Abstract
OBJECTIVES
The purpose of this study was to test whether a microcatheter can safely be advanced across the right atrial appendage to access the pericardium and then withdrawn despite subsequent high-intensity anticoagulation. We also tested whether transatrial pericardial insufflation of carbon dioxide (CO2) would enhance the safety of subxiphoid needle access to the empty pericardium by separating the heart from the anterior pericardium.
BACKGROUND
Subxiphoid needle access to the empty pericardium, required for left atrial suture ligation and epicardial ablation for rhythm disorders, risks myocardial or coronary laceration.
METHODS
A catheter from the femoral vein engaged the right atrial appendage for angiographic confirmation of position. Through that catheter, the back end of a 0.014- or 0.018-inch guidewire crossed the right atrial wall to enter the pericardium and delivered a 2.4-F microcatheter. CO2 1 to 2 ml/kg was insufflated into the pericardium immediately before subxiphoid needle access under lateral projection fluoroscopy. Thirteen patients undergoing subxiphoid suture ligation of the left atrial appendage consented to participate in this research protocol.
RESULTS
Right atrial exit succeeded in 11 subjects (85%) and failed uneventfully in 2 subjects. CO2 insufflation of 96 ± 22 ml achieved 12 ± 4 mm separation of the anterior pericardium from the myocardial wall, allowed rapid and successful subxiphoid anterior needle and guidewire entry in all 11 subjects, and did not have any evident hemodynamic effects. The immediate pericardial aspirate was serous in all but 1 subject.
CONCLUSIONS
We report the first human intentional transatrial exit procedure. Transatrial microcatheter access to the pericardium can be achieved safely. Pericardial insufflation with CO2 makes subxiphoid access to the empty pericardium rapid and safe. Although our clinical experience to date remains small, with further experience, this approach may prevent the life-threatening complications of “dry” subxiphoid pericardial access.
Keywords: epicardial ablation, left atrial appendage ligation, pericardial access, structural heart disease, transatrial exit
Subxiphoid access to the normal or “empty” pericardial space is required for therapeutic procedures, including epicardial suture ligation of the left atrial appendage (LAA) (1,2) and epicardial ablation of ventricular tachycardia (3). “Dry” subxiphoid needle access is variably described as straightforward or perilous (4–7). Inadvertent needle entry into the atria or ventricles is seldom clinically important; however, myocardial and coronary artery laceration usually causes intractable hemopericardium requiring surgical repair. These complications continue despite small-diameter needles, oblique-end-hole needles, and temporary suspension of respiration because of the small distance between visceral and parietal pericardial walls and because of continuous cardiac motion (8). In many cases, myocardium lacerates as the heart moves across the subxiphoid needle sharp tip.
Transatrial entry to the pericardial space was initially described in animals to facilitate homogeneous epicardial drug delivery (9) or chamber visualization (10). We refined transatrial pericardial entry and contrast infusion using 2.4- to 2.8-F (0.80- to 0.93-mm outer diameter) microcatheters in animals as a method to facilitate subxiphoid needle access to the anterior or posterior pericardial spaces (11). Despite high-intensity anticoagulation, we observed no important hemopericardium using careful cardiac magnetic resonance examination after simple withdrawal of these microcatheters from across the wall of the right atrial appendage.
We hypothesized that, in patients, the right atrial appendage can safely be crossed with a microcatheter and that the microcatheter can be withdrawn without causing clinically important pericardial hemorrhage. We further hypothesized that transatrial carbon dioxide (CO2) insufflation can reduce the risk of subxiphoid pericardial needle entry in patients undergoing epicardial suture ligation of the LAA by separating the visceral and parietal pericardial walls to keep the subxiphoid needle away from the heart. We describe the first human experience with intentional transcatheter right atrial exit and pericardial CO2 insufflation.
METHODS
SUBJECTS
The Henry Ford Health System Institutional Review Board approved this protocol. From June 2014 through May 2015, consecutive candidates were invited to participate who were undergoing intrapericardial suture ligation of the LAA (Lariat, Sentreheart, Redwood City, California) because of high risk of thromboembolism and intolerance of anticoagulation for atrial fibrillation. All subjects gave written informed consent.
