Abstract
A symptom of mild cognitive impairment (MCI) and Alzheimer's disease (AD) is a flat learning profile. Learning slope calculation methods vary, and the optimal method for capturing neuroanatomical changes associated with MCI and early AD pathology is unclear. This study cross-sectionally compared four different learning slope measures from the Rey Auditory Verbal Learning Test (simple slope, regression-based slope, two-slope method, peak slope) to structural neuroimaging markers of early AD neurodegeneration (hippocampal volume, cortical thickness in parahippocampal gyrus, precuneus, and lateral prefrontal cortex) across the cognitive aging spectrum [normal control (NC); (n = 198; age = 76 ± 5), MCI (n = 370; age = 75 ± 7), and AD (n = 171; age = 76 ± 7)] in ADNI. Within diagnostic group, general linear models related slope methods individually to neuroimaging variables, adjusting for age, sex, education, and APOE4 status. Among MCI, better learning performance on simple slope, regression-based slope, and late slope (Trial 2–5) from the two-slope method related to larger parahippocampal thickness (all p-values < .01) and hippocampal volume (p < .01). Better regression-based slope (p < .01) and late slope (p < .01) were related to larger ventrolateral prefrontal cortex in MCI. No significant associations emerged between any slope and neuroimaging variables for NC (p-values ≥ .05) or AD (p-values ≥ .02). Better learning performances related to larger medial temporal lobe (i.e., hippocampal volume, parahippocampal gyrus thickness) and ventrolateral prefrontal cortex in MCI only. Regression-based and late slope were most highly correlated with neuroimaging markers and explained more variance above and beyond other common memory indices, such as total learning. Simple slope may offer an acceptable alternative given its ease of calculation.
Keywords: Alzheimer's disease, Mild cognitive impairment, Structural imaging, episodic memory, Verbal learning, Prefrontal cortex, Simple slope, Hippocampus
INTRODUCTION
Alzheimer's disease (AD) is a major public health issue for older adults that is projected to worsen as the population ages (Hebert, Scherr, Bienias, Bennett, & Evans, 2003). Episodic verbal learning and memory impairments are among the earliest clinical signs of AD pathophysiology (Jedynak et al., 2012). Among episodic memory assessment tools, list-learning tests are not only sensitive in detecting AD pathology (Tierney et al., 1994), but they also predict cognitive decline and conversion to AD (Albert, Moss, Tanzi, & Jones, 2001). To date, much of the literature has focused on delayed recall (Tierney, Yao, Kiss, & McDowell, 2005). However, learning slope is an important aspect of episodic memory assessment in AD, as a flat learning slope is characteristic of a classic amnestic profile (Bondi et al., 1994).
List-learning tests involve the presentation of a list of words across several trials, with multiple methods to quantify learning slope, including simple slope (i.e., Trial 1 to Trial 5 only; Jones et al., 2005), regression-based slope (i.e., linear fit over all learning trials; Tulving, 1964), or peak slope (i.e., Trial 1 to maximum recall; McMinn, Wiens, & Crossen, 1988). It remains unclear which calculation method is optimal for assessing learning in clinical and research efforts.
Determining the neuroanatomical relevance of different slope calculations would enhance the clinical utility of list-learning measures. Episodic learning indices other than learning slope have been linked to brain structures implicated early in the pathophysiological process of AD, including the hippocampus (Petersen et al., 2000) and parahippocampal gyrus (Stout et al., 1999). Learning slope may be associated with other brain regions, such as the dorsolateral prefrontal cortex (DLPFC; D'Esposito, Postle, Ballard, & Lease, 1999), ventrolateral prefrontal cortex (VLPFC; Park & Rugg, 2008), and precuneus (Chang et al., 2010).
This study aims to identify the neuroanatomical significance of learning slope in older adults across the cognitive aging spectrum by comparing different slope calculation measures to structural neuroimaging variables. Selected neuroimaging variables include markers associated with the earliest pathological signs of AD (i.e., hippocampal volume and parahippocampal gyrus cortical thickness) and cortical thickness markers in regions-of-interest (ROIs) implicated in successful learning processes among older adults (i.e., DLPFC, VLPFC, and precuneus). We stratify the analyses by diagnosis (i.e., normal cognition, MCI, AD) to evaluate the relation between slope method and neuroimaging variable separately. The separate analysis allows for a more specific assessment of how learning slope relates to possible pathology by minimizing the heterogeneity in both cognitive performance and neuroanatomy across diagnostic categories. We do not expect differences in the relation between slope and neuroanatomical regions across diagnostic categories given the reliance on these structures for cognitive performance regardless of disease stage. Based on prior research, we hypothesize that simple slope, regression-based slope, late slope (Trials 2–5), and peak slope will correlate most strongly with hippocampus, parahippocampal gyrus, and precuneus cortical thickness. Also, we hypothesize that early slope (Trials 1–2) will be associated with DLPFC and VLPFC given lateral prefrontal associations with attention and distractibility (Chao & Knight, 1995). Secondarily, we will include other commonly used RAVLT summary score indices in the models (i.e., Total Learning, Immediate Recall, Delayed Recall) to assess the unique predictive utility of each slope method. We hypothesize that learning slope will provide valuable information above and beyond other common learning and memory indices.
METHODS
Participants
Participants were drawn from the multisite, longitudinal Alzheimer's Disease Neuroimaging Initiative (ADNI; http://adni.loni.ucla.edu/), launched in 2003 to examine neuroimaging biomarkers in the progression of AD. At the time of participant enrollment, ADNI exclusion criteria included neurological disease other than AD, history of brain lesion or head trauma, and history of psychoactive medication use (http://www.adni-info.org for full inclusion/exclusion criteria). We accessed publicly available data from the original ADNI cohort on 1/07/13, including 822 individuals aged 50 to 95 years who had a baseline diagnosis of normal cognition (NC), MCI, or AD as follows:
-
(1)
NC defined by (a) Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) score between 24 and 30; (b) Clinical Dementia Rating (CDR; (Morris, 1993) global score of 0 (no dementia); (c) preserved activities of daily living (ADLs); and (d) not meeting criteria for MCI or AD.
-
(2)
MCI, based upon Petersen et al. (2010), was defined by (a) MMSE = 24–30; (b) CDR = 0.5–1.0 (mild impairment); (b) relatively spared ADLs; (c) objective cognitive impairment as measured by education-adjusted scores on neuropsychological evaluation; (d) report of subjective cognitive change by patient or informant; and (e) not meeting criteria for AD.
-
(3)
AD, defined by (a) MMSE = 20–26; (b) CDR = 0.5–2.0; (c) objective cognitive impairment (i.e., performances >1.5 standard deviations outside the age-adjusted mean) in at least two cognitive systems; (d) cognitive impairment that directly impaired ADLs; and (e) meeting probable AD criteria (McKhann et al., 1984). For the current study, we excluded participants with severe AD (i.e., CDR = 3).
