Abstract
Background
Children with pediatric low-grade gliomas (PLGG) are known to have excellent 10-year survival rates; however the outcomes of adult survivors of PLGG are unknown. We identified patients diagnosed with PLGG diagnosed between 1973 and 2008 through the Surveillance Epidemiology and End Results (SEER) database to examine outcomes of adult survivors of PLGG.
Procedure
Four thousand and forty patients with either WHO grade I or II PLGG were identified and outcome data retrieved. Two analyses were performed to assess survival and risk of death from tumor. Competing risks analysis was conducted and cumulative incidence curves of death due to disease were generated. Cox proportional hazards regression was performed, with adjustment for non-disease death. Kaplan–Meier curves for overall cancer specific survival (OS) were also generated.
Results
The 20-year OS was 87% ± 0.8% and the 20-year cumulative incidence of death due to glioma was 12% ± 0.8%. The incidence of death after transition to adulthood (age greater than 22 years) was slightly lower, with 20-year cumulative incidence of disease death of 7% ± 1.8%. Year of diagnosis, age of diagnosis, histology, WHO grade, primary site, radiation, and degree of initial resection were prognostic in univariate analysis, while the administration of radiation was the greatest risk of death in multivariate analysis of OS (hazard ratio = 3.9).
Conclusions
PLGGs are associated with an excellent long-term survival, with a low likelihood of PLGG related death in adult survivors. Treatment strategies for pediatric tumors should therefore aim for disease control during childhood and adolescence with an emphasis on minimizing long-term treatment induced toxicities.
Keywords: outcome, pediatric low-grade glioma, SEER
INTRODUCTION
Children with PLGGs have been reported to have superior 10-year overall survival (OS) rates compared to adults diagnosed with low-grade gliomas during adulthood (ALGG) [1]. While children have an excellent 10-year OS, adult patients diagnosed with low-grade gliomas have a more aggressive clinical course and poor long-term survival rates, with a high incidence of malignant transformation and death [2,3]. PLGGs have been reported to spontaneously stabilize or regress [4,5], particularly in children with neurofibromatosis type 1 [6,7], a phenomenon which is rare in ALGG. Although small case series and anecdotal evidence have suggested that PLGGs rarely undergo malignant transformation [8–10], long-term outcomes of PLGG remain unknown. In particular, it remains unclear whether these tumors undergo malignant transformation to cause death in adulthood. We performed a comparative retrospective analysis of survival of patients with PLGG diagnosed between 1973 and 2008 as identified in the National Institute Surveillance, Epidemiology and End Results (SEER) database of the National Cancer Institute (NCI), to analyze the survival rates of adult survivors of pediatric low-grade gliomas.
MATERIALS AND METHODS
Approval to access the SEER data was granted by the NCI to use all datasets up until 2011 [11]. Pediatric patients (0–19 years of age) diagnosed with a grade I or II glioma between 1973 and 2008 were identified. Variables recorded for each patient included age, year of diagnosis, race, sex, location of tumor, histological diagnosis and grade of tumor, extent of surgery, and whether the child received radiation therapy. Histological groupings used to identify patients included pilocytic astrocytoma, diffuse astrocytoma, astrocytoma not otherwise specified (NOS), glioma NOS, mixed glioma, or unique astrocytoma variants. Only tumors classified as grade I or II were included. Outcomes were recorded for status (alive or dead), time from diagnosis to last follow-up or death, and cause of death. Deaths were classified as death due to glioma, or death not due to glioma. Secondary malignancies, such as radiation induced tumors, were not classified as deaths due to glioma.
A competing risks analysis was conducted according to the methods of Pepe and Mori [12]. With adjustment for non-disease deaths, cumulative incidence curves of death due to disease were plotted for the overall cohort and for subgroups of each potential prognostic factor: age, sex, year of diagnosis, histology, WHO grade of differentiation, primary site, extent/type of radiation therapy, and degree of initial resection. The prognostic ability of the factors was tested using a Cox proportional hazards regression model (univariate and multivariate) for competing risks, according to Rosthoj et al. [13], with adjustment for non-disease death. This method allows for separate estimation/quantification of (i) risk for death due to disease; and (ii) risk for death from non-disease causes. Kaplan–Meier curves of OS were generated to determine overall survival including all causes of death. Cancer specific survival was performed by generating Kaplan–Meier curves including glioma related deaths. Non-disease deaths were censored [3].