TECHNIQUE OF TRANSATRIAL PERICARDIAL EXIT
Intentional exit of the right atrial appendage was performed via the femoral vein. A pulmonary artery catheter was placed via a separate vein to record hemodynamics solely for this protocol. The right atrial appendage was engaged with a catheter (7-F Sones in the first subject and 7-F balloon wedge end-hole catheter in the others, inside a transseptal introducer sheath) using a sweeping ascending clockwise motion from the lateral right atrial wall. Right atrial appendage angiography was performed using a hand injection of 2 to 5 ml iodinated contrast. Through the guidewire lumen, a 2.4-F braided microcatheter (Renegade STC-18 150 cm, Boston Scientific, Marlborough, Massachusetts) was positioned at the tip of the catheter and the back end of a guidewire (initially 0.014-inch Balance Middleweight, Abbott Vascular, Santa Clara, California; later 0.018-inch V-18, Boston Scientific) advanced into the pericardial space. Next, the microcatheter was advanced into the apical pericardial space, and the guidewire was withdrawn. Out of caution, the larger transfemoral catheter was retracted to prevent inadvertent rail advancement over the microcatheter into the pericardium, which might enlarge the hole. The technique was further refined as described in the Results section.
After refinement, we used SL2 or SL3 curve introducer sheaths (St. Jude Medical, St. Paul, Minnesota) to direct a curved balloon wedge end-hole catheter (pulmonary artery balloon, Medtronic, Minneapolis, Minnesota; or Double-Lumen Hi-Shore Catheter T-Tip, Edwards Lifesciences, Irvine, California) to direct the back end of a guidewire shaft known to be polymer coated (0.014-inch run through, Terumo, Somerset, New Jersey).
TRANSATRIAL PERICARDIAL CO2 INSUFFLATION AND SUBXIPHOID NEEDLE ACCESS
The subxiphoid needle trajectory was planned according to the usual technique, aiming toward the LAA shadow on an anteroposterior projection. A coaxial long 21-gauge × 12-cm needle (Stiffen Bariatric, Galt, Harland, Texas; or Mak NV 216, Merit Medical, Chester, Virginia) was inserted alone or coaxially inside of an 18-gauge × 9-cm spinal needle (Havel, Cincinnati, Ohio) under the skin but not yet abutting the cardiac shadow.
For CO2 insufflation, a closed bag with 1-way valve and manifold (Figure 1) was filled and purged in 3 cycles using filtered pure CO2 as taught by Cho and Hawkins (12). The system stopcock was connected to the hub of the pericardial microcatheter, and serous pericardial fluid was aspirated in all patients to ensure an air-free connection and proper positioning. CO2 was injected by hand through this closed system until adequate (approximately 5 to 10 mm) anterior fluoroscopic pericardial separation was obtained. The initial gas clearance of the microcatheter was slow to avoid a high pressure “jet effect” that might injure soft tissue. Subxiphoid needle access to the anterior pericardium was obtained in the lateral projection, without using a finder syringe or contrast injections, and instead with the 0.018-inch guidewire (Steelcore, Abbott) pre-positioned inside the needle to ease advancement into the pericardium immediately upon needle entry.
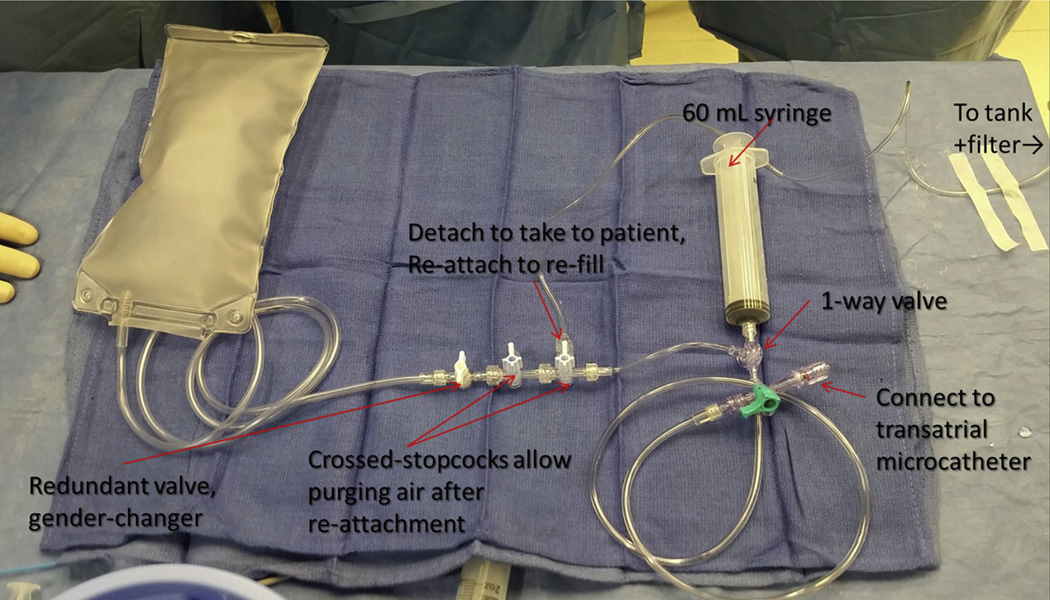
FIGURE 1. CO2 Setup for Transatrial Pericardial Insufflation.
