Among 1201 human immunodeficiency virus–infected youth followed up in 2008–2014, opportunistic infections were rare, but psychiatric, neurodevelopmental, inflammatory, metabolic, and genital tract conditions were frequent. Deaths were uncommon but mortality rates substantially higher than in the general population.
Keywords: pediatric HIV, mortality, opportunistic infections, psychiatric, pregnancy
Abstract
Background. Combination antiretroviral therapy (cART) has resulted in a dramatic decrease in human immunodeficiency virus (HIV)–related opportunistic infections and deaths in US youth, but both continue to occur.
Methods. We estimated the incidence of complications and deaths in IMPAACT P1074, a long-term US-based prospective multicenter cohort study conducted from April 2008 to June 2014. Incidence rates of selected diagnoses and trends over time were compared with those from a previous observational cohort study, P219C (2004–2007). Causes of death and relevant demographic and clinical features were reviewed.
Results. Among 1201 HIV-infected youth in P1074 (87% perinatally infected; mean [standard deviation] age at last chart review, 20.9 [5.4] years), psychiatric and neurodevelopmental disorders, asthma, pneumonia, and genital tract infections were among the most common comorbid conditions. Compared with findings in P219C, conditions with significantly increased incidence included substance or alcohol abuse, latent tuberculosis, diabetes mellitus, atypical mycobacterial infections, vitamin D deficiency or metabolic bone disorders, anxiety disorders, and fractures; the incidence of pneumonia decreased significantly. Twenty-eight deaths occurred, yielding a standardized mortality rate 31.5 times that of the US population. Those who died were older, less likely to be receiving cART, and had lower CD4 cell counts and higher viral loads. Most deaths (86%) were due to HIV-related medical conditions.
Conclusions. Opportunistic infections and deaths are less common among HIV-infected youth in the US in the cART era, but the mortality rate remains elevated. Deaths were associated with poor HIV control and older age. Emerging complications, such as psychiatric, inflammatory, metabolic, and genital tract diseases, need to be addressed.
An estimated 40 000 13–24-year-old children and youth in the United States were infected with human immunodeficiency virus (HIV) at the end of 2012 [1]. Introduction of combination antiretroviral therapy (cART) in the mid-1990s decreased the occurrence of opportunistic infections (OIs) in HIV-infected children and youth, with a subsequent decline in the mortality rate in that age group in the United States and Europe [2–8]. However, infectious (including OIs) and noninfectious conditions continue to contribute to the morbidity and mortality risk in this age group [9–11]. With improving access to cART, researchers in Asia [12–14] and Africa [15] are also reporting improved survival of HIV-infected children and youth. Treatment of aging youth with HIV in both resource-rich and resource-limited countries has led to the development of chronic and age-related problems, including dyslipidemia [16, 17], insulin resistance and diabetes mellitus [18, 19], decreased bone mineral density [20–22], renal disease [17], and psychosocial problems [23–25].
Longitudinal cohort studies have previously helped identify trends in HIV-associated infectious and noninfectious morbid conditions and deaths [7, 8]. The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network P1074 study is a US-based prospective, multicenter surveillance study of long-term outcomes in HIV-infected children and adolescents. We used data from P1074 to describe current infectious and noninfectious morbid conditions, pregnancy, and mortality rates among HIV-infected youth, in comparison with an earlier US-based cohort study, Pediatric AIDS Clinical Trials Group (PACTG) 219C [2–4, 11].
METHODS
Study Population
Data for this analysis were derived from the IMPAACT P1074 and PACTG 219C (P219C) studies. P219C was conducted between 2000 and 2007 to assess the long-term effects of cART on HIV-infected and HIV-exposed uninfected children in the United States, as described elsewhere [2–4]. To address changes in incidence over the past decade, we focused on the 2358 P219C participants with perinatally and nonperinatally acquired HIV in the study during 2004–2007. IMPAACT P1074 opened on 15 April 2009, after P219C closed, to continue long-term follow-up of those and other HIV-infected subjects. P1074 closed to accrual on 28 June 2013 and to follow-up on 30 June 2014. Of the 1201 P1074 participants, 782 (65%) had been enrolled previously in P219C, and the others had enrolled in IMPAACT interventional studies; age and other characteristics at P1074 entry were generally similar by prior P219C participation status. Institutional review board approval was obtained at all 39 participating clinical centers, and written informed consent was obtained from all participants and/or families, with assent obtained from minors according to local institutional review board guidelines.
Clinical and Laboratory Data
Clinical and laboratory data for the P1074 cohort were obtained from annual medical record reviews and included the 1-year period before study entry. Diagnoses were abstracted if resulting in hospitalization, persisting disability, or death; considered significant based on chronicity, recurrence, or major impact on quality of life; or potentially related to medication or indicative of underlying organ disease. Additional data obtained included pregnancy, height and weight, CD4 lymphocyte counts and HIV RNA viral load (VL) measurements, antiretroviral therapy (ART), and other medications used for >30 days. In P219C, medical history including infectious and noninfectious diagnoses, was recorded at enrollment and at study visits every 3 months thereafter. The HIV VL and CD4 lymphocyte counts were measured at each visit [2, 4].
Outcome Measures
The primary outcomes were occurrences of infectious or noninfectious conditions, pregnancy, and death. We considered incident events, defined as new events occurring between 1 year before study entry and the last chart abstraction. Each condition was counted only once per participant, at its earliest occurrence. Repeated pregnancies were also described. All clinical conditions were classified by the same coding team at the same data management center (Frontier Science Technology and Research Foundation) for P1074 and P219C, based on MedDRA (Medical Dictionary for Regulatory Activities, version 17.0) coding; MedDRA is a clinically validated international medical terminology created under the guidance of the International Conference on Harmonization to facilitate safety reporting [26] (see Supplementary Text 1 for further details).
Statistical Analyses
Incidence rates (IRs; per 100 person-years) and exact Poisson 95% confidence intervals (CIs) were calculated for the entire P1074 follow-up period (2008–2014, including the year prior to study entry) and during 2004–2007 for the P219C cohort. Participants with historical or prevalent events were excluded from the relevant risk sets. Subjects without a condition were censored as of their last chart abstraction date or death. We considered pregnancy only among female subjects aged ≥12.5 years, and genital tract infections among male and female subjects aged ≥12.5 years. We used Poisson regression models to evaluate trends in IRs for specific conditions over calendar years and over CD4 cell count strata for the P1074 cohort, and to adjust for age differences when comparing P1074 with P219C. The Poisson models yielded both unadjusted and age-adjusted IR ratios and corresponding 95% CIs [27]. A standardized mortality ratio was calculated comparing the mortality rate of P1074 participants with that of the general US population [28], standardized for age, sex, and race.
Demographic characteristics, HIV disease severity measures, and ART regimens at both entry and at final assessment (time of death or last chart abstraction) were compared between those who died during P1074 and those who survived. cART was defined as use of ≥3 ART drugs from ≥2 drug classes. We used χ2, Wilcoxon rank sum, and 2-sample t-tests to compare characteristics between those who died and survivors, and Cox proportional hazards models to evaluate risk factors for mortality. Sensitivity analyses were conducted, accounting for overlap in subjects participating in P1074 and P219C and restricting to perinatally HIV-infected youth, to assess consistency in results. Differences were considered statistically significant at P < .05 (2 sided). Owing to the exploratory nature of this study, no adjustments were made for multiple testing. All analyses were performed using SAS software (versions 9.2 and 9.4, SAS Institute).
