Abstract
Objective
Aortic pulse-wave velocity (aPWV) increases with age and is a strong independent predictor of incident cardiovascular diseases (CVDs) in healthy middle-aged and older adults. aPWV is lower in middle-aged and older adults who perform regular aerobic exercise than in their sedentary peers. As exercise is associated with reduced systemic inflammation, we hypothesized that suppression of the pro-inflammatory transcription factor nuclear factor κ B (NFκB) may mediate this process.
Methods
aPWV was measured in young sedentary [n =10, blood pressure (BP) 108 ± 3/59 ± 2 mmHg; mean ± SEM], middle-aged and older sedentary (n =9, 124 ± 7/73 ± 5 mmHg) and middle-aged and older aerobic exercise-trained (n =12, 110 ± 4/67 ± 2 mmHg) healthy, nonhypertensive men and women.
Results
Baseline aPWV increased with age [626 ± 14 (young sedentary) vs. 859 ± 49 (middle-aged and older sedentary) cm/s, P <0.001] but was 20% lower in middle-aged and older trained (686 ± 30 cm/s) than in middle-aged and older sedentary (P <0.005). Short-term (4 days × 2500–4500 mg) treatment with the NFκB inhibitor salsalate (randomized, placebo-controlled cross-over design) reduced aPWV (to 783 ± 44 cm/s, P <0.05) without changing BP (P =0.40) or heart rate (P =0.90) in middle-aged and older sedentary, but had no effect in young sedentary (623 ± 19) or middle-aged and older trained (699 ± 30). Following salsalate treatment, aPWV no longer was significantly different in middle-aged and older sedentary vs. middle-aged and older trained (P =0.29). The reduction in aPWV with salsalate administration was inversely related to baseline (placebo) aPWV (r = −0.60, P <0.001).
Conclusion
These results support the hypothesis that suppressed NFκB signalling may partially mediate the lower aortic stiffness in middle-aged and older adults who regularly perform aerobic exercise. Because aPWV predicts incident cardiovascular events in this population, this suggests that tonic suppression of NFκB signalling in middle-aged and older exercising adults may potentially lower cardiovascular risk.
Keywords: aerobic exercise, ageing, inflammation, pulse wave velocity
INTRODUCTION
With advancing age, even in the absence of clinical disease, the large elastic arteries progressively stiffen [1,2]. Aortic pulse wave velocity (aPWV), the gold standard measurement of large elastic artery stiffness, is an independent predictor of future cardiovascular events in middle-aged and older adults without baseline cardiovascular disease (CVD) [3–5]. Regular aerobic exercise slows the age-associated rise in arterial stiffness, as middle-aged and older adults who perform habitual aerobic exercise demonstrate lower aPWV than their sedentary peers [6,7]. However, the mechanisms contributing to the lower aPWV in exercising vs. sedentary middle-aged and older adults are largely unknown.
One possibility is that exercise induces an anti-inflammatory effect that subsequently slows the age-associated increase in large elastic artery stiffness. Acute induction of inflammation in healthy volunteers increases aPWV [8], whereas pharmacologically inhibiting tumour necrosis factor alpha (TNF-α), a pro-inflammatory cytokine, reduces aPWV in patients with rheumatoid arthritis, a chronic systemic inflammatory disorder [9]. In contrast, regular physical activity is associated with reduced systemic markers of inflammation [10,11]. We have recently shown that middle-aged and older adults who regularly exercise also have reduced vascular endothelial cell protein expression of the pro-inflammatory transcription factor nuclear factor κ B (NFκB) compared with their sedentary peers [12]. By promoting transcription of proinflammatory genes, NFκB is a key modulator of inflammation, including production of circulating pro-inflammatory cytokines [13,14].
Thus, NFκB signalling may be implicated in changes in large elastic artery stiffness with ageing and exercise. Using an established protocol of sasalate treatment to acutely inhibit NFκB signalling and shown previously to reduce vascular endothelial cell NFκB protein expression [15,16], we tested the hypothesis that regular aerobic exercise is associated with a supression of the age-associated increase in arterial stiffness in part by suppressing NFκB signalling.