The current study was limited to ADNI participants with available baseline structural neuroimaging data, which resulted in a total sample size of 739 participants (n = 198 NC, n = 370 MCI, and n = 171 AD). Apolipoprotein-E (APOE) genotyping for the ε4 allele (APOE4) was performed by the ADNI Biomarker Core at the University of Pennsylvania (http://www.adni-info.org/). All study procedures were performed in compliance with institutional research standards. All participants provided written informed consent. Analysis of ADNI's publicly available database was approved by our local Institutional Review Board before data access or analysis.
Rey Auditory Verbal Learning Test
The Rey Auditory Verbal Learning Test (RAVLT; Rey, 1964) evaluates verbal episodic memory skills. The examiner reads aloud a list of 15 nouns, after which the patient is asked to repeat as many words as s/he can remember. The list is repeated for five total learning trials followed by immediate recall of a distractor list, immediate recall, delayed (30-min) recall, and recognition. The current study focused on four methods for modeling learning slope across the initial five learning trials:
-
(1)
Simple slope, defined as the change in recall scores between Trial 1 and Trial 5, divided by four (Jones et al., 2005);
-
(2)
Regression-based slope, defined as the linear least squares regression of Trials 1–5 recall scores on the trial numbers (Tulving, 1964);
-
(3)
Peak slope, defined as the change between Trial 1 recall and the earliest peak recall on Trials 2 to 5, divided by the change in trial number (McMinn et al., 1988); and
-
(4)Two-slope method, which separately assessed learning slope between Trials 1 and 2 and between Trials 2 and 5 (Delis, Kramer, Kaplan, & Ober, 2000). Slope parameters were calculated using the formula by Delis et al. (2000), based on the Pearson product moment correlation coefficient (Rodgers & Nicewander, 1988):
where x indicates the Trial number (i.e., 1 through 5), y indicates the total number correct per trial (i.e., 0 to 15), and n is a normalization factor (i.e., total number of Trials or 5). This method, like the regression-based slope, assumes linearity between learning trials and fits a line to extract the coefficient of correlation (Jones et al., 2005).
Neuroimaging Protocol
The ADNI neuroimaging protocol has been reported elsewhere (Weiner et al., 2010). Images for the current study included original uncorrected 1.5T T1-weighted high-resolution three-dimensional structural data. Before processing, all scans were viewed on a slice-by-slice basis to confirm motion and artifacts were not present. All neuroimaging measures of interest were derived using FreeSurfer Version 5.0 (http://surfer.nmr.mgh.harvard.edu; (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999). Briefly, participant data were run through the reconstruction process (recon-all) for skull stripping, intensity normalization, and segmentation by tissue type (i.e., cerebrospinal fluid, gray matter, and white matter). White and gray matter regions were segmented using spatial intensity gradients and intensity of gray/white borders (Fischl & Dale, 2000). Contiguous ROIs were detected based on intensity similarity and spatial gradient (contour). Bias fields were modeled as a three-dimensional second order polynomial. After three iterations of likelihood maximizations of the hidden Markov field model, estimated total intracranial volume (etICV) was computed based on the transformation to standard space as outlined by Buckner et al. (2004). The cortical surface of the brain was then inflated and registered to a spherical atlas to parcellate gyral and sulcal structures (Fischl, Sereno, Tootell, & Dale, 1999). All data were manually inspected and edited to correct for registration, topological, and segmentation defects, which included inspection of white and gray surfaces in accordance with the FreeSurfer training manual (http://surfer.nmr.mgh.harvard.edu/fswiki/Edits). After these manual intervention steps, images were re-processed through FreeSurfer to update the transformation template and segmentation information. After surface generation, all surfaces were smoothed at 30 mm full-width/half-maximum Gaussian kernel to reduce the effects of noise on the results. Variables of interest for the current study were generated as follows:
Hippocampal volumetric analysis: Raw images underwent automated Talairach transformation and segmentation (Fischl et al., 2002). ICV-corrected hippocampal volume was computed as hippocampal ROI volume/etICV*100.
Cortical thickness analysis: Both intensity and continuity information were used to produce representations of cortical thickness, calculated as the closest difference from the gray/white matter boundary to the gray matter/CSF boundary at each surface vertex (Fischl & Dale, 2000). The generated values relied on spatial intensity gradients not restricted to the voxel resolution, so they were not affected by absolute signal intensity and were able to detect submillimeter features. Such cortical thickness procedures have been validated with histological (Rosas et al., 2002) and manual measurements (Salat et al., 2004). Average gray matter thickness was calculated for all cortical ROIs. For the current study, ROIs from FreeSurfer (Destrieux, Fischl, Dale, & Halgren, 2009) included the precuneus, the parahippocampal gyrus, and the VLPFC (i.e., pars orbitalis, pars triangularis, and pars opercularis). Cortical thickness of the DLPFC was based on the caudal middle frontal gyrus (Desikan et al., 2006).
Statistical Analysis
Baseline clinical characteristics were calculated and compared across the three diagnostic groups (i.e., NC, MCI, and AD) using Pearson's chi-squared test and one way analyses of variance. Characteristics included age, sex, education, APOE4 status (i.e., positive defined as carrying one or more copies of the ε4 allele or negative defined as carrying no copies of the ε4 allele), global cognition (as assessed by the MMSE), and RAVLT learning indices (i.e., Trial 1, Trial 2, Trial 3, Trial 4, Trial 5, and Trial 1–5 Total Learning). Pearson correlation analyses assessed the relatedness within the predictor set of slope variables (simple, regression-based, peak, and two-slope method where early and late slope were treated as two separate terms) and within the outcome set of neuroimaging variables (hippocampal volume, parahippocampal gyrus cortical thickness, precuneus thickness, DLPFC thickness, and VLPFC thickness) by diagnosis.
Within each diagnostic group, general linear models (GLMs) related each of the four learning slope calculation methods to each of the structural neuroimaging markers. Based on theoretical considerations, age (Salat et al., 2004; Salthouse, 1996), sex (for review, see Cosgrove, Mazure, & Staley, 2007; Herlitz, Nilsson, & Backman, 1997), and education (Apostolova et al., 2006; Stern, 2002) were selected a priori as covariates for inclusion in the GLMs. APOE4 status was also used as a covariate because APOE4 carrier status has been related to decreased memory performance and smaller brain structure (Flory, Manuck, Ferrell, Ryan, & Muldoon, 2000; O'Dwyer et al., 2012) and thus could independently relate to the predictor or outcome measures regardless of diagnostic group status. For the two-slope method, early (Trials 1–2) and late (Trials 2–5) slope were included in the model simultaneously. For each model, the R2 of the base model (with only covariates) was measured and used to calculate the incremental R2 (ΔR2) relative to the base model for each slope method. This value was used to assess the additive predictive value above and beyond the adjusting covariates. The F-test was used to conduct significance testing for ΔR2, which is equivalent to the t-test used to assess significance in the regression coefficients between learning slope methods and neuroimaging variables, resulting in equivalent p-values to the primary regression analyses. Next, semi-partial correlations were used to assess the unique contribution (i.e., variance explained) of each learning slope method, where a larger value of squared semi-partial correlation indicates greater “unique” contribution. Significance testing for the semi-partial correlations was calculated using a Fisher's Z-transformation. The exception is that the two-slope method includes two variables, so significance testing for the incremental and semi-partial correlation statistics is calculated differently from the regression analyses (i.e., significance is calculated after addition of both two-slope method variables). Secondary analyses were conducted to assess the predictive utility of each slope method compared to other commonly used RAVLT summary score indices. Specifically, significance tests on ΔR2 were conducted with models including Total Learning (i.e., Trials 1–5), Immediate Recall, and Delayed Recall to clarify if learning slope provides additional information above and beyond these more common RAVLT summary score indices. The significance threshold was set at p < .01 for primary hypothesis testing to reduce the probability of a type I error while balancing power and sample size given the number of comparisons (i.e., 20). Analyses were conducted in R (http://cran.r-project.org) and MATLAB (2012a, The MathWorks, Natick, MA) using ordinary least-squares regression and custom scripts.
