Abstract
Growing season conditions are widely recognized as the main driver for tundra shrub radial growth, but the effects of winter warming and snow remain an open question. Here, we present a more than 100 years long Betula nana ring-width chronology from Disko Island in western Greenland that demonstrates a highly significant and positive growth response to both summer and winter air temperatures during the past century. The importance of winter temperatures for Betula nana growth is especially pronounced during the periods from 1910–1930 to 1990–2011 that were dominated by significant winter warming. To explain the strong winter importance on growth, we assessed the importance of different environmental factors using site-specific measurements from 1991 to 2011 of soil temperatures, sea ice coverage, precipitation and snow depths. The results show a strong positive growth response to the amount of thawing and growing degree-days as well as to winter and spring soil temperatures. In addition to these direct effects, a strong negative growth response to sea ice extent was identified, indicating a possible link between local sea ice conditions, local climate variations and Betula nana growth rates. Data also reveal a clear shift within the last 20 years from a period with thick snow depths (1991–1996) and a positive effect on Betula nana radial growth, to a period (1997–2011) with generally very shallow snow depths and no significant growth response towards snow. During this period, winter and spring soil temperatures have increased significantly suggesting that the most recent increase in Betula nana radial growth is primarily triggered by warmer winter and spring air temperatures causing earlier snowmelt that allows the soils to drain and warm quicker. The presented results may help to explain the recently observed ‘greening of the Arctic’ which may further accelerate in future years due to both direct and indirect effects of winter warming.
Keywords: Arctic, Betula nana, dendrochronology, Greenland, shrub expansion, winter warming
Introduction
Air temperatures across the Arctic have increased markedly during the past 20 years (IPCC, 2013). Predictions of the future climate suggest that air temperatures will continue to increase, not the least during the winter season, leading to changes in snow depth and duration of snow cover (Serreze et al., 2000; Serreze & Francis, 2006; IPCC, 2013). The recent warming has increased productivity and triggered an overall greening of the Arctic (Tape et al., 2006; Epstein et al., 2012; Henry et al., 2012; Macias-Fauria et al., 2012), impacting the surface energy balance (Sturm et al., 2005a,b), the nutrient cycling and net carbon balance (Elmendorf et al., 2012a,b). The conventional explanation for this ‘greening’ is improved growing season conditions, but it has been pointed out that winter biological processes also play crucial roles with negative as well as positive effects on plant growth (Sturm et al., 2005a,b; Schmidt et al., 2006; Hallinger et al., 2010). Positive effects on shrubs growth have been described in snow fence experiments and linked to increasing nutrient availability due to enhanced decomposition during the warmer snow-protected winter months (Nobrega & Grogan, 2007; Semenchuk et al., 2015). But so far the net effects of winter conditions on annual radial growth of Arctic shrubs are uncertain.
Betula nana (dwarf birch) is one of the circumpolar Arctic shrubs that play a major role in the ‘greening of the Arctic’ and for which growth has been shown to react positively to both experimental summer warming and fertilization (Bret-Harte et al., 2001; Van Wijk et al., 2003; Wahren et al., 2005), but so far not to winter warming. Recent studies on foliar carbon cycling in Betula nana growth under long-term warming and fertilization treatments at Toolik Lake (Alaska) reveal as follows: (i) increased concentration of N and P in leaves; (ii) near-complete dominance of Betula nana at community scale; (iii) the highest (among studied species) rates of photosynthesis; and (iv) increased carbon gain efficiency (Heskel et al., 2013). This suggests a physiological advantage of Betula nana, which under continuous warming may lead to a possible dominance over other tundra species and a resulting increase in carbon accumulation (Bret-Harte et al., 2001; Deslippe & Simard, 2011; Deslippe et al., 2011; Henry et al., 2012).
Climate–growth response studies of the Arctic tundra plants require long-term observations and homogeneous data sets from the nearby climatic stations. Few Arctic sites have climate records that represent the past 100 years, but the record of air temperature observations from Ilulissat in western Greenland, starting in 1873, is among the few. The record correlates well with air temperature observations in the region, including 1991–2004 observations from our study site Arctic Station at Disko Island, 60 km west of Ilulissat (Hansen et al., 2006). Changes and trends in this long temperature record have been analysed previously, and five distinct trend periods have been identified (Putnins, 1970; Cappelen et al., 2001; Box, 2002): 1873–1895, 1896–1909, 1910–1930, 1931–1960 and 1961–1990 (Fig.1). The most consistent period was 1910–1930 where the mean winter temperature increased by 0.3 °C year−1 without a corresponding summer warming.
Fig. 1.
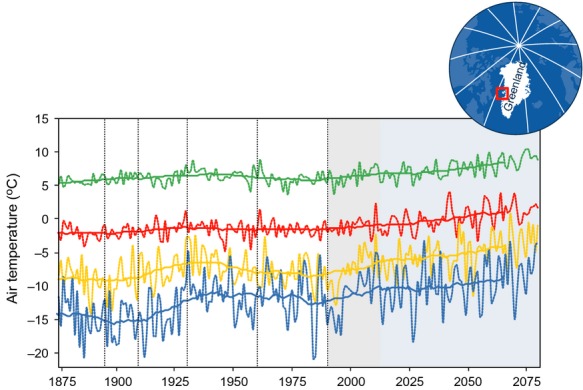
Seasonal air temperatures from Arctic Station (Disko Island, western Greenland) combined from historical climate data (1873–1992), recent observations (1991–2011) (shaded in grey) and regional climate model simulations (2012–2080). The historical data are based on data from Ilulissat and the future predictions (shaded in blue) are derived from the Danish Meteorological Institute's HIRHAM4 model downscaled for use in the Disko region. The scenario presented is based on a 2.4 °C warming over a 70-year period. Temperatures are shown for the winter (blue), spring (yellow), summer (green) and autumn (red), and the five distinct temperature trend periods that have been identified from 1873 to 1990 are separated with vertical dotted lines.
Typically, the effects of winter warming on shrub growth have been studied through primary growth or experimental treatment studies (warming and/or fertilizing) which are very useful to assess plant responses to climatic changes over short observational periods. However, dendroclimatological analyses provide an opportunity to link secondary growth of shrub with interannual climate variability over longer timescales (i.e. Forbes et al., 2010; Hallinger et al., 2010; Blok et al., 2011; Jørgensen et al., 2014). This study investigates the impact of winter conditions on the annual ring-width growth (i.e. radial growth) of Betula nana at a high intraplant resolution and over a century-long scale. To do so we: (i) investigate the climatic conditions in southern Disko in western Greenland and evaluate recent climatic trends (ii) develop a Betula nana ring-width chronology for southern Disko in Western Greenland, (iii) investigate climate–growth relationships for the last century, especially focusing on the influence of winter air temperatures on Betula nana radial growth and (iv) investigate the importance of soil temperatures, sea ice and snow depths on Betula nana radial growth during the last two decades.
Materials and methods
Study site
Arctic Station was established in 1906 by M.P Porsild near the Greenlandic settlement Qeqertarsuaq on Disko in western Greenland (69o15′N, 53o31′W) due to the high plant diversity in the area (Fig. S1 ). The site is located in the erect dwarf-shrub tundra zone (Walker et al., 2002) and is exposed to an Arctic maritime climate. The area is dominated by a low bench of bedrock (gneiss) and Tertiary breccia and plateau lava. To the North, the terrain rises gently to 200 m above sea level about 1.5 km from the coast. Further away steep mountain slopes reach 600–800 m extending east and west.
Meteorological data and sea ice observations
Arctic Stations meteorological station was established in 1990 about 300 m from the coast and 20 m above sea level (m a.s.l). Measurements include wind speed, wind direction, precipitation (rain), air temperatures and incoming and outgoing solar radiation. Ground temperatures are measured 5, 60 and 175 cm below the surface in Early Holocene marine stratified sediments. All sensors are connected to an Aanderaa datalogger programmed to log every 30 min. Annual values (1991–2004) have been reported by Hansen et al. (2006). The meteorological station has experienced several short breakdowns during its lifetime, and from July 2002 to June 2003, a long period of breakdown and unstable measurements was experienced. Furthermore, precipitation measurements are lacking 7 of the 21 observations years. Data from a nearby meteorological station (1 km away) run by Asiaq (available from: http://www.asiaq.gl) have been used to replace minor data gaps using linear correlation analyses between the two stations.
