Abstract
Altering endogenous genes in cells is an integral tool of modern cell biology. The ease-of-use of the CRISPR/Cas9 system to introduce genomic DNA breaks at specific sites in vivo has led to its rapid and wide adoption. In the absence of a DNA template, the lesion is repaired by nonhomologous end joining resolving as internal deletions. However, in the presence of a homologous DNA template, homology-directed repair occurs with variable efficiencies. Recent work has demonstrated that highly efficient gene targeting can be induced by combining CRISPR/Cas9 targeting of genomic loci with recombinant adeno-associated virus (rAAV) to provide a single-stranded homologous DNA template. Here we review the current state of CRISPR/Cas-based gene editing and provide a practical guide to applying the CRISPR/Cas and rAAV system for highly efficient, time- and cost-effective gene targeting.
Short History of Gene Editing and CRISPR Biology
In 1979, Scherer and Davis initiated the beginning of yeast genetics by demonstrating that in vitro-generated vector sequences can induce homology-directed recombination in Saccharomyces cerevisiae to delete or alter any isolated gene [1]. Soon thereafter, the process of genome editing was used in human cells [2–4]. A decade later, the discovery was made that genomic breaks can be repaired by either nonhomologous end joining (NHEJ) or homology-directed repair (HDR), with HDR being the process required for precise or template genome editing [5–9]. Around that same time, the discovery of zinc finger nucleases was made [10–12], and their potential for targeted genome editing was demonstrated [13–15]. In 2009, the transcription activator-like effector nucleases (TALENs) from the plant pathogen Xanthomonas were decoded [16–18], making in silico design and binding predictions for TALENs possible. Due to their simplicity and in silico design, many laboratories have used engineered TALENs for their genome-editing strategies.
In parallel to the field of gene editing, the molecular biology of CRISPR (clustered regularly interspaced short palindromic repeats) has opened up entirely new genome-editing possibilities. Soon after their initial description in the late 1980s by Ishino et al. [19], it was realized that these sequences are made of viral nucleotide sequences and have CRISPR-associated (cas) genes in proximity [20–27]. This work resulted in the hypothesis of CRISPR/Cas being a bacterial immune system [26,28]. Within the following years, the molecular mechanisms were determined and a type II CRISPR/Cas system was identified that cuts DNA in a programmable and sequence-specific manner [29]. The identification of trans-activating crispr RNAs (tracrRNAs) and their involvement in crRNA maturation by RNase III closed the gap between missing endoribonucleases and the maturation of crRNAs [30]. Until then, all CRISPR/Cas systems had been thought to be a multiprotein/RNA system that was difficult to assemble in vitro. However, in 2011, the work of Sapranauskas et al. [31] and that of Jinek et al. [32] demonstrated that a single cas gene (Cas9) is sufficient for a fully functional type II defense system and that Cas9 is an RNA-guided DNA endonuclease [33], respectively.
The use of CRISPR/Cas for sequence-specific gene editing has since been demonstrated in a variety of cell types and tissues in eukaryotes [34–37]. The simplicity of this technology is demonstrated by an explosion of scientific literature during the last 2 years, which includes characterization of off-target mutagenic effects [38–40], improved single-guide RNA (sgRNA) design [41–43], the activation or repression of endogenous genes [44,45], the development of Cas9 nickases or dCas9-FokI fusion proteins [46–49], genome-wide knockout libraries [50–53], CRISPR/Cas9 knockin mice [54,55], and mutation of genes in a living animal [56–58]. Taken together, these recent rapid advances in our understanding of the CRISPR/Cas9 system are indicative of its bright potential for gene therapy and utilization to uncover new genetic pathways governing cell biology.
Selecting the “Right” Cas9 for Each Application
CRISPR/Cas systems can broadly be classified into three specific types (I–III) based on the combination of Cas proteins present in the genome of the respective organism [59]. The commonly used type II system is characterized by its single CRISPR ribonucleoprotein complex (Cas9), while types I and III are multiprotein complexes comprising many different Cas subunits. Interestingly, types I and II target DNA while type III has evolved to target RNA [59,60]. The fact that type II systems contain a single Cas gene has made them ideal for a variety of applications, most notably genome engineering for the purpose of “disease in a dish” models, whole animal model generation, and drug development [61].
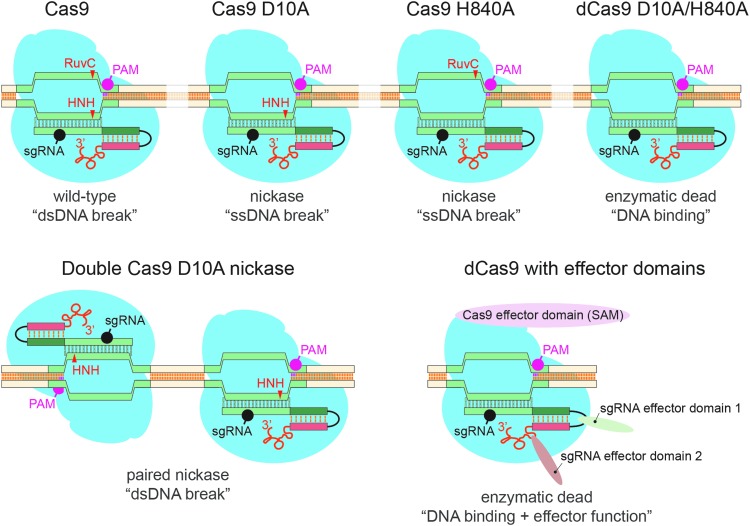
The type II ribonucleoprotein (Cas9) is a multidomain RNA-guided DNA endonuclease with two catalytic centers, HNH and RuvC [32,62]. Two amino acid residues are of particular importance, D10 (RuvC domain) and H840 (HNH domain). Mutation of either residue to alanine converts Cas9 into a nickase enzyme with only one active catalytic center [47,48] (Fig. 1). The mutation of both residues will generate a Cas9 enzyme without DNA nuclease activity, while maintaining its ability to selectively bind DNA in an RNA-guided manner.