RESULTS
Participant Characteristics
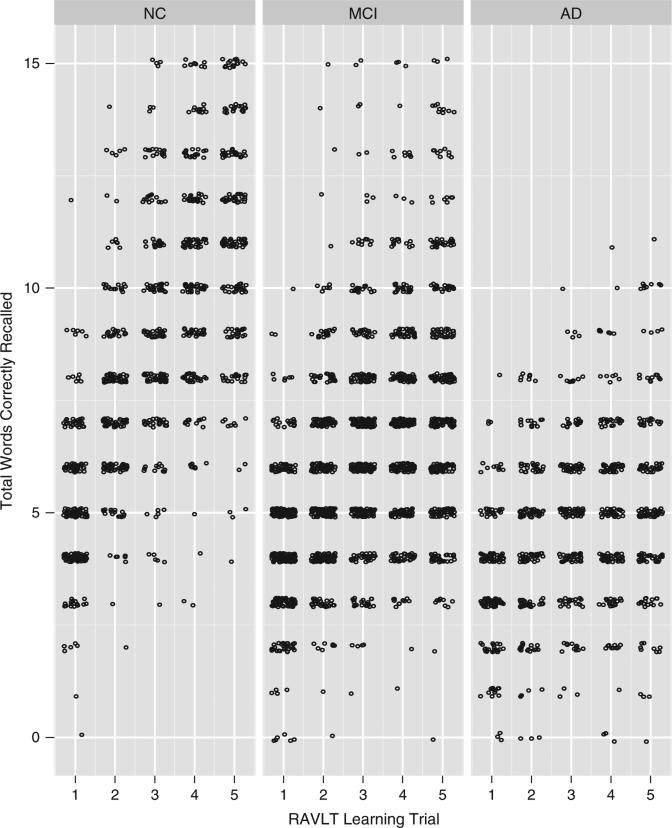
Participants included 198 NC, 370 MCI, and 171 AD individuals. Between-group comparisons by diagnosis suggested no difference in age [F(2,736) = 2.4; p = .09] but differences in sex [χ2(2) = 9.1; p = .01], education [F(2,736) = 9.8; p < .001], and APOE4 status [χ2(2) = 64.3; p < .001]. By design, there were main effects for CDR global score and all cognitive performances (see Figure 1 for total words correctly recalled by learning trial by diagnostic group). The three diagnostic groups differed on all learning slope methods, including simple slope [F(2,736) = 160.1; p < .001], regression-based [F(2,736) = 180.1; p < .001], Trials 1–2 [F(2,736) = 39.2; p < .001], Trials 2–5 [F(2,736) = 96.1; p < .001], and peak slope [F(2,736) = 40.2; p < .001].
Fig. 1.
RAVLT Learning Trial Scores by Diagnostic Group. Points are ‘jittered’ to minimize overplotting. RAVLT = Rey Auditory Verbal Learning Test, NC = normal control, MCI = mild cognitive impairment; AD = Alzheimer's disease
Similarly, the diagnostic groups differed on all neuroimaging outcomes, including parahippocampal gyrus [F(2,736) = 108.2; p < .001], hippocampal volume [F(2,736) = 124.1; p < .001], precuneus [F(2,736) = 27.6; p < .001], DPLFC [F(2,736) = 36.1; p < .001], and VLPFC [F(2,736) = 24.1; p < .001]. Post hoc analyses were completed on each demographic, predictor, and outcome variable, and all differences were in the expected direction (i.e., NC > MCI > AD; see Table 1).
Table 1.
Participant characteristics
| NC | MCI | AD | p-Value* | Pairwise comparison | |
|---|---|---|---|---|---|
| Sample size, n | 198 | 370 | 171 | — | — |
| Age, y | 76 ± 5 | 75 ± 7 | 76 ± 7 | .09 | — |
| Sex, % female | 47 | 36 | 47 | .01 | NC > MCI, MCI = AD, NC = AD |
| Education, y | 16 ± 3 | 16 ± 3 | 15 ± 3 | <.001 | NC = MCI, NC > AD, MCI > AD |
| CDR, Global Score, % | |||||
| 0.0 | 100 | 0 | 0 | ||
| 0.5 | 0 | 88 | 16 | <.001 | — |
| 1.0 | 0 | 12 | 78 | ||
| 2.0 | 0 | 0 | 7 | ||
| APOE4 positive, % | 26 | 54 | 66 | <.001 | NC < MCI < AD |
| MMSE, total score† | 29 ± 1 | 27 ± 2 | 23 ± 2 | <.001 | NC > MCI > AD |
| RAVLT Performance | |||||
| Trial 1 | 5.1 ± 1.6 | 4.2 ± 1.6 | 3.5 ± 1.4 | <.001 | NC > MCI > AD |
| Trial 2 | 7.5 ± 2.0 | 5.6 ± 1.9 | 4.4 ± 1.7 | <.001 | NC > MCI > AD |
| Trial 3 | 9.2 ± 2.4 | 6.5 ± 2.2 | 4.9 ± 1.8 | <.001 | NC > MCI > AD |
| Trial 4 | 10.3 ± 2.5 | 7.0 ± 2.3 | 5.1 ± 1.9 | <.001 | NC > MCI > AD |
| Trial 5 | 11.0 ± 2.4 | 7.5 ± 2.6 | 5.3 ± 2.1 | <.001 | NC > MCI > AD |
| Total Learning Trials 1-5 | 43.1 ± 9.1 | 30.9 ± 9.1 | 23.1 ± 7.5 | <.001 | NC > MCI > AD |
| Learning slope measures | |||||
| Simple slope | 1.5 ± 0.6 | 0.8 ± 0.6 | 0.5 ± 0.5 | <.001 | NC > MCI > AD |
| Regression-based slope | 1.5 ± 0.6 | 0.8 ± 0.6 | 0.4 ± 0.4 | <.001 | NC > MCI > AD |
| Two-slope | — | ||||
| Early slope (Trials 1–2) | 2.4 ± 1.8 | 1.4 ± 1.6 | 0.9 ± 1.4 | <.001 | NC > MCI > AD |
| Late slope (Trials 2–5) | 1.2 ± 0.7 | 0.6 ± 0.6 | 0.3 ± 0.6 | <.001 | NC > MCI > AD |
| Peak slope | 2.0 ± 0.8 | 1.5 ± 0.9 | 1.3 ± 0.8 | <.001 | NC > MCI > AD |
| Neuroimaging variables | |||||
| Parahippocampal gyrus thickness, mm | 6.04 ± 0.43 | 5.62 ± 0.61 | 5.14 ± 0.67 | <.001 | NC > MCI > AD |
| ICV-corrected hippocampal volume | 0.47 ± 0.07 | 0.40 ± 0.07 | 0.36 ± 0.06 | <.001 | NC > MCI > AD |
| Precuneus thickness, mm | 2.29 ± 0.17 | 2.21 ± 0.20 | 2.13 ± 0.23 | <.001 | NC > MCI > AD |
| DLPFC thickness, mm | 2.36 ± 0.17 | 2.28 ± 0.18 | 2.20 ± 0.21 | <.001 | NC > MCI > AD |
| VLPFC thickness, mm | 2.40 ± 0.16 | 2.33 ± 0.17 | 2.28 ± 0.18 | <.001 | NC > MCI > AD |
Note: Data presented as mean ± standard deviation.
