Abstract
The C allele of the rs2736100 single nucleotide polymorphism located in the second intron of the TERT gene has recently been identified as a susceptibility factor for myeloproliferative neoplasms (MPN) in the Icelandic population. Here, we evaluate the role of TERT rs2736100_C in sporadic and familial MPN in the context of the previously identified JAK2 GGCC predisposition haplotype. We have confirmed the TERT rs2736100_C association in a large cohort of Italian sporadic MPN patients. The risk conferred by TERT rs2736100_C is present in all molecular and diagnostic MPN subtypes. TERT rs2736100_C and JAK2 GGCC are independently predisposing to MPN and have an additive effect on disease risk, together explaining a large fraction of the population attributable fraction (PAF = 73.06%). We found TERT rs2736100_C significantly enriched (P = 0.0090) in familial MPN compared to sporadic MPN, suggesting that low-penetrance variants may be responsible for a substantial part of familial clustering in MPN. Am. J. Hematol. 89:1107–1110, 2014. © 2014 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Introduction
Myeloproliferative neoplasms (MPN) constitute a group of phenotypically diverse chronic myeloid malignancies including three major disease entities: polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Mutually exclusive oncogenic somatic mutations in three genes have been identified in more than 90% of MPN cases 1. Mutations in JAK2, most notably JAK2-V617F are present in all three disease subtypes, more frequently in PV 2–5, while MPL and CALR mutations are exclusively found in ET and PMF patients 6–8. The same somatic mutations are present also in familial MPN, which account for 5–10% of MPN cases 9–11. Previously, a common haplotype (GGCC or 46/1) at the JAK2 locus has been found to predispose to JAK2 mutation positive sporadic and familial MPN 12–15. Genome-wide association studies have successfully revealed risk loci for a series of cancers 16. A recent study identified the germline sequence variant rs2736100_C located in the second intron of the TERT gene as risk variant for MPN in the Icelandic population 17. In this study, we were seeking to confirm the TERT association in an independent cohort from a different ethnic background and evaluate the role of the TERT predisposition locus in familial clustering of MPN. Furthermore, we were testing the possibility of an interaction of TERT and JAK2 susceptibility loci in sporadic and familial MPN.
Methods
Blood samples from sporadic MPN (n = 717), familial MPN (n = 121) and control (n = 202) subjects from Italy were obtained after written informed consent. The study was approved by the institutional ethics committee (Comitato di Bioetica, Fondazione IRCCS Policlinico San Matteo) and procedures were in accordance with the Helsinki declaration. Details on patient characteristics and sample collection have been described in a previous study 15. Patients were defined as familial cases if two or more individuals within the same pedigree were affected. For each family, the proband was identified as the first affected family member seeking medical attention.
Presence of the JAK2-V617F mutation, JAK2 exon 12 mutations, MPL exon 10 mutations, and CALR exon 9 mutations was assessed in granulocyte DNA as previously described 7. Genotyping for rs2736100 (TERT) and rs10974944 (JAK2) was performed using commercially available TaqMan SNP genotyping assays (C___1844009 and C__31941696, respectively; Applied Biosystems, Foster City, CA).
Statistical analyses were performed using the R statistical software (version 3.0.3) 18 in conjunction with the R-packages “SNPassoc” and “scrime.” Population attributable fraction (PAF) and proportions of familial relative risk (FRR) explained by TERT and JAK2 loci were calculated as previously described by others 19,20, using an estimate of 5.6 for the overall MPN FRR 21. The Cochran–Armitage test of trend was applied to study differences in distribution of risk allele numbers per individual in the different cohorts. The absolute risk for developing MPN in different TERT/JAK2 genotypic classes was calculated using logistic regression.
Results
We confirmed the previously reported 17 association of rs2736100_C with the MPN phenotype in a cohort of sporadic MPN patients (n = 717) of a different ethnic background (Table1). The size of our sporadic MPN cohort allowed for comparison of genotype distributions in different molecular and diagnostic subgroups. Significant associations were detected for both JAK2-positive and CALR-positive MPN at similar strength (Table1), suggesting that there is no preferential susceptibility to any molecular subtype. A similar trend was observed for MPL-positive and triple negative MPN patients (lacking JAK2, MPL, and CALR mutations), however, statistical significance is absent, possibly due to low sample sizes (Supporting Information Table 1). As in the prior study 17, the TERT rs2736100_C association was present in PV, ET, and PMF, implying a general role in MPN pathogenesis (Supporting Information Table 1).
