Abstract
BACKGROUND
OX513A is a genetically engineered strain of Aedes aegypti carrying a repressible, dominantly inherited transgene that confers lethality in immature heterozygous progeny. Released male OX513A adults have proven to be effective for the localised suppression of wild Ae. aegypti, highlighting its potential in vector control. Mating and life-table assessments were used to compare OX513A with reared Ae. aegypti strains collected from New Delhi and Aurangabad regions in India.
RESULTS
Mating proportions of New Delhi females versus males of OX513A or New Delhi strains were 0.52 and 0.48 respectively, indicating no discrimination by females against either strain, and males of both strains were equally competitive. Developmental time from first instar to adult emergence was significantly longer for OX513A (10.7 ± 0.04 days) than for New Delhi (9.4 ± 0.04 days) and Aurangabad strains (9.1 ± 0.04 days). Differences in mean longevities, female reproductive parameters and population growth parameters between the strains were non-significant.
CONCLUSIONS
The laboratory study demonstrates that only minor life-table variations of limited biological relevance exist between OX513A and Indian Ae. aegypti populations, and males had equal potential for mating competitiveness. Thus, results support the OX513A strain as a suitable candidate for continued evaluation towards sustainable management of Ae. aegypti populations in India. © 2014 Gangabishan Bhikulal Investment and Trading Limited. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: mosquito, Aedes aegypti, RIDL, transgenic, sterile insect technique, dengue
INTRODUCTION
Aedes aegypti Linnaeus (Diptera: Culicidae), commonly referred to as the yellow fever mosquito, is well known as a vector of numerous human diseases, including dengue, chikungunya and yellow fever.1–3 Dengue, a debilitating disease present throughout the tropics, continues to increase in both geographical spread and intensity owing to the proliferation of multiple serotypes.4 A recent report suggests that India is the country with the greatest number of cases per annum, potentially contributing one-third of the global total.5 Ae. aegypti is the primary vector of dengue virus and as such is the target of intensive mosquito control programmes. Although epidemiological data on fluctuating dengue prevalence are difficult to attribute to specific vector control efforts, it is widely accepted that the present focus of larval breeding source reductions and insecticidal applications is not sufficiently effective.
Reduction of vector populations is just one of the methods used to combat the spread of public health diseases, but is of particular relevance for the mitigation of dengue, as neither therapeutic medication nor preventive vaccines are available. In recent years, genetically based insect control strategies for mosquitoes have received increasing attention, with conventional and transgenic technologies undergoing scientific appraisal.6,7 One such approach that has been successfully used to suppress localised Ae. aegypti populations is RIDL® (Release of Insects carrying a Dominant Lethal) and involves repeated, large-scale releases of genetically ‘sterilised’ male mosquitoes.8 Unlike truly sterile insects, RIDL insects can still breed and produce offspring; however, a proportion (up to 100%) of the offspring are unviable and die before attaining adulthood. Operationally, RIDL resembles the conventional sterile insect technique (SIT), although, by contrast, SIT involves the release of insects that have typically been sterilised by either irradiation or chemosterilants.9,10
OX513A is an Ae. aegypti RIDL strain in which all individuals carry a repressible, homozygous, dominantly inherited lethal gene insertion.11 Mass rearing of OX513A in the laboratory is facilitated through the addition of tetracycline as a dietary supplement that represses the lethal phenotype. Males are released into the field to compete against wild males for female mates, and, if successful, offspring inherit a copy of the lethal gene and die before adulthood. Repeated releases of OX513A males have delivered high levels of suppression of target Ae. aegypti populations in open field studies conducted in the Grand Cayman Islands8 and north-east Brazil (McKemey AR, unpublished).
Dispersal, mate seeking and mating success are core requirements for genetically based population suppression strategies, and so assessing the potential for mass-reared mosquitoes to survive and compete effectively provides invaluable information. Although the efficacy of released mosquitoes in the open field is ultimately determined by complex biological, environmental and operational interactions, controlled laboratory environments can help to isolate and identify deleterious physiological effects due to inbreeding depression or transgene insertions within the receiving organism.12 Identification of deleterious traits or parameters does not necessarily preclude the use of specific insect strains in the field, but may enable a strategic approach that maximises efficiencies and performance, and could aid the technical development of improved strains.13
With a view towards evaluation of OX513A for Ae. aegypti control within open environments in India, the work presented here is a laboratory study of mating and life-table parameters in comparison with strains recently collected from New Delhi (New Territory Capital) and Aurangabad (Maharashtra State).
MATERIALS AND METHODS
Insect strains
OX513A
OX513A Ae. aegypti eggs (supplied by Oxitec Ltd, UK) were imported during September 2011 in accordance with the import permit (No. BT/BS/17/328/2008-PID) issued by the Department of Biotechnology (DBT), Government of India, New Delhi. Development of the OX513A strain was originally described by Phuc et al.11 OX513A individuals are identifiable under fluorescent light owing to the expression of a second, dominantly inherited introduced gene that encodes for a red fluorescent protein (DsRed2). DsRed2, controlled by an Act5C promoter, is visible under a microscope equipped for fluorescence (filters for red fluorescence with excitation of 510–550 nm and emission at 600 nm).
Wild types
Aquatic and adult stages of Ae. aegypti were collected separately from New Delhi and Aurangabad during 2011. Collections from both localities were maintained under standardised rearing conditions (see Section 2.2) and were referred to as ‘wild-type’ strains after they had completed one generation in the laboratory. These strains were named DEL and AWD for the New Delhi and Aurangabad collections respectively.
