Abstract
Four anatomical patterns of hydrocephalus secondary to congenital Toxoplasma gondii infection were identified and characterized for infants enrolled in the National Collaborative Chicago-based Congenital Toxoplasmosis Study. Analysis of parasite serotype revealed that different anatomical patterns associate with Type-II vs Not-Exclusively Type-II strains (NE-II) (P = .035).
Keywords: hydrocephalus, Toxoplasma gondii, aqueductal obstruction, foraminal obstruction
(See the Major Article by Contopoulos-Ioannidis et al on pages 1815–24.)
Toxoplasma gondii is a protozoan parasite that may cause ocular and neurologic disease [1–6]. It is the most common parasitic infection in the world and causes recognizable, symptomatic disease in persons infected in utero or with compromised immune systems or when chorioretinal damage occurs in postnatal infections. Congenital toxoplasmosis may lead to a wide range of ocular and neurologic sequelae, including chorioretinitis, developmental delay, motor abnormalities, seizures and hydrocephalus [2–5].
Hydrocephalus has been estimated to affect approximately 4% of infants with congenital toxoplasmosis and is a significant cause of morbidity and mortality in this disease. In the past, hydrocephalus usually has been attributed to aqueductal obstruction [4], however, we noted other patterns in our study population, which we describe and characterize herein.
Genetic analysis of T. gondii reveals that discrete strains exist, reflecting divergent genetic lineages [7, 8]. These include clonotypes (referred to as Type I, II, III), recombinant, and other atypical lineages. It appears that infection with isolates harboring different genetics may manifest with different disease patterns. Genetic diversity of T. gondii as well as the human host may also help to explain differences in prevalence and virulence seen in different populations [7, 8].
In a previous study, we found that overall prevalence of hydrocephalus in children with congenital toxoplasmosis did not differ significantly according to parasite serotype [9]. Herein, the different patterns of hydrocephalus noted in 65 children with hydrocephalus caused by congenital T. gondii infection are described and the relationship between pattern of hydrocephalus and parasite serotype is analyzed.
METHODS
Study Population
Data were obtained from persons in the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS). This cohort consisted of 210 persons with congenital toxoplasmosis who were referred to the study between 1981 and 2010. Brain computed tomography scans were performed for all children in infancy or at time of diagnosis, and further neuroimaging was performed as clinically indicated. Neuroimages were examined for anatomy of the ventricular system and evidence of obstructive processes. Details of study design and population have been described previously [2, 5, 6].
Identification of Parasite Strain
Sera were studied for reactivity against polymorphic GRA6 and GRA7 peptides using a peptide-based enzyme-linked immunosorbent assay (ELISA), as described previously [9].
Statistical Methods
Data were analyzed for associations between patterns of hydrocephalus and parasite strains using Stata Version 13 [10]. Categorical data were analyzed using Fisher's exact test. A 2-tailed P-value ≤.05 was considered statistically significant.
Ethics
The NCCCTS was conducted with ethical standards for human experimentation established in the Declaration of Helsinki, with prior institutional review board (IRB) approval, in accordance with HIPAA (Health Insurance Portability and Accountability Act) regulations and was reviewed regularly by a Data Safety Monitoring Board. Informed consents were obtained from subjects for all aspects of the NCCCTS in accordance with IRB and National Institutes of Health (NIH) guidelines.
RESULTS
Patterns of Hydrocephalus
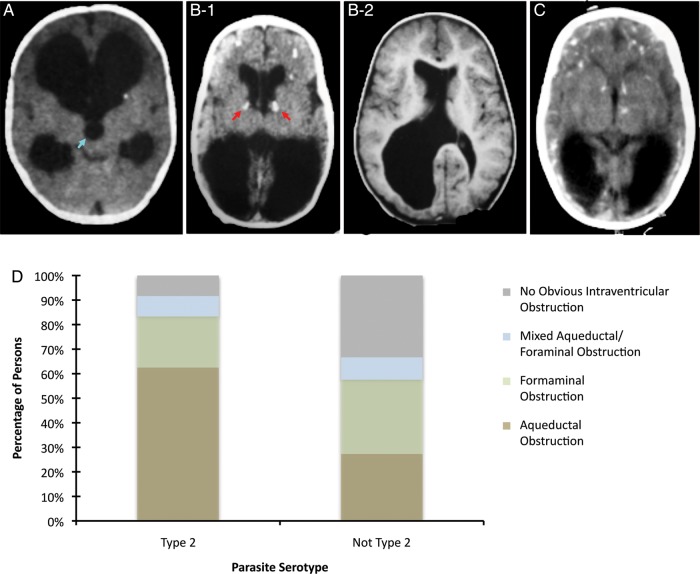
Of the 210 persons enrolled in the NCCCTS, 65 (31%) had hydrocephalus. Four distinct patterns of hydrocephalus were identified. Aqueductal obstruction with enlargement of the lateral and third ventricles and a normal fourth ventricle (Figure 1A) was the most common pattern, occurring in 28 (43%) of those with hydrocephalus. The next most common pattern, obstruction of the foramina of Monro (Figure 1B-1), was identified in 16 (25%) persons and is characterized by calcifications at the site of the foramina and often asymmetric dilatation of the lateral ventricles with normal third and fourth ventricles. The frontal horns of the ventricles often appeared fuller in an asymmetrical pattern. One person with this pattern had unilateral foraminal obstruction causing unilateral hydrocephalus (Figure 1B-2), but this pattern was usually bilateral. Seven (11%) persons had evidence of a third pattern of mixed aqueductal and foraminal obstruction. Some persons with this pattern initially presented with aqueductal obstruction and foraminal obstruction manifesting following ventriculoperitoneal shunt placement when one or both lateral ventricles remained dilated. The fourth pattern, which we describe as having no obvious evidence of intraventricular obstruction resulting in ventricular enlargement, occurred in 14 (21%) persons. This pattern is characterized by bilateral lateral ventricular dilatation, normal third and fourth ventricle size, and foramina of Monro without periforaminal calcifications or other obvious intraventricular obstructive lesions that result in marked dilatation in the frontal horns (Figure 1C). This was often associated with colpocephaly, compression of parieto-occipital cortical mantle, and less fullness of the frontal horns relative to what is often seen with foraminal obstruction. The degree of fullness of the frontal horn of the ventricle distinguishes the patterns presented in Figures 1B and 1C.