RESULTS
Characteristics of Study Participants
A total of 1201 participants enrolled in P1074 and had ≥1 chart abstraction. Demographic and HIV disease characteristics are summarized in Table 1. The overall study population was 52% female, 58% black non-Hispanic, and 28% Hispanic; the majority (87%) had acquired HIV infection perinatally. The route of transmission for the nonperinatally infected group was primarily via sexual activity (74%), with other routes including blood transfusion or blood contact (14%), and sexual abuse (4%). At the first chart abstraction, participants’ mean (standard deviation [SD]) age was 17.4 (5.4) years, their median CD4 cell count was 609 cells/mm3, and their median VL was 1.88 log copies/mL. Most (85%) were receiving cART, and CD4 and VL measures remained relatively stable during a median follow-up of 3.7 years (Table 1). Among the 2358 P219C youth followed up between 2004 and 2007, their mean (SD) age in 2004 (or at enrollment, if after 2004) was 11.9 (5.0) years, with a median follow-up of 3.0 years. The P219C cohort was 52% female, 58% black, and 26% Hispanic, with 91% having perinatally acquired HIV infection; at start of the 2004–2007 follow-up period, their median CD4 was 720 cells/mm3, their median VL 2.78 log copies/mL, and 75% were receiving cART.
Table 1.
Demographic and Human Immunodeficiency Virus Disease Characteristics, Overall and by Death Status, of P1074 Participants, Enrolled and Followed Up at 39 Sites in the United States, 2008–2014
| Characteristica | Total (N = 1201) | Patient Status |
P Valueb | |
|---|---|---|---|---|
| Alive (n = 1173) | Died (n = 28) | |||
| Female sex, No. (%) | 623 (52) | 604 (52) | 19 (68) | .09 |
| Age (mean), SD, y | ||||
| First chart review | 17.4 (5.4) | 17.3 (5.4) | 21.3 (3.0) | <.001 |
| Last chart review | 20.9 (5.4) | 20.8 (5.4) | 23.5 (3.3) | .01 |
| Duration of study follow-up, median (IQR), y | 3.7 (2.9–4.4) | 3.7 (3.0–4.4) | 2.3 (1.2–3.0) | <.001 |
| Race/ethnicity, No. (%) | ||||
| White non-Hispanic | 136 (11) | 133 (11) | 3 (11) | .98 |
| Black non-Hispanic | 698 (58) | 681 (58) | 17 (61) | |
| Hispanic | 331 (28) | 324 (28) | 7 (25) | |
| Other or >1 race | 31 (3) | 30 (3) | 1 (4) | |
| Perinatally HIV infected, No. (%) | 1040 (87) | 1015 (87) | 25 (89) | .71 |
| BMI z score, mean (SD) | ||||
| First chart review | 0.41 (1.10) | 0.41 (1.08) | 0.22 (1.53) | .72 |
| Last chart review | 0.39 (1.15) | 0.40 (1.13) | −0.01 (1.76) | .43 |
| Change during follow-up | −0.02 (0.49) | −0.01 (0.48) | −0.24 (0.78) | .61 |
| HIV disease severity measures | ||||
| CD4 cell count, median (IQR), cells/mm3 | ||||
| First chart review | 609 (399–882) | 618 (412–886) | 28 (14–338) | <.001 |
| Last chart review | 580 (325–822) | 593 (350–828) | 20 (7–271) | <.001 |
| CD4 cell count ≤200 cells/mm3, No. (%) | ||||
| First chart review | 123 (10) | 104 (9) | 19 (68) | <.001 |
| Last chart review | 171 (14) | 151 (13) | 20 (71) | <.001 |
| CD4 cell count <200 cells/mm3 during follow-up, mean (SD), %c | 12.1 (28.2) | 10.7 (26.3) | 70.7 (43.1) | <.001 |
| CD4 cell count <350 cells/mm3 during follow-up, mean (SD), %c | 22.8 (35.8) | 21.5 (34.7) | 76.0 (39.2) | <.001 |
| HIV-1 RNA VL, median (IQR), log copies/mL | ||||
| First chart review | 1.88 (1.68–3.30) | 1.88 (1.68–3.21) | 4.59 (2.69–5.14) | <.001 |
| Last chart review | 1.68 (1.30–3.47) | 1.68 (1.30–3.37) | 4.49 (2.85–5.40) | <.001 |
| VL >1000 log copies/mL during follow-up, mean (SD), %c | 30.9 (33.4) | 29.9 (32.8) | 75.1 (28.4) | <.001 |
| ART regimen, No. (%) | ||||
| First chart review | .88 | |||
| cART | 1017 (85) | 993 (85) | 24 (86) | … |
| Non-cART ART | 70 (6) | 68 (6) | 2 (7) | … |
| No ART | 114 (10) | 112 (10) | 2 (7) | … |
| Last chart review | .02 | |||
| cART | 999 (83) | 980 (84) | 19 (68) | … |
| Non-cART ART | 83 (7) | 81 (7) | 2 (7) | … |
| No ART | 119 (10) | 112 (10) | 7 (25) | … |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; cART, combination ART (≥3 antiretroviral drugs from ≥2 drug classes); HIV, human immunodeficiency virus; IQR, interquartile range (25th–75th percentiles); SD, standard deviation; VL, viral load.
a Measures were unavailable for some characteristics, including race/ethnicity (n = 5), perinatal infection status (n = 5), BMI z score at first (n = 117) and last (n = 115) chart review, CD4 cell count at first (n = 4) and last (n = 2) chart review, and ART regimen at first (n = 22) and last (n = 11) chart review. Percentages are calculated among those with available information.
b P values comparing P1074 subjects who died with those who survived, using χ2 test for categorical measures and Wilcoxon rank sum test for continuous measures.
c Mean percentage of measurements within an individual meeting the cutoff value.
Incidence of Conditions During P1074
Common incident infectious and noninfectious conditions occurring during P1074 follow-up (Table 2) included genital tract infections (including human papillomavirus [HPV]) and chlamydial infections), psychiatric conditions (particularly mood disorders and anxiety disorders), and neurodevelopmental disorders, especially learning and communication disorders. Asthma and pneumonia were also common. IRs were generally very similar when analysis was restricted to perinatally HIV-infected youth (Supplementary Table 1). First pregnancy occurred in 97 of 512 female subjects (19%); 18 pregnancies were associated with complications (preeclampsia, premature labor or delivery, fetal growth restriction, or fetal death) and 29 concluded with an abortion (elective in 13 and spontaneous in 16). Twenty-four (25%) of these 97 subjects also had ≥1 subsequent pregnancy during P1074. In P219C, first pregnancies occurred in 48 of 792 female subjects (6%) between 2004 and 2007.
Table 2.