MATERIALS AND METHODS
The study was conducted in the University of Colorado Boulder Clinical and Translational Research Center (CTRC) and the blood assays were performed by the Colorado Clinical Translational Sciences Institute CTRC Core Lab at the University of Colorado Denver Anschutz Medical Campus. Testing sessions were after an overnight fast and 24-h abstention from exercise and alcohol. All procedures were approved by the Institutional Review Board of the University of Colorado Boulder. The nature, benefits and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Participants
Study participants were recruited for groups of young (18–35 years) sedentary, middle-aged and older (50–79 years) sedentary, or middle-aged and older trained. Sedentary individuals performed no regular aerobic exercise (i.e. ≤30 min per day ≤2 days per week) for at least the last 2 years. Aerobic exercise-trained was defined as at least four sessions/week of vigorous aerobic-endurance exercise. All of the individuals had a BMI less than 35 kg/m2, were nonsmokers and free of clinical diseases as assessed by medical history, physical examination, blood chemistry and resting and exercise ECG. Individuals were not taking medications and had refrained from antioxidants (e.g. vitamins C and E) and aspirin within 2 weeks of the study.
Experimental design
The randomized cross-over study design for administering salsalate and placebo has been described previously by our group [15,16]. Participants in this study are a subgroup of individuals included in one of two previously published cross-over studies evaluating the effect of NFκB inhibition on vascular endothelial function [15,16]. We have previously shown in these two cross-over studies that the acute salsalate treatment described below inhibits NFκB, as evidenced by reduced vascular endothelial cell protein expression of total and nuclear NFκB p65, and increased expression of the inhibitor of NFκB, IκBα [15,16]. Salsalate treatment did not affect the cyclooxygenase (COX) 1/2 pathway, with no change in vascular endothelial cell expression of COX 1/2 [15]. In addition, salsalate reduced vascular endothelial cell NFκB protein expression in the middle-aged and older sedentary but not trained adults, indicating reduced NFκB signalling with regular aerobic exercise [16].
Briefly, in a double-blind, randomized, crossover design, individuals were assigned oral doses of salsalate or placebo for 3 days prior to experimental testing. Serum salicylate was measured on the morning of days 2 and 3 and the day of experimental testing. Individuals received 2500–4500 mg of salsalate each day, to account for individual variation in pharmacokinetics due to sex, size, absorption and metabolism and to result in a steady-state serum salicylate in the therapeutic range of 10–30 mg/dl. Doses were adjusted each day on the basis of serum salicylate concentration to maintain salicylate in the therapeutic range without reaching toxicity (>30 mg/dl). For the 3 days prior to each experimental testing procedure (salsalate and placebo conditions), individuals received a standardized research diet prepared by a CTRC bionutritionist.
Clinical characteristics and VO2max
BMI was calculated from height and weight to the nearest 0.1 kg. Brachial artery BP was assessed under quiet resting conditions in a supine position using a semi-automated device (Dinamap XL; Johnson and Johnson, Arlington, Texas, USA), as described previously [16]. Plasma total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides were determined using standard assays. Maximal oxygen consumption (VO2max) was assessed during incremental treadmill exercise using open-circuit spirometry as described previously [16].
Aortic pulse wave velocity
Two identical transcutaneous Doppler flowmeters (model 810-A; Parks Medical, Aloha, Oregon, USA) were used to simultaneously obtain the pulse wave at the carotid and femoral artery, as described in detail previously [6,17,18]. The distance from the suprasternal notch to the carotid was subtracted from the distance between the two recording sites, and aPWV was calculated as the distance divided by time between the foot of waveforms recorded at each site, as described previously [3]. The intraobserver coefficient of variation for aPWV in our laboratory is 7.2 ± 2.1%.
Statistics
Statistical analyses were performed with SPSS (version 20.0, Chicago, Illinois, USA) with statistical significance for all analyses set at P value less than 0.05. Group differences at baseline (i.e. placebo) were determined by one-way analysis of variance (ANOVA). In the case of significant F values, Bonferroni posthoc analyses were performed. A 3 × 2 repeated-measures ANOVA was used for between-group (young sedentary, middle-aged and older sedentary, and middle-aged and older trained) and within-group (placebo condition, salsalate condition) comparisons. When a significant condition × group interaction was revealed (P <0.05), differences within individual groups during salsalate vs. placebo were compared with paired t-tests. Bivariate Pearson correlation analyses were performed to examine relations between variables of interest. All data are reported as means ± SE.