Based on Pearson's chi-squared test for categorical variables and one-way analysis of variance for continuous variables.
MMSE score range from 0 to 30 with lower score = worse performance.
NC = cognitively normal control; MCI = mild cognitive impairment; AD = Alzheimer's disease; ICV = Intracranial Volume; CDR = Clinical Dementia Rating; APOE4 = Apolipoprotein E ε4; MMSE = Mini-Mental State Examination; DLPFC = dorsolateral prefrontal cortex, VLPFC = ventrolateral pre-frontal cortex.
Between-slope correlation analyses revealed across all diagnostic groups, the strongest positive correlations were seen between simple and regression-based slope (r ≥ 0.93; p-values <.01). Other strong positive correlations across all groups included regression-based method and late slope (Trials 2–5) from the two-slope method (r ≥ 0.79; p-values <.01), simple and late slope (Trials 2–5; r ≥ 0.66; p-values <.01), and peak and early slope (Trials 1–2 from the two-slope method; r ≥ 0.50; p-values <.01). A modest negative correlation was noted between early slope and late slope across all diagnostic groups (r ≤ − 0.16; p-values <.01) with a moderate association seen in AD participants (r = − 0.42; p < .01; see Table 2).
Table 2.
Pearson correlations between slope variables by diagnosis
| Two-slope method |
||||
|---|---|---|---|---|
| NC participants (n = 198) | Simple slope | Regression-based slope | Trials 1–2 slope | Trials 2–5 slope |
| Regression-based slope | 0.95* | — | — | — |
| Trials 1–2 slope | 0.55* | 0.36* | — | — |
| Trials 2–5 slope | 0.66* | 0.79* | –0.23* | — |
| Peak slope | 0.50* | 0.51* | 0.50* | 0.12 |
| Two-slope method |
||||
|---|---|---|---|---|
| MCI participants (n = 361) | Simple slope | Regression-based slope | Trials 1–2 slope | Trials 2–5 slope |
| Regression-based slope | 0.96* | — | — | — |
| Trials 1–2 slope | 0.55* | 0.39* | — | — |
| Trials 2–5 slope peak | 0.72* | 0.83* | –0.16* | — |
| Peak slope | 0.41* | 0.34* | 0.68* | –0.09 |
| Two-slope method |
||||
|---|---|---|---|---|
| AD participants (n = 155) | Simple slope | Regression-based slope | Trials 1–2 slope | Trials 2–5 slope |
| Regression-based slope | 0.93* | — | — | — |
| Trials 1–2 slope | 0.36* | 0.12 | — | — |
| Trials 2–5 slope peak | 0.67* | 0.83* | –0.42* | — |
| Peak slope | 0.31* | 0.15 | 0.75* | –0.30* |
Note: NC = normal control; MCI = mild cognitive impairment; AD = Alzheimer's disease.
p < .01.
Correlations between neuroimaging outcomes revealed that in all diagnostic groups, hippocampal volume was moderately correlated with parahippocampal gyrus (r ≥ 0.36; p-values <.01). Similarly, VLPFC thickness was strongly correlated to DLPFC thickness across the three groups (r ≥ 0.68; p-values <.01). Precuneus thickness was moderately related to VLPFC (r ≥ 0.56; p-values <.01) and DLPFC (r ≥ 0.63; p-values <.01) in all diagnostic groups. Across the three groups, VLPFC was modestly correlated with parahippocampal gyrus (r ≥ 0.39; p-values <.01). VLPFC was positively related to hippocampal volume (r ≥ 0.24; p-values < .01) in NC and MCI only. Hippocampal volume was weakly related to precuneus (r = 0.17; p < .01) in MCI and was related to DLPFC (r ≥ 0.20; p < .01) in NC and MCI.
Simple Slope
The simple slope method was unrelated to any neuroimaging marker in NC (p-values >0.23) or AD (p-values > .09). In MCI, a higher simple slope value (indicating better performance) was associated with larger parahippocampal gyrus thickness (β = 0.25; p < .001) and hippocampal volume (β = 0.02; p < .001; see Table 3). In NC, the absolute semi-partial correlations between simple slope and all neuroimaging outcomes were small in magnitude (r = 0.00–0.08; p-values > .05). In MCI, the absolute semi-partial correlations between simple slope and all neuroimaging outcomes were small in magnitude (r = 0.07–0.24, p > .05). Absolute semi-partial correlations in AD were small ( r = 0.02-0.13; p-values > .05; see Table 4). R2 and incremental R2 values indicated that in NC, simple slope did not explain additional variance above and beyond the covariates on any neuroimaging marker (ΔR2 = 0.00–0.01). In MCI, simple slope explained additional variance compared to the covariates and parahippocampal gyrus thickness (ΔR2 = 0.06; p < .01) and hippocampal volume (ΔR2 = 0.03; p < .01). In AD, simple slope did not explain additional variance above and beyond the covariates on any neuroimaging marker (ΔR2 = 0.00–0.02, all p-values > .09; see Table 4).
Table 3.