FIG. 1.
Available Cas9 species and their applications. Highlighted are the protospacer adjacent motif (PAM), the two Cas9 nuclease domains, RuvC and HNH, and the DNA position (arrowhead) on which they act as well as the position of the single-guide RNA (sgRNA). Mediated by the two nuclease domains, the wild-type enzyme generates a DNA double-strand break. Mutating a single nuclease domain, to either D10A or H840A, will result in a nickase enzyme that is only able to nick DNA at the sgRNA-targeted site. Nickase enzymes can be used in a paired manner to improve specificity and reduce off-target effects. The mutation of both nuclease domains results in a catalytically “dead” (dCas9) enzyme that retains RNA-guided DNA binding capacity. Combining dCas9 with either Cas9-linked or sgRNA-linked effector domains can be used for transcriptional activation, repression, or genomic visualization.
Wild-type Cas9 can be used as a single enzyme for indel generation, DNA insertions and replacements, as well as for gene editing, or when using multiple Cas9-gRNA complexes, for the deletion of large DNA sequences or the induction of DNA rearrangements (Fig. 1). Using the nickase as a single enzyme results in a single-stranded DNA nick, with lower efficiencies for indel generation, DNA insertions, or replacements than the wild-type Cas9 complex, but with a lower likelihood of inducing off-site mutations. This lower efficiency can be overcome by targeting two nickase complexes to the same genomic region with two different guide RNA sequences on opposing DNA strands. The resulting double-strand DNA break restores the efficiencies for indel generation, DNA insertions and replacements, and gene editing (Fig. 1) [46–49], while maintaining a low off-target effect.
The Cas9 double-mutant (dCas9; D10A, H840A) has no nuclease activity but retains its ability to bind DNA in an RNA-guided manner, making it useful for other applications. Fusing dCas9 N- or C-terminal to effector domains allows for gene activation (VP64 domain) [45,63,64], repression (KRAB domain) [45,63], visualization (fluorescent proteins) [65–67], or chromatin modification (histone modifier or DNA methylation regulators) [68] (Fig. 1). Interestingly, an sgRNA sequence is often not sufficient to drastically change the expression profile of a gene, instead a pool of up to five RNA guide sequences is often required to target multiple complexes to the same region. To circumvent this obstacle, structure-guided engineering of the CRISPR/Cas9 complex produced to an internal VP64 domain with additional RNA aptamer sequences into the gRNA that facilitate the recruitment of aptamer-binding domain-fused activator domains to the CRISPR/Cas9 complex [69]. This results in a dCas9-VP64:sgRNA:aptamer-effector complex that activates gene promoter transcription very efficiently with an sgRNA (Fig. 1).
How to Choose a Guide RNA?
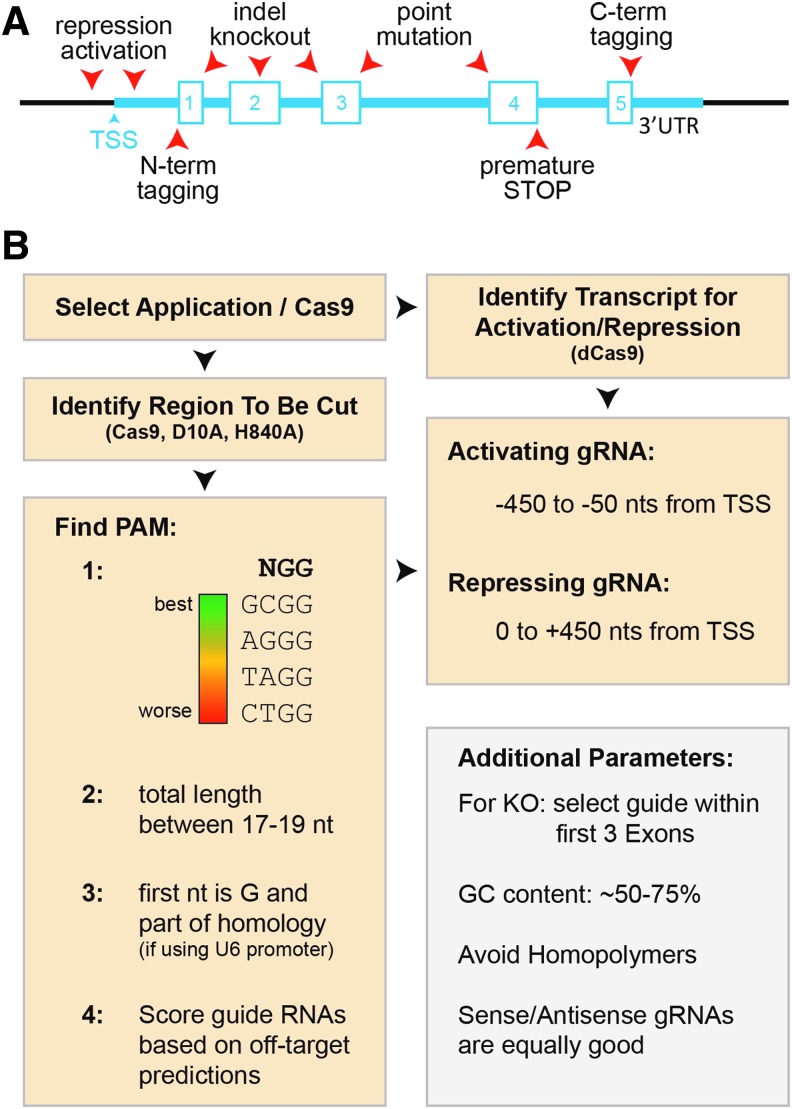
The most difficult task in the process of applying CRISPR/Cas9 technology is identifying the “best” guide RNA sequence. To increase the likelihood of selecting a highly efficient guide RNA, a few selected parameters need to be considered. The first is choosing the position of the guide sequence with respect to the region of interest (promoter, enhancer, coding region, untranslated region, miRNA, etc.), which depends on the intended application (Fig. 2A). For transcriptional activation, the guide sequence should be 450 to 50 nucleotides upstream of the transcriptional start site (TSS), whereas for transcriptional repression it should be between 50 and 450 bases downstream of the TSS [45]. To generate indel mutations for the purpose of gene disruption, the guide sequence should ideally be located within the first three exons of the gene of interest [41]. However, recent work demonstrated that guide RNAs targeting functional protein domains have a higher probability to induce gene-function disruption than other guide RNAs [70]. For gene editing in the form of introducing coding missense mutations or gene tagging, the guide sequence should be located within an adjacent intron or noncoding region to avoid inadvertent disruption of the other allele [37].
