Fathers of congenitally infected infants in the National Collaborative Chicago-Based Congenital Toxoplasmosis Study were found to have a high prevalence of Toxoplasma gondii chronic and acute infection indicating a high incidence of T. gondii infections cluster within families in North America.
Keywords: clusters, Toxoplasma gondii, toxoplasmosis, congenital infections
Abstract
Background. Family clusters and epidemics of toxoplasmosis in North, Central, and South America led us to determine whether fathers of congenitally infected infants in the National Collaborative Chicago-Based Congenital Toxoplasmosis Study (NCCCTS) have a high incidence of Toxoplasma gondii infection.
Methods. We analyzed serum samples collected from NCCCTS families between 1981 and 2013. Paternal serum samples were tested for T. gondii antibodies with immunoglobulin (Ig) G dye test and IgM enzyme-linked immunosorbent assay. Additional testing of paternal serum samples was performed with differential-agglutination and IgG avidity tests when T. gondii IgG and IgM results were positive and serum samples were collected by the 1-year visit of the congenitally infected child. Prevalence of paternal seropositivity and incidence of recent infection were calculated. We analyzed whether certain demographics, maternal parasite serotype, risk factors, or maternal/infant clinical manifestations were associated with paternal T. gondii infection status.
Results. Serologic testing revealed a high prevalence (29 of 81; 36%) of T. gondii infection in fathers, relative to the average seropositivity rate of 9.8% for boys and men aged 12–49 years in the United States between 1994 and 2004 (P < .001). Moreover, there was a higher-than-expected incidence of recent infections among fathers with serum samples collected by the 1-year visit of their child (6 of 45; 13%; P < .001). No demographic patterns or clinical manifestations in mothers or infants were associated with paternal infections, except for sandbox exposure.
Conclusions. The high prevalence of chronic and incidence of recent T. gondii infections in fathers of congenitally infected children indicates that T. gondii infections cluster within families in North America. When a recently infected person is identified, family clustering and community risk factors should be investigated for appropriate clinical management.
(See the Brief Report by Hutson et al on pages 1831–4.)
Toxoplasma gondii causes significant illness when acquired congenitally [1–20]. There have been few studies defining frequency of infection in other family members in this setting, factors that predict who may be infected, or consequences for other infected family members.
Toxoplasma gondii infection can be acquired through exposure to food and water contaminated with infectious, environmentally resistant oocysts excreted by cats and/or tissue cysts in meat not cooked to well done [21–28]. Common-source outbreaks of toxoplasmosis within communities due to contaminated food and water sources have been noted in the literature, highlighting that this parasite is a public health risk [29]. Case reports and case series of clusters of recent T. gondii infections [21–29] and a systematic evaluation of recent Toxoplasma infections among 32 families in the United States that identified 18 (56%) household clusters with ≥2 recently infected persons [30] led us to question how often fathers of children with congenital infections acquire this infection at the same time as their child's mother.
The National Collaborative Chicago-Based Congenital Toxoplasmosis Study (NCCCTS), which began in 1981 and continues to the present [1–8, 10–13, 20], offers a unique opportunity to study prevalence of within-family clusters of T. gondii infections in the United States. The NCCCTS facilitates careful evaluation of children with congenital toxoplasmosis and serum sample collection from these children and their families. In the current study, we determined how often fathers of congenitally infected infants were seropositive for T. gondii infection and also how often they had recent T. gondii infections, most likely acquired at about the time that their child's mother who was infected during gestation. We also analyzed whether there are predictive factors for paternal T. gondii infections and/or recent infections, which would refine selection of fathers or families for whom testing is advisable. Placed in this context, the presence of T. gondii in semen of animals, including humans, suggests that there might be sexual transmission [31–35].
METHODS
Study Population
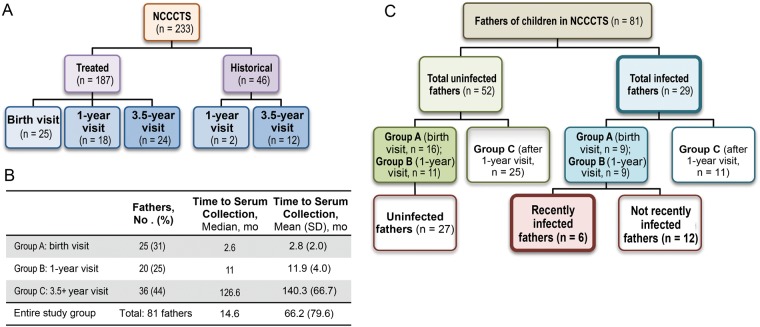
The NCCCTS consists of congenitally infected infants referred by their physicians from January 1981 to the present (March 2013). Congenitally infected infants enrolled in this study visit near birth, at 1, 3.5, 5, 7.5, 10, and 15 years, and at 5-year intervals thereafter (Figure 1A). Fathers of many infants were present for a visit to Chicago during this study period and provided a serum sample for future testing related to Toxoplasma infection . In this study, we retrospectively analyzed serum samples collected from these fathers who traveled to Chicago from throughout the US (Figure 2) as part of the NCCCTS protocol.
Figure 1.
