Abstract
Significance: Oxidative stress is suggested to be a disease mechanism common to a wide range of disorders affecting human health. However, so far, the pharmacotherapeutic exploitation of this, for example, based on chemical scavenging of pro-oxidant molecules, has been unsuccessful. Recent Advances: An alternative emerging approach is to target the enzymatic sources of disease-relevant oxidative stress. Several such enzymes and isoforms have been identified and linked to different pathologies. For some targets, the respective pharmacology is quite advanced, that is, up to late-stage clinical development or even on the market; for others, drugs are already in clinical use, although not for indications based on oxidative stress, and repurposing seems to be a viable option. Critical Issues: For all other targets, reliable preclinical validation and drug ability are key factors for any translation into the clinic. In this study, specific pharmacological agents with optimal pharmacokinetic profiles are still lacking. Moreover, these enzymes also serve largely unknown physiological functions and their inhibition may lead to unwanted side effects. Future Directions: The current promising data based on new targets, drugs, and drug repurposing are mainly a result of academic efforts. With the availability of optimized compounds and coordinated efforts from academia and industry scientists, unambiguous validation and translation into proof-of-principle studies seem achievable in the very near future, possibly leading towards a new era of redox medicine. Antioxid. Redox Signal. 23, 1113–1129.
Introduction
Oxidative stress is the production of reactive oxygen species (ROS) to high nonphysiological concentrations or at nonphysiological locations. Mechanistically, this can lead to DNA damage, lipid peroxidation (72), protein modification, and other pathological effects observed in various chronic disorders, including neurodegenerative, cardiovascular and diabetes-associated renal diseases, and cancer. Many therapeutic attempts to improve patient-relevant outcomes using exogenous small-molecule antioxidants, such as vitamins C and E, have failed (38) or even increased mortality (101) such as in the settings of diabetes mellitus (168, 169).
Possible explanations for this paradox may reside in the lack of specificity of antioxidants towards a certain cellular compartment or tissue, and/or the possibility of generating reductive stress, by increasing levels of reducing agents and therefore disturbing redox homeostasis in the opposite direction. Exogenous antioxidants are also likely to interfere with both disease-triggering and physiological ROS levels. The latter regulate extracellular matrix, control vasomotor activity, are involved in the innate immune response, and promote cell differentiation, proliferation, and migration (4, 10, 161, 163).
Another somewhat indirect type of antioxidant therapeutic strategy that could have fewer side effects relies on the activation of endogenous antioxidant responses. In this context, pharmacological activation of the transcription factor NRF2 is promising therapeutic option currently studied clinically. The conceptual difference between these two antioxidant approaches is broad unspecific scavenging versus a localized response at physiological (sub)cellular sites. Only the latter has promise in leaving physiological ROS formation and signaling intact.
Of far broader relevance is a third approach that involves the specific inhibition of the disease-relevant sources of ROS. In this case, the key question is which enzyme to target. Besides NADPH oxidases (NOXs) (10), xanthine oxidase (XO) (96), uncoupled nitric oxide synthase (uc-NOS) (155), and monoamine oxidases (MAOs) (39), other sources such as cytochrome P450 oxidases (44), lipoxygenases (170), and the mitochondrial electron transport chain (134) are all able to generate ROS. Among these, NOXs stand out as their primary function is to produce ROS. All other enzymes do not form ROS as their primary function, but only as a collateral or side product. Examples include uc-NOS, uncoupled mitochondria, and XO. Additional approaches include the inhibition of ROS-toxifying peroxidases, such as myeloperoxidase (MPO), or the functional repair of oxidatively damaged proteins, such as the redox-sensitive soluble guanylate cyclase (sGC), a principle that has already entered the clinic.
We here review the current status and outlook of the most advanced areas in the field of translational redox medicine by focusing on drugs in four categories:
• Activators of endogenous antioxidant defense systems (indirect antioxidants)
• Inhibitors of ROS formation
• Inhibitors of ROS toxification
• Compounds that allow functional repair of ROS-induced damage
Activators of Antioxidant Defense Systems
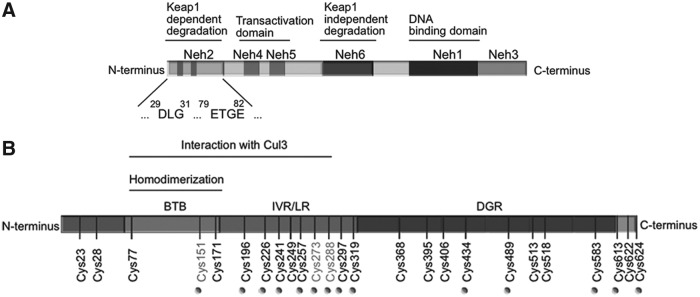
The main, if not only, representative members of this group of drugs are nuclear factor (erythroid-derived 2)-like 2 (NRF2) activators. NRF2 is a basic region-leucine zipper (bZIP) transcription factor (Fig. 1A) that forms heterodimers with other bZIP partners, of which the small musculoaponeurotic fibrosarcoma proteins are the best characterized. Together, they recognize an enhancer sequence termed Antioxidant Response Element (ARE) that is present in the regulatory regions of over 250 genes (ARE genes), including antioxidant genes such as HMOX1 (coding heme oxygenase-1) (58). These genes encode enzymes involved in antioxidant reactions, including those driven by glutathione and thioredoxin, generation of nicotinamide adenine dinucleotide phosphate (NADPH), biotransformation, proteostasis, and even DNA repair (58, 90, 135).
FIG. 1.
Domain structures of NRF2 and KEAP1. (A) Domain structure of NRF2. NRF2 possesses six highly conserved domains called NRF2-ECH homology (Neh) domains (105). The functional role of each Neh domain is specified. Within the Neh2 domain, the low-affinity (DLG) and high-affinity (ETGE) binding domains to KEAP1 are zoomed in. (B) Domain structure of a KEAP1 monomer showing the position of cysteine residues. The N-terminal BTB (bric-a-brac, tramtrack, broad-complex) domain participates in homodimerization and binding to Cul1/Rbx. The C-terminal region, Kelch repeat, DGR domain, contains a WD40 propeller that binds NRF2 at its Neh2 domain. The intervening region connects BTB and DGR domains and is particularly rich in redox-sensitive cysteine residues. C151 is targeted by some electrophiles (tert-butylhydroquinone, diethylmaleate, sulforaphane, and dimethylfumarate; see Fig. 2) disrupting the KEAP1-Cul3 interaction. Other important cysteines are C272 and C288 that react with other compounds (15-deoxy-Δ12,14-prostaglandin J2, 2-cyano-3,12 dioxooleana-1,9 diene-28-imidazolide, ebselen, and cadmium chloride; see Fig. 2) leading to a conformational distortion of the DC domain and altering the KEAP1-NRF2 interaction (147). BTB, (bric-a-brac, tramtrack, broad-complex); DGR, double glycine repeat; DLG, aspartate leucine glycine; ETGE, glutamate, threonine, glycine, glutamate; KEAP1, kelch-like ECH-associated protein 1; Rbx, ring box protein.
The main mechanism of regulation of NRF2 transcriptional activity is through control of protein stabilization by the E3 ligase adapter Kelch-like ECH-associated protein 1 (KEAP1). This is a homodimeric zinc (Zn) finger protein that bridges NRF2 with the E3 ligase complex formed by Cullin3 and Rbx proteins (Cul3/Rbx). Under homeostatic conditions, the N-terminal domain of the KEAP1 homodimer binds one molecule of NRF2 at two amino acid sequences of low (aspartate leucine glycine) and high glutamate, threonine, glycine, and glutamate (ETGE) affinity, thus presenting NRF2 to ubiquitination by Cul3/Rbx (152). However, in the presence of ectopic or endogenous electrophiles, KEAP1 is inactivated.
Mechanistically, electrophiles modify sulfhydryl groups of specific redox-sensitive cysteines of KEAP1, including C151, C273, and C288 (Fig. 1B). These modifications of KEAP1 lead to changes in NRF2 recognition, alterations in dimer conformation, or interaction with Cul3/Rbx. As a result, NRF2 escapes KEAP1-dependent degradation, accumulates in the nucleus, and activates ARE genes.
KEAP1 is one of the best-suited proteins to act as an electrophilic/redox sensor as it contains a large number of cysteine residues (27 in the human protein) and can function as an electrophile trap. However, other proteins such as phosphatase and tensin homolog (PTEN), which is mutated in a large number of human tumors, are also redox sensitive (55, 79, 84) and affect NRF2 activity. The catalytic C124 residue of PTEN can be modified through adduct formation with strong electrophiles such as synthetic triterpenoids (2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-imidazolide; CDDO-Im) (114) and tert-butylhydroquinone (121). This modification results in loss of the PTEN lipid phosphatase activity and yields a more sustained activation of signaling events downstream of phosphoinositide 3-kinase, leading to NRF2 activation by a KEAP1-independent mechanism (117, 118). Thus, electrophilic targeting of NRF2 may involve not only KEAP1 but also other redox-sensitive enzymes. Moreover, KEAP1 interacts with other proteins that also contain the high-affinity binding motif, ETGE (57), such as inhibitor of nuclear factor kappa-B kinase subunit beta and Bcl-2 (78, 109). Hence, some results obtained from KEAP1 mutant or -deficient cells may not be necessarily related to the control of NRF2.