Common Incident Conditions in P1074 Participants and Their Relationships to CD4 Levels at Study Entry
| Conditiona | Participants at Risk, No. | Events, No. | Total Person-Years | IR/100 Person-Years | P Valueb |
|---|---|---|---|---|---|
| Broad categories of conditions | |||||
| Genital tract infections | 961 | 128 | 3760 | 3.405 | .008 |
| Psychiatric disorders | 1061 | 124 | 4492 | 2.760 | .10 |
| Neurodevelopmental disorders | 816 | 41 | 3520 | 1.165 | .054 |
| Specific conditions | |||||
| Infectious conditions | |||||
| Pneumonia | 811 | 41 | 3508 | 1.169 | <.001 |
| Zoster | 1042 | 35 | 4599 | 0.761 | <.001 |
| Oropharyngeal candidiasis | 840 | 27 | 3732 | 0.723 | <.001 |
| Nongenital herpes simplex virus | 1064 | 31 | 4724 | 0.656 | <.001 |
| Cellulitis | 1082 | 24 | 4801 | 0.500 | .002 |
| Esophageal or pulmonary candidiasis | 1160 | 23 | 5185 | 0.444 | <.001 |
| Latent tuberculosis | 1196 | 21 | 5345 | 0.393 | .52 |
| Genital tract infections | |||||
| HPV | 1024 | 79 | 4155 | 1.901 | .006 |
| Organism not specified | 1035 | 48 | 4286 | 1.120 | .003 |
| Chlamydia | 1064 | 36 | 4462 | 0.807 | .66 |
| Anogenital herpes simplex virus | 1069 | 24 | 4502 | 0.533 | <.001 |
| Candidiasis | 1064 | 19 | 4498 | 0.422 | .18 |
| Syphilis | 1080 | 17 | 4571 | 0.372 | .19 |
| Gonorrhea | 1072 | 16 | 4546 | 0.352 | .25 |
| Trichomoniasis | 1072 | 16 | 4540 | 0.352 | .33 |
| Pregnancy | 512 | 97 | 1896 | 5.117 | .32 |
| Psychiatric and neurodevelopmental disorders | |||||
| Mood disorders | 1092 | 102 | 4696 | 2.172 | .04 |
| Learning and communication disorders | 962 | 44 | 4170 | 1.055 | .002b |
| Anxiety disorders | 1172 | 38 | 5195 | 0.731 | .80 |
| Substance or alcohol abuse | 1186 | 24 | 5290 | 0.454 | .53 |
| Trauma and stress-related disorders | 1189 | 16 | 5318 | 0.301 | .57 |
| Other conditions | |||||
| Asthma | 884 | 47 | 3844 | 1.223 | .78 |
| Iron deficiency anemia | 1145 | 39 | 5076 | 0.768 | <.001 |
| Hypertension | 1156 | 34 | 5147 | 0.661 | .003 |
| Eczema | 1019 | 25 | 4501 | 0.555 | .74 |
| Vitamin D deficiency/metabolic bone disorders | 1179 | 25 | 5281 | 0.473 | .29 |
| Renal disease | 1162 | 24 | 5186 | 0.463 | .54 |
| Gastroesophageal reflux disease | 1161 | 19 | 5195 | 0.366 | .07 |
| Lipodystrophy | 1140 | 19 | 5089 | 0.373 | .20 |
| Neutropenia | 1044 | 16 | 4644 | 0.345 | .68 |
| Dyslipidemia | 1174 | 18 | 5242 | 0.343 | .06 |
| Wasting/failure to thrive | 1031 | 15 | 4616 | 0.325 | <.001 |
| Fractures | 1183 | 17 | 5311 | 0.320 | .75 |
| Hepatitis, nonspecific | 1153 | 16 | 5151 | 0.311 | .06 |
Abbreviations: HPV, human papillomavirus; IRs, incidence rates.
a IRs for all infectious and noninfectious conditions were calculated, and conditions with IRs >0.3 per 100 person years are included; conditions are defined in greater detail in Supplementary Text 1.
b P value for trend test computed from Poisson regression model comparing IRs across increasing baseline CD4 cell count categories of 0–199, 200–349, 350–499, and ≥500 cells/mm3; all significant increases reflect higher IRs with lower CD4 cell counts with the exception of learning and communication disorders. (Additional details, including IRs by baseline CD4 cell count, are provided in Supplementary Table 2).
Trends in Incidence by Calendar Year and CD4 for P1074
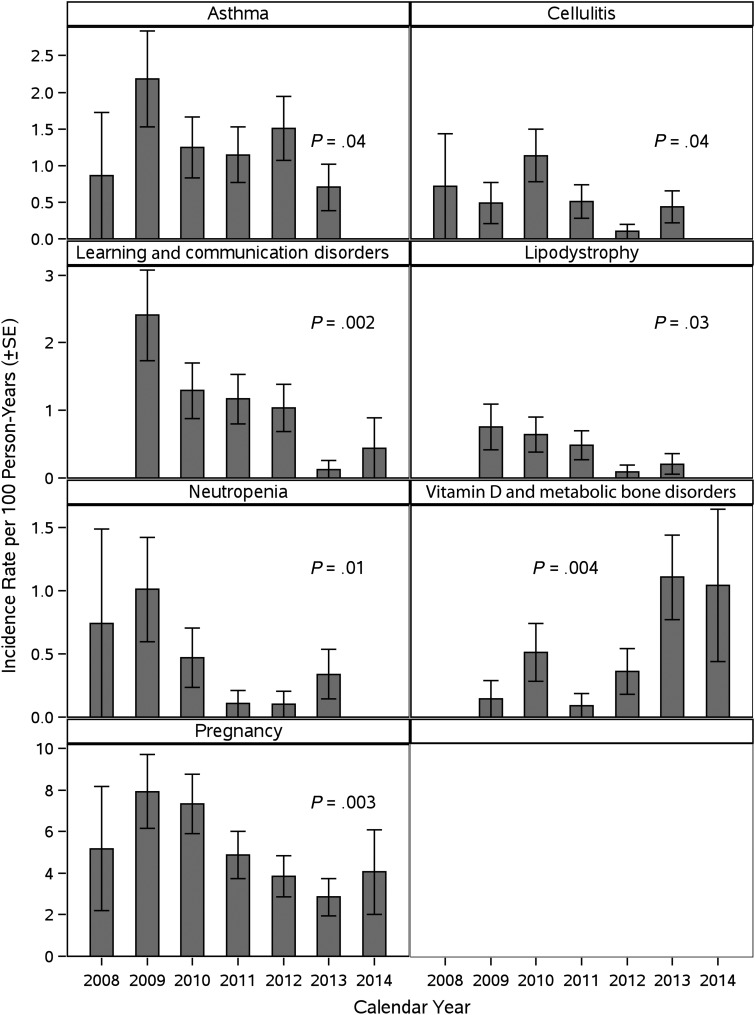
The IR for vitamin D deficiency and metabolic bone disorders in P1074 increased significantly from 2008 to 2014 (Figure 1). In contrast, IRs for asthma, cellulitis, learning and communication disorders, lipodystrophy, neutropenia, and pregnancy significantly decreased during this period. No significant temporal trends during 2008–2014 were observed for other diagnoses.
Figure 1.
Significant temporal trends in diagnoses and pregnancy among P1074 participants between 2008 and 2014. Trends are based on Poisson regression models with number of events in a given year as the outcome, calendar year as a linear predictor, and offset term equal to log person-years of follow-up during that calendar year. Abbreviation: SE, standard error.