To compare the risk of death from disease prior to transition to adulthood (age 22 years) versus after transition to adulthood, a time-dependent covariate for the occurrence of a patient's 22nd birthday was included in the Cox model. The age of 22 was chosen to allow for the examination of the natural history of PLGG after the age at which the majority of brain maturation during the transition from adolescence to adulthood has been completed [14].
RESULTS
A total of 4,040 patients aged ≤19 years of age (median age at diagnosis: 9 years, range 0–19 years) at time of diagnosis of a PLGG between 1973 and 2008 were identified (Table I). The 30-year OS of the 4,040 patients was 74.8% ± 2%, including all causes of death. We were unable to determine progression-free survival from the available data. Of the 4,040 patients, 347 patients died as a result of PLGG and 78 patients died of other causes. Cause of death was unknown in 17 patients. Median time of follow-up was 6.9 (0–36.5) years. Importantly, the cohort included 875 patients with at least 15 years of follow-up from diagnosis.
TABLE I.
Demographics of Pediatric Patients in Cohort
| Category | Number (%) |
|---|---|
| Gender | |
| Male | 2,059 (51) |
| Female | 1,981 (49) |
| Age (median) | 9 years |
| Histology | |
| Pilocytic astrocytoma | 2,648 (65) |
| Astrocytoma NOS | 841 (21) |
| NOS grade I | 227 |
| NOS grade II | 614 |
| Diffuse astrocytoma | 260 (6) |
| Glioma NOS (total) | 189 (5) |
| Grade I | 68 |
| Grade II | 121 |
| Mixed glioma (total) | 75 (2) |
| Grade I | 16 |
| Grade II | 59 |
| Unique astrocytoma variant | 27 (1) |
| Grade I | 12 |
| Grade II | 15 |
| Grade (all histologies) | |
| Grade I | 2,971 (74) |
| Grade II | 1,069 (26) |
| Location | |
| Supratentorial | 1,264 (31) |
| Ventricular | 189 (5) |
| Cerebellum | 1,170 (29) |
| Brainstem | 504 (12) |
| Overlapping or NOS | 718 (18) |
| Spinal cord | 195 (5) |
| Extent of resection | |
| No resection | 373 (9) |
| Biopsy | 9 (0.2) |
| Subtotal resection | 1,769 (44) |
| Gross total | 1,094 (27) |
| Not otherwise specified | 593 (15) |
| Unknown | 202 (5) |
| Radiation | |
| No radiation therapy | 3,235 (80) |
| Radiation therapy | 736 (18) |
| Unknown | 69 (2) |
| Number of deaths | 442 (11) |
| PLGG | 347 |
| Non-PLGG | 78 |
| Unknown | 17 |
The Kaplan–Meier estimate of overall cancer specific OS at 20 years was 87% ± 0.8% (Fig. 1A), and the 20-year overall survival of the 875 patients for which there was at least 15 years follow-up was 92% ± 3% (Fig. 1B). Pepe–Mori 20-year cumulative incidence of death due to disease was 12% ± 0.8% (Fig. 1C). We examined the overall survival of adult survivors of pediatric low-grade gliomas by calculating OS after transition to adulthood. This analysis included children diagnosed with all subtypes of PLGG. After reaching adulthood (defined as age 22), survivors of PLGG (including all histological subtypes) had a 30-year OS of 93% ± 1.7 (Fig. 1D). These patients had a 20-year cumulative incidence of death due to disease of 7% ± 1.8% (Fig. 1E). For patients prior to reaching their 22nd birthday, the risk of death due to disease is slightly higher (HR = 1.37, P = 0.24) than the risk of death from any cause (HR = 1.20, P-value = 0.40) in comparison to patients after age 22 years, though not significantly higher in either case.
Fig. 1.