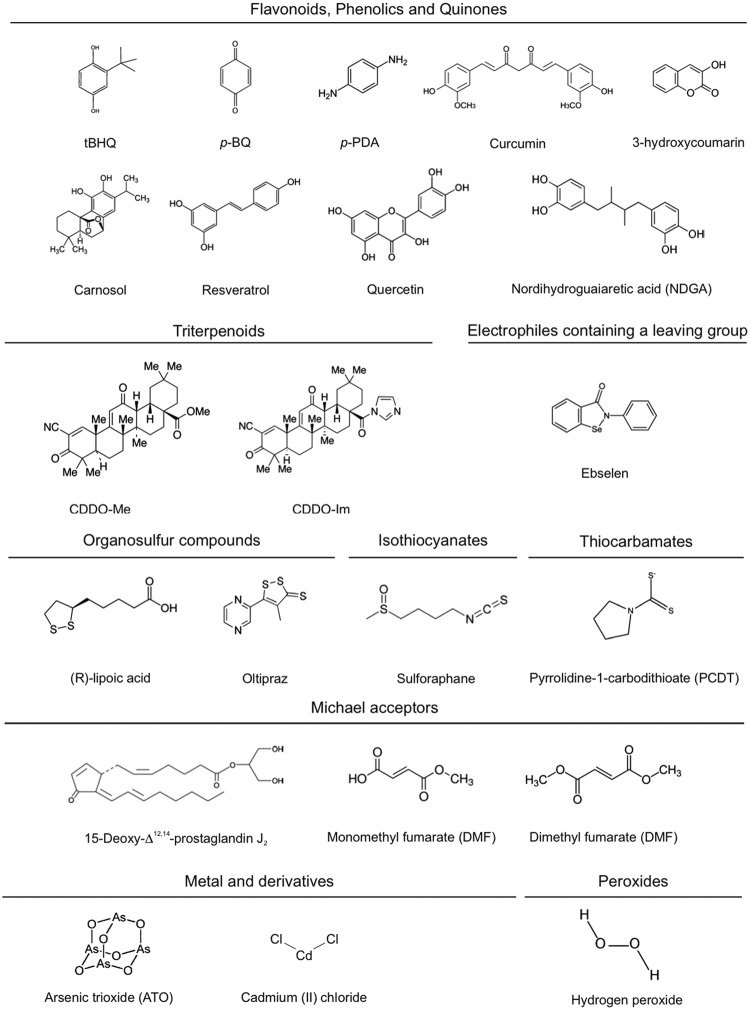
Several groups of electrophilic compounds induce NRF2 in cell culture and less frequently in animals or humans (120) [Fig. 2; for a detailed list of KEAP1 ligands, see refs. (37, 59, 91)]. Many of these compounds are used as nutraceuticals, and for some of them, there is evidence of clinical efficacy. The most successful drug of this type is the ester derivative of fumaric acid, dimethyl fumarate (DMF) (87). DMF crosses the gastrointestinal barrier where it is converted into monomethyl fumarate. The first clinical use of DMF was for the topical treatment of psoriasis in 1994 (5). More recently, an oral formulation of DMF, known as BG12, was commercialized for the treatment of relapsing–remitting multiple sclerosis (14, 76). Other autoimmune diseases such as lupus erythematosus, asthma, and arthritis are under investigation with other formulations of fumarate esters (128, 153).
FIG. 2.
Molecular formulas of some common redox-active compounds. These compounds are capable of modifying protein cysteine thiols by oxidation, reduction, alkylation, and metal chelation and presumably disrupt the KEAP1/NRF2 interaction. The classification has been simplified from Ma et al. (91).
Other lines of research have focused on targeting NRF2 in degenerative diseases where low-grade chronic inflammation is present. One very potent synthetic triterpenoid, CDDO-methyl ester, bardoxolone methyl, has been studied in great detail for treatment of diabetic nephropathy (157). The initial excitement about this compound was set back by a small yet significant increase in the risk of heart failure. Importantly though, this effect appears not to be related to NRF2 targeting, but rather to alteration of endothelin signaling, leading to reduction in urine volume and sodium excretion in some patients with advanced chronic kidney disease (26). Bardoxolone methyl is now being studied in new indications for pulmonary arterial hypertension, melanoma, and Friedreich's ataxia.
A third NRF2 inducer that has reached the level of clinical studies is the isothiocyanate sulforaphane (SFN) isolated from broccoli sprout extracts. Contrary to DMF and bardoxolone methyl, a drawback of this compound is the absence of a pure formulation that could be used clinically and the lack of commercial value. Nevertheless, SFN provided proof of concept that NRF2 targeting has a therapeutic potential (40, 129, 132). Furthermore, NRF 2 agonists in clinical development are summarized in Table 1.
Table 1.
NRF2 Activators in Clinical Development
| Compound | Chemical characteristics | Pathology | Outcome | Current situation |
|---|---|---|---|---|
| (DMF) | Methyl ester of fumaric acid | Relapsing–remitting multiple sclerosis | Phase III clinical trial showed that DMF presents anti-inflammatory activity and protects against oxidative injury. Patients have fewer relapses and improved MRI measures of disease activity (75). | TECFIDERA approved in USA, Canada, Australia, Switzerland, and the European Union. |
| Rheumatoid Arthritis | Finished last 2010. No data available. | — | ||
| FP187 | DMF combined with three other fumaric acid esters. Better pharmacological characteristics. | Psoriasis | Still open. No data available. | Phase III clinical trial ongoing. |
| ALKS8700 | Monomethyl fumarate. | — | Evaluate the safety, tolerability, and pharmacokinetics in healthy adults. | Still open, recruiting patients. |
| RTA402 | Acid oleanolic derivate. Semisynthetic triterpenoid. | Type 2 diabetes mellitus. Stage 4 chronic kidney disease. | Phase III clinical trial showed that RTA402 did not reduce the risk of end-stage renal disease or death. High rate of cardiovascular events (34). | Prompted termination of the trial. |
| RTA408 | Next-generation synthetic triterpenoid. | Radiation dermatitis and Friedreich's ataxia. | Phase II clinical trial. | Still open, recruiting patients. |
DMF, dimethyl fumarate; NRF2, nuclear factor (erythroid-derived 2)-like 2.
Inhibitors of ROS-Forming Enzymes
NADPH oxidase inhibitors
NOXs are transmembrane proteins comprising seven members (NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2). Each NOX isoform has specific tissue expression and regulation (10, 85). The catalytic core of all NOXs contains four conserved C-terminal NADPH-binding subregions and two flavin adenine dinucleotide (FAD)-binding subregions, as well as four conserved histidine residues, which coordinate two nonidentical iron heme prosthetic groups located between transmembrane domains 3 and 5. They are commonly referred to as the inner and outer heme, depending on their proximity to the cytosol and extracellular space, respectively. NOX activity was first described in neutrophils (7) where it forms superoxide anion radical (O2−) as part of the phagocytic oxidative burst of the innate immune response (65). All NOX enzymes catalyze the reduction of extracytosolic oxygen (i.e., in phagosomes, endosomes, or the extracellular space) with cytosolic NADPH serving as an electron donor. The activity of most NOX isoforms is tightly regulated: NOX1, NOX2, and NOX3 require the presence of cytosolic proteins, while NOX4 generates ROS in a constitutive manner; NOX5 and DUOX isoforms require increased cellular Ca2+ concentrations and binding to N-terminal EF-hand domains for full activity. NOX5 is also a notable exception with respect to preclinical target validation; it is present in several mammals, including humans, but not in mice and rats. Increasing evidence shows that inhibition of NOX activity can be beneficial in multiple models of human diseases [for details, see Casas et al. in the same Forum (24)]. In addition, NOX2-derived ROS can have anti-inflammatory effects under certain conditions such as rheumatoid arthritis and multiple sclerosis (67). This provides a rationale for the development of NOX activators. Many advantages and pitfalls of currently available NOX inhibitors have been recently comprehensively reviewed in (4) and (69). In this study, we focus on three chemical compound families, with one compound currently in clinical development (Table 2).
Table 2.
NADPH Oxidase Inhibitors: Chemical Properties and Treatment Indications (4)
| Compound | Chemical structure | Drug properties | Basic mechanism of action | Pathology |
|---|---|---|---|---|
| GKT136901 | Pyrazolopyridine dione derivate | Peroxynitrite scavenger activity oral administration | NOX1, NOX4, NOX5, DUOX inhibitor | Stroke (unpublished preclinical data) |
| GKT137831 | Pyrazolopyridine dione derivate | Better pharmacokinetic profile than GKT136901 oral administration | NOX1, NOX4, NOX5 inhibitor | Type 2 diabetes mellitus associated with diabetic nephropathy (50a, 70). |
| VAS2870 | Triazolo pyrimidine derivate | Low solubility, poor pharmacokinetic profile. Potential off-target effect leading to thioalkylation of cysteine residues. | NOX2, NOX4, NOX5 inhibitor | Stroke (preclinical data) (81) |
| VAS3947 | Triazolo pyrimidine derivate | Better solubility than VAS2870 with similar NOX inhibitor profile. | NOX2, NOX4, NOX5 inhibitor | — |
NO, nitric oxide; NOX, reduced nicotinamide adenine dinucleotide phosphate oxidase.
GKT136901 and GKT137831 were developed by GenKyoTex to explore structure–activity relationship along pyrazolopyridine dione compounds (82). These compounds were selected based on oral bioavailability and beneficial pharmacokinetic parameters (4, 70). They block NOX1, NOX4, NOX5, and DUOX (142) activity in the micromolar range. In terms of off-targets effects, GKT136901 also scavenges peroxynitrite (125), but no interference was identified with other redox-sensitive enzymes, G-protein-coupled receptors, kinases, ion channels, or other enzyme activity (4). However, GKT136901 interacts with Amplex Red fluorescence and dose dependently decreases the signal, thereby complicating the interpretation of in vitro results (4). Preclinical results show that GKT137831 reduces glomerular injury and structural changes, as well as macrophage infiltration and proinflammatory transcription factor expression, in models of diabetic nephropathy (54, 70). GKT137831 has entered a clinical study phase II clinical trial, testing its efficacy in diabetic type 2 patients with diabetic nephropathy (50a) (study completed 2015).