Insect rearing
All strains were maintained within an Arthropod Containment Level II facility under standardised laboratory conditions of 27 ± 2 °C, 70–80% relative humidity and a day length of 12 h.14 Eggs were submerged in tap water and maintained at reduced atmospheric pressure for synchronised hatching, and larvae were subsequently reared in tap water at a density of 1 larva mL−1. OX513A larvae only were reared using tap water containing an initial concentration of 30 µg mL−1 of tetracycline (used to repress expression of the lethal construct throughout the immature stages). Larvae were provided with Liquifry fish food (Interpret, UK) for the first day of development post-hatching. They were subsequently fed with ground Tetramin® fish food (Tetra, Germany) until pupation, with an increasing daily feeding regime of 0.06, 0.00, 0.08, 0.16, 0.32, 0.32, 0.32, 0.32 and 0.32 mg larva−1. All individuals were sexed manually at the pupal stage, based on size difference, and introduced into rearing cages (30 × 30 × 30 cm) at a 1:2 ratio (male:female) prior to adult emergence. To ensure accuracy in sex sorting of pupae, around 50 pupae of each batch were picked and observed under stereomicroscope for confirmation of sex, based on the shape of the eighth abdominal segment (i.e. pointed in male pupae and blunt in female pupae). Emerged adults were provided with 10% sucrose solution and a damp substrate for laying eggs; in addition, female adults were fed with sheep blood twice per week via a feeding membrane. Eggs were collected once per week, allowing 2 days for embryonic development prior to dry storage under standardised laboratory conditions.
Mating competitiveness
Ae. aegypti is not an indigenous species and is considered to originate from the African continent. It has invaded much of the tropical and subtropical world relatively recently, and a primary objective of this study was to analyse the mating potential of the OX513A strain in comparison with a wild strain of Indian origin. Mating competitiveness experiments were therefore done only between OX513A and the wild strain from New Delhi (DEL). This was the primary objective of the present work, supplemented with life-table studies for both DEL and an additional wild strain from Aurangabad (AWD).
In addition to standardised environmental conditions, equivalent development of individuals is essential to facilitate fair comparisons between adult mating abilities. Therefore, OX513A and DEL strains were simultaneously reared from eggs at equal densities and with equal food regimes to promote synchronised growth. Precautions were taken to ensure virgin insects alone were used for the experiments. Male and female pupae were separated according to size into batches of five individuals and adults allowed to eclose. Following emergence, the sex of each individual was confirmed, and only those batches correctly sexed as pupae were used for the mating competitiveness experiments.
All mating experiments were performed under standardised laboratory conditions (see Section 2.2) in 30 × 30 × 30 cm cages. Experiments were conducted on the fourth day post-emergence (all adults were 3 days old) at a ratio of 5:5:5 (OX513A males:DEL males:DEL females respectively). Five DEL females were introduced into each mating cage and allowed to acclimatise for 1 h. Five male OX513A adults and five male DEL adults were then released simultaneously into the cage to provide equal opportunities for mating. After 24 h, female adults were separated and housed individually in rearing boxes (6.5 cm diameter × 8.0 cm height) and provided with a blood meal and substrate for oviposition. Eggs were allowed to mature prior to hatching, and subsequent fluorescence screening was done during third larval instar. A total of 57 such caged mating experiments were conducted.
Life-table studies
Adult longevity, fecundity, offspring sex ratio and offspring survivorship
Thirty male and 30 female pupae of each strain were separately contained in small rearing cages (16 × 16 × 16 cm). Once emerged, adults were provided with cotton pads constantly saturated in 10% sucrose. On the fourth day post-emergence, male and females of each strain (60 individuals in total) were combined in a cage (30 × 30 × 30 cm) for 24 h to allow sufficient time for mating and successful insemination of females.
Female adults from each mating cage were then isolated in individual rearing boxes (6.5 cm diameter × 8.0 cm height) containing small vessels (2.7 cm diameter × 3.0 cm height) of tap water and a paper strip for oviposition. They were provided with a blood meal until fully engorged following each oviposition, and daily observations of adult survivorship and fecundity were recorded to determine longevity and reproductive parameters. Hatch rate and larval-to-adult survivorship were recorded for eggs from each oviposition event. In order to assess the adult sex ratio, eggs laid from ten individual females for each strain were randomly selected and reared to adults for sex determination.
Male adults of each strain were transferred from the mating cages to individual polypropylene tubes (50 mL capacity), confined with mesh and provided with a cotton pad saturated in 10% sucrose. Sucrose feeders were replaced every other day to prevent fungus development. Dead adults were recorded daily.
Developmental period of immature stages
Eggs were submerged in tap water containing 30 µg mL−1 of tetracycline and placed under low atmospheric pressure to synchronise hatching. Larvae were then transferred individually to plastic cups (8 cm diameter × 3.5 cm height) containing 20 mL of tap water, with 120 replicates for each of the three strains (OX513A, DEL, AWD). Observations of ecdysis were recorded twice daily at fixed times (0900 and 1700) throughout the aquatic developmental period. The larvae were provided with Liquifry for the first day, followed by Tetramin® ad libitum during subsequent days of larval development. Daily observations were done to determine the mean developmental period of each larval instar and pupal period.
Pupal size
Pupae were transferred to a small petri dish that contained a measuring scale and cold water to immobilise the pupae. Images were captured manually using a 14 megapixel digital camera. Pupal sizes based on the width (dorsal view) of the cephalothorax were recorded using Imagej 1.46r software (National Institute for Health, USA).
Population growth parameters
Fecundity, adult female longevity and offspring survivorship (see Section 2.4.1) were used to determine the following population growth parameters.15
The net reproductive rate
where R0 is the average number of female offspring that a female produces during her lifetime, lx is the proportion of females surviving to each age (x) and mx is the average number of female offspring born to the surviving females at each age. Adding the products (lxmx) of all age groups yields the per-generation growth rate of the population under defined conditions. A value of R0 > 1.0 indicates an increasing population; R0 = 1.0 indicates that a population is neither increasing nor decreasing but replacing its number exactly; R0 < 1.0 indicates a decreasing population.
The mean generation time
where Tc is the average interval between the birth of an individual and the birth of its first offspring.
The intrinsic rate of increase
where rm is the rate of increase in populations that reproduce within discrete time intervals and possess generations that do not overlap, and T is time (days).
The doubling time
where Td is the time required for a population growing at a specified rate of increase to double in size.
Statistical analysis
Statistical tests were carried out using IBM SPSS Statistics v.20 (IBM Corporation). The numbers of female adults mated with OX513A males, DEL males and double-mated females (mated with both OX513A and DEL males) were compared by one-way ANOVA using Tukey’s b-test. The chi-square test was applied to test the observed relative mating index against the expected mating index.
