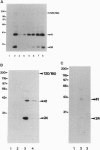
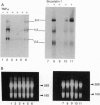
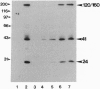
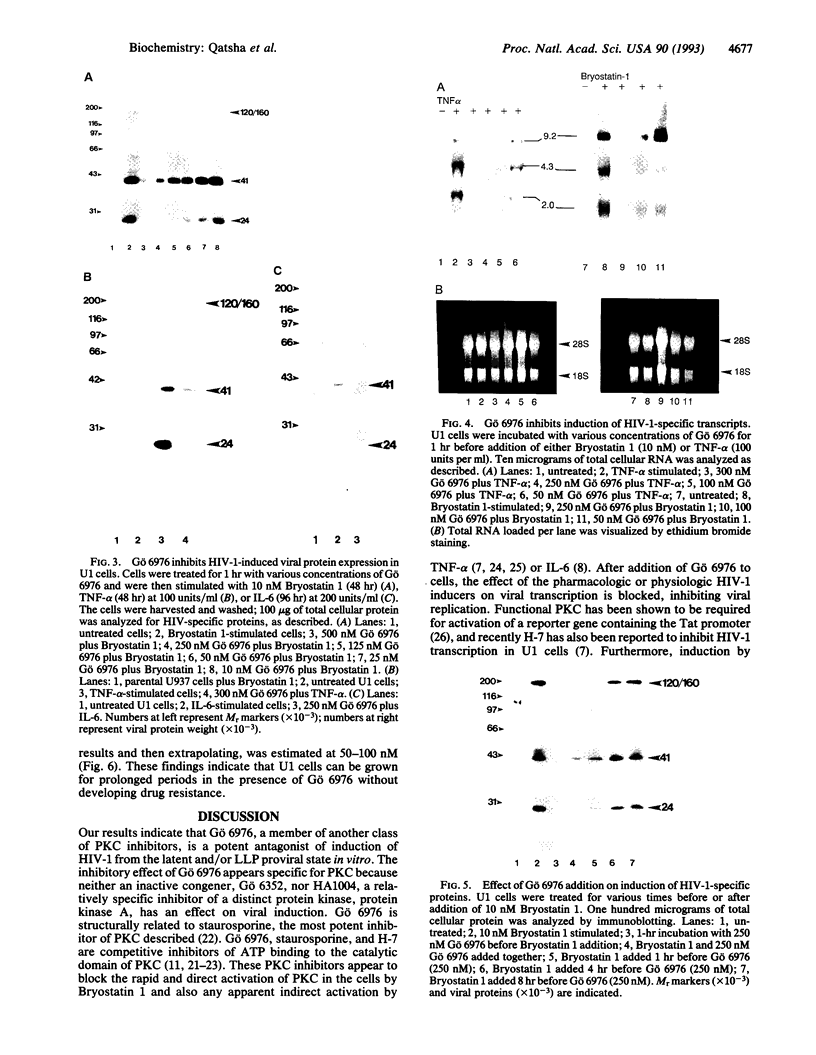
Abstract
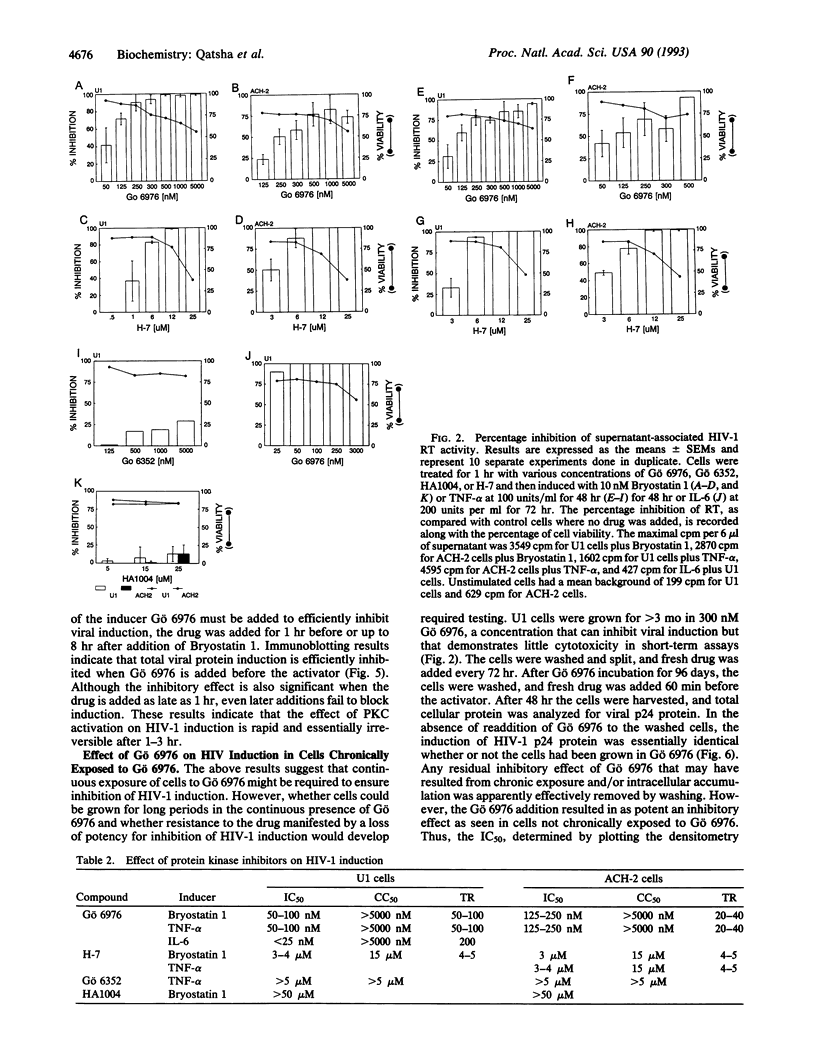
Human immunodeficiency virus (HIV-1) infection is followed by a period of latency or a low-level-persistent (LLP) state that results in an asymptomatic infection of the host. Productive viral expression may be triggered by a variety of activators including mitogens, antigens, and cytokines. Protein kinase C (PKC) has been shown to be important in the intracellular cascade of signals induced by such activators. With U1 and ACH-2 cell lines representative of an HIV-1 postintegration state, the effect of Gö 6976, a synthetic inhibitor of PKC was tested. Gö 6976 is a nonglycosidic indolocarbazole found to potently inhibit HIV-1 induction by Bryostatin 1, tumor necrosis factor alpha, and interleukin 6. Gö 6976 effectively blocks viral transcription induced by Bryostatin 1 or tumor necrosis factor alpha that leads to the inhibition of intracellular viral protein synthesis and viral shedding. Gö 6976 also blocks interleukin 6-mediated posttranscriptional induction of viral proteins. The IC50 of Gö 6976 shows a 12- to 60-fold more potent effect than for H-7, another PKC inhibitor with a similar mechanism. The inhibitory effect is reduced when Gö 6976 is not added before or within 1 hr of induction by the potent PKC activator Bryostatin 1. However, U1 cells can be grown for long periods in a nontoxic concentration of Gö 6976 (300 nM), which confers virtual inhibition of HIV-1 induction without the development of resistance. Results indicate that inhibition of HIV-1 proviral induction from latent/low-level-producing infectious states with potent PKC inhibitors like Gö 6976 may represent an additional and promising antiviral approach.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Folks T. M. Mechanisms of HIV-1 latency. AIDS. 1992 Jan;6(1):3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Mosca J. D., Raj N. B., Pitha P. M. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated alpha 2-interferon. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4958–4962. doi: 10.1073/pnas.86.13.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields A. P., Bednarik D. P., Hess A., May W. S. Human immunodeficiency virus induces phosphorylation of its cell surface receptor. Nature. 1988 May 19;333(6170):278–280. doi: 10.1038/333278a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Schnittman S., Orenstein J., Poli G., Fauci A. S. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988 Feb 15;140(4):1117–1122. [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Blanco M. A., Cullen B. R. Molecular basis of latency in pathogenic human viruses. Science. 1991 Nov 8;254(5033):815–820. doi: 10.1126/science.1658933. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D. R., Haeberle J. R. Selective purification of the 20,000-Da light chains of smooth muscle myosin. Anal Biochem. 1983 Nov;135(1):37–43. doi: 10.1016/0003-2697(83)90726-1. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Watanabe M., Hidaka H. N-(2-Aminoethyl)-5-isoquinolinesulfonamide, a newly synthesized protein kinase inhibitor, functions as a ligand in affinity chromatography. Purification of Ca2+-activated, phospholipid-dependent and other protein kinases. J Biol Chem. 1985 Mar 10;260(5):2922–2925. [PubMed] [Google Scholar]
- Jakobovits A., Rosenthal A., Capon D. J. Trans-activation of HIV-1 LTR-directed gene expression by tat requires protein kinase C. EMBO J. 1990 Apr;9(4):1165–1170. doi: 10.1002/j.1460-2075.1990.tb08223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A. L., Poli G., Maury W., Folks T. M., Fauci A. S. Direct and cytokine-mediated activation of protein kinase C induces human immunodeficiency virus expression in chronically infected promonocytic cells. J Virol. 1990 Sep;64(9):4306–4312. doi: 10.1128/jvi.64.9.4306-4312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A. S., Baker V. V., May W. S. Bryostatin induces changes in protein kinase C location and activity without altering c-myc gene expression in human promyelocytic leukemia cells (HL-60). Oncogene. 1987 May;1(2):111–118. [PubMed] [Google Scholar]