Vasopharm developed the triazolo pyrimidine, VAS2870, following a screening approach for NOX2 inhibitors (139, 148). Its derivative, VAS3947, was later generated to slightly improve VAS solubility while keeping a similar NOX inhibitory profile.
Both compounds are able to inhibit different NOX isoforms, such as NOX2 (4), NOX 4, and NOX5 (3, 81), in the micromolar range. Intrathecal injection of VAS2890 significantly reduced cerebral infarct volume and ROS production in a mouse stroke model, suggesting a crucial contribution of one or more NOX enzymes in stroke (Table 2).
However, VAS2870 presents a number of limitations: (i) it blocks NOX2-derived ROS in neutrophils; (ii) its mode of action is independent of NOX2 (50); (iii) it is cytotoxic at low concentrations (171); and (iv) it exerts thioalkylation of cysteine residues in vitro, with so far unknown functional relevance (4, 144). In terms of drug development, a proof of principle of VAS compounds in humans is currently unfeasible due to their low solubility and unknown oral pharmacokinetic profile.
Recently, GSK2795039, a novel NOX2 inhibitor, abolished NOX2-induced ROS production in a model of paw inflammation and is protective in an animal model of acute pancreatitis (62). A pharmacokinetic/pharmacodynamic evaluation indicates that GSK2795039 is suitable for in vivo use. Further assessment of this compound will provide insights regarding its possible utility for validation of NOX2 as a pharmacological target.
XO inhibitors
Xanthine oxidoreductase (XOR), a 300-kDa homodimer, can exist as an NAD-dependent dehydrogenase (XD) or as an O2-dependent oxidase (XO), depending on the oxidation state of its cysteine thiols (95). XD can be converted into the ROS-generating XO either by formation of intramolecular disulfide bonds (reversible) or by proteolytic cleavage of a loop region connecting the FAD-binding domain and the molybdenum-binding domain (irreversible) (107). While XD depends on NAD+ (31, 140), XO uses O2 as electron acceptor and generates O2− and H2O2 as products (110). As a consequence, XO conversion from XD could be a direct consequence of increased oxidative stress and results in further production of ROS by XO.
XO may contribute to the pathogenesis of various diseases, such as coronary artery disease, type 2 diabetes, and idiopathic dilated cardiomyopathy (21, 22). The XO inhibitor, allopurinol, an analog of hypoxanthine, and its active metabolite, oxypurinol, have been in clinical use for more than 40 years for the treatment of hyperuricemia and gout (41). A recent meta-analysis of 38 clinical trials with allopurinol or oxypurinol in patients with chronic heart failure and coronary artery disease has concluded that XO inhibition improves endothelial function and circulating markers of oxidative stress in patients with, or at risk of, cardiovascular disease (61). Because heterogeneity in those studies made it impossible to come to a conclusion on the effect of XO inhibitors on cardiac outcome, larger prospective multicenter trials are needed (61). Most recently, a study involving 253 high-risk heart failure patients with elevated uric acid levels failed to show improvement with allopurinol in clinical and functional parameters (53).
In 2009, the XOR inhibitor, febuxostat (TEI-6720, TMX-67), was approved by the Food and Drug Administration and marketed for gout (9) as more selective and potent than allopurinol and oxypurinol (110). In contrast to allopurinol, febuxostat has no structural similarity to a purine. Therefore, it has no effects on the activities of other enzymes involved in purine and pyrimidine metabolism, such as guanine deaminase, hypoxanthine-guanine phosphoribosyltransferase, purine nucleoside phosphorylase, orotate phosphoribosyltransferase, and orotidine-5V-monophosphate decarboxylase, compared with allopurinol (166). Contrary to allopurinol and oxypurinol, febuxostat, a potent inhibitor of both XO and XD (146), forms stable long-lasting complexes with the oxidized XOR (111). Its therapeutic application may be useful in cases of allopurinol incompatibility (8). From an experimental point of view, febuxostat may be a superior tool over allopurinol, which may have intrinsic radical scavenging properties that could make it difficult to distinguish between its antioxidant effects and XO inhibition. For example, it was proposed that the protective effects of allopurinol after hypoxia cannot be entirely explained by XO inhibition alone (104).
Another compound used in preclinical studies is BOF-4272 [sodium-8-(3-methoxy-4-phenylsulfinyl-phenyl) pyrazolo[1,5-a]-1,3,5-triazine-4-olate monohydrate] (112), which specifically inhibits XO-based O2− generation (94, 100, 123, 145). However, it could not be tested clinically because of unfavorable pharmacokinetics due to both hepatic metabolism and poor intestinal absorption (108).
Other newly introduced XO inhibitors, such as naphtoflavons, 1,3,5-triazine-based purine analogs, and topiroxostat (FYX-051, 4-[5-pyridin-4-yl-1H-[1, 2, 4] triazol-3-yl]pyridine-2-carbonitrile), are currently being tested in preclinical studies (86, 93, 108, 131). A selection of substances in clinical development is shown in Table 3.
Table 3.
Monoamine Oxidase and Xanthine Oxidase Inhibitors: Mechanism of Action and Treatment Indications
| Target | Compound | Basic mechanism of action | Pathology |
|---|---|---|---|
| MAO | Hydrazines (Phenelzine, isocarboxazid, tranylcypromine) | Nonselective and irreversible MAO inhibitors | Major depressive disorder (130) |
| Moclobemide, toloxatone, pirlindole | Selective and reversible MAO-A inhibitors | Depression, anxiety (130) | |
| Rasagiline, selegiline | Selective and irreversible MAO-B inhibitors | PD, depression, neurodegenerative diseases (30) | |
| Safinamide | Selective and reversible MAO-B inhibitor | PD (15, 16) | |
| XO | Allopurinol and oxypurinol | XOR inhibitor oxypurinol is the active metabolite of allopurinol. | Hyperuricemia and gout (46) cardiovascular disease |
| Febuxostat | Nonpurine XOR inhibitor. More selective and potent than allopurinol. Do not interfere with other metabolic enzymes. | Hyperuricemia and gout (8) more effective, safe, and well tolerated than allopurinol (8, 27). | |
| BOF-4272 | Inhibits XOR-based superoxide generation. | Impossible for clinical use due to low blood concentrations (112) | |
| Topiroxostat | XOR inhibitor | Hyperuricemia and gout (86, 93, 107) |
PD, Parkinson's Disease; MAO, monoamine oxidase; BOF-4272 sodium,7-[4-(benzenesulfinyl)-3-methoxyphenyl]-1,3,9-triaza-5-azanidabicyclo[4.3.0]nona-3,6,8-trien-2-one; XO, xanthine oxidase; XOR, xanthine oxidoreductase.
MAO inhibitors
The attention on MAO as a drug target has been driven by the serendipitous discovery of the antidepressant effect of the antitubercular agent, iproniazid, which was found to act as an MAO inhibitor (35). This observation paved the way to the clinical use of MAO inhibition in depressive disorders (130). Recently, MAO has become also a drug target for ROS-related pathologies. Due to its localization on the outer mitochondrial membrane, H2O2 and other MAO products [aldehydes and ammonia; for details, see Casas et al. in the same Forum (24)] can accumulate in the mitochondria to a significant extent and affect mitochondrial function (73). This can further lead to amplification of oxidative stress and cell damage so that the inhibition of MAO is beneficial in a number of disease models [for details, see Casas et al. in the same Forum (24); (11, 17, 18, 36, 73, 74, 98, 136, 156, 165)]. With the possible exception of NOX4 [for details, see Hirschhäuser et al. in the same Forum (63)]), MAO is the only known mitochondrial ROS source that can be inhibited pharmacologically without interfering with energy metabolism.
MAO exists in two isoforms, A and B, which generate H2O2 as a by-product during the oxidative deamination of biogenic monoamines. A wide range of MAO inhibitors are in clinical use, targeting one or both isoforms. Clorgyline is the prototypic MAO-A-specific inhibitor, while deprenyl inhibits MAO-B, and pargyline is nonselective. Recently, other more selective MAO inhibitors have been developed for the treatment of depressive disorders (130). Of those, phenelzine, isocarboxazid, and tranylcypromine are nonselective and irreversible MAO inhibitors, while moclobemide, toloxatone, and pirlindole are MAO-A selective and reversible. Selective and irreversible MAO-B inhibitors, such as selegiline and rasagiline, are widely prescribed for the treatment of affective and neurodegenerative disorders (Table 3), for example, mild symptoms of Parkinson's disease (PD) and associated motor fluctuations (30). Recently, specific and reversible MAO-B inhibitor, safinamide, has been launched in Germany for the treatment of mid- to late-stage PD in combination with levodopa or other PD therapies (15, 16). The therapeutic potential of MAO-B inhibitors is currently being evaluated also for the treatment of Alzheimer's disease. GABA formation from reactive astrocytes is mediated by MAO-B and affects synaptic plasticity, learning, and memory (71). Since astrocytic GABA and MAO-B are upregulated also in postmortem brains of individuals affected by Alzheimer's disease, MAO-B inhibition has been proposed as a potentially effective therapeutic strategy for treating memory impairment in this disease. Indeed, ladostigil, a dual acetylcholine butyrylcholine esterase and brain-selective MAO-A and -B inhibitor, was shown to antagonize scopolamine-induced impairment in spatial memory (66). More recently, a new small-molecule MAO-B inhibitor, EVT 302, is currently in phase IIb clinical trial for the treatment of Alzheimer's disease.
MAO inhibition can also be the result of an off-target effect. For example, the PPAR-gamma agonist, pioglitazone, used for the treatment of type 2 diabetes, specifically inhibits MAO-B in a reversible manner (12), a property that is not shared by other members of the glitazone family, such as troglitazone and rosiglitazone. Importantly, this off-target effect may contribute to the beneficial effects of pioglitazone in diabetic cardiomyopathy.