The relative mating index was calculated as the number of females mating OX513A males/total number of females whose mating genotype could be determined. If the OX513A males were equally competitive, then this number would on average be 0.5. A statistically significant difference from 0.5 indicates that the OX513A males were either more or less competitive than the competing males.
The relative male mating success takes into account the numbers of progeny produced and was calculated as the proportion of female progeny (fluorescence) sired by each respective genotype.16
Data obtained for longevity and developmental and reproductive parameters were compared by ANOVA for multiple comparisons by Tukey’s test to determine the significance of differences between the three strains (OX513A, DEL, AWD). The proportions of female adult emergence in relation to male adult emergence were analysed for each strain by applying the chi-square test. Male and female adult survivorships were analysed for each strain using the Wilcoxon (Gehan) test.
RESULTS
Mating competitiveness
The cephalothorax measurements (Table 1) of male pupae batches used for the mating competitiveness experiments were not significantly different between OX513A and DEL strains (F = 3.50, P > 0.05).
Table 1.
Mean dorsal cephalothorax widths (mm) of OX513A and wild-type Delhi Ae. aegypti male pupae from mating competition experimentsa
| Dorsal-side thoracic width of male pupae | |||
|---|---|---|---|
| RIDL OX513A strain | WT male | F-value | P-value |
| 1.172 ± 0.009 (n = 49) | 1.142 ± 0.012 (n = 49) | 3.50 | >0.05 |
Mean differences were non-significant at the 0.05 level by one-way ANOVA using the Bonferroni test.
Out of 57 mating cage experiments conducted, 261 female adults survived. Of those, 122 females mated with OX513A males and 113 mated with wild-type DEL males. Twenty-six were double mated, i.e. progeny sired by both OX513A and DEL males, and 24 females (8.4%) were found dead (Table 2). The percentage matings of OX513A, DEL and double matings from the total number of all females recovered were 42.8, 39.6 and 9.1 respectively (Table 2). Calculated values of the relative mating index for female adults mated with OX513A males, DEL males and double mated were found to be 0.47, 0.43 and 0.10 respectively. The relative mating index calculated when excluding females that were double mated yielded values of 0.52 and 0.48 for OX513A and DEL respectively and were not significantly different.
Table 2.
Mating values for OX513A and wild-type Delhi Ae. aegypti male adults when competing to mate with wild-type Delhi Ae. aegypti female adultsa
| Mosquito strains | OX513A ♂ (mean ± SE) | Wild-type Delhi ♂ (mean ± SE) | Double mating (wild-type Delhi and OX513A) ♂ (mean ± SE) | Number of females dead (mean ± SE) | F-value (df) | P-value |
|---|---|---|---|---|---|---|
| Number of female adults mated | 2.14 ± 0.147a (n = 122) | 1.98 ± 0.176a (n = 113) | 0.46 ± 0.109b (n = 26) | 0.42 ± 0.086 (n = 24) | 40.2 (2) | 0.0001 |
| Percentage female adults mated | 42.8 | 39.6 | 9.1 | 8.4 | — | — |
| Relative mating index | 0.47 | 0.43 | 0.10 | — | — | — |
| Relative mating index (excluding double-mated females) | 0.52 | 0.48 | — | — | — | — |
| Observed mating index | Expected mating ratio | Chi-square test (χ2) | P-value | df | — | — |
| 122:113:26 [OX513A ♂:Delhi ♂:double mating (OX513A and DEL ♂)] | 1:1:0 | 2.9 | 0.2345 | 2 | — | — |
Means (±SE) are the results of 57 mating competitiveness experiments between wild-type and OX513A strain male adults with wild-type Delhi strain female adults, conducted at a ratio of 5:5:5 respectively. Figures in parentheses represent percentage female adults mated with male adults of respective strains in the columns. Differences between mean values followed by the same letters within rows are non-significant at the 0.05 level by one-way ANOVA using Tukey’s b-test.
Analysis of mating numbers 122:113:26 (OX513A:DEL:double mated) by the chi-square test against numbers expected for equal mating proportions revealed non-significant deviation at the 0.05 level (χ2 = 0.012, P = 0.9942). Expected progeny ratios were 1:1:0, based on consideration of the fact that female Ae. aegypti are largely monogamous and double mating is rare in wild environments.
The numbers of larvae screened were from single oviposition events (following a blood meal) of individual female adults recovered from mating experiments. The total number of larvae screened for paternity, based on the presence of fluorescence emission, revealed 3086 (49.67%) and 3114 (50.32%) for OX513A and DEL respectively. An additional 600 and 524 (OX513A and DEL respectively) progeny were recorded from double-mated females. Calculation of relative mating success (i.e. total OX513A progeny:total DEL progeny) revealed a ratio of 0.98:1.01 (Table 3).
Table 3.
Total progeny sired and relative mating successes for male OX513A and wild-type Delhi Ae. aegypti adults when competing to mate with wild-type Delhi Ae. aegypti female adults
| Female adults mated by male adults of OX513A or wild-type Delhia | ||||
|---|---|---|---|---|
| Double mating | ||||
| Parameters | OX513A strain | Delhi strain | OX513A strain | Delhi strain |
| Total number of progeny | 3086 (49.67) | 3114 (50.32) | 600 | 524 |
| Relative mating successb of OX513A and wild type (Delhi strain) with respect to total progeny | 0.98 | 1.01 | — | — |
Figures in parentheses are the percentage progeny calculated for single-mated female adults.
Relative mating success is calculated as the ratio of progeny from transgenic OX513A strain male adults to wild-type Delhi strain male adults, and vice versa.
Life-table studies
Adult longevity, fecundity, sex ratio and larval-to-adult survivorship
There were no significant differences in longevities of adult females between strains, with mean survival periods of 49.3 ± 5.6 days for OX513A, 39.3 ± 2.6 days for DEL and 40.9 ± 2.8 days for AWD. Male adult longevity was found to be significantly shorter for OX513A; however, there was no significant difference observed between the wild-type strains. Adult female survival following each oviposition decreased consistently, and survival extended greater than 80 days for some females in all strains (Fig. 1). Male adult survival was significantly shorter for OX513A; variations in female adult survival were not statistically significant (Figs 2a and b).
Figure 1.