Slope and neuroimaging regression results by diagnostic group
| Two-slope method |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NC participants | Simple slope n = 198 |
Regression-based n = 198 |
Trials 1–2 slope n = 198 |
Trials 2–5 slope n = 198 |
Peak slope n = 198 |
|||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Parahippocampal gyrus thickness | –0.01 | .88 | –0.03 | .63 | 0.01 | .70 | –0.03 | .47 | 0.03 | .47 |
| Hippocampal volume** | 0.00 | .83 | 0.00 | .52 | 0.00 | .96 | 0.00 | .62 | 0.00 | .46 |
| Precuneus thickness | 0.00 | .96 | 0.00 | .92 | 0.00 | .59 | 0.00 | .98 | 0.00 | .91 |
| DLPFC thickness | –0.01 | .62 | –0.01 | .65 | 0.00 | .46 | 0.00 | .86 | 0.00 | .96 |
| VLPFC thickness | –0.02 | .23 | –0.03 | .14 | 0.00 | .92 | –0.03 | .05 | 0.00 | .88 |
| Two-slope method |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MCI participants | Simple slope n = 370 |
Regression-based n = 370 |
Trials 1–2 slope n = 370 |
Trials 2–5 slope n = 370 |
Peak slope n = 361 |
|||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Parahippocampal gyrus thickness | 0.25 | <.01* | 0.27 | <.01* | 0.06 | <.01* | 0.19 | <.01* | 0.06 | .11 |
| Hippocampal volume** | 0.02 | <.01* | 0.03 | <.01* | 0.00 | .24 | 0.02 | <.01* | 0.00 | .58 |
| Precuneus thickness | 0.04 | .03 | 0.04 | .02 | 0.01 | .32 | 0.04 | .03 | 0.01 | .36 |
| DLPFC thickness | 0.02 | .15 | 0.03 | .05 | 0.00 | .96 | 0.03 | .05 | 0.00 | .92 |
| VLPFC thickness | 0.03 | .02 | 0.05 | <.01* | 0.00 | .37 | 0.04 | .01 | 0.01 | .48 |
| Two-slope method |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AD participants | Simple slope n = 171 |
Regression-based n = 171 |
Trials 1–2 slope n = 171 |
Trials 2–5 slope n = 171 |
Peak slope n = 155 |
|||||
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Parahippocampal gyrus thickness | 0.19 | .09 | 0.26 | .02 | 0.01 | .72 | 0.21 | .03 | –0.05 | .41 |
| Hippocampal volume** | 0.01 | .40 | 0.01 | .31 | 0.00 | .94 | 0.01 | .46 | 0.00 | .88 |
| Precuneus thickness | 0.02 | .68 | 0.04 | .40 | –0.01 | .55 | 0.02 | .50 | −0.01 | .61 |
| DLPFC thickness | –0.01 | .84 | 0.01 | .79 | 0.00 | .65 | 0.01 | .79 | 0.00 | .94 |
| VLPFC thickness | 0.03 | .34 | 0.05 | .12 | 0.00 | .80 | 0.05 | .14 | –0.01 | .49 |
Note. NC = normal control, MCI = mild cognitive impairment; AD = Alzheimer's disease; DLPFC = dorsolateral prefrontal cortex, VLPFC = ventrolateral prefrontal cortex
p< .01.
Hippocampal volume was corrected by intra-cranial volume.
Table 4.
Semi-partial correlations, R2, and incremental R2 for slope × neuroimaging regression results
| NC participants | Base model |
Simple slope |
Regression-based |
Two-slope method |
Peak slope |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | |
| Parahippocampal gyrus thickness | 0.07 | 0.07 | 0.00 | 0.01 | 0.07 | 0.00 | 0.03 | 0.07 | 0.00 | 0.06 | 0.07 | 0.00 | 0.05 |
| Hippocampal volume** | 0.24 | 0.24 | 0.00 | 0.01 | 0.24 | 0.00 | 0.04 | 0.24 | 0.00 | 0.03 | 0.24 | 0.00 | 0.05 |
| Precuneus thickness | 0.08 | 0.08 | 0.00 | 0.00 | 0.08 | 0.00 | 0.01 | 0.08 | 0.00 | 0.04 | 0.08 | 0.00 | 0.01 |
| DLPFC thickness | 0.15 | 0.15 | 0.00 | 0.03 | 0.15 | 0.00 | 0.03 | 0.15 | 0.00 | 0.05 | 0.15 | 0.00 | 0.00 |
| VLPFC thickness | 0.10 | 0.10 | 0.01 | 0.08 | 0.11 | 0.01 | 0.10 | 0.12 | 0.02 | 0.13 | 0.10 | 0.00 | 0.01 |
| MCI participants | Base model |
Simple slope |
Regression-based |
Two-slope method |
Peak slope |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | |
| Parahippocampal gyrus thickness | 0.07 | 0.13 | 0.06* | 0.24 | 0.13 | 0.06* | 0.24 | 0.12 | 0.05* | 0.23 | 0.07 | 0.01 | 0.08 |
| Hippocampal volume** | 0.16 | 0.20 | 0.03* | 0.18 | 0.21 | 0.04* | 0.20 | 0.20 | 0.04* | 0.20 | 0.17 | 0.00 | 0.03 |
| Precuneus thickness | 0.02 | 0.03 | 0.01 | 0.11 | 0.03 | 0.01 | 0.12 | 0.03 | 0.01 | 0.12 | 0.02 | 0.00 | 0.05 |
| DLPFC thickness | 0.07 | 0.08 | 0.01 | 0.07 | 0.08 | 0.01 | 0.10 | 0.08 | 0.01 | 0.10 | 0.07 | 0.00 | 0.01 |
| VLPFC thickness | 0.11 | 0.12 | 0.01 | 0.12 | 0.13 | 0.02* | 0.14 | 0.13 | 0.02 | 0.13 | 0.11 | 0.00 | 0.04 |
| AD participants | Base model |
Simple slope |
Regression-based |
Two-slope method |
Peak slope |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | R2 | ΔR2 | Semi-partial | |
| Parahippocampal gyrus thickness | 0.08 | 0.09 | 0.02 | 0.13 | 0.11 | 0.03 | 0.17 | 0.11 | 0.03 | 0.17 | 0.09 | 0.00 | 0.06 |
| Hippocampal volume** | 0.14 | 0.14 | 0.00 | 0.06 | 0.14 | 0.01 | 0.07 | 0.14 | 0.00 | 0.05 | 0.13 | 0.00 | 0.01 |
| Precuneus thickness | 0.03 | 0.03 | 0.00 | 0.03 | 0.03 | 0.00 | 0.06 | 0.03 | 0.01 | 0.09 | 0.03 | 0.00 | 0.04 |
| DLPFC thickness | 0.08 | 0.08 | 0.00 | 0.02 | 0.08 | 0.00 | 0.02 | 0.08 | 0.00 | 0.05 | 0.08 | 0.00 | 0.01 |
| VLPFC thickness | 0.05 | 0.06 | 0.01 | 0.07 | 0.07 | 0.01 | 0.12 | 0.07 | 0.02 | 0.13 | 0.05 | 0.00 | 0.06 |
Note. Data presented as absolute semi-partial correlation; p-values for the incremental R2 are equivalent to the beta p-values in Table 4 except for the Two Slope Method
p < .01.
Hippocampal volume was corrected by intra-cranial volume.
NC = normal control, MCI = mild cognitive impairment; AD = Alzheimer's disease; DLPFC = dorsolateral prefrontal cortex, VLPFC = ventrolateral prefrontal cortex; Δ = Incremental.