Figure 1.
Anatomical patterns of hydrocephalus in congenital Toxoplasma gondii infection and association with parasite strain. The large dark areas in the neuroimages indicate the dilated ventricular regions and hydrocephalus. A, Obstruction of the aqueduct of Sylvius; aqueduct of Sylvius indicated by blue arrow. B1, Bilateral obstruction of the foramina of Monro; foramina of Monro indicated by red arrow. B2, Unilateral obstruction of the foramina of Monro. C, No obvious intraventricular obstruction causing ventricular dilatation. D, Twenty-four patients had antibodies to Type-II peptides, and 33 had antibodies to NE-II peptides. Type-II strains were associated with aqueductal obstruction and NE-II strains were associated with nonobstructive hydrocephalus (P = .035). % Total = (Number of patients with antibodies to parasite strain and pattern of hydrocephalus/Total number of patients with antibodies to parasite strain) × 100.
Parasite Strains Causing Infection
Serum from infected persons was tested against polymorphic peptides specific to Type-II or not exclusively-II (NE-II) strains using an ELISA assay. Of the 65 persons with hydrocephalus, 24 were found to have antibodies against Type-II peptides, 33 were found to have antibodies against NE-II peptides, 5 had sera that were not reactive to any peptide, and 3 have not yet had their sera tested.
Patterns Associated With Parasite Strain
The distribution of patterns of hydrocephalus in persons infected by Type-II or NE-II parasites is shown in Figure 1D. Of the 24 persons with hydrocephalus who were infected by Type-II parasites, 15 (62.5%) had aqueductal obstruction, 5 (20.8%) had foramina of Monro obstruction, 2 (8.3%) had mixed aqueductal and foraminal obstruction, and 2 (8.3%) had a pattern of no obvious intraventricular obstruction. Of the 33 persons with hydrocephalus who were infected by NE-II parasites, 9 (27.3%) had aqueductal obstruction, 10 (30.3%) had foramina of Monro obstruction, 3 (9.1%) had mixed aqueductal and foraminal obstruction and 11 (33.3%) had a pattern of no obvious intraventricular obstruction. Pattern of hydrocephalus was significantly associated with strain type (P = .035), with aqueductal obstruction occurring more frequently with Type-II strains and the pattern of no obvious intraventricular obstruction occurring more frequently with NE-II strains.
DISCUSSION
Hydrocephalus in persons with congenital toxoplasmosis has traditionally been attributed only to obstruction of the aqueduct of Sylvius. In this study, we found aqueductal obstruction to be the predominant cause of hydrocephalus in our cohort, accounting for 43% of cases and 54% when mixed aqueductal and foraminal obstruction are included. However, we report additional patterns of hydrocephalus that are present in a substantial proportion of our cohort. Recognition of these patterns is important when considering approaches to neurosurgical intervention, and may help to elucidate the pathophysiology of cerebral Toxoplasma infection.
To our knowledge, the pattern of bilateral obstruction of the foramina of Monro in cerebral toxoplasmosis has not been previously described. Toxoplasma infection commonly causes cerebral calcification and inflammation, and the periforaminal regions were common sites for calcification in our patients. Presence of generalized, rather than only focal, periventricular inflammation combined with focal periforaminal calcifications may contribute to the pathogenesis of this pattern of hydrocephalus, and also likely contributes to asymmetrical dilatation of the lateral ventricles observed with all patterns of hydrocephalus in these patients. It will be of interest in future studies to investigate whether parasite serotypes associate with calcification patterns.
Pathogenesis of the pattern with no obvious intraventricular obstruction remains undefined. Studies in murine models suggest that intraventricular or leptomeningeal inflammation hindering cerebrospinal fluid (CSF) resorption may contribute to the mechanism of hydrocephalus in mice [11], and this has been proposed as a mechanism of hydrocephalus in human toxoplasmosis [12]. This may be an etiology in the pattern of no obvious intraventricular obstruction described above and may ultimately also contribute to all patterns of hydrocephalus. Leptomeningeal inflammation may also be an explanation for the high failure rate of third ventriculostomy in post-toxoplasmosis aqueductal obstruction [13, 14].