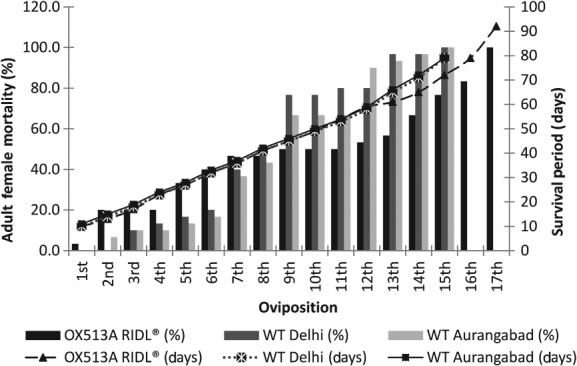
Representation of percentage survival with respect to oviposition and survival (days) for adult females of OX513A and wild-type strains of Ae. aegypti.
Figure 2.
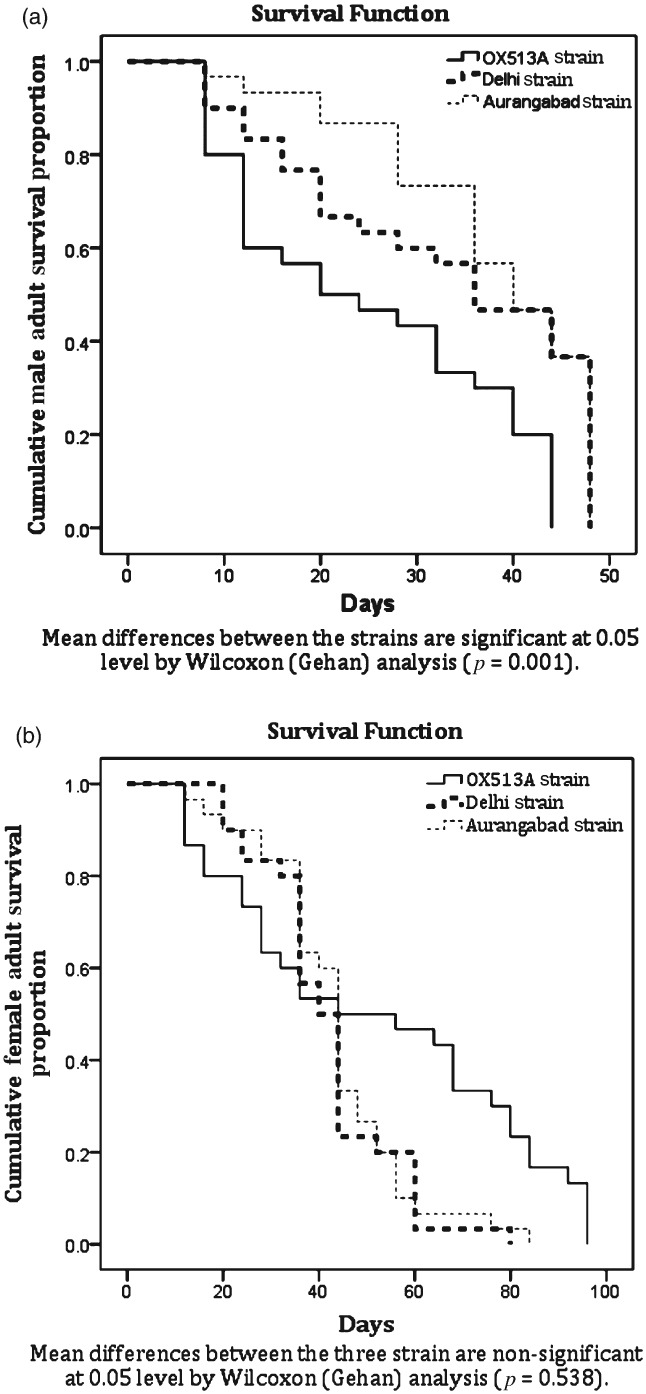
Male (a) and female (b) adult survivorship of OX513A and wild-type strains of Ae. aegypti.
Adult female reproductive parameters were generally similar between the transgenic and wild-type strains (Table 4). The mean number of blood meals taken and the mean number of times that eggs were laid for OX513A, DEL and AWD were found to be 9.5 ± 1.1, 7.6 ± 0.5, 7.5 ± 0.5 and 7.9 ± 0.9, 7.0 ± 0.5, 6.7 ± 0.5 respectively, with no significant differences. The mean numbers of eggs per oviposition per adult female decreased significantly after the sixth, tenth and eighth oviposition events for OX513A, DEL and AWD strains respectively (Table 5). The mean number of eggs laid by OX513A adult females (546.5 ± 66.9) was not statistically different to DEL (499.8 ± 41.8), yet was statistically different to the AWD strain (378.8 ± 32.1) (Table 4).
Table 4.
Reproductive parameters of OX513A and wild-type strains of Ae. aegyptia
| Wild type | |||||
|---|---|---|---|---|---|
| Reproductive parameters | OX513A strain (mean ± SE) | DEL strain (mean ± SE) | AWD strain (mean ± SE) | F-value (df) | P-value |
| Blood meals per female | 9.5 ± 1.1 a | 7.6 ± 0.5 a | 7.5 ± 0.5 a | 1.9 (2) | 0.148 |
| Oviposition events per female | 7.9 ± 0.9 a | 7.0 ± 0.5 a | 6.7 ± 0.5 a | 0.8 (2) | 0.466 |
| Eggs laid per female | 546.5 ± 66.9 b | 499.8 ± 41.8 ab | 378.8 ± 32.1 a | 3.1 (2) | 0.050 |
| Hatch rate (%) | 91.8 ± 1.24 a | 91.7 ± 0.95 a | 94.9 ± 1.09 a | 2.5 (2) | 0.088 |
| Pupation rate (%) | 81.0 ± 3.06 ab | 75.2 ± 3.16 a | 88.0 ± 1.70 b | 5.1 (2) | 0.010 |
| Adult emergence (%) | 79.0 ± 3.13 ab | 73.7 ± 3.20 a | 86.9 ± 1.62 b | 5.3 (2) | 0.009 |
Differences in the mean values indicated by the same letters within rows are non-significant at the 0.05 level by one-way ANOVA using Tukey’s b-test.