To date, MAO inhibitors have been used in patients to preserve or increase monoamine levels. It remains to be investigated clinically whether MAO inhibitors modulate oxidative stress-based pathologies and whether their use can be extended to other indications. The most relevant hurdle in the clinical development of MAO inhibitors is represented by a hypertensive reaction occurring when selective MAO-A inhibition is combined with intake of tyramine-rich food, such as aged cheese and alcoholic beverages (43). Tyramine is mostly oxidized by intestinal MAO-A; MAO-A inhibition causes an increase in circulating tyramine, which is taken up by postganglionic sympathetic neurons and induces noradrenaline release. However, MAO-B and reversible MAO-A inhibitors are devoid of this potential risk (167). Other minor contraindications and concerns related to MAO inhibitors are listed in (162).
NOS inhibitors
Nitric oxide (NO) is another ROS, although mostly with beneficial effects. However, under certain conditions, overproduction may cause cell death, for example, in neurotrauma and stroke. Most NOS inhibitors are based on displacing the substrate, arginine, off its binding site. However, none of these has been approved as a drug for any indication. The most dramatic failure was NG-mono-methyl-l-arginine (L-NMMA) in septic shock (89), where L-NMMA resulted in a 10% increase in overall mortality due to a higher proportion of cardiovascular deaths. Another analog, the amidino amino acid N6-(1-iminoethyl)-l-lysine (L-NIL), applied as its 5-tetrazole-amide prodrug (L-NIL-TA, SC-51) was tested to treat asthma. Oral administration of L-NIL-TA reduced exhaled NO levels in both healthy volunteers and asthmatics for at least 72 h without affecting blood pressure and pulse rate, but did not improve respiratory function (56). Finally, GW273629 (3-[[2-[(1-iminoethyl)amino]ethyl] sulfonyl]-l-alanine) was ineffective in the treatment of acute migraine (154).
The most advanced and currently most successful therapeutic approach is to target another and more unique binding site in NOS, the redox-sensitive cofactor, tetrahydrobiopterin (13, 48). Vasopharm's VAS203 has been successfully developed up to phase II for traumatic brain injury (141) (Table 4).
Table 4.
Nitric Oxide Synthase NOS Inhibitors: Mechanism of Action and Treatment Indications
| Compound | Chemical characteristics | Basic mechanism of action | Pathology |
|---|---|---|---|
| L-NMMA | Arginine derivate | Nonselective NO synthase inhibitor | Septic shock dramatic failure in clinical trials (89) |
| VAS203 | Tetrahydrobiopterin derivate | Nonselective NO synthase inhibitor | Traumatic brain injury (141) |
| Tilarginine acetate | Arginine derivate | Nonselective NO synthase inhibitor | Cardiogenic shock complicating—acute myocardial infarction (2) |
| L-NIL | Arginine derivate L-NIL-TA and SC-51 prodrug oral administration | Inducible NO synthase inhibitor | Asthma (56) |
| GW273629 | Alanine derivate | Inducible NO synthase inhibitor | Acute migraine (154) |
GW273629, (3-[[2-[(1-iminoethyl)amino]ethyl]sulfonyl]-l-alanine); L-NIL-TA, L-N6-(1-iminoethyl)lysine-5-tetrazole-amide; L-NMMA, 1-(4-aminopentyl)-2-methylguanidine; SC-51, L-N6-(1-iminoethyl)lysine 5-tetrazole amide.
Inhibitors of ROS Toxification
These inhibitors target enzymes that do not produce ROS but metabolize ROS to other more toxic species. The most prominent example is myeloperoxidase (MPO).
MPO inhibitors
MPO is a heme protein that can use H2O2 to oxidize Cl− to the highly reactive hypochlorous acid (HOCl), a potent oxidizing agent, but can also generate free radicals through its catalytic peroxidase cycle (77). Besides the major halide Cl−, MPO can also utilize bromide (Br−) to form brominating species, including hypobromous acid (HOBr) (60).
MPO is abundant in neutrophils and certain macrophages where it plays a role in the innate immune response. MPO-derived oxidants also have the potential to cause host tissue injury via initiation of post-translational protein modifications (i.e., chlorination) of proteins (115, 164) and lipid peroxidation (124). As a result, MPO-mediated oxidative damage is thought to contribute to a wide range of chronic inflammatory diseases, including cardiovascular and neuroinflammatory diseases (33, 106). The extracellular Br− concentration is much lower than that of Cl− (149). Thus, the physiological relevance of brominating oxidants such as HOBr, although they elicit antimicrobial effects in vitro (80, 159), has yet to be determined.
However, complete deficiency of MPO can be detrimental. For example, mice deficient in MPO and the low-density lipoprotein receptor (Ldlr), that is, Mpo−/−Ldlr−/−mice, develop larger atherosclerotic lesions compared with Ldlr−/−mice (20), and engraftment of bone marrow from Mpo−/−mice into Ldlr−/−mice increases rather than decreases the size of atherosclerotic lesions (20). Moreover, mice lacking MPO are more susceptible to experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis (19), and are protected from some features of PD (28). As a result of the implied overall benefit of phagocyte MPO, pharmacological strategies to attenuate MPO-mediated inadvertent oxidant damage aim at partial rather than complete inhibition of the enzyme.
Until recently, no specific MPO inhibitors were described that could be considered drug candidates. Although a number of commercially available compounds, including hydroxamic acids, hydrazines, and hydrazides, were used previously to inhibit the catalytic activity of MPO (92), they are not specific and also inhibit other heme peroxidases.
More recently, AstraZeneca found that 2-thioxanthines are potent and selective suicide inhibitors of MPO. Upon oxidation by MPO compound I, the thioxanthine radical forms an adduct with the heme prosthetic group of the enzyme, resulting in inactivation of MPO (150). These new compounds inhibit MPO activity in plasma, decrease protein chlorination in a mouse model of peritonitis, and elicit a range of beneficial effects in various disease models, without interfering with the killing of bacteria by neutrophils or other peroxidases, for example, thyroid peroxidase or lactoperoxidase activity (150). A number of thioxanthines have yielded positive results in preclinical and clinical studies. For example, the thioxanthine, AZD5904, stopped progression of emphysema and small airway remodeling and partially protected against pulmonary hypertension in a guinea pig model of chronic obstructive pulmonary disease (COPD) induced by exposure to cigarette smoke (29). In addition to AZD5904 entering phase I clinical trials for COPD and multiple sclerosis, AstraZeneca has completed a phase IIA clinical trial with another thioxanthine, AZD3241, in patients with PD (116) (Table 5).
Table 5.
Myeloperoxidase Inhibitors: Mechanism of Action and Treatment Indications
| Compound | Chemical characteristics | Basic mechanism of action | Pathology |
|---|---|---|---|
| AZD3241 | 2-thioxanthine derivate | MPO inhibitor by forming an adduct with the heme prosthetic group of the enzyme. | Peritonitis (preclinical data) PD (116) |
| AZD5904 | 2-thioxanthine derivate | MPO inhibitor, Similar mechanism of action as AZD3241. | Chronic obstructive pulmonary disease (29) |
MPO, myeloperoxidase.
Another small-molecule inhibitor of MPO is INV-315 with a submicromolar IC50 [0.9 μM; (88)]. INV-315 decreases plaque burden and improves endothelial function in apolipoprotein E-deficient mice fed a high-fat diet for 16 weeks, a commonly used mouse model of atherosclerosis (88). However, no direct evidence for MPO inhibition or improved endothelial function was provided in that study. Pfizer, Inc., has also implemented a discovery program targeting MPO in inflammation and has filed patents for 2-thiopyrimidones (Fig. 3), which have a structure that is similar to 2-thioxanthines, suggesting that they may also act as suicide inhibitors by forming adducts with the heme moiety of MPO (23).
FIG. 3.
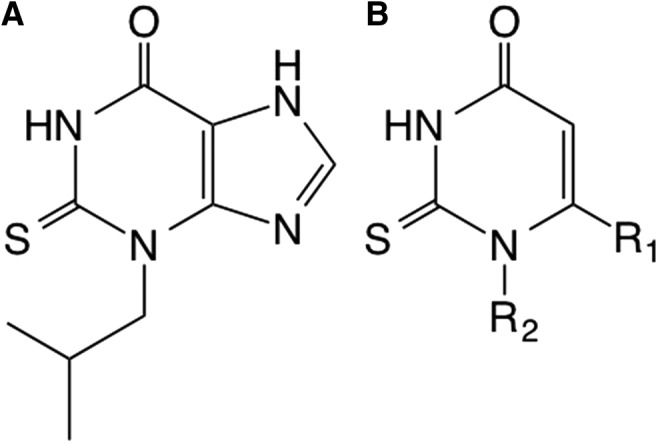
Selective myeloperoxidase inhibitors. (A) Structure of the 2-thioxanthine, TX1 (150). (B) Basic structure of 2-thiopyrimidone compounds in development by Pfizer, Inc. R1 is a five- to six-membered aromatic ring with one to three heteroatoms, while R2 is a fully saturated, partially unsaturated of fully unsaturated 1- to 14-membered straight carbon chain.
Functional Repair of ROS-Induced Protein Damage
This category of ROS-related drugs does not modulate ROS formation, but corrects some of its functional consequences. In the present review, we focus on NO-cGMP signaling, which appears to be one of the major mechanisms of deregulation initiated by oxidative stress (97). ROS can interfere with NO-cGMP signaling in three manners:
• by uncoupling NOS,
• by chemically scavenging NO, or
• by oxidatively damaging the NO receptor, sGC.