The connection to the carbon dioxide (CO2) tank should be detached before use in a patient; crossed stopcocks allow air to be purged after it is reattached to be refilled. The system is filled and purged at least 3 times before use. The syringe has a 1-way valve that allows it to fill with CO2 from the bag and to eject into a stopcock connected to the transatrial microcatheter.
The transatrial pericardial microcatheter was withdrawn over a guidewire immediately after the subxiphoid Lariat SoftTip sheath was advanced into the pericardial space. Next, transseptal access was obtained under transesophageal echocardiography guidance, and unfractionated heparin was administered immediately to achieve an activated clotting time >300 s. Then, Lariat suture ligation of the LAA was performed according to usual methods, and a subxiphoid pericardial drain was left in overnight.
DATA ANALYSIS
Data were collected using REDCap (13) and analyzed using Excel version 2010 (Microsoft, Redmond, Washington). Data were described as mean ± SD if they were normally distributed, otherwise as median (first quartile, third quartile).
RESULTS
All consecutive patients undergoing Lariat suture closure during the study period consented to participate. Of the 13 subjects enrolled in the study, the mean age was 75 ± 8 years, 46% were women, 46% had prior transient ischemic attack or stroke, all were intolerant of anticoagulation, 92% used aspirin at the time of the procedure, 0% used a P2Y12 blocker, 85% had prior hemorrhage, the average CHADS2 (congestive heart failure, hypertension, 75 years of age or older, diabetes mellitus, and previous stroke or transient ischemic attack) score was 2.8 ± 1.4, the average CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65 to 74 years, sex category) score was 4.6 ± 1.6, and the average HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) score was 3.2 ± 1.2. Baseline hemodynamics under general anesthesia were heart rate 70 ± 16 beats/min, right atrial pressure 7 ± 3 mm Hg, mean pulmonary artery 23 ± 6, and mean aortic 72 ± 17. Left ventricular ejection fraction was 0.58 ± 0.10, and 31% had moderate tricuspid regurgitation.
Right atrial exit was successful in 11 of 13 cases, and required 2.2 ± 1.7 guidewire passes. Figure 2 and Online Video 1 shows a representative sequence of right atrial angiography, transatrial guidewire exit, and transatrial microcatheter advancement into the pericardial space.
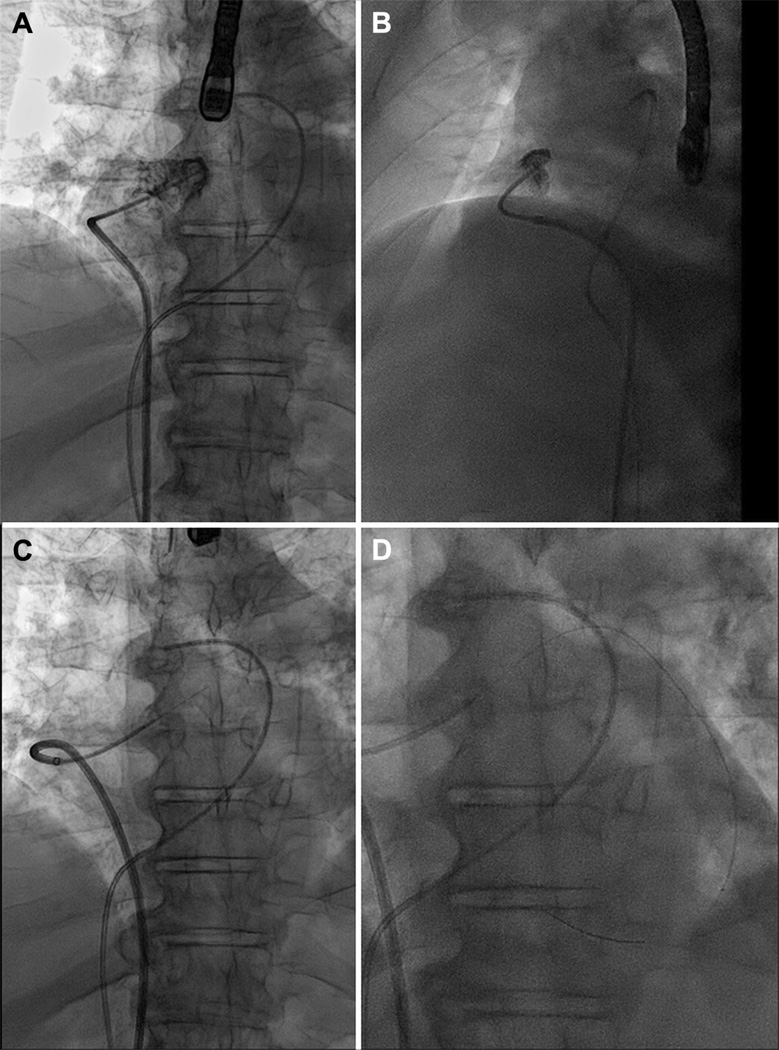
FIGURE 2. An Example of Transatrial Guidewire Exit.