Cohort structure. A, National Collaborative Chicago-Based Congenital Toxoplasmosis Study (NCCCTS) structure. Congenitally infected infants referred by their physicians were enrolled in the NCCCTS and participated in prespecified study visits with their mothers; numbers indicate the numbers of children enrolled in each group. Children who received treatment initiated within 2.5 months of birth were designated “treated,” and those who had not received treatment in their first year of life and were enrolled in the study after 2.5 months were designated “historical.” Fathers of enrolled participants often also accompany their children during their scheduled birth, 1-year, and 3.5-year visits and provide a serum sample for Toxoplasma-infection related testing; numbers indicates the number of children with fathers who provided serum samples at each time period. B, Distribution of fathers by study visit and time to paternal serum sample collection. Approximately equal numbers of fathers had their first visit at the birth, 1-year, or 3.5-year study period. The serum samples from fathers who came for the birth and 1-year visits were collected early enough to be tested for recent infection acquired near the time of maternal/congenital infection. SD, standard deviation. C, Flow chart of study subgroups. Fathers of children in the NCCCT study were tested for patterns of general Toxoplasma infection and separated into 2 groups, “infected” and “uninfected.” Fathers seropositive for immunoglobulin (Ig) G and IgM who had serum samples obtained during the birth or 1-year visit of their congenitally infected child were further examined for patterns of recent infection and subsequently subdivided into 2 groups: recently infected and not demonstrated as recently infected; uninfected fathers who had serum samples collected by the 1-year visit were also, by definition, in the latter subgroup.
Figure 2.
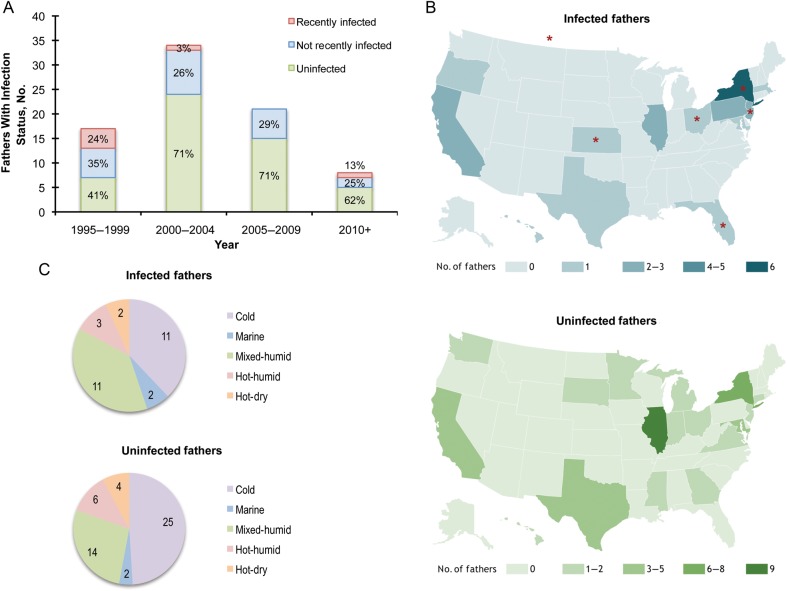
Spatiotemporal patterns of paternal infection. A, Distribution of paternal infection status by year. The seropositivity rate of fathers of congenitally infected children in this cohort was highest in 1995–1999, with 7 of 17 fathers (58.8%) infected. Seropositivity fell in the subsequent 15 years to 29.4% (n = 34) in 2000–2004, 28.6% (n = 21) in 2005–2010, and 37.5% (n = 8) from 2010 to 2013. The percentages of recently infected, chronically infected, and uninfected fathers are labeled for each bar. B, Geographic distribution of infected and uninfected fathers by US state residency. Paternal infection status and state residency were not significantly associated. State residencies of recently infected subjects are indicated by red stars. C, Distribution of infected and uninfected fathers by climate zone. Paternal infection status and climate zones were not significantly associated.
NCCCTS Data
NCCCTS data are recorded and maintained at the Toxoplasmosis Center at the University of Chicago. Visits to NCCCTS include in-person evaluations and clinical testing for congenitally infected persons and their mothers. Results of evaluations are recorded for each family on standardized forms [1–8, 10–13, 20]. Evaluations include (1) family demographic characteristics and time to serum sample collection; (2) reported maternal risk factors for T. gondii infections; (3) maternal clinical signs and symptoms during or near gestation; (4) maternal and infant serologic titers for T. gondii immunoglobulin (Ig) G, IgM, and/or IgA antibodies and, in the latter part of the study, for maternal antibodies measured in differential agglutination and IgG avidity tests; (5) general physical, neurologic, ophthalmologic, audiologic, and hematologic examination findings in congenitally infected persons; (6) review of all neuroradiologic studies, laboratory evaluations, and medical records; (7) ophthalmologic evaluation of mother and recently father; and (8) maternal T. gondii serotype (through 2012).
Serologic Screening for T. gondii Infection
Paternal serum samples were stored at −80°C at the Toxoplasmosis Center at the University of Chicago within 1 hour after being obtained, and tested at the Palo Alto Medical Foundation Toxoplasma Serology Laboratory (PAMF-TSL). Initial screening of paternal serum samples included IgG dye test and IgM enzyme-linked immunosorbent assay (ELISA). Serum samples from fathers who were IgG and IgM positive [36, 37] and had serum samples collected by the 1-year visit of their congenitally infected child were further tested with differential agglutination and IgG avidity assays to differentiate between recent and not-recent T. gondii infections. Maternal and child serum samples from time of diagnosis were analyzed as described in the Supplementary Table 1 footnote. For each eligible family, only serum samples from father, mother, and child, closest to the child's birth, were analyzed for this study (Supplementary Table 1 footnote).