FIG. 2.
A guide to selecting a guide RNA. (A) Schematic illustrating a random genomic open reading frame and the position of different guide RNAs (arrowheads), specific for the highlighted application. mRNA transcription starts at the transcriptional start site (TSS) and generates a transcript containing exons (numbered boxes) and introns as well a 5′- and 3′-untranslated regions (UTRs). (B) Flow chart highlighting the individual steps of gRNA selection and design.
Once the approximate genomic target region has been selected, identification of a specific guide sequence takes advantage of the insights gained from high-throughput studies that have identified the main parameters affecting the quality of guide sequences (Fig. 2B) [41,42,45,71]. First, and most importantly, is the identification of a protospacer adjacent motif (PAM) sequence. For simplicity, we focus on PAM sequences of the NGG format for Cas9 from Streptococcus pyogenes, the most widely used Cas9. Characterization of 1,841 coding DNA sequence-targeting guide sequences revealed that PAMs of the GCGG format have the highest probability of success, whereas gRNAs utilizing the PAM sequence CTGG are the least likely to function [41]. In terms of efficiency and off-target effects, the use of truncated guide sequences has been shown to keep on-target efficiencies high, but dramatically reduce off-target rates. Therefore, the length of the guide RNA recognizing the DNA of interest should be kept to 17–19 nts [42]. Also, the GC content should ideally be between 50% and 75% and the presence of homopolymers (GGGG, CCCC, AAAA, TTTT) should be avoided [45]. Interestingly, guide RNAs located on the sense or antisense strand work with similar efficiencies [45].
Finally, once a guide RNA sequence has been chosen, the sequence can be examined for potential off-target annealing sites by analyzing the sequence with published algorithms [41,71]. Knowing that the CRISPR/Cas system is a bacterial immune system and that the average size of a bacterial genome is 3–5 Mbp compared to the size of the human genome at 3 billion base pairs [72–74], the potential off-target cleavage effects of guide RNA sequences are increased by a factor of 1,000. Therefore, it is important to highlight that no matter how “good” the selected guide sequence is, every guide sequence will have off-target effects when used in mammalian cells.
Recombinant Adeno-Associated Virus as an Optimal Knockin Template
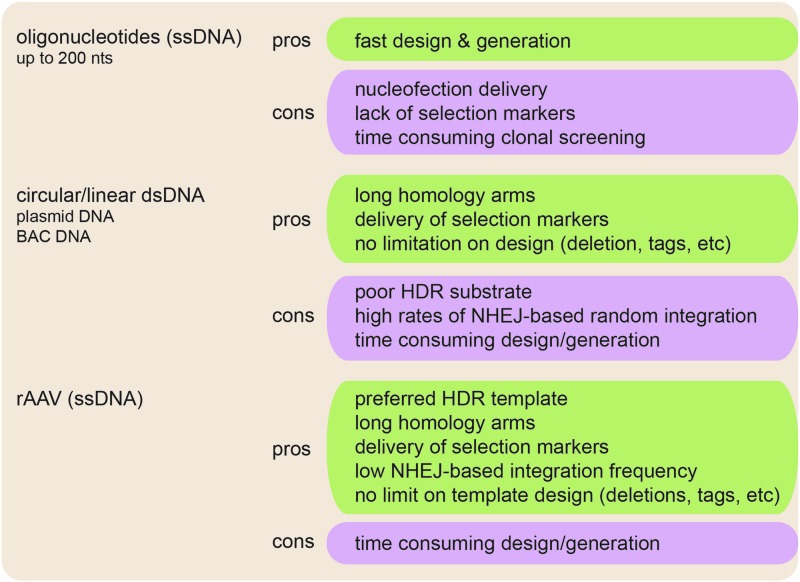
Two prerequisites are required for efficient CRISPR/Cas9 gene targeting in cells. First, a DNA double-strand break within the vicinity of the desired region. Second, a DNA template needs to be provided that can induce HDR instead of NHEJ. Different homology-directed templates have been described, among them single-stranded oligonucleotides (ssDNA) of up to 200 nts length [75,76], double-stranded circular or linearized plasmid DNA (dsDNA) with homology arms of varying length, including bacterial artificial chromosomes [77], and recombinant adeno-associated virus (rAAV) single-stranded DNA of up to 4.5 kb length [37] (Fig. 3). While short ssDNA oligonucleotides have the advantage of rapid design and generation, their disadvantages are the requirement of nucleofection for cellular delivery, poor recombination frequencies, and the inability to include selectable markers, resulting in time-consuming clonal selection and screening downstream. In contrast, long dsDNA templates with large homology arms allow the delivery of resistance genes for clonal selection. However, dsDNA has the major disadvantage of being a very poor substrate for HDR, as well as its ability to randomly integrate into the genome by NHEJ (Fig. 3). In contrast to oligonucleotides, single-stranded rAAV DNA templates retain the advantages of long homology arms and encoding resistance genes for increased recombination efficiencies with low NHEJ rates by being the preferred template for HDR, leading to high targeting frequencies [78]. However, rAAV vectors can integrate at sites of low homology in an NHEJ-dependent manner, but the process is far from being random [79]. Factors determining the rate of “random” integration include cell type and genomic sites. Interestingly, 3%–8% of all “random” integration events happen in ribosomal DNA repeats [80,81]. In addition, CpG islands and the TSS (±1,000 bp) were identified as preferred “random” integration sites [82]. Although no explanation has been given for this “nonrandom” event, it is likely that the high-CG content (>70%) of the rAAV is driving these events. To identify NHEJ-dependent rAAV integration, Southern blot, polymerase chain reaction (PCR), fluorescence in situ hybridization analysis [83,84], or rAAV shuttle vectors were used to identify the sequence of the vector:chromosome junctions [85,86].