IRs showed a significantly increasing trend with decreasing entry CD4 levels for genital tract infections overall and for several specific genital infections, nongenital infectious conditions (all those shown in Table 2, except latent tuberculosis), mood disorders, iron deficiency anemia, hypertension, and wasting or failure to thrive (Supplementary Table 2). There was a decreasing trend in IRs with decreasing CD4 levels for learning and communication disorders.
Difference in IRs Between P219C and P1074
Numerous conditions had higher incidences in P1074 than in P219C, with IRs for substance or alcohol abuse, latent tuberculosis, diabetes mellitus, atypical mycobacterial infections, vitamin D deficiency or metabolic bone disorders, anxiety disorders, and fractures all ≥5-fold in P1074 (Table 3). The IR was significantly lower in P1074 than in P219C only for pneumonia. After adjustment for differences in age distributions between the 2 study populations, IRs remained significantly lower in P1074 than in P219C for pneumonia and significantly higher for all psychiatric and neurodevelopmental conditions shown in Table 3, as well as for all other conditions with the exception of pregnancy, genital tract infections, atypical mycobacterial infection, esophageal or pulmonary candidiasis, hepatitis, diabetes mellitus, and hypertension. IRs did not differ significantly between the 2 studies for other conditions shown in Table 2. Sensitivity analyses accounting for the overlap of subjects participating in P1074 and P219C yielded almost identical results (data not shown).
Table 3.
Conditions With Significant Differences in Incidence Rates Between P1074 Participants in 2008–2014 and P219C Participants in 2004–2007a
| Categorya | P219C (2004–2007) |
P1074 (2008–2014) |
P1074 vs P219C |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants at Risk, No. | Events, No. | Total Person-Years | Rate/100 Person-Years | Participants at Risk, No. | Events, No. | Total Person-Years | Rate/100 Person-Years | IRR (95% CI) | IRR, Age-Adjustedb (95% CI) | |
| Significant Increases in IRs | ||||||||||
| Infectious conditions | ||||||||||
| Latent tuberculosis | 2350 | 2 | 6443 | 0.031 | 1196 | 21 | 5345 | 0.393 | 12.66 (2.97–53.98) | 12.62 (2.80–56.93) |
| Atypical mycobacterial infection | 2325 | 1 | 6375 | 0.016 | 1183 | 8 | 5326 | 0.150 | 9.58 (1.20–76.56) | 5.19 (.60–44.91) |
| Esophageal or pulmonary candidiasis | 2279 | 7 | 6252 | 0.112 | 1160 | 23 | 5185 | 0.444 | 3.96 (1.70–9.23) | 1.97 (.79–4.90) |
| Genital tract infections | ||||||||||
| Anogenital herpes | 1607 | 6 | 3597 | 0.167 | 1069 | 24 | 4502 | 0.533 | 3.20 (1.31–7.82) | 1.54 (.62–3.87) |
| HPV infection or disease | 1563 | 38 | 3463 | 1.097 | 1024 | 79 | 4155 | 1.901 | 1.73 (1.18–2.55) | 0.94 (.62–1.41) |
| Pregnancy | 792 | 48 | 1771 | 2.710 | 512 | 97 | 1896 | 5.117 | 1.89 (1.34–2.67) | 1.31 (.91–1.88) |
| Psychiatric and neurodevelopmental conditions | ||||||||||
| Substance abuse or alcohol abuse | 2346 | 1 | 6441 | 0.016 | 1186 | 24 | 5290 | 0.454 | 29.22 (3.95–216.00) | 10.72 (1.40–81.89) |
| Anxiety disorders | 2347 | 7 | 6435 | 0.109 | 1172 | 38 | 5195 | 0.731 | 6.72 (3.00–15.06) | 4.36 (1.83–10.42) |
| Trauma/stress-related disorders | 2351 | 4 | 6445 | 0.062 | 1189 | 16 | 5318 | 0.301 | 4.85 (1.62–14.50) | 4.27 (1.31–13.96) |
| Psychiatric disorders (any) | 2260 | 58 | 6133 | 0.946 | 1061 | 124 | 4492 | 2.760 | 2.92 (2.14–3.99) | 1.95 (1.39–2.74) |
| Disruptive/impulse control disorders | 2343 | 5 | 6422 | 0.078 | 1188 | 12 | 5318 | 0.226 | 2.90 (1.02–8.23) | 4.63 (1.59–13.48) |
| Mood disorders | 2280 | 47 | 6196 | 0.759 | 1092 | 102 | 4696 | 2.172 | 2.86 (2.03–4.05) | 1.74 (1.20–2.53) |
| Learning and communication disorders | 2046 | 26 | 5547 | 0.469 | 962 | 44 | 4170 | 1.055 | 2.25 (1.39–3.66) | 4.66 (2.83–7.66) |
| Other conditions | ||||||||||
| Diabetes mellitus | 2353 | 1 | 6454 | 0.015 | 1198 | 9 | 5381 | 0.167 | 10.79 (1.37–85.21) | 4.94 (.57–42.70) |
| Vitamin D deficiency/metabolic bone disorders | 2352 | 4 | 6444 | 0.062 | 1179 | 25 | 5281 | 0.473 | 7.63 (2.65–21.91) | 6.56 (2.16–19.97) |
| Fractures | 2343 | 4 | 6422 | 0.062 | 1183 | 17 | 5311 | 0.320 | 5.14 (1.73–15.27) | 6.43 (2.06–20.11) |
| Appendicitis | 2338 | 3 | 6407 | 0.047 | 1192 | 12 | 5349 | 0.224 | 4.79 (1.35–16.98) | 4.55 (1.20–17.19) |
| Iron deficiency anemia | 2305 | 14 | 6293 | 0.222 | 1145 | 39 | 5076 | 0.768 | 3.45 (1.88–6.36) | 3.11 (1.60–6.04) |
| Gastroesophageal reflux disease | 2313 | 7 | 6329 | 0.111 | 1161 | 19 | 5195 | 0.366 | 3.31 (1.39–7.87) | 2.62 (1.00–6.84) |
| Hepatitis, nonspecific | 2298 | 6 | 6308 | 0.095 | 1153 | 16 | 5151 | 0.311 | 3.27 (1.28–8.35) | 2.54 (.96–6.77) |
| Eczema | 2127 | 11 | 5811 | 0.189 | 1019 | 25 | 4501 | 0.555 | 2.93 (1.44–5.96) | 4.22 (1.95–9.12) |
| Dyslipidemia | 2349 | 8 | 6429 | 0.124 | 1174 | 18 | 5242 | 0.343 | 2.76 (1.20–6.35) | 3.40 (1.37–8.42) |
| Hypertension | 2328 | 16 | 6372 | 0.251 | 1156 | 34 | 5147 | 0.661 | 2.63 (1.45–4.77) | 1.38 (.73–2.62) |
| Asthma | 1935 | 27 | 5250 | 0.514 | 884 | 47 | 3844 | 1.223 | 2.38 (1.48–3.82) | 3.07 (1.84–5.13) |
| Hypothyroidism | 2356 | 0 | 6461 | 0.000 | 1191 | 8 | 5352 | 0.149 | NCc | NCc |
| Hyperthyroidism | 2358 | 0 | 6467 | 0.000 | 1200 | 5 | 5404 | 0.093 | NCc | NCc |
| Significant decreases in IRs | ||||||||||
| Pneumonia | 1571 | 73 | 4201 | 1.738 | 811 | 41 | 3508 | 1.169 | 0.67 (.46–.99) | 0.62 (.41–.95) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; IRs, incidence rates; IRRs, IR ratios; NC, not calculated.
a Conditions are defined in greater detail in Supplementary Text 1.
b Age-adjusted models fit using Poisson regression models with effects for study and age group (<10, 10–14, 14–18, or ≥18 years).
c Not calculated because there were no events in the P219C study.