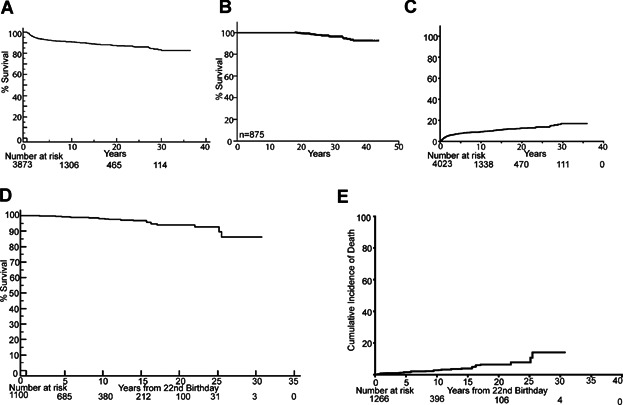
Adult survivors of pediatric low-grade gliomas have excellent overall survival with low rates of mortality after patients transition into adulthood. A: Kaplan–Meier overall survival curve of patients with PLGG including only tumor related deaths. B: Kaplan–Meier overall survival curve of patients for which there is at least 15 years of follow-up. C: Pepe–Mori cumulative incidence of tumor specific death curve of patients diagnosed with PLGG. D, A: Kaplan–Meier overall survival curve of patients with PLGG showing survival starting from the patient's 22nd birthday. E: Pepe–Mori cumulative incidence of tumor specific death curves of patients starting from patient's 22nd birthday.
Univariate analysis of risk of death identified age <2 years at diagnosis (P < 0.0001, Fig. 2A), year of diagnosis between 1970 and 1989 (P < 0.0001, Fig. 2B), histology other than pilocytic astrocytoma (P < 0.0001, Fig. 2C), tumor site other than cerebellum (P < 0.0001, Fig. 2D) and WHO grade II (P < 0.0001, Fig. 2E) as prognostic of increased risk of death. There was no significant difference in overall survival between children who had a gross total surgical resection and children with residual disease (P = 0.4, Fig. 2F).
Fig. 2.
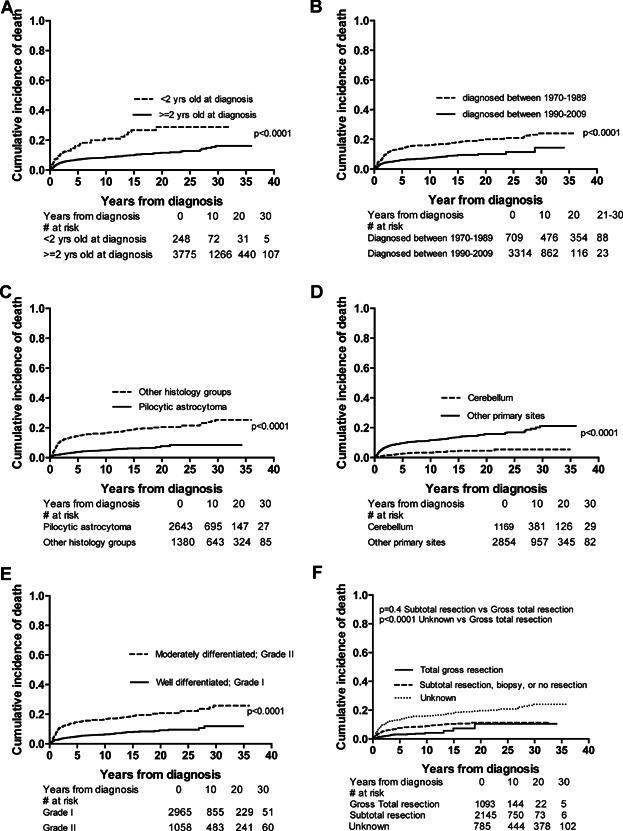
Pepe–Mori cumulative incidence of death curves depicting univariate analysis including A, age at diagnosis B, year of diagnosis C, histology D, location of primary tumor E, grade and F, extent of resection. Number of patients at each time point are shown below.
We performed a multivariate Cox model analysis (Table II) and identified an increased risk of cancer-specific death in children who were treated with radiation therapy (HR = 3.9, P < 0.0001). Children who did not have cerebellar disease (HR = 2.3, P < 0.0001) or had non-pilocytic astrocytoma histology (HR = 2.2, P < 0.0001) also had increased risk of disease-related death. Despite this, children with all histological subtypes of pediatric low-grade gliomas still had an excellent long-term overall survival (Fig. 3). Children who were less than 2 years of age had greater risk of disease-related death compared to older children (HR = 2, P < 0.0001). Although not significant on univariate analysis, patients with subtotal or no resection had a small but statistically significant increase in risk of disease-related death compared to those with total resection on multivariate analysis (HR = 1.5, 95% CI: 1.01, 2.1, P = 0.04).