FIG. 3.
Advantages and disadvantages of homology-directed repair (HDR) templates. Oligonucleotides of up to 200 bases length, circular or linear plasmid DNA, and single-stranded DNA in the form of recombinant adeno-associated virus (rAAV) are the most frequently used HDR templates. Listed are positive (green box) and negative (purple box) features of each template type.
rAAV as a DNA delivery vehicle
AAV is a single-stranded DNA virus of the Parvoviridae family. AAVs, independent of the serotype, are small icosahedral viruses with a single 4.7-kb DNA genome that contains hairpin-shaped inverted terminal repeats at the 5′ and 3′ ends. AAV contains two open reading frames, rep and cap, for nonstructural and structural genes, respectively. By deleting the rep and cap genes and replacing them with transgenic mammalian sequences and providing the cap gene in the form of a cotransfected plasmid, rAAV can be generated in cell culture for gene-editing purposes. Nine AAV serotypes have been reported (AAV1–9), with AAV2 being the most commonly found in humans (80%). In addition, a chimeric serotype (AAV-DJ) has been engineered in vitro with a capsid protein comprising a 60-amino acid hybrid derived from types 2/8/9 that outperform most natural serotypes in cell culture [87]. According to NIH guidelines, serotypes 1–4, and all recombinant rAAV constructs that do not contain potentially tumorigenic gene products or toxin molecules and that are produced in the absence of a helper virus, can be handled at biosafety level 1, making rAAV a widely useful reagent for gene editing with low to no biosafety concerns.
Selection Cassettes
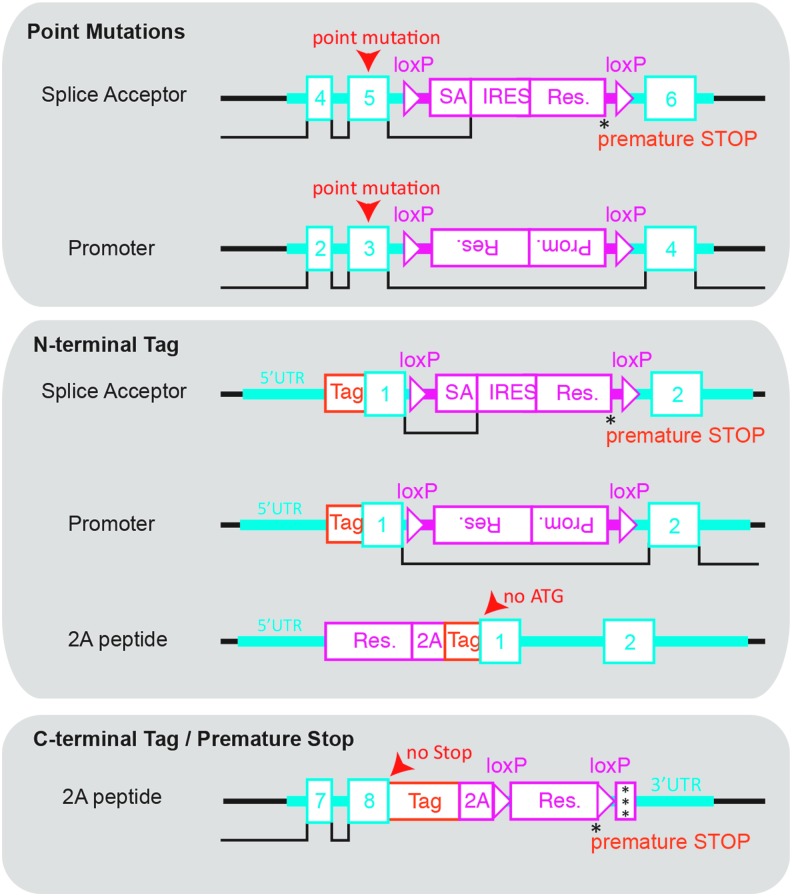
Depending on the desired gene-targeting event, there are multiple ways to design an rAAV template for HDR. Figure 4 illustrates the most commonly used targeting strategies, namely, the introduction of coding missense mutations, premature stop codons, and N- and C-terminal tagging of genes. Among others, we discuss two different approaches to introduce coding missense mutations that differ in their respective template design. The first uses a splice acceptor (SA) site linked to an internal ribosomal entry site (IRES) and a selection gene [88], either an antibiotic resistance gene or fluorescence reporter for subsequent clonal selection or single cell sorting, respectively, which results in an extremely high gene-targeting frequency of 80%–90% of clones [37]. This strategy is particularly useful if high efficiencies are required.