Mortality Rate in P1074
There were 28 deaths during P1074 follow-up (mortality rate, 0.66/100 person-years), including 3, 3, 14, 7, and 1 death in 2010, 2011, 2012, 2013, and 2014, respectively (trend P = .24). The standardized mortality ratio compared with the general US population was 31.5 (95% CI, 19.9–43.2). The mean (SD) age at death was 23.5 (3.3) years, compared with 17.8 (3.5) years in P219C; however, the mortality rate in P1074 was very similar to the rate of 0.63/100 person-years observed from 2004 to 2006 in P219C. Primary causes of death were characterized as unspecified pneumonia in 5 deaths; advanced AIDS and progressive multifocal leukoencephalopathy in 4 deaths each; sepsis in 3 deaths; Pneumocystis jiroveci pneumonia, lymphoma, and suicide in 2 deaths each; and disseminated Mycobacterium avium intracellulare infection, HIV-related cardiomyopathy, homicide, motor vehicle accident, hepatic failure, and tuberculosis meningitis in 1 death each (Supplementary Table 3). Most deaths (86%) were directly linked to infection or other HIV-associated medical conditions. Table 1 presents the characteristics of P1074 subjects who died compared with those of survivors. Subjects who died did not differ from survivors in sociodemographic background but were older and had lower CD4 cell counts and higher VLs. The percentages receiving cART at the first chart review were similar in the 2 groups, but a higher percentage of those who died were not receiving cART at the time of their death compared with survivors at their last review. (Table 1 and Supplementary Table 3).
In multivariable Cox proportional hazards models, the CD4 cell count at study entry was the strongest predictor of mortality risk, with a 22% decrease in risk of death for each 50 CD4 cell/mm3 increase (adjusted hazard ratio, 0.78), and older age and higher VL at study entry were marginally associated with higher risk of death (Table 4). Sensitivity analyses restricted to youth with perinatal HIV infection (Supplementary Table 4), and exclusion of deaths considered unrelated to HIV disease yielded similar results.
Table 4.
Association of Demographic and Health Characteristics at Study Entry With Mortality Risk in P1074 Subjects
| Characteristica | Unadjusted Models |
Adjusted Modelb (n = 1197) |
|||
|---|---|---|---|---|---|
| Subjects, No. | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at first chart review | 1201 | 1.18 (1.10–1.28) | <.001 | 1.09 (.98–1.22) | .10 |
| CD4 cell count at first chart review (for each 50-cell/mm3 increase) | 1197 | 0.71 (.64–.79) | <.001 | 0.78 (.68–.88) | <.001 |
| HIV RNA at first chart review (for each log10 copies/mL increase) | 1201 | 3.02 (2.18–4.17) | <.001 | 1.45 (.99–2.13) | .054 |
| Sex (female vs male) | 1201 | 2.02 (.91–4.46) | .08 | … | … |
| BMI z score at first chart review | 1084 | 0.86 (.61–1.22) | .40 | … | … |
| Black race | 1201 | 1.10 (.51–2.38) | .81 | … | … |
| Hispanic ethnicity | 1201 | 0.85 (.36–2.00) | .71 | … | … |
| Receiving cART at first chart review | 1179 | 0.82 (.28–2.37) | .71 | … | … |
| Perinatal HIV acquisition (yes vs no) | 1196 | 1.15 (.35–3.80) | .82 | … | … |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio.
a Characteristics were the latest available measurements prior to or on the date of the first chart abstraction.
b Adjusted model includes only those covariates with estimates provided (age, CD4 cell count, HIV RNA).
DISCUSSION
The P1074 study allowed us to compare the incidences of complications of HIV infection, pregnancy, and death in a contemporary US cohort of HIV-infected youth with those in a younger cohort during an earlier period in the cART era [2–4]. Infected youth and young adults, including many survivors of perinatally acquired HIV infection, are now dealing with problems associated with chronic HIV infection and long-term complications of cART.
In the pre-cART era, the 5 most common OIs were serious bacterial infections, zoster, disseminated M. avium intracellulare, P. jiroveci pneumonia, and candidiasis, all with IRs >1 per 100 person-years [29]. In the cART era, IRs for P. jiroveci pneumonia, M. avium intracellulare, and other infectious conditions, such as lymphoid interstitial pneumonia, systemic fungal infection, cytomegalovirus retinitis, and tuberculosis disease, decreased to <0.50 per 100 person-years [3]. Although pneumonia was significantly less frequent in the P1074 cohort than in the P219C cohort, this and other infections, such as zoster continue to remain a significant disease burden. Importantly, we found an increase in the incidence of other infections, including latent tuberculosis, esophageal or pulmonary candidiasis, and atypical mycobacterial infections. The increased incidence of latent tuberculosis may reflect increased testing. Overall, these findings indicate that certain infections continue to occur in the era of widespread cART availability.
Among infectious complications, we observed particularly high rates of genital tract infections. These infections contribute to direct morbid effects and can increase HIV transmission [30]. Compared with the P219C cohort, P1074 participants had increased rates of genital HPV infection and anogenital herpes, as well as substantial rates of syphilis, chlamydia, gonorrhea, trichomoniasis, and yeast infections. Although these higher rates largely reflected an older age distribution in the P1074 population, they raise concern for development of cervical dysplasia in women, penile squamous cell carcinoma in men, and anal squamous cell carcinoma in women and in men who have sex with men [30]. Education about risk reduction strategies, including safe sex; administration of HPV vaccine; and cervical and anal screening for dysplasia are important preventive strategies for HIV-infected adolescents and young adults.
Chronic HIV infection and long-term cART present new challenges to HIV-infected youth. For example, we found higher rates of dyslipidemia, asthma, eczema, hypertension, diabetes mellitus, and thyroid hormone abnormalities in P1074 than during the P219C period. Siberry et al [31] reported higher rates of asthma and atopic dermatitis in HIV-infected children receiving cART than in HIV-exposed children, suggesting immune dysregulation. Metabolic disorders, such as insulin resistance, diabetes mellitus, lipodystrophy, hyperlipidemia, and hypertension, probably have multiple causes, including effects of HIV and cART [32], inflammation, hypercoagulation, endothelial dysfunction [18], and genetic polymorphisms [33].
An emerging concern with chronic HIV infection and long-term cART is poor bone health [20]. We observed higher rates of vitamin D deficiency or metabolic bone disorders and fractures in P1074 than in P219C and increasing rates over time of vitamin D deficiency during P1074. These higher rates persisted after adjustment for the older age of P1074 participants. Increased osteoclast activity due to immune activation and cytokine release can lead to vitamin D deficiency and bone loss [20, 34]. ART regimens containing protease inhibitors and tenofovir may adversely affect bone mineral health [35], and tenofovir and efavirenz can contribute to vitamin D deficiency [34]. Additional causes of poor bone health and fractures include low weight, hypogonadism, hepatitis C virus infection, glucocorticoid use, and substance use. Although routine monitoring for bone loss in HIV-infected youth is not currently recommended, adequate nutrition, including sufficient calcium and vitamin D intake, exercise, and avoidance of substance abuse, may contribute to good bone health.