Table 5.
Fecundity and survivorship values for OX513A and wild-type Ae. aegypti femalesa
| Wild type | ||||
|---|---|---|---|---|
| Oviposition | Parameters | OX513A strain (mean ± SE) | DEL strain (mean ± SE) | AWD strain (mean ± SE) |
| I | Total eggs laid (no. females) | 2872 (29) | 2481 (30) | 1618 (30) |
| Mean eggs per female | 99 ± 3.8 a | 82.7 ± 4.9 a | 53.9 ± 2.6 ab | |
| Hatch rate (%) | 95.5 | 95.4 | 98.3 | |
| II | Total eggs laid (no. females) | 2166 (24) | 2004 (30) | 1687 (28) |
| Mean eggs per female | 90.3 ± 3.8 a | 66.8 ± 4.3 ab | 60.3 ± 2.9 a | |
| Hatch rate (%) | 93.9 | 90.4 | 97.5 | |
| III | Total eggs laid (no. females) | 1811 (24) | 1945 (27) | 1014 (27) |
| Mean eggs per female | 75.5 ± 5.3 ab | 72.0 ± 4.4 ab | 37.6 ± 5.4 abc | |
| Hatch rate (%) | 95.6 | 93.1 | 97.1 | |
| IV | Total eggs laid (no. females) | 2063 (24) | 1779 (26) | 1706 (27) |
| Mean eggs per female | 86.0 ± 5.4 a | 68.4 ± 3.7 ab | 63.2 ± 3.8 a | |
| Hatch rate (%) | 93.3 | 89.9 | 97.8 | |
| V | Total eggs laid (no. females) | 1512 (20) | 1729 (25) | 1012 (26) |
| Mean eggs per female | 75.6 ± 7.0 ab | 69.2 ± 3.0 ab | 38.9 ± 5.6 abc | |
| Hatch rate (%) | 95.2 | 94.6 | 92.5 | |
| VI | Total eggs laid (no. females) | 1233 (18) | 1688 (24) | 1548 (25) |
| Mean eggs per female | 68.5 ± 5.9 abc | 70.3 ± 3.0 ab | 61.9 ± 5.0 a | |
| Hatch rate (%) | 94.1 | 83.7 | 93.7 | |
| VII | Total eggs laid (no. females) | 777 (16) | 949 (18) | 956 (19) |
| Mean eggs per female | 48.6 ± 6.7 bcd | 52.7 ± 5.5 ab | 50.3 ± 5.7 ab | |
| Hatch rate (%) | 93.2 | 92.5 | 93.9 | |
| VIII | Total eggs laid (no. females) | 659 (16) | 789 (15) | 875 (17) |
| Mean eggs per female | 41.2 ± 6.3 cd | 52.6 ± 8.8 ab | 51.5 ± 5.6 ab | |
| Hatch rate (%) | 90.5 | 93.0 | 93.6 | |
| IX | Total eggs laid (no. females) | 543 (15) | 466 (7) | 220 (10) |
| Mean eggs per female | 36.2 ± 6.9 cd | 66.6 ± 5.2 ab | 22.0 ± 8.4 bc | |
| Hatch rate (%) | 91.4 | 92.1 | 82.7 | |
| X | Total eggs laid (no. females) | 628 (15) | 432 (7) | 365 (10) |
| Mean eggs per female | 41.9 ± 6.7 cd | 61.7 ± 6.4 ab | 36.5 ± 8.1 abc | |
| Hatch rate (%) | 93.6 | 93.0 | 92.9 | |
| XI | Total eggs laid (no. females) | 467 (15) | 280 (6) | 148 (10) |
| Mean eggs per female | 31.1 ± 5.5 d | 46.7 ± 12.3 b | 14.8 ± 6.5 c | |
| Hatch rate (%) | 93.8 | 96.6 | 92.3 | |
| XII | Total eggs laid (no. females) | 441 (14) | 249 (6) | 23 (3) |
| Mean eggs per female | 31.5 ± 5.8 d | 41.5 ± 13.5 b | 7.7 ± 7.6 c | |
| Hatch rate (%) | — | 93.3 | 100.0 | |
| XIII | Total eggs laid (no. females) | 474 (13) | 110 (1) | 142 (2) |
| Mean eggs per female | 36.5 ± 6.7 cd | 110.0 ± 0 | 71.0 ± 24.0 a | |
| Hatch rate (%) | 89.3 | 95.7 | 94.8 | |
| XIV | Total eggs laid (no. females) | 334 (10) | 48 (1) | 15 (1) |
| Mean eggs per female | 33.4 ± 6.4 d | 48.0 ± 0 | 15.0 ± 0 | |
| Hatch rate (%) | 92.9 | 86.7 | 98.5 | |
| XV | Total eggs laid (no. females) | 190 (7) | 46 (1) | 34 (1) |
| Mean eggs per female | 27.1 ± 7.2 d | 46.0 ± 0 | 34.0 ± 0 | |
| Hatch rate (%) | 73.6 | 86.7 | 98.5 | |
| XVI | Total eggs laid (no. females) | 158 (5) | — | — |
| Mean eggs per female | 31.6 ± 10.4 d | — | — | |
| Hatch rate (%) | 92.0 | — | — | |
| XVII | Total eggs laid (no. females) | 66 (4) | — | — |
| Mean eggs per female | 22.0 ± 11.0 d | — | — | |
| Hatch rate (%) | 94.6 | — | — | |
| F-value (df) | 17.5 (16) | 3.5 (11) | 7.3 (12) | |
| P-value | <0.0001 | <0.0001 | <0.0001 | |
Differences in the mean values indicated by the same letters within columns are non-significant at the 0.05 level by one-way ANOVA using Tukey’s b-test.
Percentage hatching, percentage pupation and percentage adult emergence for OX513A were not significantly different from either wild-type strain. No significant differences were observed between the sex ratios of adults or progenies (Table 6).
Table 6.