Assessment of Paternal T. gondii Infection Status
Paternal serologic results were evaluated independently by 2 investigators at PAMF-TSL (D. C.-I. and J. G. M.) and 1 in Chicago (R. M.) who determined whether fathers had serologic evidence of T. gondii infection and, for those who were IgG and IgM seropositive and had serum samples collected by the 1-year visit of their child, serologic evidence of recent (acquired within approximately 12 months before sample collection and probably during gestation or close to conception) or “not-recent” (acquired >12 months before sample collection and probably long before conception of the congenitally infected child) T. gondii infection. For these determinations, PAMF-TSL investigators were blinded as to the date of birth of congenitally infected infants. Time of paternal serum sample collection was estimated from the infant's date of birth. Consensus was reached between investigators for interpretation of all results.
Groups of Fathers
Fathers were classified into 3 groups. Group A included fathers with serum samples collected at the birth visit of their infant; group B, fathers with samples collected at the 1-year follow-up visit; and group C, fathers with serum samples collected after the 1-year visit.
End Points
Our primary study end points were (1) prevalence of paternal T. gondii infections (paternal seropositivity rate) based on analysis of all paternal serum samples from all 3 groups (groups A–C) and (2) incidence of paternal recent T. gondii infections (which corresponds to the incidence of within-family clusters of recent T. gondii infections), analyzing paternal serum samples from fathers from groups A and B only, collected approximately ≤12 months after their child's date of birth. In fathers with serum samples collected >12 months after the child's birth, even if they were seropositive for T. gondii, it would not have been possible to confirm or exclude that they were infected during gestation or close to conception.
T. gondii Strain–Specific Antibodies
T. gondii serotypes (exclusively type II vs nonexclusively type II) in mothers or infants were analyzed for associations with paternal T. gondii infection status. The presence of strain-specific antibodies was determined using polymorphic peptides, as described elsewhere [5]. Only persons whose serum antibody response was typable were included in analyses associating parasite serotype with prevalence of paternal seropositivity.
Data Management, Analysis, and Statistical Methods
Data management and analyses were performed using Fourth Dimension database (4D, Version 7.0.8 Mac) [5], Excel (Microsoft), and Stata 12 software (StataCorp). We compared different demographic and maternal characteristics (risk factors and clinical characteristics) between infected and uninfected fathers and between recently infected, nonrecently infected, and uninfected fathers, using Fisher exact tests. Statistical tests were 2 tailed, and differences were considered statistically significant at P ≤ .05. When multiple comparisons were made on a single data set, a Bonferroni correction on the significance threshold (0.05 divided by the number of comparisons) was used to reduce the chance of obtaining false-positive results.
Ethics
The NCCCTS was conducted with ethical standards for human experimentation established in the Declaration of Helsinki, with prior institutional review board approval and in accordance with Health Insurance Portability and Accountability Act regulations, and it was reviewed regularly by a Data and Safety Monitoring Board. Informed consents were obtained from subjects for all aspects of the NCCCTS, including collection of paternal serum samples, in accordance with institutional review board and National Institutes of Health guidelines.
RESULTS
Study Population
Comparisons With Full Cohort to Establish That Study Subset is Representative
There are 233 families enrolled in the NCCCTS at present. Demographic and clinical data obtained from each family at each visit at prespecified intervals (Tables 1–4) permitted comparison of the whole cohort to those in the subset in which fathers provided serum. Of 233 families, there are 187 whose child's infection was diagnosed before or near birth, and these children were subsequently treated as part of the study (Figure 1A). There are 46 congenitally infected children whose infection was diagnosed after they were 1 year old, thereby missing treatment in their first year of life; these children were included in the study as historical controls (Figure 1A).
Table 1.
Infection Rates of Fathers With Children Enrolled in National Collaborative Chicago-Based Congenital Toxoplasmosis Studya
| Infection Rate | Infected Fathers/ All Fathers, No. (% Infected) |
P Valueb |
|---|---|---|
| Overall infection | ||
| NCCCTS study group | 29/81 (36) | <.001 |
| Group A: birth visit | 9/25 (36) | … |
| Group B: 1-y visit | 9/20 (45) | … |
| Group C: 3.5-y visit (or later) | 11/36 (31) | … |
| United Statesc | 486/4962 (9.8) | … |
| Recent infectiond | ||
| NCCCTS study group | 6/45 (13) | <.001 |
| Group A: birth visit | 4/25 (16) | … |
| Group B: 1-y visit | 2/20 (10) | … |
| United Statese | 1/15 000–3/1000 (0.007–0.3) | … |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; NCCCTS, National Collaborative Chicago-Based Congenital Toxoplasmosis Study.
a Full serologic testing results for father, mother, and child and time to serum sample collection are included in Supplementary Table 1. To categorize recent infections, we applied criteria that have been validated and routinely used in the daily clinical practice at the Palo Alto Medical Foundation Toxoplasma Serology Laboratory (PAMF-TSL), as follows: (1) a father was considered Toxoplasma gondii infected if at least the immunoglobulin (Ig) G dye test result was positive; (2) a father was considered “recently infected” if the IgG dye test and IgM ELISA results were positive and the differential agglutination test yielded an acute or equivocal pattern; (3) a father was considered “not recently” infected if all the following were true: the IgG dye test was positive at a low titer (<1:1024), the IgM ELISA result was either negative or low positive (<3.0), the differential agglutination test (if performed) had nonacute results, and the IgG avidity (if tested) was high. A within-family cluster of recent T. gondii infections was identified when the father had serologic evidence of recent T. gondii infection, acquired both approximately ≤12 months before sample collection and near the time of his child's mother's acute T. gondii infection, which by definition occurred during gestation or very close to conception.