Our findings suggest that the inappropriate CSF volume observed in the persons in this study may be caused by either aqueductal or foraminal obstruction, “obstructive hydrocephalus,” or abnormal resorption of CSF or fibrosis, “absorptive hydrocephalus.” One of the key distinctions between these 2 patterns is degree of intracranial pressure. Obstructive hydrocephalus results in the high intracranial pressure common in congenital hydrocephalus. By contrast, hydrocephalus with abnormal CSF resorption would result in a pressure that may not be as high, similar to the normal pressure hydrocephalus seen in adults. This pattern of possible impaired absorption of CSF causing hydrocephalus does, however, still respond to interventions, such as ventriculoperitoneal shunt placement, to lower the pressure because CSF does not transit or ventricles are stiff physiologically.
Intraocular interferon γ/interleukin (IL)-17a decreased and IL-6/IL-13 increased in Colombian ocular toxoplasmosis [15]. If this occurs in brain, it might alter hydrocephalus pattern. There were insufficient numbers of mothers with residency in South America during gestation to determine whether there was any geographic association with pattern of hydrocephalus in the child.
Studies of human neuroepithelioma cells and our own work with neuronal stem and differentiated cells infected by 3 different isolates of T. gondii show that host gene expression is significantly altered by infection, and that Type-II strains alter host gene expression in a distinctly different way than Type-I and Type-III strains [16]. This may begin to explain the differences in anatomical patterns of hydrocephalus observed in this study between persons infected with NE-II and Type-II strains. Future investigation into the strain-specific pathogenesis of hydrocephalus due to T. gondii may provide further insights into how this parasite modulates its host cell and causes disease.
Notes
Acknowledgments. We thank persons in our National Collaborative Chicago-based Congenital Toxoplasmosis Study cohort for permitting us to evaluate them, and their physicians for working with us.
Financial support. This work was supported by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) Division of Microbiology and Infectious Diseases [R01 AI27530], the Intramural Research Program of the NIH, NIAID (M. E. G.), and by the Mann Cornwell Family, the Engel family and “Taking out Toxo,” the Morel family, and the Rooney-Alden Family. M. E. G. is a Scholar of the Canadian Institute for Advanced Research Integrated Microbial Biodiversity program.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet 1986; 1:254–6. [DOI] [PubMed] [Google Scholar]
- 2.McAuley J, Boyer KM, Patel D et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis 1994; 18:38–72. [DOI] [PubMed] [Google Scholar]
- 3.Swisher CN, Boyer KM, McLeod R. Congenital toxoplasmosis. Bodensteiner JB (ed). Sem Ped Neurol 1994; 1:4–25. [PubMed] [Google Scholar]
- 4.Diebler C, Dusser A, Dulac O. Congenital toxoplasmosis. Clinical and neuroradiological evaluation of the cerebral lesions. Neuroradiology 1985; 27:125–30. [DOI] [PubMed] [Google Scholar]
- 5.McLeod R, Boyer K, Karrison T et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis 2006; 42:1383–94. [DOI] [PubMed] [Google Scholar]
- 6.Boyer KM, Holfels E, Roizen N et al. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Am J Obstet Gynecol 2005; 192:564–71. [DOI] [PubMed] [Google Scholar]
- 7.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol 2002; 5:438–42. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proc Natl Acad Sci 2006; 103:11423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLeod R, Boyer KM, Lee D et al. Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009). Clin Infect Dis 2012; 54:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- 11.Stahl W, Kaneda Y. Pathogenesis of murine toxoplasmic hydrocephalus. Parasitology 1997; 114(Pt 3):219–29. [DOI] [PubMed] [Google Scholar]
- 12.Ciurea AV, Coman TC, Mircea D. Postinfectious hydrocephalus in children. In: Cinalli G, Meixner W, Sainte-Rose C, eds. Pediatric Hydrocephalus; Milano, Italy: Springer-Verlag Italia, 2004:201–18. [Google Scholar]
- 13.Javadpour M, Mallucci C, Brodbelt A, Golash A, May P. The impact of endoscopic third ventriculostomy on the management of newly diagnosed hydrocephalus in infants. Pediatr Neurosurg 2001; 35:131–5. [DOI] [PubMed] [Google Scholar]
- 14.Koch D, Wagner W. Endoscopic third ventriculostomy in infants of less than 1 year of age: which factors influence the outcome? Childs Nerv Syst 2004; 20:405–11. [DOI] [PubMed] [Google Scholar]
- 15.De-La-Torre A, Sauer A, Pfaff AW et al. Severe South American ocular toxoplasmosis is associated with decreased IFN-γ/Il-17a and increased Il-6/Il-13 intraocular levels. PLoS Negl Trop Dis 2013; 7:e2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao J, Jones-Brando L, Talbot CC Jr, Yolken RH. Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infect Immun 2011; 79:1363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]