Regression-Based Slope
The regression-based slope method was unrelated to any neuroimaging marker in NC (all p-values ≥ .14) or AD based on the a priori significance threshold (all p-values ≥ .02). In MCI, a higher regression-based slope value (indicating better performance) was associated with larger parahippocampal gyrus thickness (β = 0.27; p < .001), hippocampal volume (β = 0.03; p < .001), and VLPFC (β = 0.05; p = .003; see Table 3). In NC, the absolute semi-partial correlations between regression-based slope and all neuroimaging outcomes were small in magnitude (r = 0.01–0.10; p-values ≥ .05). In MCI, absolute semi-partial correlations between regression-based slope and all neuroimaging outcomes were relatively small in magnitude (r = 0.10–0.24; p-values > .08). Absolute semi-partial correlations in AD were small (r = 0.02–0.17; p-values ≥ .05). See Table 4 for semi-partial correlations. R2 and incremental R2 values indicated that in NC, regression-based slope did not explain additional variance (all p-values > .23). In MCI, regression-based slope explained additional variance in the parahippocampal gyrus thickness (ΔR2 = 0.06; p < .01), hippocampal volume (ΔR2 = 0.04; p < .01), and VLPFC (ΔR2 = 0.02; p < .01). In AD, regression-based slope did not explain additional variance compared to the covariates (all p-values > .02; see Table 4).
Two-Slope Method
The two-slope method was assessed using a single model with two predictors: early slope (i.e., from Trial 1 to Trial 2) and late slope (i.e., from Trial 2 to Trial 5). Conditional on the value of the late slope, early slope was not related to any neuroimaging marker in NC (all p-values > .46) or AD (all p-values > .55). In MCI, early slope was related to parahippocampal gyrus thickness (β = 0.06; p = .003).
Conditional on early slope, late slope (i.e., from Trial 2 to Trial 5) was not related to any neuroimaging marker in NC (p-values > .05) or AD (p-values > .03). In MCI, late slope was related to parahippocampal gyrus thickness (β = 0.19; p < .001), hippocampal volume (β = 0.02; p < .001), and VLPFC (β = 0.04; p = .008; see Table 3).
In NC, the absolute semi-partial correlations between two-slope method and all neuroimaging outcomes were small in magnitude (r = 0.04–0.13; p-values > .05). In MCI, the absolute semi-partial correlations between two-slope method and neuroimaging outcomes were relatively small in magnitude (r = 0.10-0.23; p-values > .05; see Table 4). R2 and incremental R2 values indicated that in NC, the two-slope method did not explain additional variance above and beyond the covariates (all p-values > .14). In MCI, the two-slope method explained additional variance beyond the covariates with respect to parahippocampal gyrus thickness (ΔR2 = 0.05; p < .01) and hippocampal volume (ΔR2 = 0.04; p < .01). In AD, the two-slope method did not explain additional variance compared to the covariates (all p-values > .07; see Table 4).
Peak Slope
Across the three diagnostic groups, peak slope was not related to any neuroimaging marker (all p-values > .11; see Table 3). The semi-partial correlations between peak slope and all neuroimaging outcomes were small across the three diagnostic groups, including NC (r = 0.00–0.05; p-values ≥ .46), MCI (r = 0.01–0.08; p-values ≥ .11), and AD (r = 0.01–0.06; p-values ≥ .41; see Table 4). R2 and incremental R2 values indicated that peak slope did not explain additional variance above and beyond the covariates on any neuroimaging marker in NC (all ΔR2 = 0.00; all p-values > .47), MCI (ΔR2 = 0.00–0.01; all p-values > .11), and AD (all ΔR2 = 0.00; all p-values > .41; see Table 4).
Secondary Analysis
For all primary analyses, regression results were unchanged when APOE4 allele status was removed from the model (data not shown). That is, for each diagnostic group all statistically significant findings persisted (and were not strengthened or weakened) when APOE4 status was removed from the model.
R2 and ΔR2 (incremental change) values for each slope method and neuroimaging variable were assessed with RAVLT Total Learning, Immediate Recall, and Delayed Recall included separately in the model to measure the additional predictive ability of slope over covariates and other common RAVLT memory indices. With Total Learning in the model, no learning slope method explained additional variance in NC (ΔR2 = 0.00–0.01; p-values ≥ .23) or AD (ΔR2 = 0.00–0.02; p-values ≥ .16). In MCI, additional predictive ability was noted for simple slope and parahippocampal gyrus (ΔR2 = 0.02; p < .01), and regression-based slope and parahippocampal gyrus (ΔR2 = 0.02; p < .01) and hippocampal volume (ΔR2 = 0.02; p < .01). When Immediate Recall was included in the model, no slope method explained additional variance in NC (ΔR2 = 0.00–0.02; p-values ≥ .09), MCI (ΔR2 = 0.00–0.01; p-values ≥ .08), or AD (ΔR2 = 0.00–0.02; p-values ≥ .12). When Delayed Recall was included in the model, no slope method explained additional variance in NC (ΔR2 = 0.00–0.02; p-values ≥ .19), MCI (ΔR2 = 0.00–0.01; p-values ≥ .05), or AD (ΔR2 = 0.00–0.02; p-values ≥ .08).
DISCUSSION
The current study advanced understanding of the neuroanatomical and clinical importance of learning efficiency by comparing different learning slope measures to structural neuroimaging markers of early AD pathology and neurodegeneration in cognitively normal, MCI, and AD individuals. Our primary results suggest that among MCI participants, stronger simple slope, regression-based slope, and two-slope method performances were associated with more robust volumes in the medial temporal lobe, including parahippocampal gyrus thickness and hippocampal volume. Stronger regression-based and two-slope method performances also related to higher VLPFC thickness values in MCI. In contrast, there were no statistically significant associations between learning performance assessed by any of the slope measures and any structural neuroimaging markers for either the NC or AD groups, suggesting that a subset of learning slope measures correspond to the structural integrity within the medial temporal lobe and prefrontal cortex in individuals with MCI but not necessarily among individuals with normal cognition or clinical AD.
The association between stronger learning slope performance and larger medial temporal lobe structures is consistent with prior work in MCI linking word list recall to hippocampal volume (Mormino et al., 2009) and parahippocampal gyrus thickness (Leube et al., 2008). The current results enhance prior literature in at least two ways. First, we report associations between specific learning slope methods and surrogate neuroimaging markers of neurodegeneration likely due to AD pathology, and we provide information about which specific calculation method may be most clinically meaningful. Second, our findings highlight that poor learning efficiency may correspond to structural brain changes in the prodromal phase of dementia (MCI) when secondary prevention methods could be most useful.
Another key finding in MCI was that two different methods (regression-based and late slope) correlated with VLPFC cortical thickness, consistent with prior work (Chang et al., 2010). The VLPFC has been implicated in memory tasks that require maintaining, retrieving, and selecting detailed item information (Blumenfeld, Parks, Yonelinas, & Ranganath, 2011), goaloriented learning (Badre & Wagner, 2007), and learning item-to-item associations between unrelated words (Park & Rugg, 2008). Successful performance on a list-learning task involves recalling an increasing number of words across consecutive learning trials, a cognitive skill that may be mediated by the VLPFC. Thus, VLPFC may be important for performance across all trials (i.e., Trials 1–5) rather than just early learning (i.e., Trials 1–2) given that both regression-based slope and late slope related to VLPFC. Future research should explore the VLPFC's involvement in list-learning paradigms before the onset of clinical dementia.