We also performed secondary analyses. We compared the paternal seropositivity rates according to predefined paternal groups (group A vs B vs C), year, and geographic region (Figures 1 and 2). We compared different factors between infected and uninfected fathers and between recently infected fathers and those who were not demonstrated as recently infected fathers (nonrecently infected plus uninfected), including demographic characteristics and maternal parasite serotype status (Table 2) and maternal risk factors for T. gondii infections and clinical characteristics of the mother and infant (Table 3). We summarized the demographic characteristics and reported maternal risk factors for the subgroup of fathers with recent T. gondii infections. Detailed descriptions of these maternal parasite serotyping results have been published elsewhere [5]; in the current study we analyzed their possible association with paternal T. gondii infection status.
b P values determined with Fisher exact tests, comparing the overall study group with the US data.
c Rate of T. gondii infection in boys and men aged 12–49 in the United States in 1999–2004, taken from the literature [38].
d Numerator represents number of fathers recently infected during their child's mother's pregnancy; denominator, total number of fathers who had serum samples collected by the 1-year study visit of their child (25 in group A and 20 in group B).
Table 4.
Summary of Within-Family Cluster Demographics and Risk Factors
| Year | Strain | State of Residence | SES Scorea | Ethnicity | Maternal Risk Factors | Maternal Illness | Infant Illness | Parityb | Other Notes |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | ND | NJ | 1 | Non-Hispanic | Cat exposure, sandbox exposure | Adenopathy | Jaundice | 1 | Father developed adenopathy after infection |
| 1996 | NE-II | NY | 1 | Non-Hispanic | Cat exposure, sandbox exposure | PPc | Rash, chorioretinitis, thrombocytopenia, petechiae | 1 | Extended maternal exposure to aerosolized excreta from wild cats in pregnancy; brother was recently infected during this time; maternal grandmother was chronically infected during this time |
| 1997 | NE-II | FL | 4 | Non-Hispanic | Cat exposure, exposure to raw or undercooked meat, gardening | PP | Jaundice, hypothermia, chorioretinitis, blindness, seizures, hydrocephalus, cerebrospinal fluid pleocytosis | 1 | Mother prepared raw deer and pork during pregnancy |
| 2000 | IId | Canada | 2 | Non-Hispanic | Cat exposure, sandbox exposure | Fever, nausea, myalgia, PPROMc | Jaundice, hydrocephalus, chorioretinitis, respiratory distress | 1 | No clear risk factors other than a single exposure to cats at a sandbox in the 1st trimester |
| 1996 | ND | OH | 2 | Hispanic | Cat exposure, sandbox exposure, gardening | Adenopathy, URIc | Hepatosplenomegaly | 1 | No clear risk factors other than a single exposure to cats at a playground in the 2nd trimester |
| 2012 | ND | KS | 1 | Non-Hispanic | Cat exposure, exposure to raw or undercooked meat | Fever, myalgia | Intracranial calcifications | 3 | Mother consumed venison tartare during pregnancy; father and other community members became ill after consuming venison tartare; father had eye disease at examination after infection |
Abbreviations: ND, not determined; NE-II, nonexclusively type II; PP, placenta previa; PPROM, preterm premature rupture of membranes; SES, socioeconomic status; URI, upper respiratory tract infection.
a SES was calculated according to the Hollingshead 4-factor socioeconomic score.
b Parity number refers to the parity of the mother at the time of her acute primary infection, which occurred during the gestational period of her congenitally infected child. Parity was evaluated because if the families already have toddler-aged children who play in sandboxes, the risk of exposure to the oocyst form of the parasite may increase for mothers, fathers, and other children. Parity at the time of acute maternal infection was therefore also reviewed as a possible risk factor for the 6 mothers in within-family clusters.
c Not known to be an associated symptom of Toxoplasma infection.
d Exclusively type II.
Paternal serum samples were collected from 81 fathers of congenitally infected infants as a part of the NCCCTS. The number of fathers who accompanied their child to a visit in Chicago and were asked to provide serum is less than the full 233 families owing to study and travel logistics. There was no identifiable, systematic bias for those asked to provide serum, and only 1 father who was asked did not provide serum samples. The proportion of fathers whose children were enrolled as historical controls (14 of 81; 17%) parallels that in the full cohort (46 of 233; 20%). There were 25 fathers whose serum samples were collected at their child's birth visit, 20 whose samples were collected at their child's 1-year visit, and 36 whose samples were collected during or after the 3.5-year visit (Figure 1B). Paternal serum samples were collected a median of 14.6 months after the birth of the congenitally infected child, although time to sample collection varied (Figure 1B).
Overall Prevalence of Paternal T. gondii Infections
Data were analyzed by (1) whether fathers were uninfected or infected and, for those who had serum samples collected by the 1-year visit of their child, (2) by whether fathers were recently infected or not demonstrated as recently infected (nonrecently infected plus uninfected) (Figure 1C). Overall 36% of tested fathers (29 of 81) were found to be seropositive for T. gondii–specific IgG antibodies. This seropositivity rate is higher than the average seroprevalence of 9.8% for boys and men aged 12–49 years in the United States, as determined by National Health and Nutrition Examination Survey data collected between 1999 and 2004, approximately the same period as our study [38] (Figure 1D; P < .001).
Seropositivity was also compared separately for fathers who had serum samples collected at their child's birth visit (group A), 1-year visit (group B), or during or after the 3.5-year visit (group C), which demonstrated that seropositivity did not differ significantly between the 3 visits when serum samples were collected (Figure 1C and 1D). The prevalence of paternal seropositivity in groups A, B, and C was 36%, 45%, and 31% respectively (Table 1). Of note, overall paternal seroprevalence was also compared separately for fathers whose child's infection was diagnosed or treated near birth (24 of 67; 36%) and fathers of historical controls (5 of 14; 36%), and seroprevalence was found to be nearly identical in the 2 groups of fathers. Based on the consistency of seropositivity between these 2 groups, data were combined in our analyses.