TABLE II.
Multivariate Analysis of Risk Factors in Patients With Pediatric Low-Grade Glioma
| Death due to disease |
Death from non-disease causes |
|||
|---|---|---|---|---|
| Factor (reference level) | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Beam radiation, with or without implants or isotopes (no radiation) | 3.9 (3.0, 4.9) | <0.0001 | 2.4 (1.4, 3.9) | 0.0006 |
| Degree of radiation unknown (no radiation) | 3.1 (1.7, 5.8) | 0.0003 | NA | 1.0 |
| Primary site (cerebellum) | 2.3 (1.6, 3.2) | <0.0001 | NA | 1.0 |
| Histology group (pilocytic astrocytoma) | 2.2 (1.7, 2.8) | <0.0001 | NA | 0.05 |
| Age of diagnosis (≥2 years old) | 2.0 (1.5, 2.8) | <0.0001 | 2.2 (1.1, 4.5) | 0.03 |
| Subtotal resection, biopsy or no resection (total resection) | 1.5 (1.01, 2.1) | 0.04 | 3.0 (1.1, 8.6) | 0.04 |
HR, hazard ratio (increased risk of death due to disease for the Factor in comparison to the reference level); CI, confidence interval.
Fig. 3.
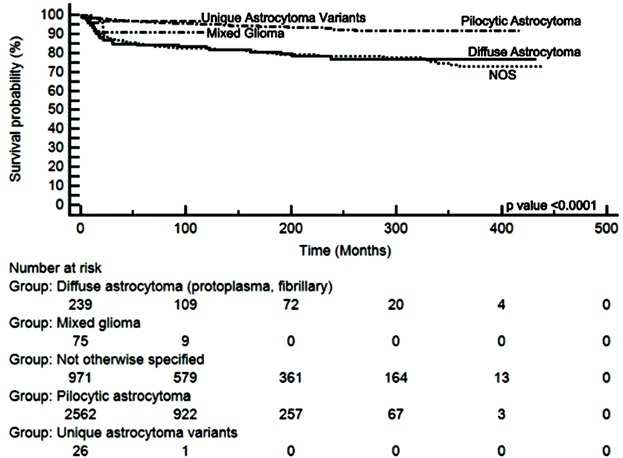
Patients with all histological subtypes of PLGG have excellent very long-term overall survival. Kaplan–Meyer curves of outcomes of patients diagnosed with different histological subtypes of PLGG.
Treatment with radiation therapy was an adverse prognostic factor. Regardless of the extent of surgical resection, children who were treated with radiation therapy had a worse OS and greater risk of disease death compared to those that were not treated with radiation therapy (HR = 3.9, P < 0.0001; Fig. 4, respectively). Within each treatment group (radiation or no radiation), there was no difference in OS or cumulative incidence of disease death in children who had received a gross total resection in comparison to children who had residual tumor. Further, children in whom only a subtotal resection was achieved but did not undergo radiation had a significantly superior outcome compared to children who had a gross total resection but treated with radiation. The hazard ratio of death due to glioma in patients who were treated with radiation was 3.9 (P-value < 0.0001). Data were not available in the SEER database to permit a determination of the incidence of radiation-induced secondary malignancies; however the hazard ratio of death due to non-glioma related causes of 2.4 (P-value = 0.0006) in patients treated with radiation suggests that radiation-induced mortality may have accounted for some of the deaths that were not directly due to glioma.
Fig. 4.
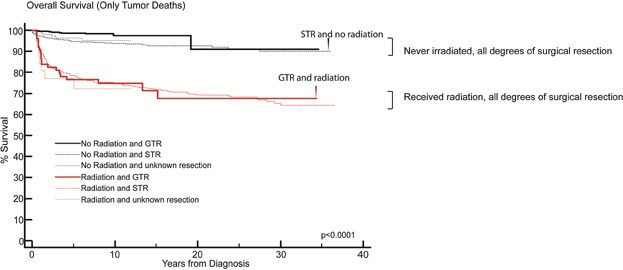
Patients with PLGG who received radiation therapy have inferior overall survival compared to patients that did not receive radiation therapy. Kaplan–Meier overall survival curve of patients with PLGG including only tumor related deaths showing outcomes of children who received radiation therapy and extent of surgical resection. Patients with a STR who did not receive radiation (curve shown by black arrow) had a superior outcome than patients with a GTR who did receive radiation (curve shown by red arrow).