Anteroposterior (A) and lateral (B) contrast angiogram of the right atrial appendage from a catheter positioned at its crest. (C) The back end of a 0.014-inch guidewire crosses the right atrial appendage. (D) A microcatheter is advanced over the guidewire into the pericardial space. See accompanying Online Video 1.
After pericardial insufflation, subxiphoid needle pericardial entry was consistently rapid and straight-forward in all cases because the pericardial layers clearly separated. Figure 3 shows a representative sequence of CO2 insufflation, subxiphoid needle, and subxiphoid guidewire delivery. These steps were associated with no change in heart rate, central aortic blood pressure, or pulmonary artery pressure.
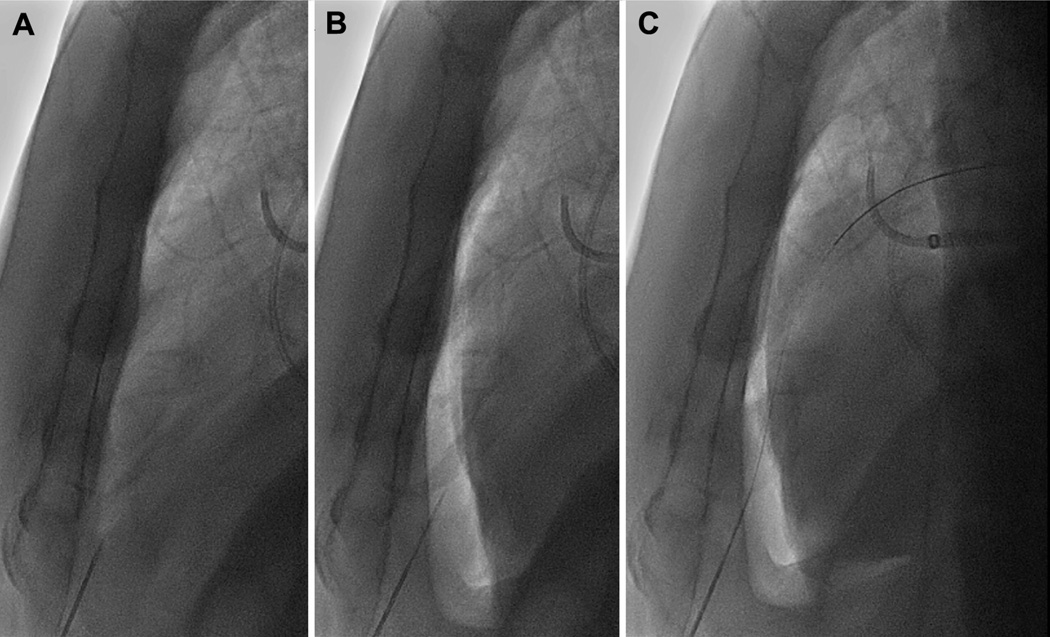
FIGURE 3. Subxiphoid Access.
After the transatrial pericardial microcatheter is placed, the subxiphoid needle is positioned outside of the heart (A). In the lateral fluoroscopic projection, the pericardium is filled with 1.0 to 1.5 ml/kg of CO2 to displace the heart posteriorly (B). The gas clearly visualizes the anterior wall of the heart to allow needle entry without cardiac contact. (C) The subxiphoid guidewire is then advanced through the needle, and the procedure continues as usual.
The mean time from right atrial angiography to right atrial exit was 1:44 ± 1:16 min and from right atrial exit to subxiphoid needle entry was 7:49 ± 2:31 min.
The right atrial exit angle relative to horizontal, in the anteroposterior projection, appeared to differ between successful and unsuccessful exits. The horizontal exit angle was 23 ± 12° when successful compared with 45 ± 14° for the 2 unsuccessful attempts. An average of 96 ± 22 ml of CO2 achieved 12 ± 4 mm anterior pericardial separation.