This essentially leads to four therapeutic options:
• to recouple uc-NOS [e.g., in peripheral arterial disease (Clinical Trials Registry ACTRN12609000882224)]
• to replenish scavenged NO via NO donor compounds
• to sensitize sGC for lower NO levels [e.g., in pulmonary arterial hypertension (52) and chronic thromboembolic pulmonary hypertension (51)]
• to (re-)activate oxidatively damaged and heme-free sGC (apo-sGC) (e.g., in calcific aortic valve stenosis [NCT02481258] and neuropathic pain [NCT00799656])
NO donors
For over 100 years, NO-releasing drugs have been in clinical use. However, they have several serious side effects that will most likely lead to their eventual replacement. First, many NO donors are subject to tolerance, leading to loss of efficacy and requiring treatment interruptions. Moreover, they can lead to systemic hypotension and reflex tachycardia. Another concern is the fact that under oxidative stress, additional NO from NO donors leads to a spillover and sGC activation, but may also be metabolized to peroxynitrite. Thus, with NO donors, NO-cGMP signaling is partially and acutely recovered, but this happens at the expense of a chronic buildup of unwanted post-translational modifications such as protein tyrosine nitration (133). In addition, NO-drug hybrid molecules comprising an established drug and an NO-releasing moiety have been developed with the aim to preserve the pharmacological activity of the lead structure and add possibly beneficial effects of NO. Of the many compounds tested and developed, a series of NO-NSAIDs (nonsteroidal anti-inflammatory drugs), nitrosylated adrenoreceptor antagonist moxisylate (S-NO-moxisylate), and latanoprostene bunod (VESNEO®), an NO-donating prostaglandin F2-alpha analog, are currently most advanced. The latter is currently in phase III clinical development for the reduction of intraocular pressure in patients with glaucoma and ocular hypertension. Results from the phase 2b study confirmed that the drug is safe (158). Whether these combinations will not have similar limitations as other NO donors remains to be seen.
HNO donors
Besides classical NO donors and NO-drug hybrid molecules, recent preclinical studies and a phase IIa study reveal the therapeutic potential of an NO-related species, nitroxyl (HNO), which is developed as an HNO-donating drug (CXL-1020) by Cardioxyl, for acute decompensated heart failure therapy (122). However, serious inflammatory irritation at the injection site led to development of a second-generation HNO donor, CXL-1427, which is currently in clinical phase II testing. In contrast to NO donors, HNO donors such as Angeli's Salt/HNO appear to not induce tolerance, at least preclinically (6, 68, 103). Interestingly, HNO seems resistant towards scavenging by superoxide and retains efficacy after repeated infusions (45, 103, 113, 122, 151). However, further proof-of-concept studies need to be performed with safe HNO donors.
Recoupling uc-NOS
Oxidative damage of NOS is seen predominately not only for NOS3/eNOS but also for NOS1/nNOS (102). For this, two reversible processes are important, the oxidation of the redox-sensitive NOS cofactor, tetrahydrobiopetrin (BH4) (119), and the accumulation of an endogenous antagonist at the arginine substrate binding site, asymmetric-dimethyl-l-arginine (ADMA). ADMA is an independent risk marker, if not a risk factor, for cardiovascular disease states [for details, see Frijhoff et al. in the same Forum (47)], which may be mechanistically related to uc-NOS.
To replenish the BH4 binding site, BH4 substitution is an option (143). However, BH4 therapy under oxidative stress may also carry the risk of leading to BH2 accumulation, a BH4 antagonist at the NOS BH4 binding site (13). The so-called salvage pathway recycles oxidized BH2 back to BH4 via dihydrofolate reductase (25). Moreover, angiotensin II type 1 receptor blockers and statins may, among other actions, increase the expression of the BH4-forming GTP cyclohydrolase 1 and therefore normalize low BH4 levels (160). High doses of l-arginine may compete off ADMA on eNOS or normalize intracellular ADMA levels. However, a direct antioxidant effect of the guanidine group is also possible (83). Moreover, important differences exist in the bioavailability of arginine in humans versus rodents. Hence, l-citrulline, which is absorbed with near 100% bioavailability, may be a better alternative in humans and is subject to ongoing trials (Australian New Zealand Clinical Trials Registry ACTRN12609000882224).
sGC stimulators and activators
Although stimulation and activation of sGC may sound similar, both innovative drug classes display entirely different modes of action and target different redox and disease states of the NO receptor, sGC. sGC stimulators (sGCs) such as riociguat (BAY 63-2521), vericiguat (BAY 1021189), BAY 41-8543, and BAY 60-4552, and YC-1 (49) bind to an allosteric binding site of Fe(II)heme-containing sGC and allosterically sensitize the enzyme for diminished biophase levels of endogenous NO. In a disease condition where biophase levels of NO are diminished, for example, by oxidative stress, higher or physiological increases in cGMP tissue levels can be achieved. Clinical indications may be similar to NO donors, but without the risk of tolerance and protein nitration as an accumulating by-product.
In contrast, sGC activators (sGCa), such as cinaciguat (BAY 58-2667), ataciguat (HMR 1766), and S3448 (126), activate only Fe(III)heme-oxidized or heme-free (apo-)sGC. They do this by either replacing the weakly bound oxidized heme in (apo-)sGC or by directly occupying the orphaned heme pocket in apo-sGC (138).
Otherwise, apo-sGC would be ubiquitinated at the empty heme binding site and degraded (64, 99). Therefore, sGC activators also stabilize apo-sGC. The ratio of oxidized or apo-sGC to Fe(II)-sGC is increased under oxidative stress conditions (42). In a condition where just NO levels are diminished, but Fe(II)sGC is intact, sGCa would be ineffective. Most recently two other sGCa, GlaxoSmithKline's GSK218123A and Boehringher-Ingelheims's Bl 703704, have been tested preclinically in different animal models of hypertension (32) and kidney diseases (137). The possible benefit of this new compound class and precise mechanism of action, as well as safety, need to be further validated.
Conclusion
In recent years, considerable data have accrued, indicating that disturbances in redox homeostasis are a common mechanism in different cardiovascular, neurological, and metabolic diseases. However, oxidative stress was hitherto not pharmacologically targetable, and the only strategy tested so far, using antioxidants, has been ineffective or even harmful. A possible reason for this is the lack of specificity for disease triggering versus physiological ROS that have a signaling, rather than pathological, role. Furthermore, ROS scavenging by antioxidants takes place in all (sub)cellular locations, not just those relevant for the disease. Innovative drugs need to target disease-relevant ROS-producing enzymes, ROS toxifying enzymes, or proteins damaged by ROS. For all of these, small molecules have become available that are able to perturb specific targets and allow for therapeutic proof-of-concept studies.
These include not only new compounds but also some well-characterized drugs, such as allopurinol and MAO isoform-selective inhibitors, which have been clinically used for decades, although not with the purpose to inhibit ROS formation. In addition, sGC stimulators (in the clinic), NOX inhibitor (entering phase III), NOS inhibitors (phase II-III), sGC activators (phase I-II), and superoxide dismutase mimetics such as GC4410 (phase I) [for details, see Schmidt et al. in the same Forum (127)] are rapidly gaining relevance. Other possible clinical candidates are, for example, mitochondria targeted antioxidants such as mitoquinone and mito TEMPO [for details, see Schmidt et al. in the same Forum (127)].
However, in several cases (e.g., NOX inhibitors), there is an unmet need for isoform-selective drugs. Finally, promising results have been obtained with activators of the transcription factor NRF2, even though in this case the mechanisms are more complex. In particular, one NRF2-activating compound, BG12, is effective and approved for the treatment of multiple sclerosis and, following its success, other NRF2 activators are currently being tested in proof-of-principle studies for various inflammatory diseases (Fig. 4).
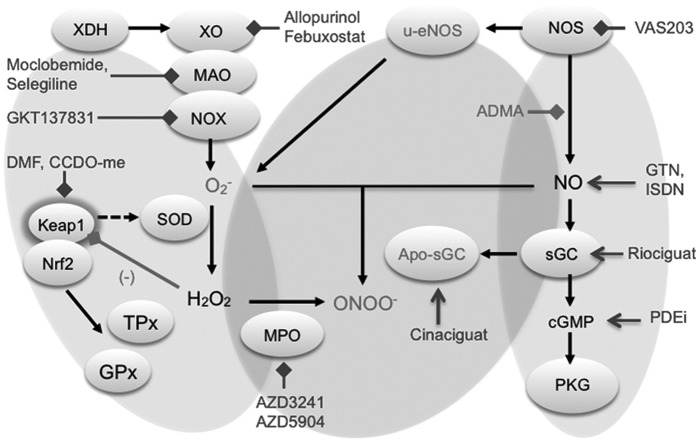
FIG. 4.
Compounds targeting ROS sources, ROS toxifiers, and enzymes damaged by ROS. The physiologic interaction of NRF2 and KEAP1 underlies a negative feedback mechanism. Disruption of this interaction can be caused by electrophilic compounds, which react with specific cysteines of Keap1. Keap 1 is a sensor for environmental and endogenous reactive oxygen and nitrogen species. In conditions of ROS overload, NRF2 escapes Keap1-dependent degradation, accumulates in the nucleus, and activates ARE genes, leading to activation of glutathione and thioredoxin metabolism, including GPx and TPx. Sources that produce O2− are NOX, XO, and MAO. Superoxide anion will be detoxified by SOD to H2O2 and further by catalase to H2O or will react with NOS-derived NO to form peroxynitrite (ONOO−). On the other side, NO activates the sGC, which results in cGMP generation and stimulates protein kinase G signaling. The NO production can be reduced by inhibition of NOS with the endogenous l-arginine analog ADMA. In oxidative stress conditions, the sGC heme can be oxidized or removed (apo-sGC), leading to NO unresponsive sGC. Selected inhibitors and activators are indicated with inhibiting or activating arrows and blocks, respectively. ADMA, asymmetric-dimethyl-l-arginine; ARE, antioxidant responsive element; apo-sGC, heme-free soluble guanylate cyclase; cGMP, cyclic guanosine monophosphate; GPx, glutathione peroxidase; MAO, monoamine oxidases; NO, nitric oxide; NOS, nitric oxide synthase; NOX, nicotinamide adenine dinucleotide phosphate oxidase; ROS, reactive oxygen species; sGC, soluble guanylate cylcase; SOD, superoxide dismutase; TPx, thioredoxin peroxidase; XO, xanthine oxidase.