The risk of death due to disease was slightly higher for patients before age 22 compared to after age 22 (hazard ratio = 1.37), although not statistically significant (P = 0.24). The risk of death from any cause was slightly higher for patients before age 22 compared to after age 22 (hazard ratio = 1.20), although not statistically significant (P = 0.40). Within the group that had received radiation, the hazard ratio of dying from causes all causes compared to patients less than 22 years of age was 1.59 (P-value = 0.1) and from tumor 1.74 (P-value = 0.1).
DISCUSSION
Our outcome analysis is the largest ever reported for very long-term survivors of PLGGs, and confirms the excellent prognosis of PLGG. While it is known that patients with PLGGs have superior 5- and 10-year OS [15,16] the data presented here confirm for the first time that adult survivors of pediatric disease have a low incidence of glioma related death. Importantly this highlights that PLGGs are very unlikely to undergo malignant transformation resulting in tumor-related death, which contrasts with the natural history of ALGGs [1,3]. Further, the data show that children who are treated with radiation therapy, regardless of their surgical status, have significantly inferior outcomes compared to those children who are not treated with radiation therapy.
We have found that children who were treated with radiation had inferior outcomes. It is however important to acknowledge that it is difficult to determine from the SEER data how much of this is due to radiation itself and how much is a reflection of selection bias. While some degree of selection bias is likely, the finding that children in whom a gross total resection was achieved and were irradiated had an inferior outcome compared to those children with a subtotal resection who were not irradiated suggests that radiation induced mortality (rather than PLGG related mortality) also accounts for a number of these deaths. The HR of dying from disease after radiation was 3.9 and the HR of dying from non-glioma related disease after radiation was 2.4. These data suggest that the observed increased in mortality in children treated with radiation therapy is likely to be a combination of both selection bias (i.e., children with harder to control disease are more likely to have received radiation) and radiation induced mortality, such as the increased risk for secondary malignancies and vasculopathy. Due to the inherent limitations associated with SEER database, we are unable to determine the proportion of children who were treated with radiation after failing chemotherapeutic approaches.
GTR of PLGGs have been associated with superior event free and overall survival [16]. In the SEER cohort of PLGG we have found that children who do not have a GTR also have an excellent overall survival. This analysis included all histological subtypes of pediatric low-grade gliomas. Thus, the aim of surgery for these children should be to resect the maximal amount of tumor that can be safely resected while minimizing the risk of long-term neurological sequalae.
The overall long-term survival of patients diagnosed with PLGGs including all causes of death is superior to the outcomes of patients diagnosed with glioma as adults [3]. Our analysis, using two methodologies to examine disease-specific deaths of patients with PLGG, confirms that PLGG infrequently causes death in adult survivors. In fact, the risk of death decreases once patients reach adulthood.
Our study reports outcomes of grade II low-grade gliomas in a large SEER-based cohort of pediatric patients, and our finding of excellent overall survival in this cohort has important clinical implications. Many physicians have been concerned that residual grade II PLGGs may undergo malignant transformation later in life, and have thus advocated potentially morbid therapies including radical surgical resection and/or radiation therapy. However, in our series, the majority of children with grade II PLGGs also had excellent overall survival, and we did not observe increased rates of death with time as have been reported in low-grade gliomas that arise in adults, and which are prone to undergo malignant transformation.
Unfortunately, data to perform progression-free survival analysis are not available from the SEER database; however, the observation of a very low mortality in adult survivors supports the hypothesis that PLGG become stable, possibly quiescent as children transition into their early adult years. It is possible that loss of follow-up of adult survivors of PLGG may have affected outcome measures in this study and may result in under-estimation of overall survival as adult survivors who are well and not requiring medical assistance may not be captured in follow-up data while adult survivors who have disease recurrence and subsequent death are more likely to have been reported.