Microcatheters were withdrawn from the right atrial appendage 11:27 ± 5:44 min before heparin was administered (during which time transseptal access was obtained) to raise the activated clotting time from 170 ± 53 s to 313 ± 14 s.
The equipment selected for right atrial exit evolved during the protocol. At first we applied stand-alone multipurpose-type catheters (Sones, multipurpose guiding catheters, balloon-wedge end-hole catheters). Then, we used stand-alone 3-dimensional curved catheters (heat-gun shaped sheaths) and multipurpose guiding catheters. Ultimately we settled on 3-dimensional curve introducer sheaths intended for atrial transseptal access (SL1, SL2, or SL3, Daig, St. Jude Medical) selected according to right atrial size, and used these to direct balloon-wedge end-hole catheters to the right atrial appendage. We found that the combination achieved a helical trajectory from the inferior vena cava along the lateral and then anterior right atrial and appendage wall, which allowed backup support for the transatrial guidewire exiting horizontally from the right atrial appendage.
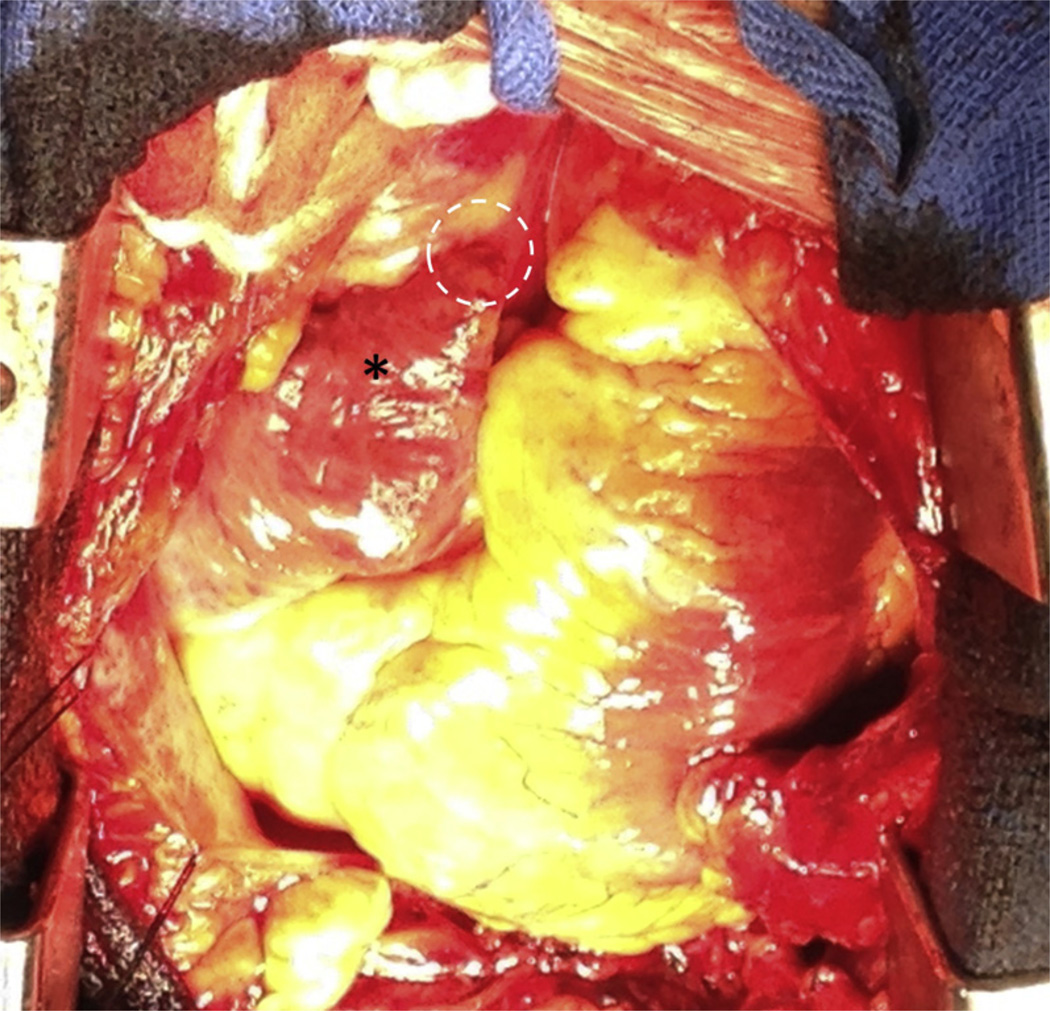
One patient (Patient #8) had a serious complication of left atrial suture ligation despite, and unrelated to, successful transatrial access. She required sternotomy for repair of visually confirmed left ventricular myocardial abrasion, most likely attributable to introducing the Sentreheart SoftTip subxiphoid sheath. In this patient, the right atrial appendage exit site was inspected directly and was found to be hemostatic and with surrounding plethora, as expected (Figure 4). The pericardium was evacuated, and she recovered uneventfully for discharge on day 5.