The null results found in the current study warrant some discussion. First, learning slope performance (regardless of calculation method) was not associated with any structural neuroimaging marker among the AD group, even though episodic learning deficits are a hallmark symptom of AD (Bondi et al., 1994). This finding is inconsistent with previous literature that links episodic memory performance (assessed by story learning) to hippocampal volume (Scheltens et al., 1992). One explanation for this disparity could be that AD participant data were confounded by a lack of variability in slope or in the amount of information learned. However, a priori, we intentionally excluded AD participants with severe dementia (CDR = 3) to minimize a potential floor effect, and post hoc visual inspection of trial-by-trial performances in the AD participants does not suggest a notable floor effect (Figure 1). Alternatively, in the AD group, the neuroimaging markers may not have had sufficient variability because of extensive atrophy. Another explanation may be that memory measures reported in the existing literature differ from the current study (i.e., story memory vs. list-learning), including the metric by which memory was measured (i.e., total score in prior literature vs. process variable in the current study). Usage of different memory measures may capture diverse learning approaches (i.e., two learning trials with contextual information in story learning versus five learning trials of unrelated words in list-learning), thus yielding different associations to neuroimaging markers of brain aging. Similarly, no learning slope model was related to any neuroimaging marker among the NC group, which is inconsistent with previous findings suggesting that poorer episodic memory (i.e., story recall) is associated with smaller hippocampal volumes (Golomb et al., 1993). These null findings may also be due to the usage of different memory measures or potential insufficient variability among the slope and neuroimaging markers. It is unlikely these null findings are due to a restriction of range as evidenced by post hoc visual inspection of trial-by-trial performances. Overall, if the current results are valid, then compromised learning slope performance may best reflect underlying neurodegeneration in individuals with mild cognitive changes but offer less information in individuals with intact cognition or frank mild to moderate dementia.
Subtle methodological differences in slope calculation methods and psychometrics may also exist as varying correlation patterns emerged between slopes. Simple slope and regression-based slope were most strongly correlated. These slopes are conceptually similar, although simple slope relies only on two data points whereas the regression-based slope incorporates five data points. Interestingly, even despite the loss of information in simple slope, the association between simple slope and neuroimaging outcomes remains strong suggesting the utility of this slope calculation method. Furthermore, each of these methods correlated with late slope regardless of diagnostic group. Associations with late slope were likely attenuated in strength because of the 20% loss of information in late slope through exclusion of Trial 1 information. Additionally, peak slope moderately correlated with early slope regardless of diagnostic group but was inconsistently and less strongly related to late slope. Early and late slope were unrelated, except in the AD group where the slopes were negatively correlated. This pattern of results may be related to several factors. First, the greatest gain in learning is thought to occur between Trials 1 and 2 (Baldo, Delis, Kramer, & Shimamura, 2002). Second, psychological factors during testing may contribute to learning pattern differences across trials. For example, compared to individuals with no depression, individuals with depression show worse immediate recall for novel information (Trial 1) in the presence of intact learning and recall abilities (Kizilbash, Vanderploeg, & Curtiss, 2002). Performance changes from Trial 1 to Trial 2 may be especially augmented in older adults with attention difficulties, concerns about their memory, or marked memory impairment. Specifically, in NC, the inverse correlation may be reflective of individuals reaching their ideal performance earlier, reflecting intact learning abilities. Similarly, in AD, this inverse correlation may relate to individuals with marked memory impairment reaching their maximum storage capacity almost immediately and being unable to recall any additional words after the initial learning trials. Overall, the pattern of results between early and late slope suggests these two slope methods may reflect different processes and predict different variances in individuals with normal cognition versus dementia.
The current study provides some clinical guidance regarding the ideal method for calculating learning slope among older adults across the cognitive aging spectrum. We conducted semi-partial correlations and R2 analyses to compare the relative contribution of each slope method to the various neuroimaging outcomes for each diagnostic group. The semi-partial correlations suggest that in MCI, simple slope, regression-based slope, and the late slope from two-slope method were uniquely related to neuroanatomical regions, whereas the other slope methods (i.e., peak slope and early slope) were not. This finding was further supported by incremental R2 results suggesting that simple, regression-based, and late slope methods may be the most clinically relevant calculations to use, as they have robust relations with key neuroanatomical structures. Because the regression-based and late slope methods require scoring software or complex mathematical calculation for computation, they may not be practical in many settings. Simple slope provides a feasible alternative for easy implementation in both clinical and research settings because it strongly correlates with both other slope methods and has significant associations with neuroanatomical regions implicated in AD and neurodegeneration. That said, it is worth noting that other methods requiring more complex calculations (i.e., regression-based, late slope) may be more highly correlated with certain neuroimaging markers. However, results suggest that peak slope and early slope from the two-slope method may be less acceptable methods given the lack of neuroanatomical correlation or additional information provided above and beyond covariates.
The current findings highlight details about the predictive utility of different slope methods above and beyond other common RAVLT learning indices, such as Total Learning (i.e., Trials 1–5). Specifically, incremental R2 analyses suggest that simple, regression-based, and two-slope methods provide a small degree of additional predictive power with respect to various neuroimaging markers (i.e., parahippocampal gyrus, hippocampal volume, VLPFC) above and beyond total learning on the RAVLT. Slope methods may lack additional predictive power above and beyond Immediate and Delayed Recall because these latter measures depend upon learning (i.e., slope) and thus share variance. This assumption is supported by the strong correlation between each slope method and the more common learning indices (i.e., Total Learning, Immediate Recall, and Delayed Recall). Given these associations, it is difficult to disentangle the specific role of these common and slope indices in relation to neuroimaging markers of atrophy. However, taken together, results suggest that in some cases slope calculation (i.e., simple, regression-based, and two-slope method) may provide some (albeit limited) additional information about cognitive and neural integrity, particularly in MCI.
The present study has several noteworthy limitations. First, ADNI participants are predominantly White and well educated, which may limit the generalizability of findings. Due to the sample selection, the age of the cohort is restricted to older adults and different results may be seen in a younger sample. Second, limited item-level data in the ADNI dataset preclude examination of anatomical associations with other learning metrics, such as clustering or error responses. Third, our analyses are cross-sectional in nature, and as such we are unable to determine temporal or causal associations about the relation of slope indices and neuroanatomical changes. A longitudinal analysis would be beneficial in further understanding how learning slope contributes to brain morphology changes over time. Next, variability in hardware and software configurations may have contributed unknown variance to the neuroimaging data. Additionally, we used the FreeSurfer estimation of ICV, which does not directly measure subarachnoid CSF. Finally, although our analytical plan was hypothesis-driven, the current study did not analyze all possible brain structures, so we may have overlooked an important association between learning slope and neuroanatomical changes.
Despite these limitations, the present study offers several strengths. We chose a comprehensive set of commonly used slope calculation methods to ensure our study's pertinence to as many clinical applications as possible. In addition, our cohort samples the entire cognitive aging spectrum, allowing us to draw more comprehensive conclusions about the nature of slope performances among older adults free of cognitive impairment as well as elders with MCI and AD. Third, ADNI itself offers a nationally representative cohort, as well as a standardized entry, diagnostic, and neuroimaging protocol.