Incidence of Paternal Recent T. gondii Infections
T. gondii–specific IgM, avidity, and differential agglutination testing revealed that among 45 fathers who had serum samples collected early enough to test for recent infection (by the 1-year visit of their child), 6 (13%) had antibody profiles consistent with recent infection likely acquired during their wife's gestational period or close to conception. This is 40–2000-fold higher than the incidence of T. gondii infection acquired during pregnancy reported in the literature, which has ranged from 0.007% to 0.3% based on different regionally based surveys [39, 40] (Table 1). The exposure period for recently infected fathers (9–12 months) exceeds the duration of pregnancy but is comparable in magnitude.
Factors Associated With Family Clusters of T. gondii Infections
Demographic Factors
Temporal analysis of paternal infection status for the 81 fathers in this analysis revealed higher seroprevalence in fathers tested in 1995–1999, which decreased substantially in the subsequent 2 decades (Figure 2A). No association was found between hometown size, socioeconomic status (SES) score, ethnicity, state residency, or climate region and paternal infection status (Table 2; Figure 2B and 2C). Family clusters were spatially and temporally diverse. Families lived primarily in nonrural hometowns, were of higher SES, and were typically non-Hispanic (Table 2). Of 6 recently infected fathers, 2 families were from a rural hometown, 1 was of Hispanic background, and 1 was of lower SES.
Table 2.
Demographics and Mother/Child Serotypes by Paternal Infection Status
| Infected Fathers, No. (%) (n = 29) |
Uninfected Fathers, No. (%) (n = 52) |
P Valuea | |
|---|---|---|---|
| Demographics for infected fathersb | |||
| Rural hometownc | 8/29 (28) | 14/52 (27) | >.99 |
| Hispanic background | 6/29 (21) | 7/52 (13) | .53 |
| SES score ≥3 | 12/28 (43) | 17/51 (33) | .47 |
| Recently Infected Fathers, No. (%) (n = 6) |
Not Demonstrated Recently Infected Fathersd, No. (%) (n = 39) |
P Valuea | |
| Demographics for recently infected fathers | |||
| Rural hometown | 2/6 (33) | 12/39 (31) | >.99 |
| Hispanic background | 1/6 (17) | 8/39 (21) | >.99 |
| SES score ≥3 | 1/6 (17) | 15/39 (38) | .40 |
| Infected Fathers, No. (%) (n = 29) |
Uninfected Fathers, No. (%) (n = 52) |
P Valuea | |
| Serotypes in NCCCTS mothers and children for infected fatherse | |||
| Type II | 8/22 (36) | 20/40 (50) | .43 |
| NE-II | 14/22 (64) | 20/40 (50) | .43 |
Abbreviations: NCCCTS, National Collaborative Chicago-Based Congenital Toxoplasmosis Study; NE-II, nonexclusively type II; SES, socioeconomic status.
a For comparing infected and uninfected fathers were P values determined with Fisher exact tests.
b Numerator represents number of infected or uninfected fathers within a particular demographic group; denominator, total number infected or uninfected.
c Rural was defined as hometown population size <1900 at the time of the child's first visit to NCCCTS. Hispanic status was self-identified at the child's first visit. SES was calculated according to the Hollingshead 4-factor socioeconomic score.
d Not demonstrated as recently infected fathers includes uninfected fathers plus those with nonrecent Toxoplasma gondii infections (acquired >12 mo before sample collection).
e Numerator represents number of infected or uninfected fathers who have a child with this serotype; denominator represents total number of fathers infected or uninfected whose children and respective mothers were serotyped through 2009.
T. gondii Serotypes in Congenitally Infected Infants and Their Mothers
Parasite serotype was previously identified [5] for 62 of 81 infected infants whose fathers were of known infection status (Table 2). Infants infected with nonexclusively type II strain seemed to have a higher incidence of seropositive fathers than infants infected with type II strain (41.2% vs 28.6%), although this difference was not statistically significant. Of the 3 mothers in the 6 within-family clusters who had their parasite serotype identified, 2 were infected with nonexclusively type II strains, and 1 with type II strain.
Maternal Epidemiologic Risk Factors and Maternal and Infant Clinical Manifestations
Analysis of reported maternal risk factors and maternal clinical data did not reveal significant associations with overall seropositivity in fathers (Table 3). Clinical manifestations in the child were analyzed because severe manifestations in newborns may reflect parasite burden or virulence, and thus likelihood that an exposed father might also be infected; however, no identifiable clinical manifestations of T. gondii infection in mothers or children were associated with significant increased seropositivity in fathers (Table 3). Maternal and infant serologic findings were also analyzed but were not associated with seropositivity in fathers (data not shown).
Table 3.