FIGURE 4. Operative Inspection of a Transatrial Exit Site.
This patient required surgical repair of left ventricular abrasion attributed to the suture delivery sheath. Incidental inspection of the right atrial appendage (asterisk) showed plethora and inflammation, but no bleeding or discrete hole at the site of right atrial exit from the crest of the right atrial appendage (dotted circle).
One patient had mild pericardial bleeding after withdrawal of the transatrial microcatheter, evident on angiography of the right atrial appendage, amounting to 125 ml total drainage. This was treated conservatively with close echocardiographic surveillance. The subject was discharged uneventfully on day 2.
After CO2 insufflation, no patient experienced right ventricular or coronary artery laceration by the subxiphoid needle or even inadvertent right ventricular entry.
DISCUSSION
To our knowledge, this is the first human experience intentionally exiting the right atrium to enter the pericardial space. We found that a catheter can intentionally traverse the right atrial appendage wall into the pericardium and be withdrawn without important sequelae as part of a therapeutic procedure that requires brief intensive anticoagulation. We infer that the small 0.8-mm hole recoils immediately. This should come as no surprise because transcameral needle perforation is common using 0.6- to 0.9-mm outer diameter needles (e.g., 18- to 21-gauge pericardial access and transseptal needles) and usually is not clinically significant. Indeed, intraoperative cardiac pressures are often recorded from plunge needles that are withdrawn despite anticoagulation. Transatrial CO2 insufflation separated the anterior pericardial walls sufficiently to keep a subxiphoid needle far from the epicardial surface throughout the cardiac and respiratory cycles, allowing safe and rapid subxiphoid needle access without changing hemodynamics.
The main shortcoming of our approach was failure to exit the right atrial appendage in 2 of 12 subjects (fourth and seventh in sequence). We attribute this to inexperience, to malalignment of the right atrial catheter with the right atrial appendage and intended empty pericardial space, and also to the subjectively greater advancement force required to perforate the appendage in humans compared with swine (11). We believe that with experience, our success rate is likely to improve, especially now that we recognize the importance of good backup support through a helical geometry of the coaxial atrial catheter system (see the following text). We were unable to obtain meaningful measurements of thickness of the right atrial appendage wall at the resolution of the available computed tomography scans to determine if thickness was a contributing factor. Fortunately, both transatrial exit failures were clinically uneventful and were addressed by switching to conventional “dry” subxiphoid pericardial access.
We refined our catheter technique during the protocol. First, we imparted a 3-dimensional curve on the right atrial appendage catheter system, extending from the cava to the superoanterior wall of the right atrial appendage. This afforded better backup support and aligned the catheter system with the crest of the appendage and the pericardial space. Second, we switched to a balloon wedge end-hole catheter, which in a cautious inflation-deflation maneuver acted to separate the anterior and posterior walls of the right atrial appendage and allowed the catheter tip to advance to the leftmost tip of the appendage despite trabeculations. The inflated balloon tip catheter may also protect against inadvertent advancement of the support catheter across the right atrial appendage wall along the microcatheter. We warn that incautious inflation-deflation may be hazardous in the right atrial appendage. Third, we began to orient our crossing system more horizontally leftward, reasoning that more vertical upward-oriented crossing may contribute to failure because of constraint from cephalad pericardial reflections as depicted in Figure 5. We took care to select transatrial exit sites near the cephalad extent of the right atrial appendage, which was consistently far from the right coronary artery on computed tomography.