In addition to the automatic meteorological measurements, measurements of snow depths and visual observations of sea ice coverage to the South have been made by the different station managers from 1991 and onward. The sea ice cover has been grouped in intervals of ‘no ice’, 0–25%, 26–50%, 51–75% and 76–100% (Hansen et al., 2006).
Climate record
Monthly mean temperatures from Arctic Station and the station in Ilulissat (1992–2012) were used to calculate linear regression equations between the two stations. The regression equations were subsequently used to calculate mean monthly air temperatures for Arctic Station for the period 1873–1991. The climate record constructed in this study is a continuation of the work made by Hansen et al. (2006) where 1991–2004 observations from Arctic Station were used to reconstruct monthly air temperatures from 1873 to 1991. Furthermore, modelled precipitation data from the NOAA (1901–2010) were used to investigate the influence of variations in precipitation on Betula nana radial growth (Schneider et al., 2011).
Dendrochronological survey
The dendrochronological survey followed the serial sectioning approach proposed by Kolishchuk (1990), which consists of repeated tree-ring width measurements and crossdating at intraplant level (Buchwal et al., 2013). Within 5 km from Arctic Station, 15 intact Betula nana individuals were collected in early August 2011 from a stable site that represented semi-erect shrub tundra conditions. The sampling only considered isolated, mature and healthy individuals to avoid competition-influenced plant structures. Although a confined number of shrubs were taken from the field, each individual was carefully inspected and sectioning was performed for both below-ground and above-ground plant segments (Fig. S2). In total, 135 cross sections were analysed from the main root and below-ground shrub compartments (41% of sections) and from stems and branches (59% of sections). The number of cross sections taken per shrub depended on plant size and morphology. On average, nine cross sections per individual were inspected but with a variation from 1 to 19 cross sections per shrub. Cross sections were collected along the main growth axis, including main roots and up along the stem and a maximum of two main branches. This approach ensured the accuracy of the ring-width dating through detection of commonly formed partially (i.e. wedging rings) and completely missing rings (Buchwal et al., 2013) and improved the common signal and correlation between individual growth series.
Thin sections of ∼15–20 μm thick were obtained using the GLS-1 sledge microtome, stained with a mixture of Safranin and Astra blue, and permanently fixed on microslides with Canada balsam (Schweingruber et al., 2011). Images were captured using a digital camera (CS30, Olympus) connected to a microscope (Olympus BX41) under 40–100 magnification. Images of a single cross section were merged in ADOBE PHOTO SHOP CS2 (Adobe Systems Incorporated, San Jose, CA, USA). Annual ring widths (RW) were measured using manual path analyses mode in the wincell software (Regent Instruments Inc, Quebec, Canada). Growth rings (i.e. ring width) were measured within each cross section along two to three radii. Altogether, detection of growth rings, missing and partially missing rings was conducted along 294 radii. Radial measurements along chosen radii were supplemented by careful visual inspection of each cross section to eliminate annual growth underestimation caused by partially missing rings, that is incomplete growth rings formed due to failure of cambial activity. The principal three stages of crossdating were based on visual comparison of growth curves (i) between 2–3 radii measured within a single cross section, (ii) among the mean growth curves of all the sections within individual shrubs and finally (iii) between the mean growth curves of all shrubs. Alignment of growth curves was based on comparison of positive and negative pointer years (Schweingruber et al., 1990). The output of visual crossdating was statistically checked in COFECHA (Holmes, 1983).
Within all 135 cross sections, it was not possible to successfully crossdate eight cross sections, mostly showing growth suppression in the above-ground shrub parts. Additionally, 11 sections were eliminated from the final data set because of their low (i.e. <0.3) correlations with the master chronology.
The raw ring-width measurements were standardized to remove a nonclimatic variance from the time series (Cook et al., 1990). Although there were no evidences of an unequivocal age trend presence in the raw Betula nana chronology, detrending was executed to account for (i) autocorrelation in the annual growth series, (ii) competition effects and (iii) a possible age trend (not visible in a raw series) superimposed by the climatic signal, that is enhanced growth over time as a result of a warming climate. Three methods of detrending of Betula nana chronology were tested using the dplR package in r (Bunn, 2008). Initially, a linear trend in the mean raw growth series was removed by subtracting a least-squares-fit straight line. Secondly, a cubic smoothing spline was used to fit and remove a nonlinear trend, which was observed in our ring-width chronology. Thirdly, a horizontal line was fitted to the raw growth series. Each detrended chronology was tested in the climate–growth relationship analyses to confirm the stability of the temperature signal obtained while using the raw (i.e. undetrended) series. Finally, the cubic smooth spline method was determined to be the most flexible and representative method for removing a possible trend in our growth rings data and further to be used for a comparable climate–growth relationship analyses. A relative flexible smooth spline function with 50% frequency response at 32 years was used to remove low-frequency variability (i.e. any possible biological effects) and act as a final standardized Betula nana ring-width chronology in our study (Fig.2).
Fig. 2.
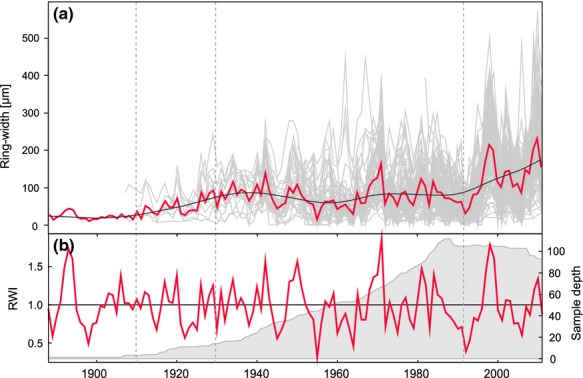
(a) Betula nana ring-width chronology (1888–2011) for southern Disko Island, western Greenland (red bold line) with fitted smoothing spline (black line), individual ring-width series (grey lines) based on three-step crossdating of 15 shrubs and 116 cross sections. (b) Standardized Betula nana chronology (RWI – ring-width index) after fitting a 32-year smoothing spline. The sample depth expresses a total number of cross section. Note the decreasing number of cross sections in the recent years due to the outermost rings missing in some individuals, both in below- and above-ground shrub parts.
The strength of the common signal of both detrended and undetrended chronologies was quantified using the mean correlation between the time series (Rbt) (Briffa & Jones, 1990) and the expressed population signal (EPS) (Wigley et al., 1984).
Climate–growth relationships
The exploration of climate–growth relationships for the Betula nana chronology was conducted in three steps using three complementary methods. First, the overall linear dependences between the available climatic variables from the study area and the Betula nana chronology were investigated. This was carried out by computing Pearson's correlation coefficients between raw (i.e. undetrended) and detrended (i.e. standardized) chronologies and monthly air temperatures (1910–2011) and precipitation rates (1910–2010) including prior dormant season (starting from previous November) and following year conditions (from January to September). Additionally for air temperature, two seasonal averages for winter (previous December and current January) and summer (current June and July) were used. The climate–growth relationship analyses were performed for both a century-long period as well as for five distinct climatic trend periods which have been identified previously (Putnins, 1970; Cappelen et al., 2001; Box, 2002). Also Pearson's correlation coefficients were calculated between detrended Betula nana chronologies and standardized air temperature data for last century. Climate data were detrended by fitting a 30-year smooth spline into a monthly average temperature records from Arctic Station. Subsequently, linear correlations were made between the raw and the standardized Betula nana chronology and 1991–2011 soil temperatures, snow depth, growing degree-days (GDD), thawing degree-days (TDD) and sea ice cover. All climate–growth relationships were performed using r software. Secondly, bootstrapped response coefficients (BRC) were calculated between monthly climatic variables (including previous and current growing season conditions) and the Betula nana chronology (raw series) to test the overall stability and significance of the obtained summer and winter climate–growth relationships. Finally, moving correlation functions (MCF) were used between the Betula nana chronology (raw series) and air temperatures (1910–2011) and precipitation rates (1910–2010) to test the seasonal variation of the climatic signal reflected in the Betula nana growth rings. The MCF were computed using a 30-year moving window, offset by 1 year, which produced a temporal set of coefficients for each monthly predictor. The analyses were conducted in R using the bootRes (Zang & Biondi, 2013) and treeclim package (Zang & Biondi, 2015). The significance of the response (BRC) and correlation (MCF) coefficients was calculated based on 1000 bootstrapped iterations, obtained by random extraction with replacement from the initial data set (Guiot, 1991).
