Abstract
BACKGROUND
In a previous trial, treatment with soy isoflavones was associated with improved nonverbal memory, construction, verbal fluency, and speeded dexterity compared to treatment with placebo in cognitively healthy older men and women.
Objective
The current trial aimed to examine the potential cognitive benefits of soy isoflavones in patients with Alzheimer’s disease.
METHODS
Sixty-five men and women over the age of 60 were treated with 100mg/day soy isoflavone, or matching placebo capsules for six months. APOE genotype was determined for all participants. Cognitive outcomes and plasma isoflavone levels were measured at Baseline, and at two additional time points.
RESULTS
Fifty-nine subjects completed all study visits. Thirty-four were women (52.3%); average age was 76.3 (SD=7.2) years, and 31 (47.7%) were APOE4 positive. Plasma isoflavone levels increased in subjects treated with soy isoflavones compared to Baseline and to placebo, although intersubject variability in plasma levels was large. No significant differences in treatment effects emerged between treatment groups or genders. Analyses of associations between changes in cognition and plasma isoflavone levels revealed an association between equol levels and speed dexterity and verbal fluency.
CONCLUSIONS
Six months of 100mg/day treatment with soy isoflavones did not benefit cognition in older men and women with Alzheimer’s disease. However, our results suggest the need to examine the role of isoflavone metabolism, i.e., the ability to effectively metabolize soy isoflavones by converting daidzen to equol when attempting fully clarify the cognitive effects of isoflavones.
Keywords: Soy Isoflavones, Alzheimer’s disease, cognition, genistein, daidzein, equol, phytoestrogens, clinical trial
1. INTRODUCTION
Studies reporting on the cognitive effects of soy isoflavones are mixed. Observational research studies suggest a range of effects, including benefit, [1] benefit for subgroups only,[2] no effect,[3–5] and possible harm.[6] For example, data from the Honolulu-Asia Aging Study [6] suggested that consumption of tofu, a whole soybean protein rich in isoflavones was associated with brain atrophy in older Japanese Americans. A number of intervention studies have been conducted, and provide similarly conflicting results. Several small clinical trials suggested a beneficial cognitive effect,[7–10] a mixed or partial benefit,[11–14] or no effect.[15–17] A large trial enrolling postmenopausal women age 45 to 92 found a benefit but only for a visual memory.[18] All but a few clinical trials [9, 11, 17] excluded men, and most enrolled younger, postmenopausal women. Altogether, there is little know about soy isoflavone’s effect on cognition in older adults, especially older adults with Alzheimer’s disease (AD).
Preliminary findings from a previous study by our group enrolling cognitive healthy older adults found that treatment with soy isoflavones was associated with improved performance across several cognitive domains, including nonverbal memory, construction, verbal fluency, and speeded dexterity compared to treatment with placebo.[11] Given the potential for soy isoflavones to improve thinking skills in cognitive healthy individuals, we explored whether soy isoflavones may influence cognition in individuals with cognitive impairment, such as Alzheimer’s disease. Among animal models, evidence suggests a neuroprotective effect of soy isoflavones on spatial learning and memory in a rat model of Alzheimer’s disease.[20] Similarly, Ma et al.[21] found a neuroprotective effect of soy isoflavones in combination with folic acid on learning and memory in an amyloid β rat model of Alzheimer’s disease. In both studies, rats injected with amyloid β performed more poorly than healthy controls; however, exposure to soy isoflavones mitigated the negative effects of β-amyloid on learning and memory performance.
Human studies of soy isoflavones in Alzheimer’s disease are sparse. Jefremov et al.[22] found that both estrogens and phytoestrogens exerted a protective effect on post-mortem human brain tissues in hippocampal and frontal cortices. To our knowledge, however, no studies have been published examining the effect of soy isoflavones on living individuals diagnosed with Alzheimer’s disease. Accordingly, the present study was designed to evaluate whether dietary intake of soy isoflavones may benefit cognitive functioning among individuals diagnosed with Alzheimer’s disease. Based on our previous work [11] and that of others,[18] we hypothesized that older adults with AD, who were treated with soy isoflavones would demonstrate domain-specific cognitive changes, specifically benefits in visual spatial abilities, language fluency, and speeded dexterity, rather than global cognitive effects. Furthermore, we speculated that the soy isoflavone supplement would be well tolerated with minimal safety concerns.