RESULTS
Clinical characteristics of study participants
Resting heart rate, DBP and low-density lipoprotein cholesterol were all higher in middle-aged and older sedentary than in young adults (Table 1). Maximal aerobic capacity, as assessed by VO2max, was reduced in middle-aged and older sedentary adults, but not in middle-aged and older trained adults, as compared with young individuals. In addition to a lower VO2max, middle-aged and older sedentary individuals had a higher resting heart rate, were heavier and had a higher BMI than their trained peers. Other individual characteristics did not significantly differ among groups. None of these characteristics changed significantly in response to salsalate treatment.
TABLE 1.
Clinical characteristics
| Variable | YS (young sedentary)
|
OS (middle-aged and older sedentary)
|
OT (middle-aged and older trained)
|
|||
|---|---|---|---|---|---|---|
| Placebo | Salsalate | Placebo | Salsalate | Placebo | Salsalate | |
| N (men/women) | 10 (9/1) | – | 9 (5/4) | – | 12 (8/4) | – |
|
| ||||||
| Age (years) | 23±1 | – | 61±2* | – | 58±2* | – |
|
| ||||||
| Mass (kg) | 75.2±2.3 | – | 80.6±6.3** | – | 66.0±2.9 | – |
|
| ||||||
| BMI (kg/m2) | 24.0±0.8 | – | 26.1±1.3** | – | 22.2±0.8 | – |
|
| ||||||
| VO2max (ml/kg per min) | 45.9±2.3 | – | 29.1±1.6*,***,† | – | 43.4±2.4 | – |
|
| ||||||
| Resting HR (bpm) | 52±3 | 54±2 | 64±3***,† | 64±3**,† | 50±2 | 51±2 |
|
| ||||||
| SBP (mmHg) | 108±3 | 108±3 | 124±7 | 121±5 | 110±4 | 109±4 |
|
| ||||||
| DBP (mmHg) | 59±2 | 58±2 | 73±5† | 70±4† | 67±2 | 67±2 |
|
| ||||||
| Total cholesterol (mg/dl) | 136±9 | 128±8 | 179±6† | 166±5† | 196±10* | 179±10* |
|
| ||||||
| LDL cholesterol (mg/dl) | 75±8 | 72±6 | 111±5† | 104±7† | 121±8* | 112±9* |
|
| ||||||
| HDL cholesterol (mg/dl) | 45±3 | 45±4 | 49±5 | 47±5 | 56±5 | 56±5 |
|
| ||||||
| Triglycerides (mg/dl) | 84±24 | 59±13 | 95±11 | 67±6 | 93±7 | 61±5 |
Values are mean ± SE. HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; VO2max, maximal oxygen consumption; YS, young sedentary.
P <0.005 vs. YS.
P <0.05 vs. OT.
P <0.005 vs. OT.
P <0.05 vs. YS (same condition).
Nuclear factor κ B inhibition and aortic pulse wave velocity
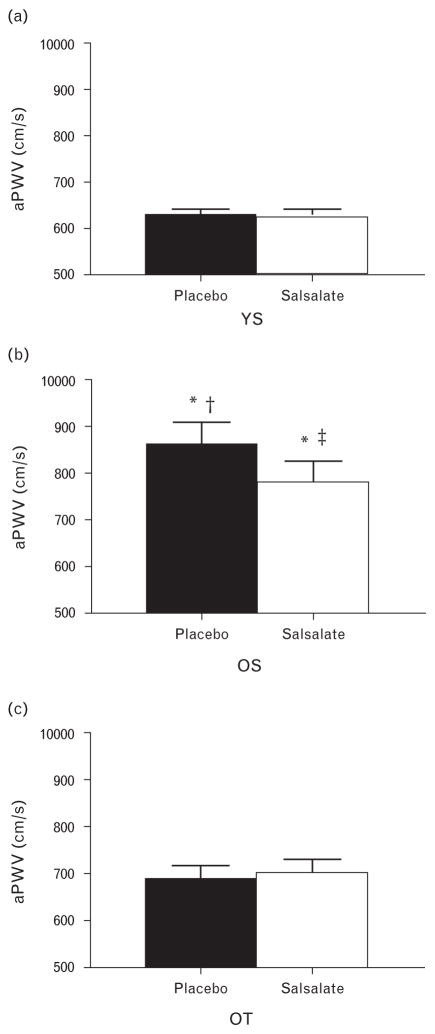
During placebo treatment, aPWV was higher in middle-aged and older sedentary (859 ± 49 cm/s) than young sedentary adults (626 ± 14 cm/s). However, middle-aged and older trained adults had lower aPWV (686 ± 30 cm/s) than middle-aged and older sedentary adults (Fig. 1). Short-term salsalate administration lowered aPWV by nearly 10% in middle-aged and older sedentary adults (to 783 ± 44 cm/s) without changing aPWV velocity in middle-aged and older trained or young sedentary adults (P ≥ 0.5). Under the salsalate treatment condition, aPWV no longer differed between middle-aged and older sedentary and middle-aged and older trained adults (P =0.21), but was still higher in the middle-aged and older sedentary than young sedentary adults (P <0.01). The reduction in aPWV with sal-salate administration was inversely related to baseline (placebo condition) aPWV (r =−0.60, P <0.001). This is consistent with the lack of change with salsalate in young sedentary and middle-aged and older trained individuals, as these groups had lower baseline aPWV.