Results
Meteorological measurements and sea ice observations
The mean annual air temperature for the period 1991–2011 was −3.0 °C ± 1.8 °C (± 1 SD) (Fig.3a). The warmest month was July with a mean temperature of 7.9 °C ± 1.6 °C, and the coldest month was March with a mean of −14.0 °C ± 5.0 °C. Air temperatures ranged from an absolute maximum of 21.9 °C (June 23, 2003) to a minimum of −32.9 °C (March 22, 1996). During the observation period, the mean annual air temperature at the station increased by 0.2 °C year−1 but with a strong seasonal variation, as winter temperatures (Dec to Feb) increased by 0.4 °C year−1, spring temperatures (March to May) by 0.3 °C year−1, summer temperatures (June to Aug) by 0.1 °C year−1 and autumn temperatures by 0.1 °C year−1 (Fig.3a). When comparing the first 10 years (1991–2000) with the last 10 years (2002–2011), the mean annual number of freezing degree-days (FDD) decreased from 2240 to 1585 FDD and the number of thawing (TDD) and growing degree-days (GDD) increased from 680 to 912 TDD and from 160 to 284 GDD (Fig.3b).
Fig. 3.
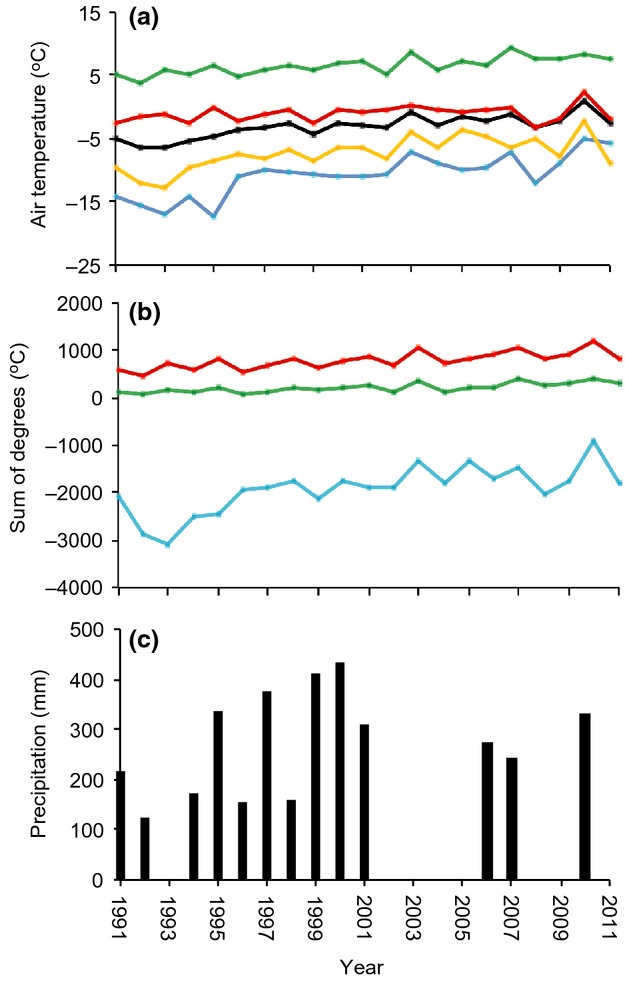
(a) Mean annual air temperatures and seasonal temperature trends at Arctic Station (Disko Island, western Greenland) from 1991 to 2011. Temperatures are shown for the whole year (black), the winter (blue), spring (yellow), summer (green) and autumn (red). (b) Freezing degree-days <0 °C (blue), thawing degree-days >0 °C (red) and growing degree-days >5 °C (green) at Arctic Station. (c) Annual precipitation rates measured near Arctic Station.
The mean annual amount of rain measured from 1994 to 2006 was 273 mm ± 100 mm (Fig.3c), with September (63 mm) being the wettest and March the driest month (15 mm). At Arctic Station, ∼60% of the total precipitation has been estimated to fall as rain (Hansen et al., 2006) and hence the mean annual amount precipitation (rain and snow) is estimated to be ∼400 mm. As precipitation data are lacking in part of the observation period, it was not possible to report the overall trends in precipitation from 1991 to 2011.
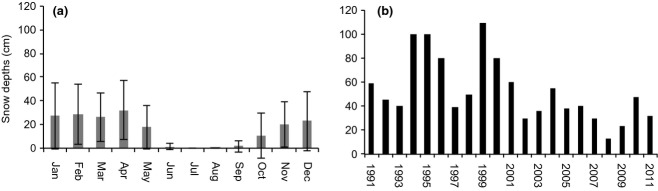
The soil was covered with snow from October to May with an average snow cover of 16 cm (Fig.4a). There was a large year-to-year variation in both the timing of the snow cover and in the snow thickness and a general trend towards a decrease in the maximum snow depth in recent years (Fig.4b). Furthermore, there has been an increase in the duration of the snow-free period (SFP) from 120 days the first 10 years (1991–2000) to 145 days the last 10 years (2002–2011), and the onset of the SFP overall has shifted from the beginning of June to mid/early May (Fig. S3).
Fig. 4.
Snow depths measured at Arctic Station (Disko Island, western Greenland) for the period 1991–2011 (a) mean monthly snow depths (line with error bars indicating ± 1 SD) and (b) maximum yearly snow depth.
Temperature readings from the meteorological station show average soil temperatures of −0.9, −0.4 and −0.5 °C at depths 5, 60 and 175 cm, respectively (Fig. S4a). Throughout the observation period, temperatures above 0 °C were recorded at all depths confirming that the active layer depth in the coarse marine stratified sediments at Arctic Station is deeper than 175 cm. Soil temperatures have been following the same increasing trend as air temperatures both on an annual and seasonal scale (Fig. S4b). Correlation analyses show that soil temperature in the upper part of the soil is positively related to air temperatures (Fig. S5) but not to snow cover (Fig. S6). The results also show that winter soil temperatures in recent years have become more directly linked to air temperatures as a consequence of the decreasing snow depths (Fig. S7). When comparing the first 10 years (1991–2000) to the last 10 years (2002–2011), it is also clear that winter and spring soil temperatures have become markedly warmer despite of less snow (Fig. S8).
In average, a sea ice cover of more than 50% was observed for 95 days each winter (1991–2011). The longest period with a sea ice cover below 50% was in 1993 with 167 days and the shortest in 2010 with 0 days. From 1991 to 2011, the sea ice cover was roughly reduced by 50% (Fig.5) with a tendency of the sea ice being formed later and disappearing earlier in the season. There was a negative correlation between winter air temperatures (December–February) and sea ice cover (r = −0.65, P < 0.05).
Fig. 5.
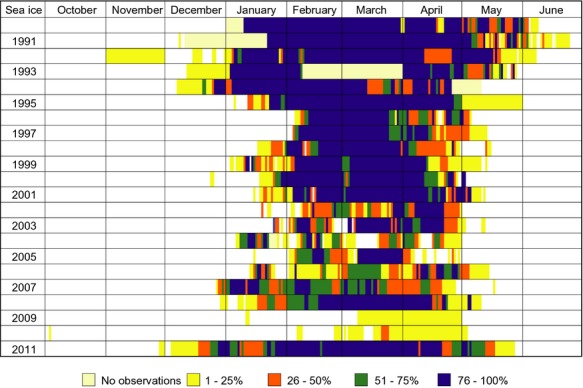
Daily observations of per cent sea ice cover as observed from Arctic Station (Disko Island, western Greenland) from 1991 to 2011.