Adding to challenges facing HIV-infected youth are high rates of psychiatric and neurodevelopmental problems, which not only directly contribute to morbid conditions but also have secondary consequences such as medication nonadherence, risk-taking behavior, and increased HIV transmission. The mental health and neurodevelopmental conditions with the largest increases from P219C to P1074 are substance or alcohol abuse, anxiety disorders, trauma or stress-related disorders, impulse control and mood disorders, and learning and communication disorders. Previous studies showed high rates of mental health diagnoses, affecting as many as 30%–70% of youth with perinatally acquired HIV infection [36, 37]. Multiple biological and environmental risk factors for development of mental health problems occur in aging children with perinatally acquired HIV [36, 37]. A decreasing trend in IRs with decreasing CD4 levels for learning and communication disorders may reflect increased ascertainment among healthier youth. The 2 suicides in our cohort underscore the importance of recognizing depression and providing timely intervention. Strategies such as incorporating mental health assessments into routine health care, early linkage to mental health professionals, and cognitive behavioral treatment, together with structured medication algorithms, are important interventions that warrant further study [36, 38].
A higher rate of pregnancy was observed in P1074 than in P219C, as expected for a cohort reaching child-bearing age. Compared with the US population, P1074 pregnancy rates were lower for female subjects aged >20 years (61.3 vs 163.0 per 1000 women), similar for those aged 15–19 years (65.8 vs 69.8 per 1000), but higher for those aged <15 years (19.5 vs 1.4 per 1000) [39]. The frequent occurrence of pregnancy complications points to the need for early linkage to prenatal care.
The majority of subjects who died had infectious and other HIV-related conditions at the time of death, similar to previous findings in deaths among HIV-infected children [2, 5]. Although subjects who died were older in P1074 than in P219C, mortality rates in the 2 studies were similar, >30 times that in the general population. Subjects who died had low CD4 cell counts, higher VLs, and lower usage of cART than survivors, presumably owing to lack of engagement in care, poor adherence to therapy, and/or limited treatment options [9, 40].
Strengths of our study include participation of a large number of experienced academic institutions and a 6-year follow-up period in P1074. Limitations include variability among sites in reporting significant diagnoses, lack of information about medication adherence, and a high proportion of subjects with perinatal HIV infection, which might limit generalizability to behaviorally infected populations. The frequency of screening for certain diagnoses (eg, tuberculosis, dyslipidemia, vitamin D deficiency, and genital tract infections) may have changed over time. Although a substantial proportion of the P219C and P1074 study populations overlapped, comparisons accounting for this overlap yielded almost identical results. In addition, although the 2 cohorts had different age profiles, most differences in IRs between P1074 and P219C persisted after adjustment for age. Furthermore, conditions that increase in incidence with increasing age require greater attention with the aging of the perinatally infected population.
HIV-associated OIs characteristic of earlier periods in the HIV epidemic have become uncommon in HIV-infected youth. Nevertheless, infections continue to occur and contribute to morbidity and mortality rates. Additional morbid conditions, including metabolic abnormalities, sexually transmitted infections, and psychiatric and neurodevelopmental disorders, are becoming more common and may reflect aging, chronic HIV infection, cART-related toxic effects, and chronic inflammation. Despite advances in cART, most deaths remain due to HIV-related conditions associated with virologic failure and immune suppression.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. MedDRA, the Medical Dictionary for Regulatory Activities terminology [26], is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
We thank the children and families for their participation in P1074 and the individuals and institutions involved in the conduct of P1074, as follows: New Jersey Medical School: Arry Dieudonne, MD, Linda Bettica, LPN, Anthony Scolpino, BA, James Oleske MD, MPH; UCLA–Los Angeles/Brazil AIDS Consortium: Yvonne Bryson, MD, Michele Carter, RN, Jaime Deville, MD, Karin Nielsen, MD; Texas Children's Hospital: Michelle Del Rey, RN, Chivon McMullen-Jackson, BSN, RN, ADN, William Shearer, MD, PhD, Mary Paul, MD; Lurie Children's Hospital of Chicago: Ram Yogev, MD, Margaret Ann Sanders, MPH, Ruth Williams, RN, Lynn Heald, PNP; Columbia University Medical Center (staff not specified); University of Miami Pediatric Perinatal HIV/AIDS: Gwendolyn Scott, MD; Charles Mitchell, MD, Claudia Florez, MD, Grace Alvarez, MD; University of California, San Diego, Mother-Child-Adolescent Program: Stephen Spector, MD, Rolando Viani, MD, MTP, Kimberly Norris, RN, BSN, Lisa Stangl, RN, NP; Duke University Medical Center: John Swetnam, Margaret Donnelly, PA-C, Joan Wilson, RN, MSN, Sunita Patil, PhD; Metropolitan Hospital: Mahrukh Bamji, MD, Indu Pathak, MD, Savita Manwanim, MD, Ekta Patel, MD; Children's Hospital of Boston: Sandra Burchett, MD, MS, Nancy Karthas, RN, MS, CPNP, Catherine Kneut, RN, MS, CPNP, Charlotte Mao, MD, MPH; Boston Medical Center Pediatric HIV Program: Ellen Cooper, MD, Diana Clarke, PharmD, Debra McLaud, RN, Pablo Leitz, MPH; New York University, New York: Aditya Kaul, MD, Nagamah Deygoo, MS, William Borkowsky, MD, Siham Akleh, RN; Jacobi Medical Center Bronx: Michael Rosenberg, MD, Joanna Dobroszycki, MD, Karen Kassen, RN, BSN, Marlene Burey, NP; Children's National Medical Center, Washington, DC: Steven Zeichner, MD, PhD, Connie Trexler, RN; Seattle Children's Hospital: Ann Melvin, MD, MPH, Gloria Bowen, MA, Amanda Robson Nuss, BS, Carrie