Table 1.
Association of TERT rs2736100 with Sporadic and Familial MPN and Molecular Subtypes
| Genotype frequency (%) case population | Genotype frequency (%) control population | Odds ratio (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case population | Control population | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | P value |
| Sporadic MPN(n = 717) | Control(n = 202) | 11.3(81) | 46.2 (331) | 42.5 (305) | 23.3 (47) | 43.6 (88) | 33.2 (67) | 1 | 2.18 (1.42–3.35) | 2.64 (1.69–4.13) | 1.15 × 10−4 |
| Sporadic MPN JAK2+ (n = 516) | Control (n = 202) | 10.7(55) | 44.8 (231) | 44.6 (230) | 23.3 (47) | 43.6 (88) | 33.2 (67) | 1 | 2.24 (1.42–3.55) | 2.93 (1.82–4.72) | 5.55 × 10−5 |
| Sporadic MPN CALR+ (n = 126) | Control (n = 202) | 11.9 (15) | 46.8 (59) | 41.3 (52) | 23.3 (47) | 43.6 (88) | 33.2 (67) | 1 | 2.10 (1.08–4.10) | 2.43 (1.23–4.82) | 0.0270 |
| Familial MPN (n = 121) | Control (n = 202) | 5.0 (6) | 39.7 (48) | 55.4 (67) | 23.3 (47) | 43.6 (88) | 33.2 (67) | 1 | 4.27 (1.7–10.72) | 7.83 (3.14–19.55) | 1.10 × 10−6 |
| Familial MPN probands (n = 75) | Control (n = 202) | 5.3 (4) | 36.0 (27) | 58.7 (44) | 23.3 (47) | 43.6 (88) | 33.2 (67) | 1 | 3.61 (1.19–10.92) | 7.72 (2.60–22.94) | 2.65 × 10−5 |
| Familial MPN (n = 121) | Sporadic MPN (n = 717) | 5.0 (6) | 39.7 (48) | 55.4 (67) | 11.3 (81) | 46.2 (331) | 42.5 (305) | 1 | 1.96 (0.81–4.73) | 2.97 (1.24–7.08) | 0.0090 |
| Familial MPN probands (n = 75) | Sporadic MPN (n = 717) | 5.3 (4) | 36.0 (27) | 58.7 (44) | 11.3 (81) | 46.2 (331) | 42.5 (305) | 1 | 1.65 (0.56–4.85) | 2.92 (1.02–8.37) | 0.0180 |
We next studied the combined effects of the two known MPN risk loci at TERT and JAK2 as well as their possible interaction. Conditional logistic regression analysis revealed that TERT and JAK2 loci are independently predisposing to MPN, the total combined risk approximating the sum of the two genotypes (Fig. 1; Supporting Information Table 2). Direct testing for interaction was negative (Supporting Information Table 3). The combined effects of TERT and JAK2 risk loci are stronger in JAK2-positive MPN (Fig. 1). This finding is compatible with the JAK2 risk haplotype predisposing primarily for the acquisition of somatic mutations in the JAK2 gene.
Figure 1.