FIG. 4.
HDR template design strategies. The complete rAAV HDR template consists of left and right homology arm (light blue) containing exons (numbered boxes) and introns, as well as a selection cassette (red) that is separated from the homology arms by loxP sites for subsequent Cre recombinase excision of the selection cassette. Point mutations (red arrowhead), tags (orange), and premature stop codons (asterisk) can directly be cloned into the homology arm to obtain the final rAAV HDR template.
However, due to premature termination of transcription at the selection marker's stop codon, it is important to highlight that integration of the selection cassette temporarily inactivates the allele. The selection cassette is flanked by loxP sites, allowing for Cre recombinase-mediated excision of the selection cassette and reactivation of the targeted allele. If essential genes are targeted with this strategy, only heterozygous clones can be obtained and a second round of gene targeting will be required to obtain clones homozygous for the desired mutation [37]. If the essential gene is haploinsufficient, an alternative strategy needs to be applied. In this case, an inverse cassette comprising a promoter and selection gene can be introduced into an intron adjacent to the site of interest. This strategy avoids the temporal inactivation of the allele but has the disadvantage of delivering a promoter-driven selection, which in the case of off-site template integration will result in a higher incidence of viable false-positive clones and a more time-consuming clone selection process. Similar to the SA-IRES cassette, the promoter-driven selection cassette can be excised by Cre recombinase.
One very useful gene-targeting application is the introduction of N- or C-terminal high avidity epitope or fluorescent tags. Different strategies are required for N-terminal versus C-terminal tagging. Similar to the introduction of point mutations, the introduction of N-terminal tags uses either an SA-IRES or inverted promoter for resistance gene expression (Fig. 4). The major difference compared to the introduction of point mutations is that the desired tag is fused to the first exon during the process of homology arm generation. Despite that difference, N-terminal tags and point mutations use a similar template design. In addition, a new selection cassette design has been proposed, but waits for laboratory testing. In this new design, the left homology arm, which encodes the endogenous promoter, drives a resistance gene that is separated from exon 1 of the gene of interest by a 2A sequence and the desired tag sequence (Fig. 4). Combined with a 5′ or 3′ death cassette such as diphtheria toxin A, this approach, theoretically, reduces the rate of false-positive clones significantly by maintaining the rate of true-positive clones. Probably the biggest advantage of this design is the fact that the tag sequence is not considered a break in homology, making it feasible to integrate even large tags such as fluorescent proteins. In contrast, introduction of C-terminal tags yields high targeting rates without having to resort to an SA-IRES or a promoter to drive expression of the resistance gene. Instead, the last exon, which is contained in the left homology arm, is fused in frame to the desired tag—thereby removing the endogenous stop codon. The tag is followed by an in-frame 2A peptide sequence [89], a loxP site, and the selection gene for antibiotic selection. Another loxP site followed by a triple stop codon sequence completes the selection cassette (Fig. 4). It is noteworthy that this strategy does not inactivate the allele, making it possible to target essential genes without affecting cell viability. Like the previous template designs, the selection cassette can be excised by Cre recombinase treatment.
Designing Homology Arms
Careful design of the left and right homology arms of the template is essential for successful gene targeting. In general, longer template DNA will result in higher targeting efficiency [90]. Also, if the template DNA contains breaks in homology, for example, missense mutations, tags, or the selection cassette, the homology break should be designed to be centered within the overall template [90]. Because the endogenous AAV genome is 4.7 kb and is packed with maximal efficiencies by the capsid proteins, the length of the rAAV template should be close to 4.5 kb and never exceed 5.2 kb. Templates exceeding 5.2 kb will be truncated at the 5′ end during the packaging process, resulting in a heterogeneous rAAV particle population with reduced transgene delivery efficiencies [91]. In sum, the overall rAAV template size should be designed around 4–4.5 kb with breaks in homology as close to the center of the template as possible.
In addition to these general rules, here are some empirically derived guidelines. First, the 5′ and 3′ ends of the left and right homology arm should, if possible, start and end within noncoding regions, respectively. Although the process of HDR is highly efficient and mostly scarless, this design avoids the potential introduction of coding region errors during the recombination process. To make the PCR-based identification of faithfully recombined clones during the clone selection process as sensitive as possible, one homology arm should be kept less than 1 kb, and the other arm to be made as long as necessary to “fill up” the rAAV. To avoid single nucleotide breaks in homology, all homology arms should be amplified using proofreading polymerases from genomic DNA isolated from the tissue or cell line that will be used for the genome editing.
Combining CRISPR/Cas9 with rAAV Templates for Highly Efficient Gene Editing
Combined use of CRISPR/Cas with rAAV donors effects high frequency and scarless gene targeting [37]. Specifically, rAAV donors yield high on-target integration rates while providing little off-target effects, and offering precise gene editing on actively transcribed open reading frames [37]. The successful combination of both technologies depends on a few parameters. In addition to the design of guide RNA and HDR donor template, efficiently introducing the individual components into cells is very important. While the delivery of HDR templates is “limited” to single-stranded rAAV, the components of the CRISPR/Cas system can be delivered in multiple ways. Most cell types can be transfected with chimeric plasmid constructs. If this yields insufficient recombination frequencies, transfecting Cas9 mRNA [92] or whole protein can increase targeting frequency [93]. Most recently, the components of the CRISPR/Cas system have been delivered by rAAV [54,58,94]. However, how this Cas9/gRNA delivery method affects the correct recombination rate during gene editing remains to be seen.