Outlook
New, more specific pharmacological agents and future drugs are likely to transform the field of oxidative stress, with its many potential medical implications. Indirectly acting compounds (e.g., sGCs) have already provided proof of concept. The final breakthrough will be achieved when inhibitors of ROS-forming enzymes will enter evidence-based medicine.
Abbreviations Used
- ADMA
asymmetric-dimethyl-l-arginine
- apo-sGC
heme-free soluble guanylate cyclase
- ARE
antioxidant responsive element
- BH4
tetrahydrobiopterin
- bZIP
basic region-leucine zipper
- CDDO
2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid
- cGMP
cyclic guanosine monophosphate
- COPD
chronic obstructive pulmonary disease
- CYBA
cytochrome b alpha subunit
- DGR
double glycine repeat
- DLG
aspartate leucine glycine.
- DMF
dimethyl fumarate
- DUOX
dual oxidase
- eNOS
endothelial NO synthase
- ETGE
glutamate, threonine, glycine, and glutamate
- FAD
flavin adenine dinucleotide
- GPx
glutathione peroxidase
- H2O2
hydrogen peroxide
- HOBr
hypobromous acid
- IC50
half maximal inhibitory concentration
- KEAP1
kelch-like ECH-associated protein 1
- LDL
low-density lipoprotein
- L-NIL
N6-(1-iminoethyl)-l-lysine
- L-NMMA
1-(4-aminopentyl)-2-methylguanidine
- MAO
monoamine oxidases
- MPO
myeloperoxidase
- NAD
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- NRF2
nuclear factor (erythroid-derived 2)-like 2
- O2−
superoxide anion
- PD
Parkinson's disease
- PTEN
phosphatase and tensin homolog
- Rbx
ring box protein
- ROS
reactive oxygen species
- SFN
isothiocyanate sulforaphane
- sGC
soluble guanylate cyclase
- SOD
superoxide dismutase
- TPx
thioredoxin peroxidase
- uc-NOS
uncoupled nitric oxide synthase
- XD
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
Acknowledgments
Several authors of this review were supported by the European Cooperation in Science and Technology (COST Action BM1203/EU-ROS). H.H.H.W.S. is the recipient of an ERC Advanced Grant and Marie-Curie IRG and co-leads a EUROSTARS program. R.S. is supported by a Senior Principal Research Fellowship from the National Health and Medical Research Council of Australia N.K. is supported by an EFSD/Sanofi Award.
Author Disclosure Statement
Vincent Jaquet holds shares in Genkyotex SA and Harald H.H.W. Schmidt in Vasopharm GmbH. For the remaining authors, no competing financial interests exist.
References
- 1.This reference has been deleted.
- 2.Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, and Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA 297: 1657–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, and Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal 23: 406–427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, Wassilew SW, Horn T, Kreysel HW, Lutz G, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol 30: 977–981, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Andrews KL, Lumsden NG, Farry J, Jefferis AM, Kemp-Harper BK, and Chin-Dusting JP. Nitroxyl: a vasodilator of human vessels that is not susceptible to tolerance. Clin Sci (Lond) 129: 179–187, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Baldrige CW. and Gerard RW. The extra respiration of phagocytosis. Am J Physiol 103: 235–236, 1932 [Google Scholar]
- 8.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, and Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353: 2450–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Becker MA, Schumacher HR, MacDonald PA, Lloyd E, and Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 36: 1273–1282, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, and Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112: 3297–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Binda C, Aldeco M, Geldenhuys WJ, Tortorici M, Mattevi A, and Edmondson DE. Molecular insights into human monoamine oxidase B inhibition by the glitazone anti-diabetes drugs. ACS Med Chem Lett 3: 39–42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bommel HM, Reif A, Frohlich LG, Frey A, Hofmann H, Marecak DM, Groehn V, Kotsonis P, La M, Koster S, Meinecke M, Bernhardt M, Weeger M, Ghisla S, Prestwich GD, Pfleiderer W, and Schmidt HH. Anti-pterins as tools to characterize the function of tetrahydrobiopterin in NO synthase. J Biol Chem 273: 33142–33149, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Bomprezzi R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 8: 20–30, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P, and Anand R. Randomized trial of safinamide add-on to levodopa in Parkinson's disease with motor fluctuations. Mov Disord 29: 229–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P, and Anand R. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson's disease. Mov Disord 29: 1273–1280, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Bortolato M, Chen K, and Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev 60: 1527–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45: 847–848, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Brennan M, Gaur A, Pahuja A, Lusis AJ, and Reynolds WF. Mice lacking myeloperoxidase are more susceptible to experimental autoimmune encephalomyelitis. J Neuroimmunol 112: 97–105, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, and Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest 107: 419–430, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler R, Morris AD, Belch JJ, Hill A, and Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35: 746–751, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, and Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 104: 2407–2411, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Carpino PA, Conn EL, Dow RL, Dowling MS, Hepworth D, Kung DWS, Orr S, Rocke BN, Ruggeri RB, and Sammons MF. 2-Thiopyrimidinones. edited by USPTO. U.S. Patent 8,835,449: Pfizer Inc.; 2013
- 24.Casas A, Dao VT, Daiber A, Maghzal GJ, Di Lisa F, Kaludercic N, Leach S, Jaquet V, Seredenina T, Krause KH, López MG, Stocker R, Ghezzi P, and Schmidt HH. Reactive oxygen-related diseases: Therapeutic targets and emerging clinical indications. Antioxid Redox Signal 23: 1171–1185, 2015 [DOI] [PMC free article] [PubMed]
- 25.Chalupsky K. and Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 102: 9056–9061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin MP, Reisman SA, Bakris GL, O'Grady M, Linde PG, McCullough PA, Packham D, Vaziri ND, Ward KW, Warnock DG, and Meyer CJ. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol 39: 499–508, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Chohan S, Becker MA, MacDonald PA, Chefo S, and Jackson RL. Women with gout: efficacy and safety of urate-lowering with febuxostat and allopurinol. Arthritis Care Res (Hoboken) 64: 256–261, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, Jackson-Lewis V, Vila M, Vonsattel JP, Heinecke JW, and Przedborski S. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J Neurosci 25: 6594–6600, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churg A, Marshall CV, Sin DD, Bolton S, Zhou S, Thain K, Cadogan EB, Maltby J, Soars MG, Mallinder PR, and Wright JL. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am J Resp Crit Care 185: 34–43, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Connolly BS. and Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 311: 1670–1683, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Corte ED. and Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J 126: 739–745, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costell MH, Ancellin N, Bernard RE, Zhao S, Upson JJ, Morgan LA, Maniscalco K, Olzinski AR, Ballard VL, Herry K, Grondin P, Dodic N, Mirguet O, Bouillot A, Gellibert F, Coatney RW, Lepore JJ, Jucker BM, Jolivette LJ, Willette RN, Schnackenberg CG, and Behm DJ. Comparison of soluble guanylate cyclase stimulators and activators in models of cardiovascular disease associated with oxidative stress. Front Pharmacol 3: 128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies MJ, Hawkins CL, Pattison DI, and Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 1199–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 34.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, and Chertow GM. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delay J, Laine B, and Buisson JF. [The action of isonicotinyl-hydrazide used in the treatment of depressive states]. Ann Med Psychol (Paris) 110: 689–692, 1952 [PubMed] [Google Scholar]
- 36.Di Lisa F, Kaludercic N, Carpi A, Menabo R, and Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol 104: 131–139, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, and Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A 98: 3404–3409, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dotan Y, Lichtenberg D, and Pinchuk I. No evidence supports vitamin E indiscriminate supplementation. Biofactors 35: 469–473, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Edmondson DE, Mattevi A, Binda C, Li M, and Hubalek F. Structure and mechanism of monoamine oxidase. Curr Med Chem 11: 1983–1993, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, and Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 7: 813–823, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elion GB. The purine path to chemotherapy. Science 244: 41–47, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, and Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finberg JP. and Gillman K. Selective inhibitors of monoamine oxidase type B and the “cheese effect.” Int Rev Neurobiol 100: 169–190, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, and Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88: 44–51, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Flores-Santana W, Salmon DJ, Donzelli S, Switzer CH, Basudhar D, Ridnour L, Cheng R, Glynn SA, Paolocci N, Fukuto JM, Miranda KM, and Wink DA. The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid Redox Signal 14: 1659–1674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fravel MA. and Ernst ME. Management of gout in the older adult. Am J Geriatr Pharmacother 9: 271–285, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, Gasparovic AC, Cuadrado A, Weber D, Poulsen HE, Grune T, Schmidt HHHW, and Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 23: 1144–1170, 2015 [DOI] [PMC free article] [PubMed]
- 48.Frohlich LG, Kotsonis P, Traub H, Taghavi-Moghadam S, Al-Masoudi N, Hofmann H, Strobel H, Matter H, Pfleiderer W, and Schmidt HH. Inhibition of neuronal nitric oxide synthase by 4-amino pteridine derivatives: structure-activity relationship of antagonists of (6R)-5,6,7,8-tetrahydrobiopterin cofactor. J Med Chem 42: 4108–4121, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Galle J, Zabel U, Hubner U, Hatzelmann A, Wagner B, Wanner C, and Schmidt HH. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol 127: 195–203, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatto GJ, Jr, Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, and Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem 28: 95–104, 2013 [DOI] [PubMed] [Google Scholar]
- 50a.Genkyotex Innovation SAS. 2015. Safety and Efficacy of Oral GKT137831 in Patient With Type 2 Diabetes and Albuminuria. National Institutes of Health, USA. https://clinicaltrials.gov/ct2/show/NCT02010242 (last accessed October29, 2015)