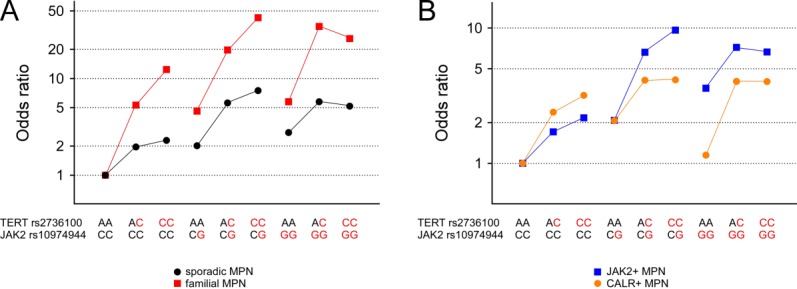
Combined effects of TERT and JAK2 MPN predisposition loci. Genotypic odds ratios for MPN in respect to the nine genotypic combinations at TERT (rs2736100) and JAK2 (rs10974944) loci are shown for (A) sporadic and familial total cohorts as well as for (B) sporadic JAK2-positive and CALR-positive subcohorts. Risk alleles are marked in red. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To evaluate the role of TERT in familial MPN, we determined the rs2736100 genotype in 121 affected members of 75 Italian families with two or more MPN cases within first and/or second degree relatives. The TERT rs2736100_C association is significantly stronger in familial MPN compared to sporadic MPN (P = 0.009; Table1), indicating a role of the TERT locus in MPN familial clustering. Proband-based analysis confirmed this observation (P = 0.018; Table1). As previously reported on a smaller familial MPN cohort 15, such a significant difference in association strength is absent for JAK2 GGCC when directly comparing sporadic and unfiltered familial JAK2-positive MPN cases (Supporting Information Table 4). However, MPN families consisting of both JAK2-positive and JAK2-negative members might be confounding the possible association, as JAK2 GGCC predisposes mainly to JAK2-positive MPN. Indeed, when restricting analysis to families exclusively consisting of JAK2-positive members, we observed a significant trend toward JAK2 GGCC enrichment in familial MPN compared to sporadic MPN (P = 0.046; Supporting Information Table 4). Moreover, the familial MPN cohort exhibits enrichment for individuals carrying more than three risk alleles (counting one for heterozygotes and two for homozygotes; Supporting Information Figure 1) and a trend for odds ratios increasing toward double positive (TERT/JAK2) individuals (Supporting Information Table 2), further supporting the possibility of JAK2 GGCC contributing to MPN familial clustering. Independence of TERT and JAK2 loci in mediating MPN susceptibility, as observed in sporadic MPN, is also true for familial MPN (Fig. 1).
Similar to that reported in the Icelandic study 17, in this study cohort TERT rs2736100_C is estimated to account for 51.89% of PAF. In concert with JAK2 GGCC (PAF = 44.01%), they explain a large part (combined PAF = 73.06%) of the population susceptibility for MPN. The absolute risk for developing MPN in different TERT/JAK2 genotypic classes is calculated in Supporting Information Table 5. As the risk variant rs2736100_C is present at high frequency (55%; Table1) in the general population, only 2.01% of the FRR can be attributed to the TERT rs2736100_C variation. In contrast, 5.15% of FRR can be explained by the JAK2 GGCC risk haplotype which is present at lower frequency (27%; Supporting Information Table 4) in the general population.
Discussion
The TERT gene encodes the reverse transcriptase of the telomerase complex, essential for maintaining telomere length 22. The C allele of rs2736100 has been linked to longer telomeres 23,24, compatible with a direct regulatory effect of rs2736100 genotype on TERT expression. TERT rs2736100_C was previously shown to also associate with elevated risk for several other cancers, albeit with lower effect 25–28. Furthermore, rs2736100_C is linked to increased blood cell count values 17,29, a hallmark of MPN. The fact that TERT rs2736100_C predisposes to all MPN subtypes implies a generic role in MPN predisposition, possibly through affecting blood cells counts.
Enrichment of common susceptibility loci in familial forms of predominantly sporadic cancers has been reported previously 20, and contribution of low-penetrance risk loci to familial clustering is well acknowledged 19,30. Notably, both TERT rs2736100_C for all MPN subtypes as well as JAK2 GGCC for JAK2-positive MPN exhibit effect sizes stronger than typically observed for common cancer-predisposing variants 16. This might explain the significance of familial enrichment for TERT rs2736100_C (Table1). The implication of JAK2 GGCC in familial clustering remains to be confirmed in a larger set of JAK2-positive MPN families.