Maternal Risk Factors and Clinical Manifestations in the Mother and Infant by Paternal Infection Status
| Risk Factors and Clinical Manifestations | No. With Risk Factor or Manifestation/Fathers in Infection Group, No. (%)a |
P Valueb | No. With Risk Factor or Manifestation/Fathers in Infection Group, No. (%)a |
P Valueb | ||
|---|---|---|---|---|---|---|
| Infected Fathers (n = 29) | Uninfected Fathers (n = 52) | Recently Infected Fathers (n = 6) | Fathers ND as Recently Infected (n = 39) | |||
| Risk factors | ||||||
| Gardening | 8/29 (28) | 9/52 (17) | .39 | 2/6 (33) | 5/39 (13) | .23 |
| Sandbox | 6/29 (21) | 4/52 (8) | .16 | 4/6 (67) | 3/39 (8) | .003c |
| Exposure to cats | 20/29 (69) | 28/52 (54) | .24 | 6/6 (100) | 23/39 (59) | .07 |
| Eating undercooked/raw meat | 12/29 (41) | 16/52 (31) | .34 | 2/6 (33) | 9/39 (23) | .62 |
| Preparing raw meat | 8/29 (28) | 9/52 (17) | .39 | 1/6 (17) | 4/39 (10) | .52 |
| Drinking raw milk | 3/29 (10) | 5/52 (10) | >.99 | 0/6 (0) | 3/39 (8) | >.99 |
| Clinical manifestations in mother | ||||||
| Maternal illness | 18/29 (62) | 34/50 (68) | .63 | 4/6 (67) | 21/39 (54) | .68 |
| Lymphadenopathy | 9/24 (38) | 11/38 (29) | .58 | 2/6 (33) | 11/36 (31) | >.99 |
| Fever | 8/24 (33) | 7/38 (18) | .23 | 2/6 (33) | 7/36 (19) | .59 |
| Night sweats | 3/24 (13) | 4/38 (11) | >.99 | 0/6 (0) | 5/36 (14) | >.99 |
| Myalgia | 3/24 (13) | 4/38 (11) | >.99 | 2/6 (33) | 3/36 (8) | .14 |
| Atypical lymphocytes | 0/24 (0) | 1/38 (3) | >.99 | 0/6 (0) | 1/36 (3) | >.99 |
| Headache | 2/24 (8) | 5/38 (13) | .70 | 0/6 (0) | 5/36 (14) | >.99 |
| Visual disturbance | 0/24 (0) | 2/38 (5) | .52 | 0/6 (0) | 1/36 (3) | >.99 |
| Clinical manifestations in infant | ||||||
| Illness in newborn period | 16/21 (76) | 29/40 (73) | >.99 | 6/6 (100) | 22/35 (63) | .15 |
| Jaundice | 5/21 (24) | 12/40 (30) | .77 | 3/6 (50) | 10/35 (29) | .36 |
| Respiratory distress | 5/21 (24) | 8/40 (20) | .75 | 1/6 (17) | 7/35 (20) | >.99 |
| Hepatosplenomegaly | 7/21 (33) | 5/40 (13) | .09 | 1/6 (17) | 2/35 (6) | .39 |
| Petechiae | 2/21 (10) | 1/40 (3) | .27 | 1/6 (17) | 0/35 (0) | .15 |
| Thrombocytopenia | 4/21 (19) | 9/40 (23) | >.99 | 1/6 (17) | 4/35 (11) | .57 |
| Chorioretinitis | 11/21 (52) | 17/40 (43) | .59 | 3/6 (50) | 11/35 (31) | .39 |
| Blindness | 2/21 (10) | 5/40 (13) | >.99 | 1/6 (17) | 3/35 (9) | .48 |
| Hydrocephalus | 7/21 (33) | 10/40 (25) | .55 | 2/6 (33) | 5/35 (14) | .27 |
| Seizures | 2/21 (10) | 5/40 (13) | >.99 | 1/6 (17) | 3/35 (9) | .48 |
| Intracranial calcifications | 8/21 (38) | 19/40 (48) | .59 | 1/6 (17) | 13/35 (37) | .64 |
| CSF pleocytosis | 5/21 (24) | 6/40 (15) | .49 | 1/6 (17) | 5/35 (14) | >.99 |
Abbreviations: CSF, cerebrospinal fluid; ND, not demonstrated.
a Numerators represent number of families exposed to risk factor or number of mothers or infants with clinical manifestation. Risk factor information was obtained only from mothers and not from fathers, according to the original study protocol. Denominators may vary owing to uncompleted surveys. “ND as recently infected” includes both nonrecently infected and uninfected fathers.
b P values determined with Fisher exact tests. Bonferroni correction was used to reduce the chances of obtaining false-positive results because multiple comparisons were performed. The correction was made by dividing the significance threshold, .05, by the number of comparisons. Significance thresholds with the Bonferroni correction are .008 for risk factors, .006 for clinical manifestations in mothers, and .004 for clinical manifestations in infants.
c Significant difference after Bonferroni correction.
Analysis of maternal risk factors revealed that all 6 mothers in recent family clusters reported exposure to cats. These family clusters also shared exposure to sandboxes (4 of 6 mothers; P < .006; Table 3). Further analysis of parity of the 6 mothers at the time of their acute infection (during gestation of their congenitally infected child) in recent family clusters revealed that all 6 acutely infected fathers of congenitally infected children also had older, toddler-aged children, supporting a possible common exposure source via T. gondii–contaminated sandboxes (Table 4). Vacation and travel to rural areas or areas different than primary residence were not included in a systematic questionnaire in early phases of the study or in this analysis, as the importance of such factors were not appreciated at the time. These factors were often noted in narrative analyses (Table 4) and are available for families with recently infected fathers who did travel to such areas. Analysis of clinical data of within-family clusters revealed that 4 (67%) of 6 mothers who were part of a recent family cluster experienced illness indicative of T. gondii infection during pregnancy (Table 3), consistent with the overall rate of maternal illness for this study subpopulation (52 of 79; 66%).