Climate record
Linear regression analyses between 1992 and 2011 monthly air temperatures from Arctic Station and Ilulissat were highly significant (R2 = 0.99) (Fig. S9). Based on the regression, an 1873–2011 record of monthly air temperatures was calculated for Arctic Station (Fig.1). The record shows that warming periods of 10–20 years have been noted at least twice in the past 100 years – from 1910 to 1930 and from 1991 to 2011, and for both of these intervals, the greatest warming occurred during the winter (>0.3 °C year−1).
Linear correlation analyses were made between measured and modelled precipitation data (Schneider et al., 2011). Because the weather station at Arctic Station only measures rain, this could only be performed for the three summer months. The results showed that the modelled values are within range of the measured values although not on a significant level (Fig. S10 and 11). The limitation and complications associated with using the modelled precipitation rates are considered in the discussion.
Chronology characteristics and quality
After crossdating, ring-width chronologies (RW) for the below-ground and above-ground parts were completed. Based on all 116 sections, a final Betula nana chronology for Southern Disko was established for the period 1888–2011 (Fig.2). The chronology was characterized by a high common signal represented by overall mean interseries correlation of 0.59 (raw RW data) and an average mean sensitivity of 0.47. The mean interseries correlation calculated for the detrended chronology was 0.42 and the expressed population signal (EPS), which is a function of mean interseries correlation and series replication, varied from 0.75 to 0.99 (mean = 0.97) (Fig. S12). Annual Betula nana radial growth rates at the root collar base were in the order of 50–400 microns. Wedging rings were common, which often corresponded to a missing ring in some part of the same plant.
Temperature-controlled growth relationships
The three complementary climate–growth relationship analyses all revealed summer and winter temperature sensitivity of the Betula nana chronology during the last century (Figs8; Table S1). The highest positive Pearson's product–moment correlation coefficients for the long time series (1910–2011) were found between the raw Betula nana chronology and seasonal mean temperatures of June and July (JunJul, r = 0.53, P < 0.05) and previous December and current year January (pDecJan, r = 0.42, P < 0.05). The highest significant positive correlations between radial growth and winter temperatures were seen for the periods 1910–1930 and 1991–2011 dominated by winter warming (Fig.6; Fig S13; Table S1). For the period 1910–1930, correlations between Betula nana growth and air temperatures were 0.49 (P < 0.05) and 0.56 (P < 0.05) for pDec and current January compared to 0.45 (P < 0.05) for June and 0.55 (P < 0.05) for July. The winter signal was even stronger for the last 20 years with r = 0.61 (P < 0.05) and 0.69 (P < 0.05) for pDec and current January, respectively, and dominated over the summer signal (0.44 (P < 0.05) for June and 0.36 (P < 0.1) for July). Pearson's correlation coefficients obtained for the standardized Betula nana chronology confirmed the overall significant and positive relationship with both summer (JunJul) and winter (pDecJan) for the period 1910–2011 (Fig.6). The result was approved by comparing different detrending methods (Fig. S14). The positive and significant correlation coefficients for both summer and winter periods were confirmed also by computing climate–growth relationships between raw and detrended Betula nana chronology and detrended air temperatures for the period 1910–2011 (Fig. S15). Furthermore, a comparison of climate–growth response of different shrub parts revealed that the importance of winter air temperatures was seen for both below-ground and above-ground plant growth (Fig. S16).
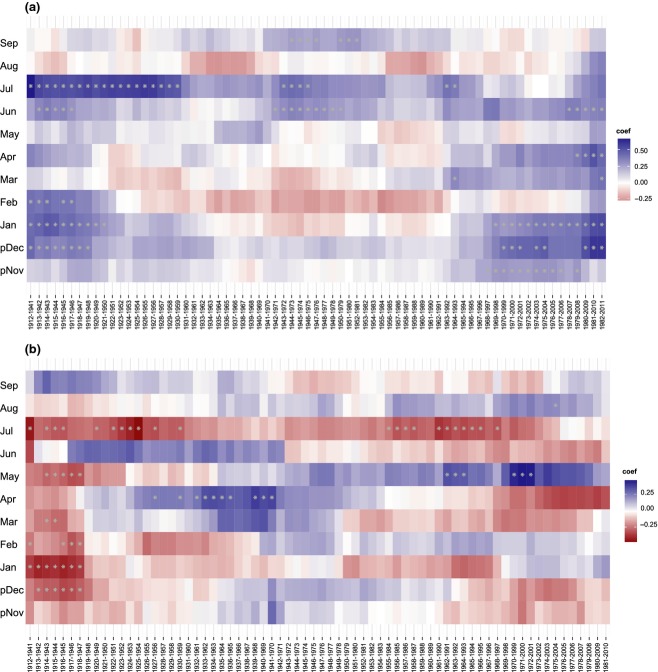
Fig. 8.
Moving correlation functions (MCF) relating the Betula nana chronology (raw series) from Disko Island, western Greenland to (a) average monthly air temperatures for the period (1910–2011) and (b) average monthly precipitation rates (from previous November to current September). The moving correlation was computed using a 30-year moving window, offset by 1 year. Significant correlations (P < 0.05) are marked by asterisks.
Fig. 6.
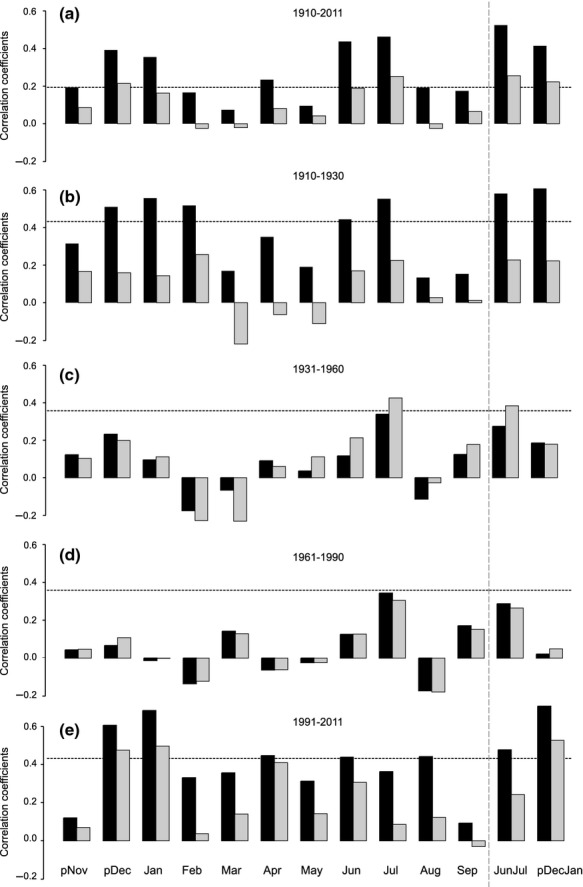
Pearson's correlation coefficients between Betula nana ring-width chronology (black = undetrended; grey = detrended) and monthly air temperature, including two seasonal averages for winter (previous December and current January) and summer (current June and July), derived from Arctic Station (Disko Island, western Greenland) for (a) 1910–2011, (b) 1910–1930, (c) 1931–1960, (d) 1961–1990 and (e) 1991–2011. Significance levels (P < 0.05) are indicated by horizontal dashed lines.
The positive long-term correlation between Betula nana radial growth and air temperature established by Pearson's correlation coefficients was further examined by calculating bootstrapped response coefficients (BRC). The results confirmed that both summer and winter air temperatures had a significant controlling influence on Betula nana growth over a last century (Fig.7a). The highest BRC were obtained for average monthly air temperature of previous December (r = 0.228, P < 0.05), current June and July (r = 0.209 and r = 0.205 respectively, P < 0.05).
Fig. 7.