In conclusion, common variation at TERT and JAK2 loci explains most of the population risk for developing MPN. Enrichment of the TERT risk variant in familial MPN suggests the possibility of random accumulation of several common high frequency variants being responsible for parts of the elevated risk underlying familial clustering. More common low penetrance and/or higher penetrance rare mutations remain to be discovered to explain the missing heritability in sporadic MPN and the missing excess familial risk in MPN.
Author Contributions
R. J., A. S. H., and R. K. designed and planned the study. E. R. and M. C. recruited the patients and recorded clinical data. R. J., D. P., T. B., and D. O. performed experiments for this study. R. J., A. S. H., and E. R. performed data analyses. R. J., A. S. H., R. S. H., and R. K. contributed to data interpretation. R. J., A. S. H., and R. K. wrote the paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
References
- Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: The clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014;123:3714–3719. doi: 10.1182/blood-2014-03-530865. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Levine RL, Loriaux M, Huntly BJ, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–3379. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood. 2008;112:141–149. doi: 10.1182/blood-2008-01-131664. [DOI] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi E, Passamonti F, Della Porta MG, et al. Familial chronic myeloproliferative disorders: Clinical phenotype and evidence of disease anticipation. J Clin Oncol. 2007;25:5630–5635. doi: 10.1200/JCO.2007.12.6896. [DOI] [PubMed] [Google Scholar]
- Harutyunyan AS, Kralovics R. Role of germline genetic factors in MPN pathogenesis. Hematol Oncol Clin North Am. 2012;26:1037–1051. doi: 10.1016/j.hoc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Rumi E, Harutyunyan AS, Pietra D, et al. CALR exon 9 mutations are somatically acquired events in familial cases of essential thrombocythemia or primary myelofibrosis. Blood. 2014;123:2416–2419. doi: 10.1182/blood-2014-01-550434. [DOI] [PubMed] [Google Scholar]
- Olcaydu D, Harutyunyan A, Jäger R, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- Jones AV, Chase A, Silver RT, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpivaara O, Mukherjee S, Schram AM, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcaydu D, Rumi E, Harutyunyan A, et al. The role of the JAK2 GGCC haplotype and the TET2 gene in familial myeloproliferative neoplasms. Haematologica. 2010;96:367–374. doi: 10.3324/haematol.2010.034488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher O, Houlston RS. Architecture of inherited susceptibility to common cancer. Nat Rev Cancer. 2010;10:353–361. doi: 10.1038/nrc2840. [DOI] [PubMed] [Google Scholar]
- Oddsson A, Kristinsson SY, Helgason H, et al. The germline sequence variant rs2736100_C in TERT associates with myeloproliferative neoplasms. Leukemia. 2014;28:1371–1374. doi: 10.1038/leu.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2013. [Google Scholar]
- Di Bernardo MC, Crowther-Swanepoel D, Broderick P, et al. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40:1204–1210. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- Lubbe SJ, Di Bernardo MC, Broderick P, et al. Comprehensive evaluation of the impact of 14 genetic variants on colorectal cancer phenotype and risk. Am J Epidemiol. 2012;175:1–10. doi: 10.1093/aje/kwr285. [DOI] [PubMed] [Google Scholar]
- Landgren O, Goldin LR, Kristinsson SY, et al. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen SE. Telomeres and human health. J Intern Med. 2013;274:399–413. doi: 10.1111/joim.12083. [DOI] [PubMed] [Google Scholar]
- Bojesen SE, Pooley KA, Johnatty SE, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384, 384e1–384e2. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427, 427e1–427e2. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JD, Hung RJ, Gaborieau V, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40:1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Broderick P, Webb E, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008;40:1407–1409. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago-Dominguez M, Jiang X, Conti DV, et al. Genetic variations on chromosomes 5p15 and 15q25 and bladder cancer risk: Findings from the Los Angeles-Shanghai bladder case-control study. Carcinogenesis. 2011;32:197–202. doi: 10.1093/carcin/bgq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatani Y, Matsuda K, Okada Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42:210–215. doi: 10.1038/ng.531. [DOI] [PubMed] [Google Scholar]
- Houlston RS, Peto J. The future of association studies of common cancers. Human Genet. 2003;112:434–435. doi: 10.1007/s00439-002-0902-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