Mean proportions of emerging adult females relative to males for OX513A and wild-type strains of Ae. aegypti
| Strains | Adult female proportiona (mean ± SE) | χ2 | P-value (df) |
|---|---|---|---|
| OX513A | 0.48 ± 0.03 (n = 594) | 0.198 | 0.999 (9) |
| Wild-type DEL | 0.50 ± 0.02 (n = 551) | 0.061 | 0.999 (8) |
| Wild-type AWD | 0.47 ± 0.02 (n = 460) | 0.099 | 0.999 (9) |
Proportion of female adult emergence was the same with respect to male adults in each strain by the chi-square test. Values in parentheses indicate the total number (n) of adults (male/female).
Developmental period of immature stages
Stage-specific developmental times for OX513A versus wild-type strains revealed significant variations (Table 7). The total larval developmental period for OX513A (8.5 ± 0.05 days) was found to be significantly longer compared with those of DEL and AWD (7.4 ± 0.04 and 7.1 ± 0.03 days respectively, P < 0.0001). The pupal period varied from 2.1 to 2.2 days between wild-type and OX513A strains (P < 0.05). The overall developmental period (first instar to adult emergence) for the OX513A strain was found to be 10.7 ± 0.04 days, and this differed significantly from the DEL and AWD strains which had developmental periods of 9.4 ± 0.04 and 9.1 ± 0.04 days respectively (P < 0.0001). Observations on stage-specific mortalities revealed 18% for OX513A during the second instar. Contrastingly, during the fourth instar, DEL and AWD strains had 8 and 10% mortalities respectively.
Table 7.
Stage-specific developmental periods of OX513A and wild-type strains of Ae. aegyptia
| Wild type (days) | |||||
|---|---|---|---|---|---|
| Developmental period | OX513A strain (days) (mean ± SE) | DEL strain (mean ± SE) | AWD strain (mean ± SE) | F-value (df) | P-value |
| First instar | 2.3 ± 0.01 b (n = 120) | 2.3 ± 0.01 b (n = 120) | 1.9 ± 0.009 a (n = 120) | 447.0 (2) | <0.0001 |
| Second instar | 2.0 ± 0.01 b (n = 98) | 2.0 ± 0.01 b (n = 120) | 1.9 ± 0.01 a (n = 119) | 12.8 (2) | <0.0001 |
| Third instar | 1.7 ± 0.04 b (n = 97) | 1.4 ± 0.00 a (n = 119) | 1.4 ± 0.00 a (n = 118) | 128.4 (2) | <0.0001 |
| Fourth instar | 2.4 ± 0.04 b (n = 96) | 1.8 ± 0.04 a (n = 109) | 1.8 ± 0.03 a (n = 106) | 90.6 (2) | <0.0001 |
| Instars 1 to 4 | 8.5 ± 0.05 c (n = 96) | 7.4 ± 0.04 b (n = 109) | 7.1 ± 0.03 a (n = 106) | 285.2 (2) | <0.0001 |
| Pupa | 2.2 ± 0.02 b (n = 96) | 2.1 ± 0.02 a (n = 109) | 2.1 ± 0.02 a (n = 106) | 4.8 (2) | 0.009 |
| First instar to adult | 10.7 ± 0.04 c (n = 96) | 9.4 ± 0.04 b (n = 109) | 9.1 ± 0.04 a (n = 106) | 374.8 (2) | <0.0001 |
| Adult female lifespan | 49.3 ± 5.6 a (n = 30) | 39.3 ± 2.6 a (n = 30) | 40.9 ± 2.8 a (n = 30) | 1.9 (2) | 0.159 |
| Adult male lifespan | 22.3 ± 2.7 a (n = 30) | 30.8 ± 2.8 b (n = 30) | 35.5 ± 2.0 b (n = 30) | 7.1 (2) | 0.001 |
Differences in the mean values indicated by the same letters within rows are non-significant at the 0.05 level by one-way ANOVA using Tukey’s b-test. Figures in the parentheses indicate the number (n) of individuals tested.
Pupal size
Observations on pupal size, based on the dorsal cephalothorax width, revealed no significant difference in male pupal sizes between the transgenic and wild-type strains; however, female OX513A pupae were slightly but significantly larger compared with female pupae of both wild-type strains (Table 8).
Table 8.
Dorsal cephalothorax widths (mm) of OX513A and wild-type Delhi Ae. aegypti male and female pupae for life-table studies
| Mean cephalothorax widths (±SE) | ||
|---|---|---|
| Strains | ♂ | ♀ |
| OX 513A | 0.923 ± 0.016 a (n = 17) | 1.251 ± 0.019 a (n = 12) |
| Wild-type DEL | 0.942 ± 0.013 a (n = 14) | 1.162 ± 0.025 b (n = 14) |
| Wild-type AWD | 0.902 ± 0.008 a (n = 18) | 1.171 ± 0.015 b (n = 16) |
| F-value (df) | 2.17 (2) | 5.29 (2) |
| P-value | 0.1248 | 0.0092 |
Differences in the mean values indicated by the same letters within columns are non-significant at the 0.05 level by one-way ANOVA using Tukey’s b-test.
Population growth parameters
All population growth parameters (net reproductive rate, mean generation time, intrinsic rate of increase and doubling time) were similar, except for the doubling time of AWD, which was slightly but significantly higher (F = 5.72, P < 0.005) compared with the OX513A and DEL strains (Table 9). Although the reproductive rate for AWD was lower than for the OX513A and DEL strains, differences were not statistically significant. Mean generation times and intrinsic rates of increase for all three strains also did not show statistically significant differences (Table 9).
Table 9.
Mean demographic growth parameters for OX513A and wild-type strains of Ae. aegyptia
| Strains | Net reproductive rateb R0 = Σ lxmx (mean ± SE) | Mean generation timec Tc = Σ xlxmx/R0 (mean ± SE) | Intrinsic rate of increasec rm = ln R0/T (mean ± SE) | Doubling timec Td = ln 2/rm (mean ± SE) |
|---|---|---|---|---|
| OX513A | 362.8 ± 38.3 a | 19.43 ± 1.4 a | 0.300 ± 0.017 a | 2.30 ± 0.13 a |
| Wild-type DEL | 365.9 ± 22.8 a | 20.26 ± 0.7 a | 0.298 ± 0.009 a | 2.40 ± 0.07 a |
| Wild-type AWD | 293.3 ± 20.8 a | 22.26 ± 0.8 a | 0.259 ± 0.008 a | 2.75 ± 0.07 b |
| F-value (df) | 2.08 (2) | 1.87 (2) | 3.49 (2) | 5.72 (2) |
| P-value | 0.130 | 0.160 | 0.035 | 0.005 |
Differences in the mean values indicated by the same letters within columns are non-significant at the 0.05 level by one-way ANOVA using Tukey’s b-test.