2. MATERIALS AND METHODS
2.1 Subjects
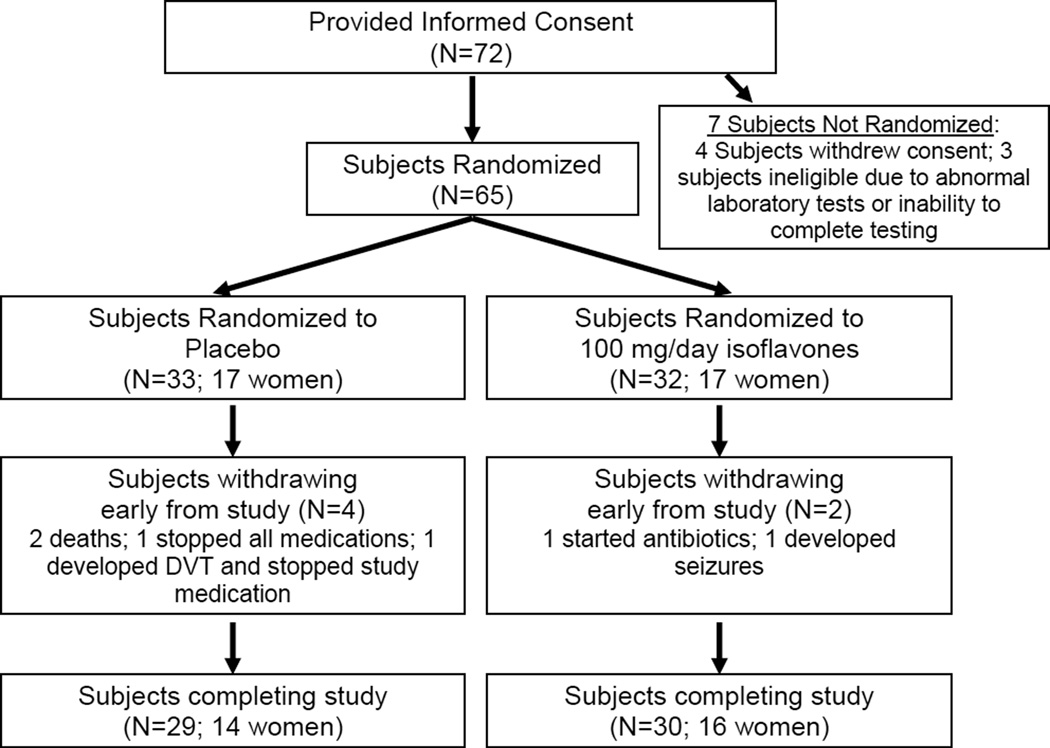
Seventy-two men and women over the age of 60, recruited from the community provided informed consent for study procedures. All participants had study partners who also provided informed consent. The research was performed in accordance with the Helsinki Declaration of 1975. The University of Wisconsin-Madison Health Science Institutional Review Board reviewed and approved of all study procedures. Seven subjects were excluded prior to randomization: four subjects withdrew consent, another was excluded due to an abnormal lipase laboratory value, and two participants were excluded due to their inability to complete cognitive testing. Thus, sixty-five volunteers were randomized to treatment with 100mg/day soy isoflavone, or matching placebo capsules for 6 months. Fifty-nine participants completed 6 months of treatment; two in the isoflavone arm and four participants in the placebo arm discontinued study participation early. See Figure 1 for illustration of the study’s participant flow.
Figure 1.
Participant recruitment, randomization, and follow-up.
Other than Alzheimer’s disease, subjects were free of major medical, neurological, and psychiatric illnesses. Individuals with diabetes, colon disease, and cancer other than basal or squamous cell carcinomas were excluded. Chronic, well-controlled medical conditions such as hypertension were permitted for entry into the study. All women were postmenopausal, and had not used hormone therapy for at least six months prior to enrollment in the study. Medication doses, including those of approved treatments for AD were stable for two months prior to randomization. Extensive laboratory and cognitive screenings were performed along with a medical examination to screen for exclusionary conditions. Female subjects underwent additional mammographic examination to screen for any pre-existing pathology.
2.2 Study design and data collection
Study procedures included cognitive and mood assessment; blood collection for safety laboratory testing (amylase, lipase, and phosphate levels) and isoflavone assays; symptom interview; and brief medical evaluation. Subjects also completed a standardized and validated food frequency questionnaire (FFQ) [23] in order to estimate weekly dietary intake of soy isoflavones. Study procedures were performed at Baseline and repeated at three and 6 months after treatment initiation.
2.3 Study medications
At Baseline subjects were randomized to receive either capsules containing 50 mg/day of purified isoflavones (approximately 85% daidzin and genistin, mainly as glycosides; Novasoy® brand isoflavones; Archer Daniels Midland Co., Decatur, IL) a matching placebo capsules containing maltodextrin and caramel food color, or in this double-blind, parallel-group design study. The specific content of the isoflavone and placebo capsules are provided as supplemental data in Supplementary Table A.
Subjects and study partners were provided written instructions for dosing and a list of foods to avoid while enrolled in the study. Specifically, subjects were instructed to take one capsule twice daily, with carbohydrate-rich, low fat meals. On study visit days, subjects were instructed to take the study medication 4h prior to their visit in order to standardize the time between dosing and blood draw. However, because there are individual differences in ability to derive aglycone forms of isoflavones from glycosides, exposure to bioactive forms of the soy isoflavones was estimated by measuring plasma levels of aglycones.