FIGURE 1.
Aortic pulse-wave velocity in young sedentary (YS; a), middle-aged and older sedentary (OS; b) and middle-aged and older trained (OT; c) adults under conditions of placebo (black bars) or salsalate (white bars). Data are mean ± SE; *P <0.01 vs. YS of the same condition; †P <0.01 vs. OT of the same condition; ‡P <0.05 vs. placebo of the same group.
DISCUSSION
We have shown for the first time that suppression of NFκB signalling plays a major mechanistic role in the lower aPWV, and perhaps, therefore, lower cardiovascular risk, in regularly exercising middle-aged and older adults than sedentary peers. Consistent with previous evidence [6,7], aPWV was greater in the middle-aged and older sedentary but not middle-aged and older trained adults in comparison to the young sedentary adults. Short-term treatment with salsalate, which we have previously shown suppresses vascular NFkB signalling in the larger cohort these participants were included from [15,16], selectively reduced aPWV in the middle-aged and older sedentary group, with no effect in middle-aged and older trained or young sedentary groups. This reduction in aPWV was observed in the absence of changes in blood pressure, heart rate or other assessed individual characteristics. Following salsalate treatment, aPWV was no longer significantly different between the middle-aged and older sedentary and middle-aged and older trained groups, indicating that reduced NFkB signalling contributed to the lower baseline arterial stiffness in the trained group. This is consistent with evidence we have published previously that middle-aged and older adults performing regular aerobic exercise have reduced NFkB signalling [16]. Our results are novel, as there is currently surprisingly little evidence available regarding the physiological mechanisms by which aerobic exercise reduces arterial stiffness, particularly in humans [19,20].
Evidence from patients with chronic systemic inflammatory diseases such as rheumatoid arthritis supports a role of inflammation in modulating arterial stiffness [9,21,22]. Similarly, acutely inducing inflammation in healthy adults via vaccination results in an increase in aPWV [8]. Our findings are consistent with this relation between chronic systemic inflammation and arterial stiffness, as regular exercise reduces systemic markers of inflammation [10,11].
Although statistically and clinically significant, the reduction in aPWV in the middle-aged and older sedentary group (~10%) with salsalate was somewhat modest compared with the improvements we have previously shown in brachial artery flow-mediated dilation (FMDBA) following salsalate treatment [15,16]. Similar to our findings with FMDBA, the response to salsalate was greatest in those individuals with the greatest baseline aPWV. Specifically, aPWV was reduced in seven of the nine middle-aged and older sedentary individuals, although the degree of reduction was small in three of these individuals, and aPWV was not reduced to that of the young sedentary group. Collectively, this suggests that mechanisms beyond suppressed NFkB signalling contribute to the attenuation of the age-associated increase in arterial stiffness with habitual aerobic exercise.