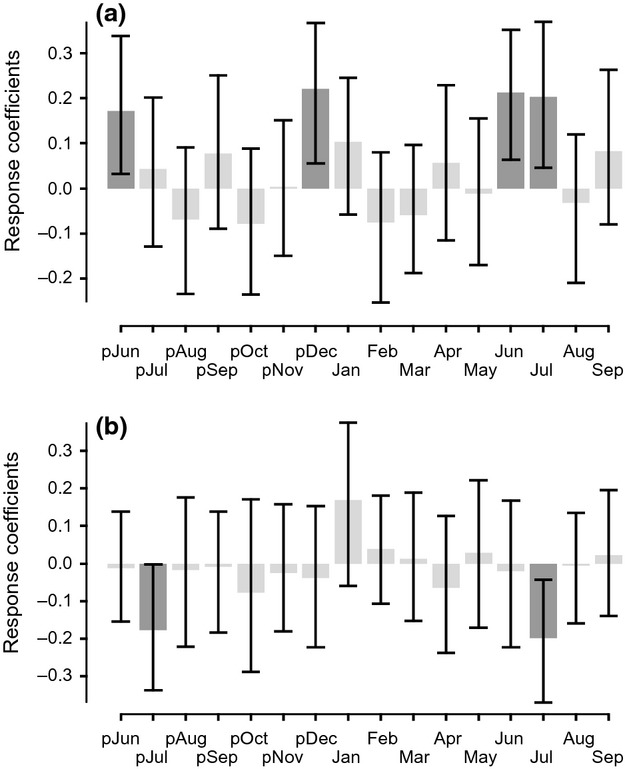
Bootstrapped response coefficients (BRC) between the Betula nana chronology (raw series) from Disko Island and (a) average monthly air temperatures for the period 1910–2011 and (b) average monthly precipitation rates for the period 1910–2010 computed from previous June to current September. Previous year months are given in lowercase letters and current year ones in uppercase letters. Significant coefficients (P < 0.05) are marked by dark grey bars, and the lines represent the 95% confidence intervals.
The century-long variability of climate–growth relationship reflected in the Betula nana growth rings was assessed by performing moving correlation functions (MCF) between the Betula nana chronology (raw series) and air temperatures (Fig.8a). The MCF applied for the whole study period (i.e. last 100 years) indicated a predominantly positive correlation with previous December, current January and February, particularly in the periods 1912–1951 and 1970–2011 (except for February). For summer temperatures, the signal was predominantly positive for current June and July (Fig.8a) and revealed moderate seasonal variability of the significance levels over time. The MCF revealed that the positive correlation between shrub radial growth and winter temperatures (previous December and current January) within last two decades has become so pronounced that it is prevailing over the summer signal. This was not the case of early study period (i.e. 1912–1950), where the summer signal (mainly July) dominated over the winter signal although both correlation coefficients were significant (P < 0.05). Additionally, the MCF indicated an increasing importance of spring temperatures (March and April) during the recent period (Fig.8a).
Precipitation and other climatic variables
Positive and significant correlations were also observed when using other measures such as growing degree-days (pDec r = 0.67 and Aug r = 0.47, P < 0.05) and thawing degree-days (pDec r = 0.72 and Aug r = 0.46, P < 0.05) (Fig. S17 a,b). Long time series do not exist for soil temperatures, but for the last 20 years (1991–2011), significant positive correlations with winter and spring soil temperatures at 60 cm depth were noted (Fig. S17c; Table S2). The highest positive correlation was found between Betula nana growth and April and May soil temperatures.
No correlation was found between Betula nana growth and winter precipitation in the period 1910–2011 (Fig. S18), whereas a negative correlation between July precipitation and Betula nana ring-width growth was observed (r = −0.22, P < 0.05). Based on average monthly precipitation, the BRC revealed a significant negative correlation between current and previous July precipitation (r = −0.215 and r = −0.172 respectively, P < 0.05) and Betula nana radial growth for the century-long period (Fig.7b). Results of MCF revealed weakening of this signal within the last years, as well as indicated an increasing positive importance of May precipitation (Fig.8b).
For the period 1991–2011, a negative but not significant correlation between Betula nana radial growth and average monthly snow depths was obtained for the winter months as well as current April. (pDec-Feb r = −0.41 and Apr r = −0.41, P < 0.06). A remarkable shift in the amount of snow was noticed within the last 20 years from a period with thick snow depths (1991–1996) and a positive effect on Betula nana radial growth to a recent period (1997–2011) with very shallow snow depth and no significant growth response towards snow depths (Fig. S19).
The influence of sea ice on shrub growth for the last 20 years was assessed using measurements of sea ice cover from Arctic Station (Fig.5). The analysis revealed a highly significant negative correlation between winter and spring sea ice extent and Betula nana radial growth, which was the highest for January (r = −0.70, P < 0.001) and May (r = −0.62, P < 0.01) (Fig. S20).
Discussion
Chronology quality
Although limited amount of very old Betula nana individuals were available at the study site, a detailed serial sectioning and crossdating analyses at the shrub level enabled the elaboration of a robust ring-width chronology that was characterized by high common signal. This is often hard to obtain dealing with shrub species which often reproduce vegetatively and form multiply branches and adventitious roots. Nevertheless, we found wedging and missing rings to be a common phenomenon in our Betula nana individuals. Three of 15 shrubs were lacking from four to 23 annual rings at the stem base and, respectively, in the root part of the same individual. This phenomenon has recently been described for Betula nana from Abisko (Wilmking et al., 2012) and thus emphasizes the importance of using the serial sectioning method when dealing with Betula nana radial growth measurements. This study reveals that smaller amount of sampled material, combined with a multistep intraplant crossdating, allows better understanding of shrub annual growth performance and significantly enhances dating quality.
From 1923 to 2011, the expressed population signal (EPS) was above 0.85, which is generally considered as the acceptable threshold for a reliable chronology (Wigley et al., 1984), confirming the robustness of the chronology. However, before 1923 (i.e. form 1910–1923), the EPS varied between 0.75 and 0.85. We tested the reliability of this early part of the chronology (i.e. represented by a minimum of three shrubs growth series) by independently comparing climate–growth correlations for the periods 1910–1930 and 1920–1930 and found that both seasonal summer and winter signals were indicated for both periods (Fig. S21 and Table S1).
The ring-width chronology for Betula nana was used for climate–growth analyses for the period 1910–2011. Application of bootstrapped resampling confirmed the significance of the obtained response coefficients between Betula nana shrubs growth and both summer and winter temperatures over the last century. Moreover using MCF, it was possible to investigate changes in the significance levels of shrub sensitivity to summer and winter temperatures over time. This helped to reveal a rather low-frequency variability of the winter signal primarily associated with the periods dominated by warm winters previously identified (i.e. 1910–1930 and after 1991), whereas sensitivity of Betula nana radial growth to summer temperatures seemed to be more stable over time.
Climate change and Betula nana radial growth
The Betula nana ring-width chronology was combined with one of the longest temperature records available in Greenland to investigate the effects of air temperatures on its radial growth over a century-long time scale. Overall, we found a strong correlation between Betula nana radial growth and winter temperatures as well as summer temperatures. This relationship was indicated for both below-ground and above-ground shrub radial growth (Fig. S16) as was well confirmed in all of the three different time series detrending methods applied (Fig. S14 and S15). Comprehensive and thoroughly tested climate–growth relationship analyses revealed that during the last century Betula nana radial growth has primarily been driven by temperatures during summer (June and July) and prior winter (previous December) (Fig.8). These results are in contrast to a Betula nana study from north-eastern Siberia, which revealed only a significant correlation between growth and early summer air temperatures (Blok et al., 2011). In addition to winter air temperatures, we also found significant and positive correlations between Betula nana radial growth with respect to both growing degree-days (GDD) and thawing degree-days (TDD) (Fig. S17 a,b).