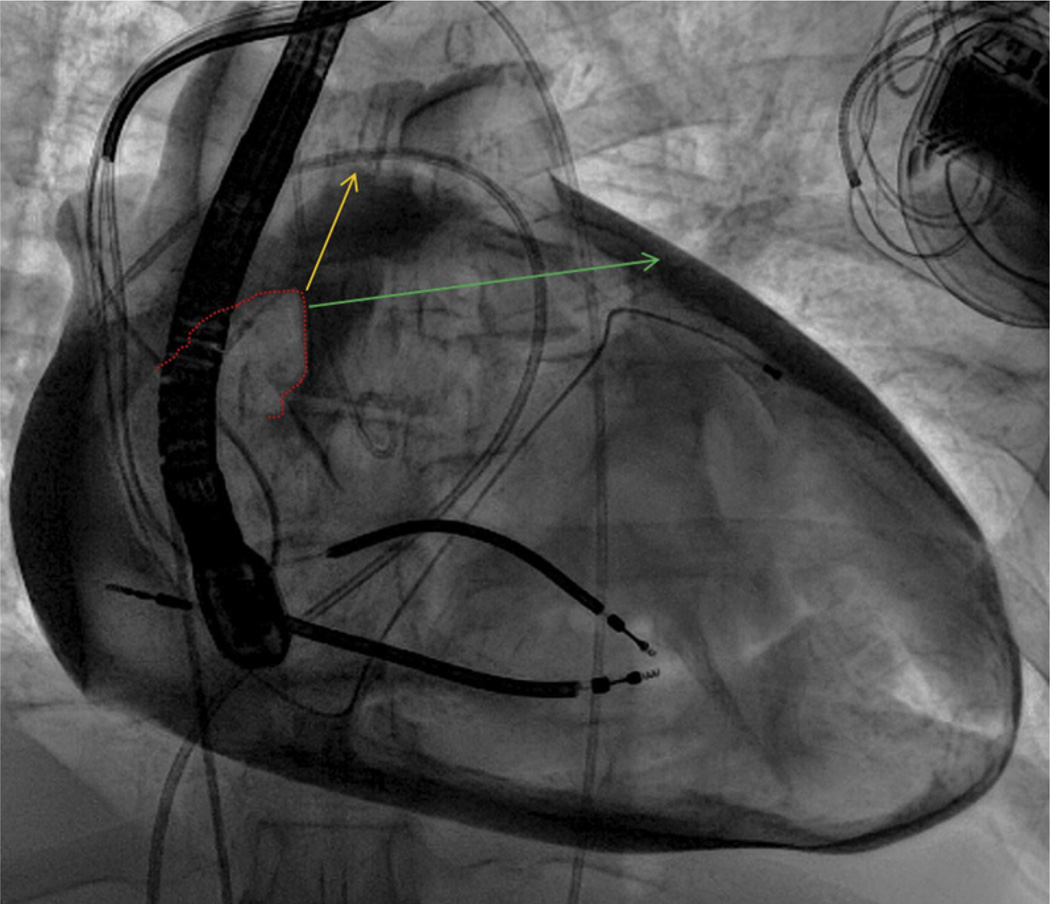
FIGURE 5. Exit Angle and Success.
In this contrast pericardiogram from another patient, the right atrial appendage border (dotted red line) is depicted along with 2 theoretical wire exit trajectories. Trajectory 1 (yellow) is aligned with the main axis of the right atrial appendage and oriented superiorly; failed right atrial exit along this trajectory may be related to the superior pericardial reflection. By contrast, trajectory 2 (green) is oriented more horizontally and leftward.
Transatrial pericardial insufflation may have value in other applications. These include conventional applications requiring subxiphoid access to the pericardium, such as epicardial mapping and ablation of ventricular tachycardia. Right atrial appendage exit alone also may have value in more novel applications, such as transatrial intrapericardial tricuspid annuloplasty (14) and emergency anterior pericardial access in acute low-volume tamponade when conventional access fails. Transatrial instillation of iodinated radiocontrast accumulates in the posterior pericardium and may enhance the safety of subxiphoid posterior pericardial access (11) if desired, although this was not tested here.
STUDY LIMITATIONS
First, pericardial fluid infusion has been shown to increase defibrillation thresholds in animals (15), and we speculate that nonconductive CO2 may increase defibrillation thresholds by surrounding the heart with an insulator. We found the typical pericardial insufflation of 100 ml (1 to 1.5 ml/kg) CO2 accumulated anteriorly over <10% of the anteroposterior dimension of the heart, and defibrillation is likely attainable using laterally positioned electrodes. CO2 need only be insufflated a few seconds before the subxiphoid needle is advanced in the lateral fluoroscopic projection. In our pre-clinical experience, much larger volumes (3 to 5 ml/kg) of CO2 gas spontaneously absorb in 5 to 10 min. Overall, the risk of ventricular fibrillation during subxiphoid access is low. Second, transatrial access requires an extra step and extra equipment beyond simple subxiphoid access. This is offset by the more simple and perhaps safer subxiphoid needle access with the pericardial walls separated. Third, transatrial separation of the pericardial walls does not address all of the risks of the Lariat suture ligation procedure, including sheath injury (which required surgical bailout in 1 patient in our series), perforation of the LAA by the docking magnet wires, and avulsion of the LAA by the suture delivery apparatus. Fourth, our series included only patients with relatively preserved myocardial function and relatively normal right atrial pressure, so our findings should not be extended to patients with more severe cardiomyopathy until after further investigation. Last, a larger clinical experience obtained from more than 1 medical center will be required to more convincingly prove the safety of this technique beyond the data presented here.