The confirmation that the majority of adult survivors of PLGG do not eventually succumb to their disease is an important observation that must be taken into account when considering therapeutic options for children with low-grade gliomas. Given these children are expected to survive into adulthood, the aim of treatment should be to control disease and minimize long-term morbidity from both the tumor itself and treatment. This can be achieved with surgery if the tumor can be safely resected without resulting in neurological sequelae, chemotherapy or novel targeted therapeutic agents. Treatment strategies should aim to minimize treatment induced long-term morbidity such as those associated with radiation therapy, including neuro-cognitive deficits [17–20], hormone deficiencies and secondary malignancies [20,21]. Although some groups are currently using radiation therapy as first-line therapy [22], the data we present suggest that treatment with radiation therapy should be reserved for the small number of children in whom tumor control cannot be achieved with surgery, chemotherapy or targeted agents.
These data confirm that the natural history of PLGGs is distinct from their adult counterparts [1,3], and it is likely that this is a reflection of differences in the underlying biology of the tumors. Although histologically these tumors have the same characteristics in children and adults, the higher rate of genetic alterations observed in ALGGs compared to pediatric low-grade gliomas suggest a difference in genomic stability. While mutations of IDH [23] and p53 [24] are reported in ALGGs and predict malignant transformation, these are rare in their pediatric counterparts, which more commonly harbor alterations of BRAF [25–28]. While the propensity of ALGGs to slowly progress and undergo malignant transformation is secondary to the acquisition of driver mutations, it remains to be determined why PLGGs do not also acquire these alterations with time to cause transformation. It can be hypothesized that normal processes that guide development and maturation of the brain, for example, epigenetic processes, may cause PLGGs to become quiescent as children transition into adulthood. The factors that govern the stability of PLGG remain unknown.
There are limitations to the data obtainable from the SEERs database. In particular, the quality of the data extracted is dependent on how the data are entered into the database, histology and radiology results have not been centrally reviewed, and interpretation of the grading of low-grade gliomas according to the current WHO classification can vary. The limitations of the WHO classification are highlighted by the finding that a significant proportion of PLGG in the SEER database are designated as “not otherwise specified,” rather than given a distinct classification according to the WHO criteria. This suggests a need for better molecular markers to diagnose and stratify these gliomas. Further, it is possible that some of the tumors that have been designated as pediatric low-grade gliomas not otherwise specified may have represented higher-grade gliomas, thus accounting for the inferior survival of those children whose tumors could not be classified according to the WHO classification. This may have resulted in an over-estimation of the actual death rate of children with true low-grade gliomas.
In addition, the SEER database lacks information about chemotherapy regimens that were used, and how many courses of chemotherapy children were treated with prior to radiation therapy. This information would have been important for those patients in whom a subtotal resection was achieved, in determining whether radiation therapy was used more frequently in tumors in which control had not been achieved with radiation therapy.
In conclusion, we show that PLGGs are not the cause of death in the majority of adult survivors and that patients diagnosed with PLGGs have excellent long-term survival. This holds true for both grade (I or II) and degree of resection (GTR or STR) in pediatric low-grade gliomas. Therapeutic strategies should be designed to provide tumor control while avoiding those that cause irreversible long-term toxicity should be avoided as patients can be expected to survive long into adulthood.
Acknowledgments
Support for this study included the Stop&Shop Pediatric Brain Tumor Program (PB, NR, SNC, MWK, PEM), Andrysiak Fund for LGG (PB, MWK, PEM), Pediatric Low-Grade Astrocytoma Foundation (PB, GB, RB, MWK), Friends of DFCI (PB), Nuovo-Soldati Foundation (GB), Philippe Foundation (GB), and St Baldrick's Foundation (AMLM). This work has been presented in part at the 13th International Conference of Long-Term Complications of Treatment of Children and Adolescents for Cancer, Memphis, TN, 2013.