- 51.Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, and Wang C. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 369: 319–329, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, and Rubin LJ. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369: 330–340, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WH, Dunlap ME, LeWinter MM, Mann DL, Felker GM, O'Connor CM, Goldsmith SR, Ofili EO, Saltzberg MT, Margulies KB, Cappola TP, Konstam MA, Semigran MJ, McNulty SE, Lee KL, Shah MR, and Hernandez AF. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation 131: 1763–1771, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, and Abboud HE. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol 308: F1276–87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han SJ, Ahn Y, Park I, Zhang Y, Kim I, Kim HW, Ku CS, Chay KO, Yang SY, Ahn BW, Jang DI, and Lee SR. Assay of the redox state of the tumor suppressor PTEN by mobility shift. Methods 77–78: 58–62, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, and Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J 17: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Hast BE, Goldfarb D, Mulvaney KM, Hast MA, Siesser PF, Yan F, Hayes DN, and Major MB. Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Res 73: 2199–2210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayes JD. and Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39: 199–218, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Hayes JD, McMahon M, Chowdhry S, and Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal 13: 1713–1748, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Henderson JP, Byun J, Williams MV, Mueller DM, McCormick ML, and Heinecke JW. Production of brominating intermediates by myeloperoxidase. A transhalogenation pathway for generating mutagenic nucleobases during inflammation. J Biol Chem 276: 7867–7875, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, and Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther 30: 217–226, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, Ramachandran S, Bridges A, Chaudry L, Pettman G, Allan C, Duncan S, Lee KC, Lim J, Ma MT, Ong AB, Ye NY, Nasir S, Mulyanidewi S, Aw CC, Oon PP, Liao S, Li D, Johns DG, Miller ND, Davies CH, Browne ER, Matsuoka Y, Chen DW, Jaquet V, and Rutter AR. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid Redox Signal 23: 358–374, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirschhäuser C, Bornbaum J, Reis A, Böhme S, Kaludercic N, Menabò R, Di Lisa F, Boengler K, Shah AM, Schulz R, and Schmidt HH. NOX4 in mitochondria: yeast two-hybrid-based interaction with complex I without relevance for basal reactive oxygen species? Antioxid Redox Signal 23: 1106–1112, 2015 [DOI] [PMC free article] [PubMed]
- 64.Hoffmann LS, Schmidt PM, Keim Y, Schaefer S, Schmidt HH, and Stasch JP. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br J Pharmacol 157: 781–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hohn DC. and Lehrer RI. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest 55: 707–713, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong-Qi Y, Zhi-Kun S, and Sheng-Di C. Current advances in the treatment of Alzheimer's disease: focused on considerations targeting Abeta and tau. Transl Neurodegener 1: 21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hultqvist M, Olofsson P, Wallner FK, and Holmdahl R. Pharmacological potential of NOX2 agonists in inflammatory conditions. Antioxid Redox Signal 23: 446–459, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Irvine JC, Kemp-Harper BK, and Widdop RE. Chronic administration of the HNO donor Angeli's salt does not lead to tolerance, cross-tolerance, or endothelial dysfunction: comparison with GTN and DEA/NO. Antioxid Redox Signal 14: 1615–1624, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Jaquet V, Scapozza L, Clark RA, Krause KH, and Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HH, and Jandeleit-Dahm KA. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25: 1237–1254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee da Y, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, Shim I, Bae YC, Cho J, Kowall NW, Ryu H, Hwang E, Kim D, and Lee CJ. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med 20: 886–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jomova K. and Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 283: 65–87, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal 20: 267–280, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106: 193–202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kappos L, Gold R, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Sarda SP, Agarwal S, Zhang A, Sheikh SI, Seidman E, and Dawson KT. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: the DEFINE study. Mult Scler 20: 243–252, 2014 [DOI] [PubMed] [Google Scholar]
- 76.Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M, Eraksoy M, Meluzinova E, Rektor I, Dawson KT, Sandrock AW, and O'Neill GN. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372: 1463–1472, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Kettle AJ, van Dalen CJ, and Winterbourn CC. Peroxynitrite and myeloperoxidase leave the same footprint in protein nitration. Redox Rep 3: 257–258, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Kim JE, You DJ, Lee C, Ahn C, Seong JY, and Hwang JI. Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell Signal 22: 1645–1654, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Kitagishi Y. and Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging (Review). Int J Mol Med 31: 511–515, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol 95: 2131–2138, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, and Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: pii: , 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Lass A, Suessenbacher A, Wolkart G, Mayer B, and Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol Pharmacol 61: 1081–1088, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, and Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277: 20336–20342, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Leto TL, Morand S, Hurt D, and Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 2607–2619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim FP. and Dolzhenko AV. 1,3,5-Triazine-based analogues of purine: from isosteres to privileged scaffolds in medicinal chemistry. Eur J Med Chem 85: 371–390, 2014 [DOI] [PubMed] [Google Scholar]
- 87.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, and Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134: 678–692, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Liu C, Desikan R, Ying Z, Gushchina L, Kampfrath T, Deiuliis J, Wang A, Xu X, Zhong J, Rao X, Sun Q, Maiseyeu A, Parthasarathy S, and Rajagopalan S. Effects of a novel pharmacologic inhibitor of myeloperoxidase in a mouse atherosclerosis model. PLoS One 7: e50767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, and Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 32: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma Q. and He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev 64: 1055–1081, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malle E, Furtmuller PG, Sattler W, and Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol 152: 838–854, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsumoto K, Okamoto K, Ashizawa N, and Nishino T. FYX-051: a novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther 336: 95–103, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Matsumura F, Yamaguchi Y, Goto M, Ichiguchi O, Akizuki E, Matsuda T, Okabe K, Liang J, Ohshiro H, Iwamoto T, Yamada S, Mori K, and Ogawa M. Xanthine oxidase inhibition attenuates kupffer cell production of neutrophil chemoattractant following ischemia-reperfusion in rat liver. Hepatology 28: 1578–1587, 1998 [DOI] [PubMed] [Google Scholar]
- 95.McManaman JL. and Bain DL. Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. J Biol Chem 277: 21261–21268, 2002 [DOI] [PubMed] [Google Scholar]
- 96.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, and Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 97.Melichar VO, Behr-Roussel D, Zabel U, Uttenthal LO, Rodrigo J, Rupin A, Verbeuren TJ, Kumar HSA, and Schmidt HH. Reduced cGMP signaling associated with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis. Proc Natl Acad Sci U S A 101: 16671–16676, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Menazza S, Blaauw B, Tiepolo T, Toniolo L, Braghetta P, Spolaore B, Reggiani C, Di Lisa F, Bonaldo P, and Canton M. Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum Mol Genet 19: 4207–4215, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch JP, Schmidt HH, and Muller-Esterl W. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 105: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, and Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett 427: 225–228, 1998 [DOI] [PubMed] [Google Scholar]
- 101.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, and Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Miller RT, Martasek P, Roman LJ, Nishimura JS, and Masters BS. Involvement of the reductase domain of neuronal nitric oxide synthase in superoxide anion production. Biochemistry 36: 15277–15284, 1997 [DOI] [PubMed] [Google Scholar]
- 103.Miranda KM, Yamada K, Espey MG, Thomas DD, DeGraff W, Mitchell JB, Krishna MC, Colton CA, and Wink DA. Further evidence for distinct reactive intermediates from nitroxyl and peroxynitrite: effects of buffer composition on the chemistry of Angeli's salt and synthetic peroxynitrite. Arch Biochem Biophys 401: 134–144, 2002 [DOI] [PubMed] [Google Scholar]
- 104.Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, and Gutteridge JM. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett 213: 23–28, 1987 [DOI] [PubMed] [Google Scholar]
- 105.Motohashi H. and Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Nicholls SJ. and Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25: 1102–1111, 2005 [DOI] [PubMed] [Google Scholar]
- 107.Nishino T. The conversion from the dehydrogenase type to the oxidase type of rat liver xanthine dehydrogenase by modification of cysteine residues with fluorodinitrobenzene. J Biol Chem 272: 29859–29864, 1997 [DOI] [PubMed] [Google Scholar]
- 108.Nishino T. and Okamoto K. Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout. J Biol Inorg Chem 20:195–207, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niture SK. and Jaiswal AK. INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ 18: 439–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, and Nishino T. An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem 278: 1848–1855, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Okamoto K, Eger BT, Nishino T, Pai EF, and Nishino T. Mechanism of inhibition of xanthine oxidoreductase by allopurinol: crystal structure of reduced bovine milk xanthine oxidoreductase bound with oxipurinol. Nucleosides Nucleotides Nucleic Acids 27: 888–893, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Okamoto K. and Nishino T. Mechanism of inhibition of xanthine oxidase with a new tight binding inhibitor. J Biol Chem 270: 7816–7821, 1995 [DOI] [PubMed] [Google Scholar]
- 113.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, and Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A 98: 10463–10468, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pitha-Rowe I, Liby K, Royce D, and Sporn M. Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci 50: 5339–5347, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Podrez EA, Abu-Soud HM, and Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med 28: 1717–1725, 2000 [DOI] [PubMed] [Google Scholar]