2.4 Safety Monitoring
The most common adverse effects reportedly associated with high doses of isoflavones included asymptomatic hypophosphatemia, and elevated levels of plasma lipase and amylase.[129, 130] Thusly, non-fasting, safety laboratory tests included measurement of serum phosphate, and plasma levels of amylase and lipase.
2.5 Plasma isoflavone measurement and APOE genotyping
Isoflavone assays were performed on non-fasting blood samples collected at baseline, and after months 3 and 6. Concentrations of total daidzein, genistein and equol in plasma (0.5 mL) were measured after enzymatic hydrolysis of the glucuronide and sulfate conjugates and by a stable-isotope dilution GC-MS method that has been described previously.[24] Concentrations of daidzein, and genistein, and the metabolite, equol were expressed as nmol/L and the assays were conducted under Good Laboratory Practice (GLP) procedures and with quality assurance by incorporating within-batch and between-batch plasma samples quality control specimens. The within day reproducibility for repeat analysis of the same plasma sample expressed as %CV was 0.5% for daidzein and 1.0% for genistein and the mean between-batch reproducibility over 19 separate analytical runs was 5% (range 1.0–11.9%) for daidzein and 7% (range 1–17%) for genistein at concentrations of 100–200 ng/mL.[25]
Determination of apolipoprotein E (APOE) genotype was performed on a non-fasting blood sample collected at Baseline, using standard PCR and DNA sequencing techniques in a CLIA certified laboratory. DNA extracted from whole blood was amplified by PCR using specific primers for the ApoE gene and the DNA then sequenced and analyzed for genotype using the FinchTV program (Version 1.3; Geospiza, Inc.).
2.6 Neuropsychological Battery
At every study visit, a trained psychometrician administered a battery of neuropsychological measures designed to thoroughly sample memory and executive function, following standardized testing procedures. Tests included measures of verbal and visuospatial memory (List Learning,[26] Paragraph Recall,[27] Benton Visual Retention test,[28] Complex Figure Recall [26]); language/executive function (phonemic fluency,[26] animal fluency [26]); working memory/executive function (Digit Symbol,[27] Digit Span [27]); executive function (Stroop Color Word test [29], Mazes [30] and Trail Making Test A and B [26]); and visual-motor function (Complex Figure Copy,[26] Grooved Peg Board [31]).
2.7 Assessment of Mood
To assess psychiatric symptoms, the Profile of Mood States [32] and the Geriatric Depression Scale-Short Form [33] were administered at Baseline, and months three and six following randomization.
2.8 Statistical Analysis
The primary objective of this pilot study was to examine the safety and feasibility of soy isoflavone supplement administration in men and women over the age of 60 years. Data on plasma levels of isoflavones following 1 and 6 months administration of 100mg/day purified isoflavones are presented along with data on side-effects, safety laboratory tests, and study and medication adherence estimated by FFQ and pill count. A second major objective was to examine the potential efficacy of a soy isoflavone supplement to favorably alter cognition in older adults with dementia due to Alzheimer’s disease. Neuropsychological data were compared for an effect of soy administration across visits. The effect of soy isoflavone withdrawal was also determined by comparing neuropsychological performance at month 6 to performance at month 8 (washout visit). Two additional exploratory analyses were conducted. First, data from baseline cognitive testing was compared to baseline FFQ data in order to estimate the effect of prior exposure to dietary soy isoflavones. Secondly, correlations between plasma levels of the soy isoflavones (genistein and daidzein, and the metabolite of daidzein, equol) while on study medication and change on neuropsychological variables were examined in order to estimate the relationship between soy metabolism and efficacy of intervention.
Given the small sample-size, nonparametric methods were employed for all comparisons. Chi-square tests were used to examine the association between categorical variables (e.g., sex and treatment group). Comparisons between continuous variables (e.g. age) were computed using the nonparametric Wilcoxon rank-sum test. Cognitive function test p-values were adjusted for age and years of education using nonparametric Wilcoxon score general linear models.[34] Correlations between continuous variables were computed using Spearman rank-order correlations. These analyses were considered exploratory; thus, corrections for multiple comparisons were not applied.
3. RESULTS
3.1 Subjects
Fifty-nine subjects finished all study visits; partial data were available from sixty-five participants. Among participants completing the trial, thirty were women (50.8%); average age was 76.3 (SD=7.2) years, and 31 (47.7%) were APOE e4 positive.