DISCUSSION
We identified an overall prevalence of paternal T. gondii infections of 36% and an overall incidence of within-family clusters of recent T. gondii infections of 13% among fathers who were tested within 1 year after their child's birth. Both are far higher than the reported seroprevalence among boys and men aged 12–49 years in the general US population between 1999 and 2004 and estimates of the rate of T. gondii infection acquired during pregnancy in the current era [38–40]. These data are consistent with the conclusion that many fathers and mothers became infected with T. gondii around the same time and that T. gondii infections cluster within families.
The incidence of recent T. gondii infections among fathers of congenitally infected children was lower than a previously reported rate of within-family clusters [30], which included 2 NCCCTS families. In this earlier analysis, among 32 families of persons with recent toxoplasmosis, 18 (56%) had ≥1 additional family/household member beyond the index case patients with recently diagnosed infection [30]. In the current study, exclusive focus on fathers (and not on additional family/household members) and collection of paternal serum samples at varying time intervals after recent T. gondii infection of their child's mother might underestimate true incidence of within-family clusters of recent T. gondii infections. Moreover, in the families analyzed in the earlier study [30], true prevalence of within-family clusters of acute or recent infections might have been overestimated owing to family selection-referral bias; only 4% of persons who had acute toxoplasmosis diagnosed at PAMF-TSL [30] during the 20-year study period had samples sent from additional household members for T. gondii testing. In the current study, paternal serum samples were collected in nonselected consecutive families of infants with congenital toxoplasmosis referred to the NCCCTS. Furthermore, although serologic testing can provide an estimate for when a person became infected, as time to paternal serum sample collection increases, it becomes increasingly difficult to prove serologically that paternal infection occurred at the same time as maternal infection.
Exact timing of infection is known for 1 recent cluster identified herein, which is part of a recent toxoplasmosis outbreak in the Midwest (R. M., unpublished data). This community-wide common-source epidemic was traced back to ingestion of contaminated venison tartare. Several other toxoplasmosis outbreaks have been associated with consumption of undercooked meat [22] and oocyst exposure through contaminated reservoirs and food products [23–28].
During an outbreak, the source of infection is often clearly identifiable and can provide clues to common sources of infection and why infections cluster within families. Because ingestion of infectious sporozoites within oocysts in contaminated water or bradyzoites within tissue cysts in undercooked contaminated meat are common recognized routes for T. gondii infection, families who eat meals together, have the same water source, or play with their children in the same sandbox or garden may be exposed to parasites at the same time.
However, although common routes of T. gondii infection are known, a previous study found that only 48% of women actually recognize epidemiologic risk factors (direct or indirect exposure to raw or undercooked meat or cat excrement) or patterns of gestational illnesses compatible with diagnosis of recent acquired toxoplasmosis during pregnancy [29]. To reconcile this discrepancy between time of maternal illness and seeming lack of exposure to well-known sources of infection, we hypothesize there might be at least one other possible mechanism of acquisition not studied in humans—sexual transmission. Studies have already demonstrated that T. gondii tachyzoites are present in seminal fluid and/or testes of infected rams, goats, dogs, and humans [31–35]. In sheep and dogs, females were infected through sexual contact or artificial insemination with semen from an infected male. This hypothesis could account for high rates of paternal infection observed in this study and will be of interest to investigate further in a larger study. If T. gondii is sexually transmitted, men and women should be educated about risks associated with unprotected sex with persons of positive or unknown infection status, particularly during pregnancy or in those who are immunocompromised.
At least 1 father experienced recent Toxoplasma-associated symptoms, and another developed retinal lesions, highlighting the importance of testing families of persons with Toxoplasma infections [39]. Furthermore, this new epidemiologic observation of within-family clusters of T. gondii infections in families of congenitally infected children emphasizes the risk of concurrent infection in family members exposed to contaminated food or water.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank persons in our National Collaborative Chicago-Based Congenital Toxoplasmosis Study cohort for permitting us to evaluate them, and their physicians for working with us.
Financial support. This work was supported by the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID) (grant R01 AI27530); the Intramural Research Program of the NIAID (M. E. G.); and the Mann Cornwell family, Engel family (and “Taking Out Toxo”), and Morel and Rooney-Alden families. M. E. G. is a scholar in the Canadian Institute for Advanced Research Integrated Microbial Biodiversity program.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McAuley J, Boyer KM, McLeod R et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis 1994; 18:38–72. [DOI] [PubMed] [Google Scholar]
- 2.Boyer K, Roizen N, McLeod R et al. The child with congenital toxoplasmosis. Curr Clin Top Infect Dis 2000; 20:189–208. [PubMed] [Google Scholar]
- 3.Swisher CN, Boyer K, McLeod R The Toxoplasmosis Study Group. Congenital toxoplasmosis. Semin Pediatr Neurol 1994; 1:4–25. [PubMed] [Google Scholar]