The meteorological measurements from Arctic Station show, as expected, a strong correlation between air temperatures and soil temperatures with a tendency of mild winters increasing winter and spring soil temperatures (Fig. S5). Results reveal a significant negative correlation between air temperatures and sea ice which highlights a possible linkage between mild winters and less sea ice or vice versa (Bhatt et al., 2010). Furthermore, we found a highly significant negative correlation between sea ice extent and plant growth (r = −0.70 for January) (Fig. S20). This indicates a possible link between warm winters, that is, expressed in the lack of sea ice with an increased Betula nana growth on a local scale which is in good agreement with investigations by Bhatt et al. (2010), showing a linkage between sea ice concentrations and tundra productivity in the High Arctic. Recently, late-spring tundra productivity (i.e. alder and willow) and temperatures were also found to be largely linked to declining sea ice extent adjacent to north-western Eurasian tundra (Macias-Fauria et al., 2012).
Betula nana is considered as an early flowering species (Molau et al., 2005) for which growth depends much on early summer thermal conditions, which was confirmed in this study by a significant correlation with June air temperatures (Figs6 and 7) and spring soil temperatures (Fig. S17c). Mild winters may thereby have a direct positive effect on Betula nana growth. The current understanding of winter conditions positively affecting growth is closely linked to the snow cover reducing the chances for frost damage, warming up the soil, and thereby increasing the mineralization and release of nutrients (Schimel et al., 2004; Inouye, 2008; Morgner et al., 2010). Over the whole period from 1991 to 2011, we found a weak negative correlation between growth and the snow depths measured at Arctic Station for the winter months as well as for April (Fig. S19). However, our data showed a clear shift within the period 1991–2011 from a period with thick snow depths (1991–1996) having a positive effect on Betula nana radial growth, to a period with very shallow snow depths (1997–2011) and no significant growth response towards snow (Fig. S19). At Arctic Station, both snow depths and the duration of the snow cover have decreased markedly over the last 20 years and the results suggest that snow depths are getting too small to effectively decouple the soil thermal regime from air temperatures (Fig. S7). Nevertheless, winter and spring soil temperatures have been increasing significantly (Fig. S8) which suggests that the recent increase in Betula nana growth is not directly controlled by snow but is rather a direct effect of warmer winter and spring air temperatures causing the snow to melt earlier and allowing the soil to drain and warm faster. Furthermore, it seems that the negative effects linked with early snowmelt, such as frost damage, are overcome by positive effects, such as increased growing season length and greater amount of growing degree-days, which previously has been shown to enhance Arctic shrub growth in snow manipulation studies (Wipf et al., 2006).
No snow data exist for the study area before 1991, and hence, it is not possible to get a 100 year perspective on the effect of site-specific snow conditions on Betula nana growth. Instead, modelled precipitation data from 1910 to 2010 were used showing a significant negative correlation between July precipitation and Betula nana ring-width growth but no correlation with winter precipitation (Fig.7b; 8b; S18). This does not necessarily exclude an effect on winter precipitation on shrub growth on a local scale. It is well known that snow–shrub growth relations are highly heterogeneous and complex (Sturm et al., 2001, 2005a,b; Liston et al., 2002; Wipf & Rixen, 2010; Rumpf et al., 2014) and often limited by inaccurate snow cover measurements over a vast and diverse shrub cover. Using modelled regional precipitation data to represent site-specific condition is difficult, especially in Arctic areas where local precipitation patterns, wind, topography and aspect, as well as density of shrub cover may have a great influence on snow depths.
An indirect effect of warmer winters positively linked with Betula nana growth might be associated with soil nutrient availability. Milder winters (i.e. warmer and characterized by lower snow depths and earlier onset of the snowmelt) might largely contribute to higher nutrient availability for shrub growth, including nitrogen availability resulting from both higher biogeochemical cycle and litter decomposition in the warmer dormant season. Numerous studies on shrub growth responses to elevated temperature (mainly through open-top chambers, OTCs) or nutrient availability have revealed a significant increase in deciduous shrub growth resulting from both higher dormant and growing season temperature (Henry & Molau, 1997; Elmendorf et al., 2012a). Particularly, Betula nana growth has been shown to be highly promoted by nutrient addition (Chapin et al., 1995; Shaver et al., 2001; Bret-Harte et al., 2008). These commonly conducted primary growth studies on tundra shrubs revealed the importance of winter conditions on tundra plant performance (Wipf & Rixen, 2010; Rayback et al., 2011).
Implications and conclusions
Compared to previous investigations, this study shows a strong winter relationship with secondary growth at a unique high resolution over more than 100 years including both below-ground and above-ground shrub parts. Thus, the developed ring-width chronology provides a unique retrospective view of more than 100 years of Betula nana growth in western Greenland and shows that both summer and winter conditions have had strong positive effect on radial growth during the warming periods of the last century. Hence, our results strongly suggest that not only summer but also winter biological processes play a crucial role in the current ‘greening’ of the Arctic tundra and that the Betula nana increase in growth may continue if the current winter warming in the region continue as predicted by climate models (Elberling et al., 2010) (Fig.1).
Warm winters may have an indirect effect on temperatures of the following summer making it difficult to differentiate between summer and winter effects. There was no correlation revealed between winter air temperatures and the following summer temperatures in the study area for the period 1991–2011, whereas winter temperatures correlated significantly with summer temperatures for the period 1910–1930 (Fig. S22 & Table S3). This correlation indicates that the importance of winter conditions for plant growth may be overlooked in periods that are characterized by warmer winters followed by a warmer summer.
The obtained ‘winter dependence’ between Betula nana ring-width growth and temperature cannot be treated as a direct growth trigger but rather as a sign of a complex relationship in the tundra ecosystem and shrubs growing under recent Arctic climate shift associated in many sites with warmer and shorter winters (Cooper, 2014). Future investigations are needed as follows: (i) to verify if the observations for Betula nana are valid for other tundra plant species, as well as Betula species from other locations (ii) to quantify the combination of biogeochemical processes which can explain the positive effect of warmer winters and its positive feedbacks for shrub growth.
We conclude that winter processes are as important as summer processes in controlling the Betula nana radial growth. This includes a positive growth response to winter air temperatures during the past century, especially during the periods from 1910–1930 to 1990–2011 that were dominated by significant winter warming. For the last two decades, we found a strong positive growth response to the amount of thawing and growing degree-days as well as towards winter and spring soil temperatures. In addition, we found a strong negative growth response to the sea ice extent indicating a possible link between warm winters, lack of sea ice and increased Betula nana growth. Snow depths have been decreasing markedly from 1990 to 2011 lowering the insulating effect of the snow pack and causing winter and spring soil temperatures to be closer related to air temperatures. Despite of this, both winter and spring soil temperatures have been increasing significantly during this period and therefore, the recent increase in Betula nana growth seems to be triggered directly by warmer winter and spring air temperatures. As far as we know, this study provides the most robust documentation for that winter processes on a century-long time scale are as important as summer processes in controlling and enhancing the overall, that is, below- and above-ground radial growth pattern of one of the dominating shrub species in the Arctic.
Acknowledgments
We gratefully acknowledge financial support from the Danish National Research Foundation (CENPERM DNRF100) as well as support from the European Union Seventh Framework Programme [FP7/2007–2013] under grant agreement no 262693 [INTERACT]. Thanks are extended to ASIAQ for providing climatic data and to the staff at Arctic Station for taking daily observations and providing assistance: O. Frimer, M. Rasch, P. Funch, S. Bernstein, C. Schander, B.J. Graae, R. Ejrnæs, H. Sulsbrück and O.M. Tervo, as well as technical assistance by U. Thomas. Finally, we would like to thank reviewers’ for very helpful and constructive suggestions.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Location of the Arctic Station 2 km east of Qeqertarsuaq at Disko Island in western Greenland.
Figure S2. An example of serial sectioning of Betula nana individual collected near Arctic Station on Disko Island.
Figure S3. Duration (A) and onset (B) of the snow free period (SFP) at Arctic Station.
Figure S4. (A) mean annual soil temperatures at 0.05 m (black), 0.60 m (blue) and 1.70 m (grey) at Arctic Station.
