M., Zubieta J-K. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.B. Springer; 1992. Timeline Follow-back. Measuring Alcohol Consumption; pp. 41–72. [Google Scholar]
- Chung T., Maisto S.A., Cornelius J.R., Martin C.S. Adolescents’ alcohol and drug use trajectories in the year following treatment. J. Stud. Alcohol Drugs. 2004;65(1):105. doi: 10.15288/jsa.2004.65.105. [DOI] [PubMed] [Google Scholar]
- Donohue B., Azrin N.H., Strada M.J., Silver N.C., Teichner G., Murphy H. Psychometric evaluation of self-and collateral timeline follow-back reports of drug and alcohol use in a sample of drug-abusing and conduct-disordered adolescents and their parents. Psychol. Addict. Behav. 2004;18(2):184. doi: 10.1037/0893-164X.18.2.184. [DOI] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Stephens R.S., Roffman R.A., Curtin L. Comparison of extended versus brief treatments for marijuana use. J. Consult. Clin. Psychol. 2000;68(5):898. [PubMed] [Google Scholar]
- Zuckerman M. 2002. Zuckerman-Kuhlman Personality Questionnaire (ZKPQ): an alternative five-factorial model; pp. 377–396. (Big Five Assessment). [Google Scholar]
- Zuckerman M. Cambridge University Press; New York, NY: 1994. Behavioral Expressions and Biosocial Biases of Sensation Seeking. [Google Scholar]
- Silverman M.H., Krueger R.F., Iacono W.G., Malone S.M., Hunt R.H., Thomas K.M. Quantifying familial influences on brain activation during the monetary incentive delay task: an adolescent monozygotic twin study. Biol. Psychol. 2014;103:7–14. doi: 10.1016/j.biopsycho.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. 1988. Co-planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Mayer A.R., Teshiba T.M., Franco A.R., Ling J., Shane M.S., Stephen J.M. Modeling conflict and error in the medial frontal cortex. Hum. Brain Mapp. 2012;33(12):2843–2855. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W.-Y.W., Zubieta J.-K., Weiland B.J., Samudra P.G., Zucker R.A., Heitzeg M.M. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neurosci. 2012;32(7):2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland B.J., Welsh R.C., Yau W-YW, Zucker R.A., Zubieta J.-K., Heitzeg M.M. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128(1):130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., Stevens M.C., Pearlson G.D., Potenza M.N. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol. Psychiatry. 2012;71(8):749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.J., Delgado M.R., McKee S.A., Grigson P.S., MacLean R.R., Nichols T.T. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn. Affect. Behav. Neurosci. 2014:1–12. doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan M.P., Watkins S.S., Koob G.F., Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Hildebrand B.E., Nomikos G.G., Hertel P., Schilström B., Svensson T.H. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779(1):214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Zhang L., Dong Y., Doyon W.M., Dani J.A. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol. Psychiatry. 2012;71(3):184–191. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinou M., Duchemin A.M., Zhang H., Neff N.H. Enhanced dopamine transporter function in striatum during nicotine withdrawal. Synapse. 2011;65(2):91–98. doi: 10.1002/syn.20820. [DOI] [PubMed] [Google Scholar]
- Jacobus J., McQueeny T., Bava S., Schweinsburg B., Frank L., Yang T. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol. Teratol. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell R., Ryabinin A. Interactive effects of nicotine and alcohol co-administration on expression of inducible transcription factors in mouse brain. Neuroscience. 2001;103(4):941–954. doi: 10.1016/s0306-4522(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Penetar D.M., Kouri E.M., Gross M.M., McCarthy E.M., Rhee C.K., Peters E.N. Transdermal nicotine alters some of marihuana's effects in male and female volunteers. Drug Alcohol Depend. 2005;79(2):211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wakefield M.A., Durkin S., Spittal M.J., Siahpush M., Scollo M., Simpson J.A. Impact of tobacco control policies and mass media campaigns on monthly adult smoking prevalence. Am. J. Public Health. 2008;98(8):1443–1450. doi: 10.2105/AJPH.2007.128991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati A., Wickramaratne P.J., Keyes K.M., Hasin D.S., Levin F.R., Weissman M.M. Smoking and psychopathology increasingly associated in recent birth cohorts. Drug Alcohol Depend. 2013;133(2):724–732. doi: 10.1016/j.drugalcdep.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Ryan Bogdan A. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Detloff A., Benson B., Nelson E.E., Perez-Edgar K. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am. J. Psychiatry. 2012;169(2):205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A., Bleicher C., Aston J.A., Gunn R.N., Clarke P., Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42(1):48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- White J.L., Moffitt T.E., Caspi A., Bartusch D.J., Needles D.J., Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. J. Abnorm. Psychol. 1994;103(2):192. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- Chassin L. Juvenile justice and substance use. Future Child. 2008;18(2):165–183. doi: 10.1353/foc.0.0017. [DOI] [PubMed] [Google Scholar]
- Redonnet B., Chollet A., Fombonne E., Bowes L., Melchior M. Tobacco, alcohol, cannabis and other illegal drug use among young adults: the socioeconomic context. Drug Alcohol Depend. 2012;121(3):231–239. doi: 10.1016/j.drugalcdep.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Batalla A., Bhattacharyya S., Yücel M., Fusar-Poli P., Crippa J.A., Nogué S. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLOS ONE. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya J.G., Block R.I., O’Leary D.S., Ponto L.B., Ghoneim M.M., Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012;37(3):618–629. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland B.J., Thayer R.E., Depue B.E., Sabbineni A., Bryan A.D., Hutchison K.E. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J. Neurosci. 2015;35(4):1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., Aslan S., Calhoun V.D., Spence J.S., Damaraju E., Caprihan A. Long-term effects of marijuana use on the brain. Proc. Natl. Acad. Sci. 2014;111(47):16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J.M., Kuster J.K., Lee S., Lee M.J., Kim B.W., Makris N. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J. Neurosci. 2014;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D.B., Johnson J.G., Bird H.R., Canino G., Goodman S.H., Lahey B.B. Psychiatric disorders associated with substance use among children and adolescents: findings from the methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) study. J. Abnorm. Child Psychol. 1997;25(2):121–132. doi: 10.1023/a:1025779412167. [DOI] [PubMed] [Google Scholar]
- Smith R.A. The effect of unequal group size on Tukey's HSD procedure. Psychometrika. 1971;36(1):31–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.