- Liu A. Y., Miskovsky E. P., Stanhope P. E., Siliciano R. F. Production of transmembrane and secreted forms of tumor necrosis factor (TNF)-alpha by HIV-1-specific CD4+ cytolytic T lymphocyte clones. Evidence for a TNF-alpha-independent cytolytic mechanism. J Immunol. 1992 Jun 15;148(12):3789–3798. [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Poli G., Bressler P., Kinter A., Duh E., Timmer W. C., Rabson A., Justement J. S., Stanley S., Fauci A. S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990 Jul 1;172(1):151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Schächtele C., Wagner B., Rudolph C. Effect of Ca2+ entry blockers on myosin light-chain kinase and protein kinase C. Eur J Pharmacol. 1989 Apr 12;163(1):151–155. doi: 10.1016/0014-2999(89)90410-x. [DOI] [PubMed] [Google Scholar]
- Schütze S., Nottrott S., Pfizenmaier K., Krönke M. Tumor necrosis factor signal transduction. Cell-type-specific activation and translocation of protein kinase C. J Immunol. 1990 Apr 1;144(7):2604–2608. [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Steffens U., Bessler W., Hauschild S. B cell activation by synthetic lipopeptide analogues of bacterial lipoprotein bypassing phosphatidylinositol metabolism and proteinkinase C translocation. Mol Immunol. 1989 Sep;26(9):897–904. doi: 10.1016/0161-5890(89)90146-6. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Vlach J., Pitha P. M. Activation of human immunodeficiency virus type 1 provirus in T-cells and macrophages is associated with induction of inducer-specific NF-kappa B binding proteins. Virology. 1992 Mar;187(1):63–72. doi: 10.1016/0042-6822(92)90295-z. [DOI] [PubMed] [Google Scholar]
- Walter U., Miller P., Wilson F., Menkes D., Greengard P. Immunological distinction between guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1980 Apr 25;255(8):3757–3762. [PubMed] [Google Scholar]