Figure S5. Correlation between seasonal air temperatures and soil temperatures at Arctic Station.
Figure S6. Correlation between seasonal air temperatures and soil temperatures at Arctic Station.
Figure S7. The upper red line shows a 14 days running average of the soil temperature.
Figure S8. Soil temperatures (A) and snow depts (B) measured at Arctic Station.
Figure S9. (A) correlation between monthly air temperatures measured at Arctic Station.
Figure S10. Annual precipitation rates for the period 1901-2010 derived from the NOAA precipitation model.
Figure S11. Correlation between monthly precipitation rates from 1991-2010.
Figure S12. (A) Betula nana ring-width chronology for southern Disko Island (western Greenland).
Figure S13.Betula nana ring-width chronology for southern Disko Island (western Greenland).
Figure S14. Pearson's correlation coefficients between raw (black bars) and standardized (grey bars) Betula nana chronologies.
Figure S15. Pearson's correlation coefficients between standardized Betula nana chronologies.
Figure S16. Correlation between Betula nana ring-width (1910–2011).
Figure S17. Pearson's correlation coefficients between Betula nana ring-width chronology for Arctic Station (Disko Island, western Greenland).
Figure S18. Pearson's correlation coefficients between Betula nana ring-width chronology for Arctic Station (Disko Island, western Greenland).
Figure S19. Correlations between Betula nana ring-width chronology for southern Disko Island.
Figure S20. Pearson's correlation coefficients between Betula nana tree-ring width chronology.
Figure S21. Pearson's correlation coefficients between Betula nana ring-width chronology.
Figure S22. Relationships between mean monthly air temperatures for previous December and current January and current June and July derived from Arctic Station.
Table S1. Significance (P-values) resulting from Pearson's correlations between Betula nana ring-width chronology and mean monthly air temperatures for Arctic Station.
Table S2. Significance (P-values) resulting from correlations between Betula nana ring-width chronology and mean monthly soil temperatures (°C) for Arctic Station.
Table S3. Correlation coefficients and significance (P-values) resulting from correlations between curremt June and July.
References
- Bhatt US, Walker DA, Raynolds MK, et al. Circumpolar arctic tundra vegetation change is linked to sea ice decline. Earth Interactions. 2010;14:1–20. [Google Scholar]
- Blok D, Sass-Klaassen U, Schaepman-Strub G, Heijmans MMPD, Sauren P, Berendse F. What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences. 2011;8:1169–1179. [Google Scholar]
- Box JE. Survey of Greenland instrumental temperature records: 1873-2001. International Journal of Climatology. 2002;22:1829–1847. [Google Scholar]
- Bret-Harte MS, Shaver GR, Zoerner JP, et al. Developmental plasticity allows Betula nana to dominate tundra subjected to an altered environment. Ecology. 2001;82:18–32. [Google Scholar]
- Bret-Harte MS, Mack MC, Goldsmith GR, et al. Plant functional types do not predict biomass responses to removal and fertilization in Alaskan tussock tundra. Journal of Ecology. 2008;96:713–726. doi: 10.1111/j.1365-2745.2008.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa K, Jones P. Basic dendrochronology statistics and assessment. In: Cook ER, Kairiukstis LA, editors. Methods in Dendrochronology: Applications in the Environmental Sciences. Dordrecht: Kluwer Academic Publishers; 1990. pp. 137–153. [Google Scholar]
- Buchwal A, Rachlewicz G, Fonti P, Cherubini P, Gartner H. Temperature modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard) Polar Biology. 2013;36:1305–1318. [Google Scholar]
- Bunn AG. A dendrochronology program library in R (dplR) Dendrochronologia. 2008;26:115–124. [Google Scholar]
- Cappelen J, Jørgensen BV, Laursen EV, Stannius LS, Thomsen RS. The Observed Climate of Greenland, 1958–99 with Climatological Standard Normals, 1961–90. Danish: Meteorological Institute; 2001. pp. 1–152. [Google Scholar]
- Chapin FS, III, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of Arctic tundra to experimental and observed changes in climate. Ecology. 1995;76:694–711. [Google Scholar]
- Cook ER, Briffa K, Shiyatov S, Mazepa V. Tree-rings standardization and growth-trend estimation. In: Cook ER, Kairiukstis LA, editors. Methods of Dendrochronology: Applications in the Environmental Sciences. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1990. pp. 104–123. [Google Scholar]
- Cooper EJ. Warmer shorter winters disrupt arctic terrestrial ecosystems. The Annual Review of Ecology, Evolution, and Systematics. 2014;45:271–291. [Google Scholar]
- Deslippe JR, Simard SW. Below-ground carbon transfer among Betula nana may increase with warming in Arctic tundra. New Phytologist. 2011;192:689–698. doi: 10.1111/j.1469-8137.2011.03835.x. [DOI] [PubMed] [Google Scholar]
- Deslippe JR, Hartmann M, Mohn WW, Simard SW. Long-term experimental manipulation of climate alters the ectomycorrhizal community of Betula nana in Arctic tundra. Global Change Biology. 2011;17:1625–1636. [Google Scholar]
- Elberling B, Christiansen HH, Hansen BU. High nitrous oxide production from thawing permafrost. Nature Geoscience. 2010;3:332–335. [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, et al. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecology Letters. 2012a;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Climate Change. 2012b;2:453–457. [Google Scholar]
- Epstein HE, Raynolds MK, Walker DA, Bhatt US, Tucker CJ, Pinzon JE. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environmental Research Letters. 2012;7:1–12. [Google Scholar]
- Forbes BC, Fauria MM, Zetterberg P. Russian arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biology. 2010;16:1542–1554. [Google Scholar]
- Guiot J. The bootstrapped response function. Tree-Ring Bulletin. 1991;51:39–41. [Google Scholar]
- Hallinger M, Manthey M, Wilmking M. Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist. 2010;186:890–899. doi: 10.1111/j.1469-8137.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- Hansen BU, Elberling B, Humlum O, Nielsen N. Meteorological trends (1991-2004) at Arctic Station, Central West Greenland (69°15′N) in a 130 years perspective. Danish Journal of Geography. 2006;106:45–55. [Google Scholar]
- Henry GHR, Molau U. Tundra plants and climate change: the International Tundra Experiment (ITEX) Global Change Biology. 1997;3:1–9. [Google Scholar]
- Henry GHR, Harper KA, Chen WJ, et al. Effects of observed and experimental climate change on terrestrial ecosystems in northern Canada: results from the Canadian IPY program. Climatic Change. 2012;115:207–234. [Google Scholar]
- Heskel M, Greaves H, Kornfeld A, et al. Differential physiological responses to environmental change promote woody shrub expansion. Ecology and Evolution. 2013;3:1149–1162. doi: 10.1002/ece3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin. 1983;43:69–78. [Google Scholar]
- Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology. 2008;89:353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, IPCC, editors. Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK & New York, NY, USA: Cambridge University Press; 2013. [Google Scholar]
- Jørgensen RH, Hallinger M, Ahlgrimm S, Friemel J, Kollmann J, Meilby H. Growth response to climatic change over 120 years for Alnus viridis and Salix glauca in West Greenland. Journal of Vegetation Science. 2014;26:155–165. [Google Scholar]
- Kolishchuk V. Dendroclimatological study of prostrate woody plant. In: Cook ER, Kairiukstis LA, editors. Methods of Dendrochronology Applications in the Environmental Sciences. Dordrecht: Kluwer Academic Publishers; 1990. pp. 51–55. [Google Scholar]
- Liston GE, McFadden JP, Sturm M, Pielke RA. Modelled changes in arctic tundra snow, energy and moisture fluxes due to increased shrubs. Global Change Biology. 2002;8:17–32. [Google Scholar]
- Macias-Fauria M, Forbes BC, Zetterberg P, Kumpula T. Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems. Nature Climate Change. 2012;2:613–618. [Google Scholar]