- 4.Roizen N, Swisher CN, McLeod R et al. Neurologic and developmental outcome in treated congenital toxoplasmosis. Pediatrics 1995; 95:11–20. [PubMed] [Google Scholar]
- 5.McLeod R, Boyer K, Lee D et al. Prematurity & severity associate with T. gondii alleles (NCCCTS, 1981–2009). Clin Infect Dis 2012; 54:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delair E, McLeod R, Brezin A et al. Clinical manifestations of ocular toxoplasmosis. Ocul Immunol Inflamm 2011; 2:91–102. [DOI] [PubMed] [Google Scholar]
- 7.Mets MB, Holfels E, McLeod R et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol 1996; 122:309–24. [DOI] [PubMed] [Google Scholar]
- 8.Noble G, Latkany P, McLeod R et al. Chorioretinal lesions in mothers of children with congenital toxoplasmosis in the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Scientia Medica 2010; 20:20–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts F, McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol Today 1999; 15:51–7. [DOI] [PubMed] [Google Scholar]
- 10.Phan L, Kasza K, McLeod R et al. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology 2008; 115:553–9. [DOI] [PubMed] [Google Scholar]
- 11.Olariu TR, Remington JS, Montoya JG et al. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 2011; 30:1056–61. [DOI] [PubMed] [Google Scholar]
- 12.Phan L, Kasza K, McLeod R et al. Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol 2008; 146:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington J, Klein J, eds. Infectious diseases of the fetus and newborn infant. 7th ed Philadelphia, PA: WB Saunders, 2011. [Google Scholar]
- 14.Wallon M, Peyron F, Binquet C et al. Congenital Toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 2013; 56:1223–31. [DOI] [PubMed] [Google Scholar]
- 15.Thulliez P. Commentary: efficacy of prenatal treatment for toxoplasmosis: a possibility that cannot be ruled out. Int J Epidemiol 2001; 30:1315–6. [DOI] [PubMed] [Google Scholar]
- 16.Brezin AP, Thulliez P, Mets MB et al. Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol 2003; 135:779–84. [DOI] [PubMed] [Google Scholar]
- 17.Hohlfeld P, Daffos F, Vidaud M et al. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. New Engl J Med 1994; 331:695–9. [DOI] [PubMed] [Google Scholar]
- 18.Hotop A, Hlobil H, Gross U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2012; 54:1545–52. [DOI] [PubMed] [Google Scholar]
- 19.Boyer KM, Holfels E, McLeod R et al. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. Am J Obstet Gynecol 2005; 192:564–71. [DOI] [PubMed] [Google Scholar]
- 20.McLeod R, Kieffer F, Sautter M et al. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz 2009; 104:320–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torrey FE, Yolken RH. Toxoplasma oocysts as a public health problem. Trends Parasitol 2013; 29:380–4. [DOI] [PubMed] [Google Scholar]
- 22.Kean BH, Kimball AC, Christenson WN. An epidemic of acute toxoplasmosis. JAMA 1969; 208:1002–4. [PubMed] [Google Scholar]
- 23.Bowie WR, King AS, Marion SA et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet 1997; 350:173–7. [DOI] [PubMed] [Google Scholar]
- 24.Benenson MW, Takafuji ET, Lemon SM et al. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med 1982; 307:666–9. [DOI] [PubMed] [Google Scholar]
- 25.Silveira C, Muccioli C, Holland GN et al. Ocular involvement following an epidemic of Toxoplasma gondii infection in Santa Isabel do Ivaí, Brazil. Am J Ophthalmol 2015; 159:1013–21. [DOI] [PubMed] [Google Scholar]
- 26.Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol 2010; 128:28–32. [DOI] [PubMed] [Google Scholar]
- 27.Doganci L, Tanyuksel M, Ciftcioglu A et al. A probable outbreak of toxoplasmosis among boarding school students in Turkey. Clin Microbiol Infect 2006; 12:672–4. [DOI] [PubMed] [Google Scholar]
- 28.Teutsch SM, Juranek DD, Sulzer A et al. Epidemic toxoplasmosis associated with infected cats. N Engl J Med 1979; 300:695–9. [DOI] [PubMed] [Google Scholar]
- 29.Boyer K, Hill D, McLeod R et al. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis 2011; 53:1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contopoulos-Ioannidis DG, Maldonado Y, Montoya JG. Acute Toxoplasma gondii infection among family members in the United States. Emerg Infect Dis 2013; 19:1981–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flegr J, Klapilová K, Kaňková Š. Toxoplasmosis can be a sexually transmitted infection with serious clinical consequences. Not all routes of infection are created equal. Med Hypotheses 2014; 83:286–9. [DOI] [PubMed] [Google Scholar]
- 32.Lopes WD, Rodriguez JD, Souza FA et al. Sexual transmission of Toxoplasma gondii in sheep. Vet Parasitol 2013; 195:47–56. [DOI] [PubMed] [Google Scholar]
- 33.Arantes TP, Lopes WD, Ferreira RM et al. Toxoplasma gondii: evidence for the transmission by semen in dogs. Exp Parasitol 2009; 123:190–4. [DOI] [PubMed] [Google Scholar]
- 34.Santana LF, Rossi GAM, Gaspar RC et al. Evidence of sexual transmission of Toxoplasma gondii in goats. Small Rumin Res 2013; 115:130–3. [Google Scholar]
- 35.Santana LF, Da Costa AJ, Pieroni J et al. Detection of Toxoplasma gondii in the reproductive system of male goats. Revista Brasileira De Parasitologia Veterinária 2010; 19:179–82. [DOI] [PubMed] [Google Scholar]
- 36.Press C, Montoya JG, Remington JS. Use of a single serum sample for diagnosis of acute toxoplasmosis in pregnant women and other adults. J Clin Microbiol 2005; 43:3481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong SY, Remington JS. Toxoplasmosis in pregnancy. Clin Infect Dis 1994; 18:853–62. [DOI] [PubMed] [Google Scholar]
- 38.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg 2007; 77:405–10. [PubMed] [Google Scholar]
- 39.Stillwaggon E, Carrier CS, Sautter M, Mcleod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis 2011; 5:E1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papoz L, Simondon F, Saurin W, Sarmini H. A simple model relevant to toxoplasmosis applied to epidemiologic results in France. Am J Epidemiol 1986; 123:154–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.