- 116.Posener JA, Hauser RA, Stieber M, Leventer SM, Eketjäll S, Minkwitz MC, Ingersoll EW, and Kugler AR. Safety, tolerability, and pharmacodynamics of AZD3241, a myeloperoxidase inhibitor, in Parkinson's disease [abstract]. Movement Disord 29 (Suppl 1): 698, 2014 [Google Scholar]
- 117.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, and Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31: 1121–1133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H, Garcia-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, and Cuadrado A. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol Cell Biol 32: 3486–3499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reif A, Frohlich LG, Kotsonis P, Frey A, Bommel HM, Wink DA, Pfleiderer W, and Schmidt HH. Tetrahydrobiopterin inhibits monomerization and is consumed during catalysis in neuronal NO synthase. J Biol Chem 274: 24921–24929, 1999 [DOI] [PubMed] [Google Scholar]
- 120.Rojo AI, Medina-Campos ON, Rada P, Zuniga-Toala A, Lopez-Gazcon A, Espada S, Pedraza-Chaverri J, and Cuadrado A. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: role of glycogen synthase kinase-3. Free Radic Biol Med 52: 473–487, 2012 [DOI] [PubMed] [Google Scholar]
- 121.Rojo AI, Rada P, Mendiola M, Ortega-Molina A, Wojdyla K, Rogowska-Wrzesinska A, Hardisson D, Serrano M, and Cuadrado A. The PTEN/NRF2 axis promotes human carcinogenesis. Antioxid Redox Signal 21: 2498–2514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sabbah HN, Tocchetti CG, Wang M, Daya S, Gupta RC, Tunin RS, Mazhari R, Takimoto E, Paolocci N, Cowart D, Colucci WS, and Kass DA. Nitroxyl (HNO): a novel approach for the acute treatment of heart failure. Circ Heart Fail 6: 1250–1258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanders SA, Eisenthal R, and Harrison R. NADH oxidase activity of human xanthine oxidoreductase—generation of superoxide anion. Eur J Biochem 245: 541–548, 1997 [DOI] [PubMed] [Google Scholar]
- 124.Savenkova ML, Mueller DM, and Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem 269: 20394–20400, 1994 [PubMed] [Google Scholar]
- 125.Schildknecht S, Weber A, Gerding HR, Pape R, Robotta M, Drescher M, Marquardt A, Daiber A, Ferger B, and Leist M. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr Med Chem 21: 365–376, 2013 [DOI] [PubMed] [Google Scholar]
- 126.Schindler U, Strobel H, Schonafinger K, Linz W, Lohn M, Martorana PA, Rutten H, Schindler PW, Busch AE, Sohn M, Topfer A, Pistorius A, Jannek C, and Mulsch A. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol 69: 1260–1268, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Schmidt HH, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, and Cuadrado A. Antioxidants in translational medicine. Antioxid Redox Signal 23: 1130–1143, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seidel P. and Roth M. Anti-inflammatory dimethylfumarate: a potential new therapy for asthma? Mediators Inflamm 2013: 875403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, Yoshida T, Iyo M, and Hashimoto K. An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharmacol Neurosci 13: 62–67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shulman KI, Herrmann N, and Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27: 789–797, 2013 [DOI] [PubMed] [Google Scholar]
- 131.Singh H, Sharma S, Ojha R, Gupta MK, Nepali K, and Bedi PM. Synthesis and evaluation of naphthoflavones as a new class of non purine xanthine oxidase inhibitors. Bioorg Med Chem Lett 24: 4192–4197, 2014 [DOI] [PubMed] [Google Scholar]
- 132.Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, and Zimmerman AW. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci U S A 111: 15550–15555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Skatchkov M, Larina LL, Larin AA, Fink N, and Bassenge E. Urinary NItrotyrosine content as a marker of peroxynitrite-induced tolerance to organic NItrates. J Cardiovasc Pharmacol Ther 2: 85–96, 1997 [DOI] [PubMed] [Google Scholar]
- 134.Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys 29: 169–202, 1996 [DOI] [PubMed] [Google Scholar]
- 135.Slocum SL. and Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol 85: 273–284, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Sorato E, Menazza S, Zulian A, Sabatelli P, Gualandi F, Merlini L, Bonaldo P, Canton M, Bernardi P, and Di Lisa F. Monoamine oxidase inhibition prevents mitochondrial dysfunction and apoptosis in myoblasts from patients with collagen VI myopathies. Free Radic Biol Med 75: 40–47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stasch JP, Schlossmann J, and Hocher B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr Opin Pharmacol 21c: 95–104, 2015 [DOI] [PubMed] [Google Scholar]
- 138.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY H S. AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, and Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, and Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344: 200–205, 2006 [DOI] [PubMed] [Google Scholar]
- 140.Stirpe F. and Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem 244: 3855–3863, 1969 [PubMed] [Google Scholar]
- 141.Stover JF, Belli A, Boret H, Bulters D, Sahuquillo J, Schmutzhard E, Zavala E, Ungerstedt U, Schinzel R, and Tegtmeier F. Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo-controlled randomized Phase IIa trial (NOSTRA). J Neurotrauma 31: 1599–1606, 2014 [DOI] [PubMed] [Google Scholar]
- 142.Strengert M, Jennings R, Davanture S, Hayes P, Gabriel G, and Knaus UG. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxid Redox Signal 20: 2695–2709, 2014 [DOI] [PubMed] [Google Scholar]
- 143.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, and Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest 99: 41–46, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun QA, Hess DT, Wang B, Miyagi M, and Stamler JS. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic Biol Med 52: 1897–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, Suematsu M, Zweifach BW, and Schmid-Schonbein GW. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci U S A 95: 4754–4759, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, and Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci 76: 1835–1847, 2005 [DOI] [PubMed] [Google Scholar]
- 147.Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, and Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med 53: 817–827, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, and Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 149.Thomas EL, Bozeman PM, Jefferson MM, and King CC. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J Biol Chem 270: 2906–2913, 1995 [DOI] [PubMed] [Google Scholar]
- 150.Tiden AK, Sjogren T, Svensson M, Bernlind A, Senthilmohan R, Auchere F, Norman H, Markgren PO, Gustavsson S, Schmidt S, Lundquist S, Forbes LV, Magon NJ, Paton LN, Jameson GN, Eriksson H, and Kettle AJ. 2-thioxanthines are mechanism-based inactivators of myeloperoxidase that block oxidative stress during inflammation. J Biol Chem 286: 37578–37589, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tocchetti CG, Stanley BA, Murray CI, Sivakumaran V, Donzelli S, Mancardi D, Pagliaro P, Gao WD, van Eyk J, Kass DA, Wink DA, and Paolocci N. Playing with cardiac “redox switches”: the “HNO way” to modulate cardiac function. Antioxid Redox Signal 14: 1687–1698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, and Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol 27: 7511–7521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsianakas A, Herzog S, Landmann A, Patsinakidis N, Perusquia Ortiz AM, Bonsmann G, Luger TA, and Kuhn A. Successful treatment of discoid lupus erythematosus with fumaric acid esters. J Am Acad Dermatol 71: e15-7, 2014 [DOI] [PubMed] [Google Scholar]
- 154.Van der Schueren BJ, Lunnon MW, Laurijssens BE, Guillard F, Palmer J, Van Hecken A, Depre M, Vanmolkot FH, and de Hoon JN. Does the unfavorable pharmacokinetic and pharmacodynamic profile of the iNOS inhibitor GW273629 lead to inefficacy in acute migraine? J Clin Pharmacol 49: 281–290, 2009 [DOI] [PubMed] [Google Scholar]
- 155.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, and Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Villeneuve C, Guilbeau-Frugier C, Sicard P, Lairez O, Ordener C, Duparc T, De Paulis D, Couderc B, Spreux-Varoquaux O, Tortosa F, Garnier A, Knauf C, Valet P, Borchi E, Nediani C, Gharib A, Ovize M, Delisle MB, Parini A, and Mialet-Perez J. p53-PGC-1alpha pathway mediates oxidative mitochondrial damage and cardiomyocyte necrosis induced by monoamine oxidase-A upregulation: role in chronic left ventricular dysfunction in mice. Antioxid Redox Signal 18: 5–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang YY, Yang YX, Zhe H, He ZX, and Zhou SF. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug Des Devel Ther 8: 2075–2088, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weinreb RN, Ong T, Scassellati Sforzolini B, Vittitow JL, Singh K, and Kaufman PL. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol 99: 738–745, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weiss SJ, Test ST, Eckmann CM, Roos D, and Regiani S. Brominating oxidants generated by human eosinophils. Science 234: 200–203, 1986 [DOI] [PubMed] [Google Scholar]
- 160.Wenzel P, Schulz E, Oelze M, Muller J, Schuhmacher S, Alhamdani MS, Debrezion J, Hortmann M, Reifenberg K, Fleming I, Munzel T, and Daiber A. AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med 45: 619–626, 2008 [DOI] [PubMed] [Google Scholar]
- 161.Williams HC. and Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol 50: 9–16, 2007 [DOI] [PubMed] [Google Scholar]
- 162.Wimbiscus M, Kostenko O, and Malone D. MAO inhibitors: risks, benefits, and lore. Cleve Clin J Med 77: 859–882, 2010 [DOI] [PubMed] [Google Scholar]
- 163.Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, and Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol 164: 866–883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Winterbourn CC. and Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med 29: 403–409, 2000 [DOI] [PubMed] [Google Scholar]
- 165.Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li Y, Chen YT, Yin F, Liao CP, Stiles BL, Zhau HE, Shih JC, and Chung LW. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J Clin Invest 124: 2891–2908, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yamamoto T, Moriwaki Y, Fujimura Y, Takahashi S, Tsutsumi Z, Tsutsui T, Higashino K, and Hada T. Effect of TEI-6720, a xanthine oxidase inhibitor, on the nucleoside transport in the lung cancer cell line A549. Pharmacology 60: 34–40, 2000 [DOI] [PubMed] [Google Scholar]
- 167.Youdim MB. and Weinstock M. Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase A and B that do not cause significant tyramine potentiation. Neurotoxicology 25: 243–250, 2004 [DOI] [PubMed] [Google Scholar]
- 168.Yusuf S, Dagenais G, Pogue J, Bosch J, and Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 342: 154–160, 2000 [DOI] [PubMed] [Google Scholar]
- 169.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, and Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 170.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, and Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 277: 46116–46122, 2002 [DOI] [PubMed] [Google Scholar]
- 171.Zielonka J, Cheng G, Zielonka M, Ganesh T, Sun A, Joseph J, Michalski R, O'Brien WJ, Lambeth JD, and Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J Biol Chem 289: 16176–16189, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]