- Molau U, Nordenhäll U, Eriksen B. Onset of the flowering and climate variability in an alpine landscape: a 10-year study from Swedish Lapland. American Journal of Botany. 2005;92:422–431. doi: 10.3732/ajb.92.3.422. [DOI] [PubMed] [Google Scholar]
- Morgner E, Elberling B, Strebel D, Cooper EJ. The importance of winter in annual ecosystem respiration in the High Arctic: effects of snow depth in two vegetation types. Polar Research. 2010;29:58–74. [Google Scholar]
- Nobrega S, Grogan P. Deeper snow enhances winter respiration from both plant-associated and bulk soil carbon pools in birch hummock tundra. Ecosystems. 2007;10:419–431. [Google Scholar]
- Putnins P. The climate of Greenland. In: Orvig S, editor. Climates of the Polar Regions. Amsterdam-London-New York: Elsevier Publ. Comp; 1970. pp. 3–128. World Survey of Climatology, 14. [Google Scholar]
- Rayback SA, Lini A, Henry GHR. Spatial variability of the dominant climate signal in Cassiope tetragona from sites in Arctic Canada. Arctic. 2011;64:98–114. [Google Scholar]
- Rumpf AB, Semenchuk PR, Dullinger S, Cooper E. Idiosyncratic responses of High Arctic plants to changing snow regimes. PLoS One. 2014;9:e86281. doi: 10.1371/journal.pone.0086281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimel JP, Bilbrough C, Welker JA. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biology & Biochemistry. 2004;36:217–227. [Google Scholar]
- Schmidt NM, Baittinger C, Forchhammer MC. Reconstructing century-long snow regimes using estimates of High Arctic Salix arctica radial growth. Arctic, Antarctic and Alpine Research. 2006;38:257–262. [Google Scholar]
- Schneider U, Becker A, Finger P, Meyer-Christoffer A, Rudolf B, Ziese M, Global Precipitation Climatology Centre (Gpcc HGDDaDW) GPCC Full Data Reanalysis Version 6.0 (at 0.5°, 1.0°, 2.5°): Monthly Land-Surface Precipitation from Rain-Gauges Built on GTS-based and Historic Data. Germany: Offenbach/Main; 2011. [Google Scholar]
- Schweingruber FH, Eckstein D, Serre-Bachet F, Bräker O. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia. 1990;8:9–38. [Google Scholar]
- Schweingruber FH, Börner A, Schultze ED. Atlas of Stem Anatomy in Herbs, Shrubs and Trees. Vol. 1. Berlin-Heidelberg: Springer verlag; 2011. [Google Scholar]
- Semenchuk PR, Elberling B, Amtorp C, Winkler J, Rumpf S, Michelsen A, Cooper EJ. Deeper snow alters soil nutrient availability and leaf nutrient status in high Arctic tundra. Biogeochemistry. 2015 doi: 10.1007/s10533-015-0082-7 (in press) [Google Scholar]
- Serreze MC, Francis JA. The arctic amplification debate. Climatic Change. 2006;76:241–264. [Google Scholar]
- Serreze MC, Walsh JE, Chapin FS, et al. Observational evidence of recent change in the northern high-latitude environment. Climatic Change. 2000;46:159–207. [Google Scholar]
- Shaver GR, Bret-Harte MS, Jones MH, Johnstone J, Gough L, Laundre J, Chapin FS., III Species changes interact with fertilizer addition to control 15 years of change in tundra. Ecology. 2001;82:3163–3181. [Google Scholar]
- Sturm M, McFadden JP, Liston GE, Chapin RS, III, Racine CH, Holmgren J. Snow-shrub interactions in Arctic Tundra: a hypothesis with climatic implications. Journal of Climate. 2001;14:336–344. [Google Scholar]
- Sturm M, Schimel J, Michaelson G, et al. Winter biological processes could help convert arctic tundra to shrubland. BioScience. 2005a;55:17–26. [Google Scholar]
- Sturm M, Douglas T, Racine C, Liston GE. Changing snow and shrub conditions affect albedo with global implications. Journal of Geophysical Research. 2005b;110:1–13. [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. [Google Scholar]
- Van Wijk MT, Williams M, Gough L, Hobbie SE, Shaver GR. Luxury consumption of soil nutrients: a possible competitive strategy in above-ground and below-ground biomass allocation and root morphology for slow-growing arctic vegetation? Journal of Ecology. 2003;91:664–676. [Google Scholar]
- Wahren CHA, Walker MD, Bret-Harte MS. Vegetation responses in Alaska arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Global Change Biology. 2005;11:537–552. [Google Scholar]
- Walker DA, Gould WA, Maier HA, Raynolds MK. The Circumpolar Arctic Vegetation Map: AVHRR-derived base maps, environmental controls, and integrated mapping procedures. International Journal of Remote Sensing. 2002;23:4551–4570. [Google Scholar]
- Wigley TML, Briffa KR, Jones PD. On the average value of correlated time-series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology. 1984;23:201–213. [Google Scholar]
- Wilmking M, Hallinger M, van Bogaert R, et al. Continuously missing outer rings in woody plants at their distributional margins. Dendrochronologia. 2012;30:213–222. [Google Scholar]
- Wipf S, Rixen C. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Research. 2010;29:95–109. [Google Scholar]
- Wipf S, Rixen C, Mulder CPH. Advanced snowmelt causes shifts towards positive neighbour interactions in a subarctic tundra community. Global Change Biology. 2006;12:1496–1506. [Google Scholar]
- Zang C, Biondi F. Dendroclimatic calibration in R: the bootRes package for response and correlation function analysis. Dendrochronologia. 2013;31:68–74. [Google Scholar]
- Zang C, Biondi F. treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography. 2015;38 doi: 10.1111/ecog.01335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Location of the Arctic Station 2 km east of Qeqertarsuaq at Disko Island in western Greenland.
Figure S2. An example of serial sectioning of Betula nana individual collected near Arctic Station on Disko Island.
Figure S3. Duration (A) and onset (B) of the snow free period (SFP) at Arctic Station.
Figure S4. (A) mean annual soil temperatures at 0.05 m (black), 0.60 m (blue) and 1.70 m (grey) at Arctic Station.
Figure S5. Correlation between seasonal air temperatures and soil temperatures at Arctic Station.
Figure S6. Correlation between seasonal air temperatures and soil temperatures at Arctic Station.
Figure S7. The upper red line shows a 14 days running average of the soil temperature.
Figure S8. Soil temperatures (A) and snow depts (B) measured at Arctic Station.
Figure S9. (A) correlation between monthly air temperatures measured at Arctic Station.
Figure S10. Annual precipitation rates for the period 1901-2010 derived from the NOAA precipitation model.
Figure S11. Correlation between monthly precipitation rates from 1991-2010.
Figure S12. (A) Betula nana ring-width chronology for southern Disko Island (western Greenland).
Figure S13.Betula nana ring-width chronology for southern Disko Island (western Greenland).
Figure S14. Pearson's correlation coefficients between raw (black bars) and standardized (grey bars) Betula nana chronologies.
Figure S15. Pearson's correlation coefficients between standardized Betula nana chronologies.
Figure S16. Correlation between Betula nana ring-width (1910–2011).
Figure S17. Pearson's correlation coefficients between Betula nana ring-width chronology for Arctic Station (Disko Island, western Greenland).
Figure S18. Pearson's correlation coefficients between Betula nana ring-width chronology for Arctic Station (Disko Island, western Greenland).
Figure S19. Correlations between Betula nana ring-width chronology for southern Disko Island.
Figure S20. Pearson's correlation coefficients between Betula nana tree-ring width chronology.
Figure S21. Pearson's correlation coefficients between Betula nana ring-width chronology.
Figure S22. Relationships between mean monthly air temperatures for previous December and current January and current June and July derived from Arctic Station.
Table S1. Significance (P-values) resulting from Pearson's correlations between Betula nana ring-width chronology and mean monthly air temperatures for Arctic Station.
Table S2. Significance (P-values) resulting from correlations between Betula nana ring-width chronology and mean monthly soil temperatures (°C) for Arctic Station.
Table S3. Correlation coefficients and significance (P-values) resulting from correlations between curremt June and July.