REFERENCES
- 1.Rees J, Watt H, Jager HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72:54–64. doi: 10.1016/j.ejrad.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Youland RS, Brown PD, Giannini C, et al. Adult low-grade glioma: 19-year experience at a single institution. Am J Clin Oncol. 2013;36:612–619. doi: 10.1097/COC.0b013e31825d580a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DR, Brown PD, Galanis E, et al. Pilocytic astrocytoma survival in adults: Analysis of the surveillance, epidemiology, and end results program of the National Cancer Institute. J Neurooncol. 2012;108:187–193. doi: 10.1007/s11060-012-0829-0. [DOI] [PubMed] [Google Scholar]
- 4.Gunny RS, Haywood RD, Phipps KP, et al. Spontaneous regression of residual low-grade cerebellar pilocytic astrocytomas in children. Pediatr Radiol. 2005;35:1086–1091. doi: 10.1007/s00247-005-1546-z. [DOI] [PubMed] [Google Scholar]
- 5.Rozen WM, Joseph S, Lo PA. Spontaneous regression of low-grade gliomas in pediatric patients without neurofibromatosis. Pediatr Neurosurg. 2008;44:324–328. doi: 10.1159/000134925. [DOI] [PubMed] [Google Scholar]
- 6.Perilongo G, Moras P, Carollo C, et al. Spontaneous partial regression of low-grade glioma in children with neurofibromatosis-1: A real possibility. J Child Neurol. 1999;14:352–356. doi: 10.1177/088307389901400602. [DOI] [PubMed] [Google Scholar]
- 7.Schmandt SM, Packer RJ, Vezina LG, et al. Spontaneous regression of low-grade astrocytomas in childhood. Pediatr Neurosurg. 2000;32:132–136. doi: 10.1159/000028917. [DOI] [PubMed] [Google Scholar]
- 8.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 9.Afra D, Osztie E, Sipos L, et al. Preoperative history and postoperative survival of supratentorial low-grade astrocytomas. Br J Neurosurg. 1999;13:299–305. doi: 10.1080/02688699943727. [DOI] [PubMed] [Google Scholar]
- 10.Parsa CF, Givrad S. Juvenile pilocytic astrocytomas do not undergo spontaneous malignant transformation: Grounds for designation as hamartomas. Br J Ophthalmol. 2008;92:40–46. doi: 10.1136/bjo.2007.125567. [DOI] [PubMed] [Google Scholar]
- 11. National Cancer Institute, Surveillance, Epidemiology and End Results (SEER) program SEER*Stat database:incidence: SEER 17 Regs research data + hurricane katrina impacted Louisiana cases, Nov 2010 Sub (1973–2008 varying): Linked to County Attributes, DCCPS, Surveillance Research Program, Cancer Statistics Branch. 2011.
- 12.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data. Stat Med. 1993;12:737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 13.Rosthoj S, Andersen PK, Abildstrom SZ. SAS macros for estimation of the cumulative incidence functions based on a Cox regression model for competing risks survival data. Comput Methods Programs Biomed. 2004;74:69–75. doi: 10.1016/S0169-2607(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 14.Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 15.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265–1284. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keene DL, Johnston DL, Grimard L, et al. Vascular complications of cranial radiation. Childs Nerv Syst. 2006;22:547–555. doi: 10.1007/s00381-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 18.Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43:3035–3040. doi: 10.1161/STROKEAHA.112.661561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: The experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14:298–303. doi: 10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galloway TJ, Indelicato DJ, Amdur RJ, et al. Second tumors in pediatric patients treated with radiotherapy to the central nervous system. Am J Clin Oncol. 2012;35:279–283. doi: 10.1097/COC.0b013e318210f533. [DOI] [PubMed] [Google Scholar]
- 22.Müller K, Gnekow A, Falkenstein F, et al. Radiotherapy in pediatric pilocytic astrocytomas. A subgroup analysis within the prospective multicenter study HIT-LGG 1996 by the German Society of Pediatric Oncology and Hematology (GPOH) Strahlenther Onkol. 2013;189:647–655. doi: 10.1007/s00066-013-0357-7. [DOI] [PubMed] [Google Scholar]
- 23.Juratli TA, Kirsch M, Robel K, et al. IDH mutations as an early and consistent marker in low-grade astrocytomas WHO grade II and their consecutive secondary high-grade gliomas. J Neurooncol. 2012;108:403–410. doi: 10.1007/s11060-012-0844-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177:2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacConaill LE, Campbell CD, Kehoe SM, et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS ONE. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549: BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian Y, Rich BE, Vena N, et al. Detection of KIAA 1549-BRAF fusion transcripts in formalin-fixed paraffin-embedded pediatric low-grade gliomas. J Mol Diagn. 2011;13:669–677. doi: 10.1016/j.jmoldx.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]