Large elastic artery stiffness is modulated by both functional (i.e. vascular tone) and structural (i.e. arterial wall proteins) influences [19,23]. Inflammation may modulate either of these components; however, acute inhibition of NFkB with salsalate would affect only the functional component, likely by enhancing nitric oxide bioavailability [23,24]. In contrast, long-term aerobic exercise may also modify structural properties influencing arterial stiffness. Following exercise training in rodents, the reduction in arterial inflammation [25] is also associated with structural modifications in the vasculature, including decreased collagen I, collagen III and transforming growth factor β [26]. However, analogous assessments are experimentally challenging in humans, and future studies are needed to examine the contribution of any structural changes to reduced arterial stiffness with exercise in humans. In addition, exercise may also induce functional changes to the vasculature via mechanisms other than reduced NFkB signalling, such as alterations in the sympathetic nervous system [27], TNF-α [28], endothelin-1 [29] signalling or the COX 1/2 pathway [30].
We recognize that it is not possible to completely separate inflammation and oxidative stress, as inflammatory signalling stimulates oxidant enzyme systems to produce reactive oxygen species [31], and reactive oxygen species also promote a pro-inflammatory cascade [32,33]. In our previously published cross-over study evaluating the effect of NFκB inhibition on vascular endothelial function [16], an acute infusion of ascorbic acid known to inhibit superoxide production selectively improved FMDBA in middle-aged and older sedentary adults during the placebo but not salsalate condition. These findings support that the improvement in FMDBA with NFκB inhibition was in part due to reduced vascular oxidative stress. However, we have previously shown that unlike with vascular endothelial function (FMDBA), an acute infusion of ascorbic acid is not effective at reducing aPWV [34]. Thus, we elected not to use this approach to evaluate the role of oxidative stress in reduced aPWV with NFκB inhibition, and cannot discern the potential role of oxidative stress to reduced aPWV.
We acknowledge that there some additional limitations to our findings. Our results are a cross-sectional group comparison, thus cannot discern the effects of an exercise intervention on NFkB modulation of large elastic artery stiffness. In addition, our sample size was small, but adequate to detect changes in our primary outcome of aPWV using a cross-over design. Future studies will be needed to confirm and expand upon these findings.
Our findings are strengthened by the fact that we directly inhibited NFkB to assess the contribution of this signalling pathway to arterial stiffness with sedentary ageing. To the best of our knowledge, we have provided the first evidence that suppressed NFkB signalling may mediate in part the lower aortic stiffness in middle-aged and older adults who regularly exercise as compared with their sedentary peers. Because aPWV predicts incident cardiovascular events in this population, this suggests that tonic suppression of NFκB signalling in middle-aged and older exercising adults may potentially contribute to lower cardiovascular risk.
Acknowledgments
The authors thank Phillip Rhodes and the staff of the University of Colorado-Boulder CTRC for their technical assistance. This work was supported by National Institutes of Health awards NIH AG031141, AG033994, AG031617, AG013038, AG006537 and TR000154 and the American Heart Association award 12POST11920023.
Abbreviations
- aPWV
aortic pulse-wave velocity
- CTRC
Clinical and Translational Research Center
- NFκB
nuclear factor κ B
- TNF-α
tumournecrosis factor α
- VO2max
maximal oxygen consumption
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–2010. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 5.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arteriscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- 7.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 9.Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by antitumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–1192. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 10.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritch-evsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 11.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 12.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arteriscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 14.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 15.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-kappa B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin Sci (Lond) 2014;127:645–654. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol. 2013;8:1952–1959. doi: 10.2215/CJN.00900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 19.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587:5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. 2010;55:333–338. doi: 10.1161/HYPERTENSIONAHA.109.143982. [DOI] [PubMed] [Google Scholar]
- 22.Wong M, Oakley SP, Young L, Jiang BY, Wierzbicki A, Panayi G, et al. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:1277–1284. doi: 10.1136/ard.2007.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 24.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, et al. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011;301:H1025–H1032. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor beta 1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;15:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, et al. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009;135:346–352. doi: 10.1016/j.ijcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-alpha inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis. 2013;230:390–396. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, et al. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol. 2003;95:336–341. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 30.Carroll CC, O’Connor DT, Steinmeyer R, Del Mundo JD, McMullan DR, Whitt JA, et al. The influence of acute resistance exercise on cyclo-oxygenase-1 and -2 activity and protein levels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2013;305:R24–R30. doi: 10.1152/ajpregu.00593.2012. [DOI] [PubMed] [Google Scholar]
- 31.Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 32.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappa B. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 33.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8(3–4):572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 34.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004;286:H1528–H1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]