Number of offspring.
Number of days.
DISCUSSION
The potential of transgenic mosquitoes to combat specific pest species and their associated pathogen transmission has been the subject of much debate.16–19 Laboratory and field-cage data may not be perfectly predictive owing to differences in environmental conditions; for example, the adult lifespans observed here are far higher than expected under more challenging conditions in the field. Nonetheless, it is widely recognised that transition to the field benefits from prior laboratory evaluation of parameters with relevance to field efficacy.20–22 Several successful field trials have now been conducted with transgenic mosquitoes,8,23,24 and, although translation of previously acquired laboratory data to those field settings has not been possible, their value as indicators of key performance parameters remains worthwhile. This study contributes to such assessment with data pertinent to deployment within Indian environments.
The caged mating experiments between OX513A and DEL males revealed that they were equally competitive under laboratory conditions. 9.1% of females produced offspring of both genotypes, indicating double mating; as only those females that mate males of different genotypes are detected as double mating by this approach, the best estimate of the multiple mating rate is approximately 18% (assuming no changed bias as to male genotype on remating, and that females rarely mate more than twice). This figure may not be representative of double-mating frequencies in open environments, as the restriction of adults in cages could have influenced courtship behaviours and/or rates of copulation.25–27 The phenomenon of single mating has been documented and is thought to be a consequence of a substance termed ‘matrone’, which is transferred in semen by male adults and renders female adults unreceptive to second matings and refractory to further copulation.28
Under these laboratory conditions, the proportional mating successes of OX513A and DEL male adults with respect to total progeny were found to be similar. Under semi-field conditions in Malaysia, OX513A males were previously found to compete equally against males from a strain of Malaysian origin.29 It should be noted that the results presented do not necessarily reflect mating following OX513A male releases in open environments, where a wider range of characteristics (e.g. dispersal, mate seeking and mate recognition) may play more significant roles.
Under the conditions of the present study, any inherent differences between these strains as a result of factors such as transgene insertion or prior geographical isolation of the background genotypes did not manifest themselves by hindering mating compatibility; given an equal choice of both male adults (OX513A and DEL), DEL females did not discriminate against either strain.
Fitness assessments between strains were largely similar for parameters relating to adult longevity, reproduction and population growth. Developmental periods were found to be somewhat longer for OX513A compared with the wild-type strains; the relative contributions to this effect of the transgene, of strain background and of environmental effects (e.g. presence of tetracycline) were not determined. Perhaps unsurprisingly there are reports of transgenic insects intrinsically burdened with substantial costs to fitness,12,15 but also several reports of transgenic insects where few or no significant fitness costs were observed.30–33 However, earlier life-table studies for the OX513A strain in comparison with wild-type Malaysian Ae. aegypti reported similarity for several parameters involving pre-oviposition period, lifetime fecundity, offspring sex ratio and female sterility.34 In addition, three transgenic strains of Anopheles stephensi (Liston) were shown to have low fitness loads, indicating that fitness should be assessed for each strain of transformed insect.16 OX513A has also previously been studied for life-history characteristics in comparison with an unmodified laboratory-adapted Malaysian strain. Parameters including larval mortality, developmental rate (i.e. time to pupation), adult size and longevity revealed a 5% lower larval survival as well as reduced adult longevity for OX513A compared with the unmodified counterpart.35
When OX513A is being used for its intended purpose, i.e. suppression of wild mosquito populations, the practical consequences of specific fitness traits are not necessarily without contradiction. For example, in the present study, the longevity of male adults was found to be significantly shorter than that of both Indian wild-type comparators, and if this also translated to field environments it would have implications for both performance of the strain and persistence of the transgene. Decreased longevity may negatively influence mating opportunities and/or mating capacity, potentially reducing field performance of OX513A males. Correspondingly, decreased longevity could reduce persistence of the transgene and result in selection against released males in an open environment, thereby offering safeguards that may be perceived as a significant biosafety benefit. It should be noted that, after reaching adulthood, the lack of exposure to the lethal transgene repressor tetracycline may contribute to a reduced lifespan in OX513A relative to wild type.35,36 Understanding how insect transformation technologies can affect fitness at these different levels may in the future improve the ability to rationally design competitive transgenic insects.37
In this study, the transgenic OX513A strain of Ae. aegypti was similar to wild-type populations that were recently collected from two geographically distinct Indian regions for a range of key developmental, reproductive and population growth parameters relevant to field performance. As such it is a suitable candidate with which to continue a phased evaluation towards open release within Indian environments.
Acknowledgments
The authors would like to thank Oxitec colleagues Sian Morgan, Zoe Curtis and Kelly Matzen for the prior validation of experimental protocols and constructive discussion throughout. They would especially like to thank the Institutional Biosafety Committee (IBSC) members of GBIT – Sarala K Subbarao, Vijay Veer, Anant Pandhare, Girish Rao, Bharat Char and Vinay Shenoy – for their suggestions and productive views.