CONCLUSIONS
We report the first human intentional transatrial exit procedure. Transatrial access to the pericardium can be achieved safely. Pericardial insufflation with CO2 makes subxiphoid access to the empty anterior pericardium rapid and safe. With further experience, this technique might prevent life-threatening complications of “dry” subxiphoid pericardial access.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
It is feasible to exit the right atrial appendage with a microcatheter and to withdraw the catheter as part of an interventional procedure without significant hemorrhage. The pericardium can be insufflated through this microcatheter with CO2 to separate the walls of the pericardium and allow rapid and safe subxiphoid needle access to the otherwise empty pericardial space.
TRANSLATIONAL OUTLOOK
On the basis of this early feasibility experience, intentional right atrial exit can be further explored for other applications, such as epicardial rhythm mapping and ablation, and by other medical centers. Infused CO2 facilitates anterior pericardial access and iodinated contrast facilitates posterior pericardial access. In the future, intentional right atrial exit access may enable other pericardial therapeutic procedures including circumferential annuloplasty.
Acknowledgments
This study was supported by the Institute for Structural Heart Disease, Division of Cardiology, Henry Ford Health; and the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (Z01-HL006040). Dr. Greenbaum has served as a proctor for Sentreheart, which commercializes the Lariat suture closure device; and has served on the scientific advisory board of Transmural Systems, Inc. Drs. Greenbaum, Rogers, O’Neill, and Lederman are inventors on a patent application, assigned to their employers, on catheters to facilitate right atrial exit.
ABBREVIATIONS AND ACRONYMS
- CO2
carbon dioxide
- LAA
left atrial appendage
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For a supplemental video and its legend, please see the online version of this article.
REFERENCES
- 1.Bartus K, Bednarek J, Myc J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011;8:188–193. doi: 10.1016/j.hrthm.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Price MJ, Gibson DN, Yakubov SJ, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. Transcatheter LAA Ligation Consortium. J Am Coll Cardiol. 2014;64:565–572. doi: 10.1016/j.jacc.2014.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 4.Sacher F, Roberts-Thomson K, Maury P, et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 5.Koruth JS, Aryana A, Dukkipati SR, et al. Un-usual complications of percutaneous epicardial access and epicardial mapping and ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol. 2011;4:882–888. doi: 10.1161/CIRCEP.111.965731. [DOI] [PubMed] [Google Scholar]
- 6.Boyle NG, Shivkumar K. Epicardial interventions in electrophysiology. Circulation. 2012;126:1752–1769. doi: 10.1161/CIRCULATIONAHA.111.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim HS, Sacher F, Cochet H, et al. Safety and prevention of complications during percutaneous epicardial access for the ablation of cardiac arrhythmias. Heart Rhythm. 2014;11:1658–1665. doi: 10.1016/j.hrthm.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Maisch B, Ristić AD, Seferović PM, et al. Interventional Pericardiology. Heidelberg: Springer Medizin Verlag; 2011. [Google Scholar]
- 9.Verrier RL, Waxman S, Lovett EG, Moreno R. Transatrial access to the normal pericardial space: a novel approach for diagnostic sampling, pericardiocentesis, and therapeutic interventions. Circulation. 1998;98:2331–2333. doi: 10.1161/01.cir.98.21.2331. [DOI] [PubMed] [Google Scholar]
- 10.Cohn WE, Winkler JA, Tuzun E, et al. Contrast pericardiography facilitates intrapericardial navigation under fluoroscopy. Ann Thorac Surg. 2010;90:1537–1540. doi: 10.1016/j.athoracsur.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Rogers T, Ratnayaka K, Schenke WH, et al. Intentional right atrial exit for microcatheter infusion of pericardial carbon dioxide or iodinated contrast to facilitate sub-xiphoid access. Catheter Cardiovasc Interv. 2015;86:E111–E118. doi: 10.1002/ccd.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho KJ, Hawkins IF. Carbon Dioxide Angiography: Principles, Techniques, and Practices. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers T, Ratnayaka K, Sonmez M, et al. Transatrial intrapericardial tricuspid annuloplasty. J Am Coll Cardiol Intv. 2015;8:483–491. doi: 10.1016/j.jcin.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada T, McElderry HT, Platonov M, Doppalapudi H, Kay GN. Aspirated air in the pericardial space during epicardial catheterization may elevate the defibrillation threshold. Int J Cardiol. 2009;135:e34–e35. doi: 10.1016/j.ijcard.2008.03.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.