In conclusion, we systematically evaluated different learning slope calculation methods in relation to neuroimaging markers associated with AD pathology and neurodegeneration. Although results are correlative in nature, they suggest a neuroanatomical association by which impaired verbal learning slope is related to reductions in hippocampal volume and cortical thinning in medial temporal and ventrolateral prefrontal regions among MCI participants.
ACKNOWLEDGMENTS
This research was supported by K12- HD043483 (KAG); Alzheimer's Association NIRG-13-283276 (KAG); K24-AG046373 (ALJ); Alzheimer's Association IIRG-08-88733 (ALJ); R01-AG034962 (ALJ); R01-HL111516 (ALJ); and the Vanderbilt Memory & Alzheimer's Center.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare;; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Alzheimer's Disease Neuroimaging Intitative (ADNI) (National Institutes of Health Grant U01 AG024904)
Footnotes
Disclosure Statement: No relevant financial or nonfinancial relationship exists for any authors, including Katherine A. Gifford, Jeffrey S. Phillips, Elizabeth M. Lane, Susan P. Bell, Dandan Liu, Raymond R. Romano, Laura R. Fritzsche, Zengqi Lu, Lauren R. Samuels, Timothy J. Hohman, and Angela L. Jefferson.
REFERENCES
- Albert MS, Moss M, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. [Research Support, U.S. Gov't, P.H.S.]. Journal of the International Neuropsychological Society. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. Brain. 2006;129(Pt 11):2867–2873. doi: 10.1093/brain/awl274. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review]. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test-II: Findings from patients with focal frontal lesions. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Journal of the International Neuropsychological Society. 2002;8(4):539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: The role of dorsolateral prefrontal cortex in relational memory encoding. [Research Support, N.I.H., Extramural]. Journal of Cognitive Neuroscience. 2011;23(1):257–265. doi: 10.1162/jocn.2010.21459. doi: 10.1162/jocn.2010.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Galasko D, Butters N, Salmon DP, Delis DC. Preclinical cognitive markers of dementia of the Alzheimer type. Neuropsychology. 1994;8(3):374–384. [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ, Jr., Jacobson MW, Alzheimer's Disease Neuroimaging, I. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. Neuropsychologia. 2010;48(5):1237–1247. doi: 10.1016/j.neuropsychologia.2009.12.024. doi: 10.1016/j.neuropsychologia.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. [Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. Neuroreport. 1995;6(12):1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Biological Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Brain and Cognition. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. doi: S1053-8119(98)90395-0 [pii] 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. doi: S1053-8119(06)00043-7 [pii] 10.1016/j.neuroimage.2006. 01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. A sulcal depth-based anatomical parcellation of the cerebral cortex. Neuroimage. 2009;47(S1):S151. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. doi: S089662730200 569X [pii]. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. doi: S1053-8119(98)90396-2 [pii] 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. doi: 10.1002/(SICI)1097-0193(1999)8:4 < 272::AID-HBM10 >3.0. CO;2-4 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory JD, Manuck SB, Ferrell RE, Ryan CM, Muldoon MF. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. American Journal of Medical Genetics. 2000;96(6):707–711. doi: 10.1002/1096-8628(20001204)96:6<707::aid-ajmg1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging. An association with recent memory impairment. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Archives of Neurology. 1993;50(9):967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Nilsson LG, Backman L. Gender differences in episodic memory. [Research Support, Non-U.S. Gov't]. Memory & Cognition. 1997;25(6):801–811. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT, Prince JL. A computational neurodegenerative disease progression score: Method and results with the Alzheimer's disease Neuroimaging Initiative cohort. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Neuro-image. 2012;63(3):1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. doi: 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Rosenberg AL, Morris JN, Allaire JC, McCoy KJ, Marsiske M, Malloy PF. A growth curve model of learning acquisition among cognitively normal older adults. [Clinical Conference Comparative Study Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.]. Experimental Aging Research. 2005;31(3):291–312. doi: 10.1080/03610730590948195. doi: 10.1080/03610730590948195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizilbash AH, Vanderploeg RD, Curtiss G. The effects of depression and anxiety on memory performance. Archives of Clinical Neuropsychology. 2002;17(1):57–67. [PubMed] [Google Scholar]
- Leube DT, Weis S, Freymann K, Erb M, Jessen F, Heun R, Kircher TT. Neural correlates of verbal episodic memory in patients with MCI and Alzheimer's disease–A VBM study. International Journal of Geriatric Psychiatry. 2008;23(11):1114–1118. doi: 10.1002/gps.2036. doi: 10.1002/gps.2036. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McMinn M, Wiens A, Crossen J. Rey auditory-verbal learning test: Development of norms for healthy young adults. Clinical Neuropsychologist. 1988;2(1):67–87. doi: 10.1080/13854048808520087. [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. [Comparative Study Research Support, N.I.H., Extramural]. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- O'Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J, Hampel H. Reduced hippocampal volume in healthy young ApoE4 carriers: An MRI study. [Research Support, Non-U.S. Gov't]. PLoS One. 2012;7(11):e48895. doi: 10.1371/journal.pone.0048895. doi: 10.1371/journal.pone.0048895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Rugg MD. Neural correlates of successful encoding of semantically and phonologically mediated inter-item associations. [Research Support, N.I.H., Extramural]. Neuro-image. 2008;43(1):165–172. doi: 10.1016/j.neuroimage.2008.06.044. doi: 10.1016/j.neuroimage.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Weiner MW. Alzheimer's Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. doi: WNL.0b013e3181cb3e25 [pii] 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr., Xu YC, Waring SC, O'Brien PC, Smith GE, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Neurology. 2000;54(3):581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- Rodgers J, Nicewander A. Thirteen ways to look at the correlation coefficient. The American Statistician. 1988;42(1):59–66. doi: 10.2307/2685263. [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. doi: 10.1093/cercor/bhh032 bhh032 [pii] [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Valk J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: Diagnostic value and neuropsychological correlates. [Research Support, Non-U.S. Gov't]. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(10):967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stout JC, Bondi MW, Jernigan TL, Archibald SL, Delis DC, Salmon DP. Regional cerebral volume loss associated with verbal learning and memory in dementia of the Alzheimer type. [Clinical Trial Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. Neuropsychology. 1999;13(2):188–197. doi: 10.1037//0894-4105.13.2.188. [DOI] [PubMed] [Google Scholar]
- Tierney M, Nores A, Snow W, Fisher R, Zorzitto M, Reid D. Use of the Rey Auditory Verbal Learning Test in differentiating normal aging from Alzheimer's and Parkinson's dementia. Psychological Assessment. 1994;6(2):129–134. doi: 10.1037-/1040-3590.6.2.129. [Google Scholar]
- Tierney M, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64(11):1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- Tulving E. Intratrial and intertrial retention: Notes towards a theory of free recall verbal learning. Psychology Review. 1964;71:219–237. doi: 10.1037/h0043186. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Aisen PS, Jack CR, Jr., Jagust WJ, Trojanowski JQ, Shaw L, Schmidt M. The Alzheimer's disease neuroimaging initiative: Progress report and future plans. Alzheimer's & Dementia. 2010;6(3):202–211. e207. doi: 10.1016/j.jalz.2010.03.007. doi: S1552-5260(10)00067-1 [pii] 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]