Pettler, MPH; University of South Florida–Tampa: Carina Rodriguez, MD, Patricia Emmanuel, MD, Denise Casey, RN, Alicia Marion, ARNP; San Juan City Hospital: Nicolas Rosario-Matos, MD, Wanda Marrero-Figueroa, BSN-RN, Carlos Ortega, BA, Lizbeth Fabregas, BS, MS; SUNY Stony Brook: Sharon Nachman, MD, Denise Ferraro, FNP, Erin Infanzon, Michele Kelly, NP; Children's Hospital of Michigan: Chokechai Rongkavilit, MD, Ayanna Walters, RN, Eric McGrath, MD; Howard University, Washington DC: Sohail Rana, MD, Chandni Parikh, PNP, Caroline Reed, FNP, Patricia Houston, MS; Harbor UCLA Medical Center: Margaret Keller, MD, Michael Bolaris, MD, Judy Hayes, RN, Yolanda Gonzalez, RN; University of Southern California School of Medicine–Los Angeles County: Eva Operskalski, PhD, MBA, James Homans, MD, MPH, LaShonda Spencer, MD, Andrea KovacS, MD; University of Florida Health Science Center: Mobeen Rathore, MD, Nizar Maraqa, MD, Saniyyah Mahmoudi, MSN, ARNP, Tabetha Gayton, PhD, ARNP; University of Colorado Denver: Hannah Bernath, MPH, Kerry Hahn, CCRP, Jennifer Dunn, MS, RN, FNP, Jennifer Englund, BS; South Florida Children's Diagnostic and Treatment Center Fort Lauderdale: Ana Puga, MD, Amy Inman, Zulma Eysallenne, RN, James Blood, MSW; Strong Memorial Hospital, University of Rochester Medical Center: Geoffrey Weinberg, MD, Barbra Murante, MS, RN, PNP; Rush University Cook County Hospital, Chicago: Kenneth Boyer, MD, Jamie Martinez, MD, James McAuley, MD, Maureen Haak; Children's Hospital of Los Angeles: Nancy Flores, Diane Tucker, MSN, Julie McAvoy, MPH, Marvin Belzer, MD; University of California San Francisco: Diane Wara, MD, Theodore Ruel, MD, Mica Muskat, NP, Nicole Tilton, NP; Johns Hopkins University Baltimore: Allison Agwu, MD, ScM, Thuy Anderson, RN, BSN, Aleisha Collinson-Streng, RN, BSN, ACRN, Kaye Park, MPH; Miller Children's Hospital: Audra Deveikis, MD, Jagmohan Batra, MD, Tempe Chen, MD, David Michalik, DO; University of Maryland Baltimore: Douglas Watson, MD, Maria Johnson, DDS, Susan Lovelace, MSN, Corinda Hilyard; Tulane University New Orleans: Russell Van Dyke, MD, Margarita Silio, MD, Thomas Alchediak, MD, Sheila Bradford, RN; University of Alabama, Birmingham: Dorothy Shaw, BA, Sharan Robbins, BA, MAE, Newana Beatty, CCRC, Marilyn Crain, MD, MPH; The Children's Hospital of Philadelphia: Carol Vincent, PhD, CRNP, Richard Rutstein, MD, Steven Douglas, MD, Sheri McDougall, MSHed, CCRC; Bronx-Lebanon Hospital: Anna Marie Emeh, MD, Mary Elizabeth Vachon, MPH, Levi Cherian, Murli Purswani, MD, FAAP; St Jude's Children's Hospital: Katherine Knapp, MD, Patricia Flynn, MD, Judy Glenn, LPN, Thomas Wride, MS; University of Puerto Rico Pediatric HIV/AIDS Research Program: Irma Febo, MD, Ruth Santos-Otero, RN, MPH, Maritza Cruz-Rodriguez, BA; Western New England Maternal Pediatric Adolescent AIDS: Katherine Luzuriaga, MD, Christina Hermos, MD, Jesica Pagano-Therrien, CPNP, Donna Picard BSN, RN, BC.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group was provided by the National Institute of Allergy and Infectious Diseases of the NIH under (grants UM1AI068632 [IMPAACT Leadership and Operations Center], UM1AI068616 [IMPAACT Statistical and Data Management Center], and UM1AI106716 [IMPAACT Laboratory Center]), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health.
Potential conflicts of interest. M. J. L. serves as a consultant for Merck Sharp & Dohme and receives research funding from Merck Sharp & Dohme and GlaxoSmithKline. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the IMPAACT P1074 Study Team, Arry Dieudonne, Linda Bettica, Anthony Scolpino, James Oleske, Yvonne Bryson, Michele Carter, Jaime Deville, Karin Nielsen, Michelle Del Rey, Chivon McMullen-Jackson, William Shearer, Mary Paul, Ram Yogev, Margaret Ann Sanders, Ruth Williams, Lynn Heald, Gwendolyn Scott, Charles Mitchell, Claudia Florez, Grace Alvarez, Stephen Spector, Rolando Viani, Kimberly Norris, Lisa Stangl, John Swetnam, Margaret Donnelly, Joan Wilson, Sunita Patil, Mahrukh Bamji, Indu Pathak, Savita Manwanim, Ekta Patel, Sandra Burchett, Nancy Karthas, Catherine Kneut, Charlotte Mao, Ellen Cooper, Diana Clarke, Debra McLaud, Pablo Leitz, Aditya Kaul, Nagamah Deygoo, William Borkowsky, Siham Akleh, Michael Rosenberg, Joanna Dobroszycki, Karen Kassen, Marlene Burey, Steven Zeichner, Connie Trexler, Ann Melvin, Gloria Bowen, Amanda Robson Nuss, Carrie Pettler, Carina Rodriguez, Patricia Emmanuel, Denise Casey, Alicia Marion, Nicolas Rosario-Matos, Wanda Marrero-Figueroa, Carlos Ortega, Lizbeth Fabregas, Sharon Nachman, Denise Ferraro, Erin Infanzon, Michele Kelly, Chokechai Rongkavilit, Ayanna Walters, Eric McGrath, Sohail Rana, Chandni Parikh, Caroline Reed, Patricia Houston, Margaret Keller, Michael Bolaris, Judy Hayes, Yolanda Gonzalez, Eva Operskalski, James Homans, LaShonda Spencer, Andrea KovacS, Mobeen Rathore, Nizar Maraqa, Saniyyah Mahmoudi, Tabetha Gayton, Hannah Bernath, Kerry Hahn, Jennifer Dunn, Jennifer Englund, Ana Puga, Amy Inman, Zulma Eysallenne, James Blood, Geoffrey Weinberg, Barbra Murante, Kenneth Boyer, Jamie Martinez, James McAuley, Maureen Haak, Nancy Flores, Diane Tucker, Julie McAvoy, Marvin Belzer, Diane Wara, Theodore Ruel, Mica Muskat, Nicole Tilton, Allison Agwu, Thuy Anderson, Aleisha Collinson-Streng, Kaye Park, Audra Deveikis, Jagmohan Batra, Tempe Chen, David Michalik, Douglas Watson, Maria Johnson, Susan Lovelace, Corinda Hilyard, Russell Van Dyke, Margarita Silio, Thomas Alchediak, Sheila Bradford, Dorothy Shaw, Sharan Robbins, Newana Beatty, Marilyn Crain, Carol Vincent, Richard Rutstein, Steven Douglas, Sheri McDougall, Anna Marie Emeh, Mary Elizabeth Vachon, Levi Cherian, Murli Purswani, Katherine Knapp, Patricia Flynn, Judy Glenn, Thomas Wride, Irma Febo, Ruth Santos-Otero, Maritza Cruz-Rodriguez, Katherine Luzuriaga, Christina Hermos, Jesica Pagano-Therrien, and Donna Picard
References
- 1. Centers for Disease Control and Prevention. HIV surveillance report, 2011; vol. 23. Available at: http://www.cdc.gov/hiv/library/reports/surveillance/2011/surveillance_report_vol_23.html Accessed 24 August 2015.