Concluding Remarks
Performing gene editing in human cells to generate disease models, to recapitulate the effect of known mutations in vitro, or to study protein function with mutant protein expression levels at physiologic levels remains a major challenge. Initial work with rAAV demonstrated its potential as an HDR template, but low targeting efficiencies prevented the technology from being widely used in cell biology laboratories. The underlying reason for the low targeting efficiency is the requirement of a DNA double-strand break in proximity of the targeted region that induces HDR. The CRISPR/Cas9 system closes this gap by enabling researchers to specifically target DNA and induce DNA breaks wherever a PAM sequence is present. Indeed, the combination of both technologies has been shown to result in high gene-targeting rates for heterozygous and homozygous targeting strategies [37]. However, several open questions remain: (1) How to identify and validate off-target effects? (2) How to avoid the CRISPR/Cas9-mediated cut on the second strand for heterozygous targeting strategies? (3) How to increase the overall targeting efficiency with HDR templates that do not contain selectable markers?
First, how to identify off-target effects, two methods are commonly used: (1) the use of “off-target” prediction tools with subsequent conventional sequencing and TIDE analysis [95], Surveyor or T7 endonuclease I (T7EI) assays [34,38], and (2) deep sequencing on predetermined target sequences [39,56,96,97]. While Surveyor and T7EI assays can be routinely performed on a selected set of genomic regions, it is not feasible to perform these assays genome wide. In contrast, whole genome (deep) sequencing can identify all potential off-target effects, but it is not cost-efficient enough to be performed on a regular basis at this point in time. However, methods such as GUIDE-seq, Digenome-seq, or BLESS are potential tools for the unbiased detection of off-target cleavage by CRISPR/Cas9 [43,98,99]. In addition, combining CRISPR/Cas9 and rAAV HDR templates makes the generation of gene-recombined resources easy and fast, demanding methods other than deep sequencing to assess all potential off-target effects. In addition to CRISPR/Cas9-mediated off-target effects, the HDR template can induce off-target random integration in an NHEJ-dependent manner. As expected, these off-target effects are more difficult to dissect since the integration is more or less random. However, selected assays can be used to identify this effect. The most widely used approach is Southern blot analysis [100]. Another approach is represented by PCR-based detection of the HDR template in predicted guide RNA off-target sites [101], although it is less comprehensive.
Second, targeting a single copy of a gene with CRISPR/Cas9 is challenging. Indeed, when gene-targeting strategies are used to mutate a single copy of an allele, the second copy, even if not recombined by rAAV, is very likely to be targeted by CRISPR/Cas9, resulting in indel formation with potential phenotypic consequences. While the distinction between heterozygous and homozygous allele targeting can be made by performing allele-specific PCRs, limiting the presence of the CRISPR/Cas9 components may help to solve this problem. In fact, the use of a doxycycline-inducible CRISPR/Cas9 system regulates the frequency and size of target gene modifications [55]. Alternative CRISPR/Cas9 component delivery methods, in the form of protein [102,103], mRNA [103,104], or as split Cas9 [105,106], may also improve the temporal control over Cas9 activity.
The use of selectable markers in rAAV HDR templates has been shown to result in extremely high gene-editing rates of selectable clones. However, relative to the total number of cells that need to be transfected and transduced with the CRISPR/Cas9 components and the rAAV template, the rate is shockingly low. Given the fact that there are four populations of cells during those experiments (CRISPR/Cas9 positive, rAAV template template positive, and double positive and double negative) and only the double-positive population contains the cells that can undergo faithful recombination, the following question remains: how to increase the double-positive population? One interesting possibility may be the use of rAAV, not only to deliver the HDR template but also to deliver the Cas9 components. Cas9 and gRNA delivery by rAAV have recently been shown to work very effectively in whole animals by direct injection [54,58]. However, whether this approach, together with rAAV template delivery, results in higher “overall” gene-targeting rates without the use of selectable markers remains to be tested. All told, CRISPR/Cas9 systems have turned what was previously a specialist-only field of genome editing into a commonly used molecular cell biology approach on par with PCR and RNAi.