REFERENCES
- Gubler DJ. Epidemic dengue/dengue haemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:2. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Pialoux G, Gaüzère B-A, Jauréguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Tabachnick WJ, Wallis GP, Aitken THG, Miller BR, Amato GD, Lorenz L, et al. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am J Trop Med Hyg. 1985;34(6):1219–1224. doi: 10.4269/ajtmh.1985.34.1219. [DOI] [PubMed] [Google Scholar]
- Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerging Themes Epidemiol. 2005;2(1):1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, McKemey A, Nimmo D, Oveido MN, Lacroix R, Matzen K, et al. Genetic control of Aedes mosquitoes. Path Glob Hlth. 2013;107:170–179. doi: 10.1179/2047773213Y.0000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol. 2012;30(9):828–830. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- Dame DA, Curtis CF, Benedict MQ, Robinson AS, Knols BGJ. Historical applications of induced sterilisation in field populations of mosquitoes. Malar J. 2009;8(Suppl 2):S2. doi: 10.1186/1475-2875-8-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, Benedict MQ, Bellini R, Clark GG, Dame DA, Service MW, et al. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonot Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, et al. Late-acting dominant lethal genetic systems and mosquito control. BioMed Cent Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catteruccia F, Godfray HCJ, Crisanti A. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299:1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- Scott TW, Rasgon JL, Black WCIV, Gould F. Fitness studies: developing a consensus methodology. In: Wageningen UR, editor; Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Dordrecht, The Netherlands: Frontis Series, Springer; 2005. pp. 171–181. ed. by, Ch. 16) [Google Scholar]
- Arthropod containment levels. Vector Borne Zoonot Dis. 2003;3:75–90. [Google Scholar]
- Irvin N, Hoddle MS, O’Brochta DA, Carey B, Atkinson PW. Assessing fitness costs for transgenic Aedes aegypti expressing the GFP marker and transposase genes. PNAS. 2004;101(3):891–896. doi: 10.1073/pnas.0305511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Marrelli MT, Yan G, Jacobs-Lorena M. Fitness of transgenic Anopheles stephensi mosquitoes expressing the SMI peptide under the control of a vitellogenin promoter. J Hered. 2008;99(3):275–282. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CF. Measuring public-health outcomes of release of transgenic mosquitoes. In: Takken W, Scott TW, editors. Ecological Aspects for Application of Genetically Modified Mosquitoes. Dordrecht, The Netherlands: Wageningen UR Frontis Series, Springer; 2003. p. 244. ed. by, pp. ( ) [Google Scholar]
- Sperança MA, Capurro ML. Perspectives in the control of infectious diseases by transgenic mosquitoes in the post-genomic era: a review. Mems Inst Oswaldo Cruz. 2007;102(4):425–433. doi: 10.1590/s0074-02762007005000054. [DOI] [PubMed] [Google Scholar]
- Stone CM. Transient population dynamics of mosquitoes during sterile male releases: modelling mating behaviour and perturbations of life history parameters. PLoS ONE. 2013;8(9):e76228. doi: 10.1371/journal.pone.0076228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CF, Sinkins SP. Wolbachia as a possible means of driving genes into populations. Parasitol. 1998;116(Suppl):S111–S115. doi: 10.1017/s0031182000084997. [DOI] [PubMed] [Google Scholar]
- Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nat Rev Genet. 2006;7:427–435. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- Huang Y, Magori K, Lloyd AL, Gould F. Introducing desirable transgenes into insect populations using Y-linked meiotic drive – a theoretical assessment. Evolution. 2007;61(4):717–726. doi: 10.1111/j.1558-5646.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- Harris AF, Nimmo D, McKemey AR, Kelly N, Scaife S, Donnelly CA, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. 2011;29:1034–1037. doi: 10.1038/nbt.2019. [DOI] [PubMed] [Google Scholar]
- Lacroix R, McKemey AR, Norzahira R, Lim KW, Wong HM, Teoh GN, et al. Open field release of genetically engineered sterile male Aedes aegypti in Malaysia. PLoS ONE. 2012;7(8):e42771. doi: 10.1371/journal.pone.0042771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadz RW, Craig GB., Jr Female polygamy due to inadequate semen transfer in Aedes aegypti. Mosq News. 1970;30:355–360. [Google Scholar]
- Williams RW, Berger A. The relation of female polygamy to gonotrophic activity in the ROCK strain of Aedes aegypti. Mosq News. 1980;40:597–604. [Google Scholar]
- Yound ADM, Downe AER. Renewal of sexual receptivity in mated female mosquitoes. Aedes aegypti. Physiol Entomol. 1982;7:467–471. [Google Scholar]
- Craig GB., Jr Mosquitoes: female monogamy induced by male accessory gland substance. Science. 1967;156(781):1499–1501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- Lee HL, Vasan SS, Ahmad NW, Idris I, Hanum N, Selvi S, et al. Mating compatibility and competitiveness of transgenic and wild type Aedes aegypti (L.) under contained semi-field conditions. Transgen Res. 2013;22(1):47–57. doi: 10.1007/s11248-012-9625-z. [DOI] [PubMed] [Google Scholar]
- Allen M, Berkebile D, Skoda S. Postlarval fitness of transgenic strains of Cochliomyia hominivorax (Diptera: Calliphoridae) J Econ Entomol. 2004;97:1181–1185. doi: 10.1603/0022-0493(2004)097[1181:pfotso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Isaacs AT, Jasinskiene N, Tretiakov M, Thiery I, Zettor A, Bourgouin C, et al. Transgenic Anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. Proc Natl Acad Sci USA. 2012;109:E1922–E1930. doi: 10.1073/pnas.1207738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci USA. 2007;104:5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Kizito C, Rasgon JL, Jacobs-Lorena M. Transgenic mosquitoes expressing a phospholipase a2 gene have a fitness advantage when fed Plasmodium falciparum-infected blood. PLoS ONE. 2013;8:e76097. doi: 10.1371/journal.pone.0076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Joko H, Nazni WA, Vasan SS. Comparative life parameters of transgenic and wild strain of Aedes aegypti in the laboratory. Dengue Bull. 2009;33:103–114. [Google Scholar]
- Bargielowski I, Nimmo D, Alphey L, Koella JC. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Ae. aegypti. PLoS ONE. 2011;6(6):1–7. doi: 10.1371/journal.pone.0020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massonnet-Bruneel B, Corre-Catelin N, Lacroix R, Lees RS, Hoang KP, Nimmo D, et al. Fitness of transgenic mosquito Aedes aegypti males carrying a dominant lethal genetic system. PLoS ONE. 2013;8:e62711. doi: 10.1371/journal.pone.0062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW. Current thoughts about the integration of field and laboratory sciences in genetic control of disease vectors. In: Knols BGJ, Louis C, editors. Strategic Plan to Bridge Laboratory and Field Research in Disease Vector Control. Dordrecht, The Netherlands: 2005. pp. 67–76. ed. by. Wageningen UR Frontis Series, Springer) [Google Scholar]