- 2.Brady MT, Oleske JM, Williams PL et al. . Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr 2010; 53:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gona P, Van Dyke RB, Williams PL et al. . Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA 2006; 296:292–300. [DOI] [PubMed] [Google Scholar]
- 4.Patel K, Hernan MA, Williams PL et al. . Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis 2008; 46:507–15. [DOI] [PubMed] [Google Scholar]
- 5.Kapogiannis BG, Soe MM, Nesheim SR et al. . Mortality trends in the US perinatal AIDS collaborative transmission study (1986–2004). Clin Infect Dis 2011; 53:1024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesheim SR, Kapogiannis BG, Soe MM et al. . Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the Perinatal AIDS Collaborative Transmission Study, 1986–2004. Pediatrics 2007; 120:100–9. [DOI] [PubMed] [Google Scholar]
- 7.Judd A, Doerholt K, Tookey PA et al. . Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis 2007; 45:918–24. [DOI] [PubMed] [Google Scholar]
- 8.Dollfus C, Le Chenadec J, Faye A et al. . Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10). Clin Infect Dis 2010; 51:214–24. [DOI] [PubMed] [Google Scholar]
- 9.Foster C, Judd A, Tookey P et al. . Young people in the United Kingdom and Ireland with perinatally acquired HIV: the pediatric legacy for adult services. AIDS Patient Care STDS 2009; 23:159–66. [DOI] [PubMed] [Google Scholar]
- 10.Nachman SA, Chernoff M, Gona P et al. . Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era. Arch Pediatr Adolesc Med 2009; 163:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ylitalo N, Brogly S, Hughes MD et al. . Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med 2006; 160:778–87. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishna M, Durga K, Rao RK, Reddy DM, Kondapi AK. Factors associated with conversion of long-term non-progressors to progressors: a prospective study of HIV perinatally infected pediatric survivors. Indian J Med Res 2013; 138:322–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Prasitsuebsai W, Kariminia A, Puthanakit T et al. . Impact of antiretroviral therapy on opportunistic infections of HIV-infected children in the therapeutic research, education and AIDS training Asia pediatric HIV observational database. Pediatr Infect Dis J 2014; 33:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chokephaibulkit K, Kariminia A, Oberdorfer P et al. . Characterizing HIV manifestations and treatment outcomes of perinatally infected adolescents in Asia. Pediatr Infect Dis J 2014; 33:291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marazzi MC, De Luca S, Palombi L et al. . Predictors of adverse outcomes in HIV-1-infected children receiving combination antiretroviral treatment: results from a DREAM cohort in sub-Saharan Africa. Pediatr Infect Dis J 2014; 33:295–300. [DOI] [PubMed] [Google Scholar]
- 16.Papi L, Menezes AC, Rocha H et al. . Prevalence of lipodystrophy and risk factors for dyslipidemia in HIV-infected children in Brazil. Braz J Infect Dis 2014; 18:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortuny C, Deya-Martinez A, Chiappini E, Galli L, de Martino M, Noguera-Julian A. Metabolic and renal adverse effects of antiretroviral therapy in HIV-infected children and adolescents. Pediatr Infect Dis J 2015; 34(5 suppl 1):S36–43. [DOI] [PubMed] [Google Scholar]
- 18.Miller TI, Borkowsky W, DiMeglio LA et al. . Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med 2012; 13:264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipshultz SE, Williams PL, Wilkinson JD et al. . Cardiac status of children infected with human immunodeficiency virus who are receiving long-term combination antiretroviral therapy: results from the Adolescent Master Protocol of the Multicenter Pediatric HIV/AIDS Cohort Study. JAMA Pediatr 2013; 167:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warriner AH, Mugavero M, Overton ET. Bone alterations associated with HIV. Curr HIV/AIDS Rep 2014; 11:233–40. [DOI] [PubMed] [Google Scholar]
- 21.DiMeglio LA, Wang J, Siberry GK et al. . Bone mineral density in children and adolescents with perinatal HIV infection. AIDS 2013; 27:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puthanakit T, Saksawad R, Bunupuradah T et al. . Prevalence and risk factors of low bone mineral density among perinatally HIV-infected Thai adolescents receiving antiretroviral therapy. J Acquir Immune Defic Syndr 2012; 61:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folayan M, Morolake O, Brown B, Harrison A. Addressing the socio-development needs of adolescents living with HIV/AIDS in Nigeria: a call for action. Afr J Reprod Health 2014; 18:93–101. [PMC free article] [PubMed] [Google Scholar]
- 24.Fish R, Judd A, Jungmann E, O'Leary C, Foster C. Mortality in perinatally HIV-infected young people in England following transition to adult care: an HIV Young Persons Network (HYPNet) audit. HIV Med 2014; 15:239–44. [DOI] [PubMed] [Google Scholar]
- 25.Chernoff M, Nachman S, Williams P et al. . Mental health treatment patterns in perinatally HIV-infected youth and controls. Pediatrics 2009; 124:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maintenance and Support Services Organization (MSSO). MedDRA introductory guide version 17.1. McLean, VA: MSSO, 2014.
- 27.Ingelfinger JA, Mosteller F, Thibodeau LA, Ware JH. Biostatistics in clinical medicine. New York, NY: McGraw-Hill, Inc, 1994. [Google Scholar]
- 28. Centers for Disease Control and Prevention. Health, United States, 2014. Available at: http://www.cdc.gov/nchs/hus.htm Accessed 24 August 2015.
- 29.Dankner WM, Lindsey JC, Levin MJ. Correlates of opportunistic infections in children infected with the human immunodeficiency virus managed before highly active antiretroviral therapy. Pediatr Infect Dis J 2001; 20:40–8. [DOI] [PubMed] [Google Scholar]
- 30.Tassiopoulos K, Moscicki AB, Mellins C et al. . Sexual risk behavior among youth with perinatal HIV infection in the United States: predictors and implications for intervention development. Clin Infect Dis 2013; 56:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siberry GK, Leister E, Jacobson DL et al. . Increased risk of asthma and atopic dermatitis in perinatally HIV-infected children and adolescents. Clin Immunol 2012; 142:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geffner ME, Patel K, Miller TL et al. . Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the pediatric HIV/AIDS cohort study. Horm Res Paediatr 2011; 76:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Almeida ER, Reiche EM, Kallaur AP, Flauzino T, Watanabe MA. The roles of genetic polymorphisms and human immunodeficiency virus infection in lipid metabolism. Biomed Res Int 2013; doi:10.1155/2013/836790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panayiotopoulos A, Bhat N, Bhangoo A. Bone and vitamin D metabolism in HIV. Rev Endocr Metab Disord 2013; 14:119–25. [DOI] [PubMed] [Google Scholar]
- 35.Mondy K, Tebas P. Emerging bone problems in patients infected with human immunodeficiency virus. Clin Infect Dis 2003; 36(suppl 2):S101–5. [DOI] [PubMed] [Google Scholar]
- 36.Mellins CA, Tassiopoulos K, Malee K et al. . Behavioral health risks in perinatally HIV-exposed youth: co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDS 2011; 25:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadow KD, Angelidou K, Chernoff M et al. . Longitudinal study of emerging mental health concerns in youth perinatally infected with HIV and peer comparisons. J Dev Behav Pediatr 2012; 33:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennard B, Brown L, Hawkins L et al. . Development and implementation of health and wellness CBT for individuals with depression and HIV. Cogn Behav Pract 2014; 21:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep 2012; 60:1–21. [PubMed] [Google Scholar]
- 40.Castro H, Judd A, Gibb DM et al. . Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet 2011; 377:1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.