Acknowledgments
We are thankful to A. Springer, A. Kacsinta, and S. Heinz for critical comments. The preparation of this article was supported, in part, by grants from the NIH (CA185589).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Scherer S. and Davis RW. (1979). Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A 76:4951–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smithies O, Gregg RG, Boggs SS, Koralewski MA. and Kucherlapati RS. (1985). Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature 317:230–234 [DOI] [PubMed] [Google Scholar]
- 3.Thomas KR, Folger KR. and Capecchi MR. (1986). High frequency targeting of genes to specific sites in the mammalian genome. Cell 44:419–428 [DOI] [PubMed] [Google Scholar]
- 4.Mansour SL, Thomas KR. and Capecchi MR. (1988). Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336:348–352 [DOI] [PubMed] [Google Scholar]
- 5.Rong YS. and Golic KG. (2000). Gene targeting by homologous recombination in Drosophila. Science 288:2013–2018 [DOI] [PubMed] [Google Scholar]
- 6.Rudin N, Sugarman E. and Haber JE. (1989). Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics 122:519–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plessis A, Perrin A, Haber JE. and Dujon B. (1992). Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics 130:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouet P, Smih F. and Jasin M. (1994). Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol 14:8096–8106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choulika A, Perrin A, Dujon B. and Nicolas JF. (1995). Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 15:1968–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevalier BS, Kortemme T, Chadsey MS, Baker D, Monnat RJ. and Stoddard BL. (2002). Design, activity, and structure of a highly specific artificial endonuclease. Mol Cell 10:895–905 [DOI] [PubMed] [Google Scholar]
- 11.Miller J, McLachlan AD. and Klug A. (1985). Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J 4:1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavletich NP. and Pabo CO. (1991). Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science 252:809–817 [DOI] [PubMed] [Google Scholar]
- 13.Kim YG, Cha J. and Chandrasegaran S. (1996). Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 93:1156–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bibikova M, Beumer K, Trautman JK. and Carroll D. (2003). Enhancing gene targeting with designed zinc finger nucleases. Science 300:764. [DOI] [PubMed] [Google Scholar]
- 15.Bibikova M, Golic M, Golic KG. and Carroll D. (2002). Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161:1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A. and Bonas U. (2009). Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326:1509–1512 [DOI] [PubMed] [Google Scholar]
- 17.Moscou MJ. and Bogdanove AJ. (2009). A simple cipher governs DNA recognition by TAL effectors. Science 326:1501. [DOI] [PubMed] [Google Scholar]
- 18.Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ. and Voytas DF. (2010). Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186:757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishino Y, Shinagawa H, Makino K, Amemura M. and Nakata A. (1987). Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169:5429–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mojica FJ, Diez-Villasenor C, Soria E. and Juez G. (2000). Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol 36:244–246 [DOI] [PubMed] [Google Scholar]
- 21.Makarova KS, Aravind L, Grishin NV, Rogozin IB. and Koonin EV. (2002). A DNA repair system specific for thermophilic Archaea and bacteria predicted by genomic context analysis. Nucleic Acids Res 30:482–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolotin A, Quinquis B, Sorokin A. and Ehrlich SD. (2005). Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151:2551–2561 [DOI] [PubMed] [Google Scholar]
- 23.Mojica FJ, Diez-Villasenor C, Garcia-Martinez J. and Soria E. (2005). Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 60:174–182 [DOI] [PubMed] [Google Scholar]
- 24.Pourcel C, Salvignol G. and Vergnaud G. (2005). CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151:653–663 [DOI] [PubMed] [Google Scholar]
- 25.Haft DH, Selengut J, Mongodin EF. and Nelson KE. (2005). A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol 1:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarova KS, Grishin NV, Shabalina SA, Wolf YI. and Koonin EV. (2006). A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen R, Embden JD, Gaastra W. and Schouls LM. (2002). Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43:1565–1575 [DOI] [PubMed] [Google Scholar]
- 28.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA. and Horvath P. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712 [DOI] [PubMed] [Google Scholar]
- 29.Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadan AH. and Moineau S. (2010). The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468:67–71 [DOI] [PubMed] [Google Scholar]
- 30.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J. and Charpentier E. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471:602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P. and Siksnys V. (2011). The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res 39:9275–9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA. and Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasiunas G, Barrangou R, Horvath P. and Siksnys V. (2012). Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A 109:E2579–E2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA. and Zhang F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinek M, East A, Cheng A, Lin S, Ma E. and Doudna J. (2013). RNA-programmed genome editing in human cells. Elife 2:e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE. and Church GM. (2013). RNA-guided human genome engineering via Cas9. Science 339:823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaulich M, Lee YJ, Lonn P, Springer AD, Meade BR. and Dowdy SF. (2015). Efficient CRISPR-rAAV engineering of endogenous genes to study protein function by allele-specific RNAi. Nucleic Acids Res 43:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK. and Sander JD. (2013). High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuscu C, Arslan S, Singh R, Thorpe J. and Adli M. (2014). Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol 32:677–683 [DOI] [PubMed] [Google Scholar]
- 40.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, et al. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doench JG, Hartenian E, Graham DB, Tothova Z, Hegde M, Smith I, Sullender M, Ebert BL, Xavier RJ. and Root DE. (2014). Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol 32:1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Y, Sander JD, Reyon D, Cascio VM. and Joung JK. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, Hwang J, Kim JI. and Kim JS. (2015). Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods 12:237–243 [DOI] [PubMed] [Google Scholar]
- 44.Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH. and Joung JK. (2013). CRISPR RNA-guided activation of endogenous human genes. Nat Methods 10:977–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, et al. (2014). Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L. and Church GM. (2013). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y. and Zhang F. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, Wang L, Hodgkins A, Iyer V, Huang X. and Skarnes WC. (2014). Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods 11:399–402 [DOI] [PubMed] [Google Scholar]
- 49.Guilinger JP, Thompson DB. and Liu DR. (2014). Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Zhu S, Cai C, Yuan P, Li C, Huang Y. and Wei W. (2014). High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature 509:487–491 [DOI] [PubMed] [Google Scholar]
- 51.Wang T, Wei JJ, Sabatini DM. and Lander ES. (2014). Genetic screens in human cells using the CRISPR-Cas9 system. Science 343:80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C. and Yusa K. (2014). Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol 32:267–273 [DOI] [PubMed] [Google Scholar]
- 53.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG. and Zhang F. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343:84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, et al. (2014). CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159:440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dow LE, Fisher J, O'Rourke KP, Muley A, Kastenhuber ER, Livshits G, Tschaharganeh DF, Socci ND. and Lowe SW. (2015). Inducible in vivo genome editing with CRISPR-Cas9. Nat Biotechnol 33:390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, Cai W, Yang G, Bronson R, et al. (2014). CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514:380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T. and Anderson DG. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol 32:551–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M. and Zhang F. (2015). In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 33:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, et al. (2011). Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Oost J, Westra ER, Jackson RN. and Wiedenheft B. (2014). Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat Rev Microbiol 12:479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu PD, Lander ES. and Zhang F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157:1262–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, et al. (2014). Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343:1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, et al. (2013). CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154:442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng AW, Wang H, Yang H, Shi L, Katz Y, Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB. and Jaenisch R. (2013). Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res 23:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS. and Vale RD. (2014). A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159:635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS. and Huang B. (2013). Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155:1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S. and Pederson T. (2015). Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci U S A 112:3002–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hilton IB, D'Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE. and Gersbach CA. (2015). Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, et al. (2015). Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB. and Vakoc CR. (2015). Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol 33:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA. and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCutcheon JP. and von Dohlen CD. (2011). An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr Biol 21:1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang YJ, Land M, Hauser L, Chertkov O, Del Rio TG, Nolan M, Copeland A, Tice H, Cheng JF, et al. (2011). Non-contiguous finished genome sequence and contextual data of the filamentous soil bacterium Ktedonobacter racemifer type strain (SOSP1-21). Stand Genomic Sci 5:97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pruitt KD, Brown GR, Hiatt SM, Thibaud-Nissen F, Astashyn A, Ermolaeva O, Farrell CM, Hart J, Landrum MJ, et al. (2014). RefSeq: an update on mammalian reference sequences. Nucleic Acids Res 42:D756–D763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, et al. (2015). Cancer-associated protein kinase C mutations reveal kinase's role as tumor suppressor. Cell 160:489–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bottcher R, Hollmann M, Merk K, Nitschko V, Obermaier C, Philippou-Massier J, Wieland I, Gaul U. and Forstemann K. (2014). Efficient chromosomal gene modification with CRISPR/cas9 and PCR-based homologous recombination donors in cultured Drosophila cells. Nucleic Acids Res 42:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rong Z, Zhu S, Xu Y. and Fu X. (2014). Homologous recombination in human embryonic stem cells using CRISPR/Cas9 nickase and a long DNA donor template. Protein Cell 5:258–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasileva A. and Jessberger R. (2005). Precise hit: adeno-associated virus in gene targeting. Nat Rev Microbiol 3:837–847 [DOI] [PubMed] [Google Scholar]
- 79.Deyle DR. and Russell DW. (2009). Adeno-associated virus vector integration. Curr Opin Mol Ther 11:442–447 [PMC free article] [PubMed] [Google Scholar]
- 80.Miller DG, Trobridge GD, Petek LM, Jacobs MA, Kaul R. and Russell DW. (2005). Large-scale analysis of adeno-associated virus vector integration sites in normal human cells. J Virol 79:11434–11442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakai H, Wu X, Fuess S, Storm TA, Munroe D, Montini E, Burgess SM, Grompe M. and Kay MA. (2005). Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol 79:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inagaki K, Lewis SM, Wu X, Ma C, Munroe DJ, Fuess S, Storm TA, Kay MA. and Nakai H. (2007). DNA palindromes with a modest arm length of greater, similar 20 base pairs are a significant target for recombinant adeno-associated virus vector integration in the liver, muscles, and heart in mice. J Virol 81:11290–11303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLaughlin SK, Collis P, Hermonat PL. and Muzyczka N. (1988). Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol 62:1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lebkowski JS, McNally MM, Okarma TB. and Lerch LB. (1988). Adeno-associated virus: a vector system for efficient introduction and integration of DNA into a variety of mammalian cell types. Mol Cell Biol 8:3988–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rutledge EA. and Russell DW. (1997). Adeno-associated virus vector integration junctions. J Virol 71:8429–8436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller DG, Rutledge EA. and Russell DW. (2002). Chromosomal effects of adeno-associated virus vector integration. Nat Genet 30:147–148 [DOI] [PubMed] [Google Scholar]
- 87.Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA. and Kay MA. (2008). In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol 82:5887–5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JS, Bonifant C, Bunz F, Lane WS. and Waldman T. (2008). Epitope tagging of endogenous genes in diverse human cell lines. Nucleic Acids Res 36:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF. and Vignali DA. (2004). Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 22:589–594 [DOI] [PubMed] [Google Scholar]
- 90.Hirata RK. and Russell DW. (2000). Design and packaging of adeno-associated virus gene targeting vectors. J Virol 74:4612–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Z, Yang H. and Colosi P. (2010). Effect of genome size on AAV vector packaging. Mol Ther 18:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, et al. (2014). Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156:836–843 [DOI] [PubMed] [Google Scholar]
- 93.Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY. and Liu DR. (2015). Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol 33:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, et al. (2015). In vivo genome editing using Staphylococcus aureus Cas9. Nature 520:186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brinkman EK, Chen T, Amendola M. and van Steensel B. (2014). Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA. and Liu DR. (2013). High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veres A, Gosis BS, Ding Q, Collins R, Ragavendran A, Brand H, Erdin S, Talkowski ME. and Musunuru K. (2014). Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell 15:27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, et al. (2015). GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol 33:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crosetto N, Mitra A, Silva MJ, Bienko M, Dojer N, Wang Q, Karaca E, Chiarle R, Skrzypczak M, et al. (2013). Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods 10:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR. and Ploegh HL. (2015). Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol 33:538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Auer TO, Duroure K, De Cian A, Concordet JP. and Del Bene F. (2014). Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res 24:142–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D'Astolfo DS, Pagliero RJ, Pras A, Karthaus WR, Clevers H, Prasad V, Lebbink RJ, Rehmann H. and Geijsen N. (2015). Efficient intracellular delivery of native proteins. Cell 161:674–690 [DOI] [PubMed] [Google Scholar]
- 103.Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, Steinfeld I, Lunstad BD, Kaiser RJ, et al. (2015). Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol 33:985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F. and Jaenisch R. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zetsche B, Volz SE. and Zhang F. (2015). A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat Biotechnol 33:139–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wright AV, Sternberg SH, Taylor DW, Staahl BT, Bardales JA, Kornfeld JE. and Doudna JA. (2015). Rational design of a split-Cas9 enzyme complex. Proc Natl Acad Sci U S A 112:2984–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]