Table 1 lists demographic data by randomization group. There were no significant differences between isoflavone and placebo-treated groups in age, education, or APOE e4 status. Furthermore, dietary intake of isoflavones, global cognition, and self-report of mood symptoms were similar between treatment groups at Baseline. These data suggest that subjects were in the early stages of AD, non-depressed and generally naïve to dietary isoflavones. Finally, the groups did not differ in use of concomitant therapies or in anthropomorphic characteristics. Nearly all participants were prescribed a single standard cholinergic therapy, and about half were taking a combination of a cholinesterase inhibiter and memantine.
Table 1.
Subject characteristics at entry into study.
| SUBJECT BASELINE CHARACTERISTICS | Placebo Treated (N=33) |
Isoflavone Treated (N=32) |
P-value |
|---|---|---|---|
| Age in years: mean (SD) | 76.8 (6.8) | 75.7 (7.7) | 0.54 |
| Gender: Percent and number of Women | 51.5% (n=17) | 53.1% (n=17) | 0.90 |
| Education in years: mean (SD) | 14.2 (2.5) | 14.8 (2.7) | 0.38 |
| ApoE4 Status1: Percent and number of ApoE4 carriers | 45.4% (n=15) | 50.0% (n=16) | 0.71 |
| Total weekly Isoflavone intake at Baseline2: mean mg/week (SD) | 6.5 (34.1) | 16.5 (66.3) | 0.45 |
| Global Cognition: Mini-Mental State Examination score mean (SD) | 22.4 (5.3) | 23.5 (4.0) | 0.35 |
| Mood Symptoms: Geriatric Depression Scale-Short Form score mean (SD) | 2.6 (2.4) | 3.2 (3.3) | 0.40 |
| Concomitant treatment: Percent and number on AchE-I3 | 90.9% (n=30) | 96.9% (n=31) | 0.32 |
| Concomitant treatment: Percent and number on NMDA-receptor blocker4 | 42.4% (n=14) | 50.0% (n=16) | 0.54 |
| Body Mass Index | 27.9 (5.4) | 27.5 (5.0) | 0.75 |
One subject in each group declined to undergo ApoE genotyping
Established with Food Frequency Questionnaire (FFQ)
Acetylcholinesterase-Inhibitors included Donepezil, Galantamine Hydrobromide, and Rivastigmine
NMDA-Receptor Blocker was Memantine
3.2 Adherence and Safety Outcomes; and Isoflavone assays
Table 2 provides data related to study adherence and safety outcomes. Overall, there was good adherence to study instructions as measured with pill counts and reports of dietary soy intake. For example, medications counts suggest adequate adherence with 97–98% of pill counts aligned with expectations. No subjects were discontinued due to abnormal safety laboratory values, mean values are provided in Table 2.
Table 2.
Data on participant adherence to study procedures, and vitals and safety laboratory values after 6 months of treatment with Soy Isoflavones or Placebo.
| Placebo Treated MEAN VALUE (SE) |
Isoflavone Treated MEAN VALUE (SE) |
P-value | |
|---|---|---|---|
| Adherence to study protocol | |||
| Average weekly dietary soy intake1 during study participation; mg/week (SD) (N=55) | 19.9 (34.7) | 4.9 (10.9) | 0.76 |
| Pill Count – Average percent of expected number of pill used between visits (SD) (N=55) | 97.5% (2.2%) | 98.1% (5.7%) | 0.68 |
| Vital signs after 6 months of treatment (N=58) | |||
| Average systolic blood pressure (SD) mmHg | 126.9 (15.7) | 128.8 (18.5) | 0.67 |
| Average diastolic blood pressure (SD) mmHg | 66.8 (7.2) | 69.3 (9.3) | 0.26 |
| Safety Laboratory Tests after 6 months of treatment (N=59) | |||
| Amylase – Average blood level (SD) U/L | 69.9 (36.6) | 58.5 (14.9) | 0.12 |
| Lipase – Average blood level (SD) U/L | 287.8 (222.3) | 267.5 (103.0) | 0.65 |
| Phosphate – Average blood level (SD) mmol/L | 3.5 (1.2) | 3.3 (0.4) | 0.39 |
Plasma isoflavone concentrations increased in all subjects treated with soy isoflavones compared to Baseline levels and to placebo-treated subjects. Table 3 lists genistein, daidzein and equol concentrations in plasma in the isoflavone - and placebo-treated participants at 3 time points: at Baseline, and after 3 and 6 months of therapy.
Table 3.
Plasma Isoflavones at Baseline, Month 3 and Month 6.
| Placebo Treated (N=33) |
Isoflavone Treated (N=32) |
P-value | |
|---|---|---|---|
| Genistein ng/mL (SD) | |||
| Baseline1 | 4.8 (11.4) | 1.5 (2.6) | 0.12 |
| Month 32 | 2.1 (3.7) | 277.7 (188.4) | 0.00 |
| Month 63 | 15.6 (35.4) | 338.6 (308.8) | 0.00 |
| Daidzein ng/mL (SD) | |||
| Baseline1 | 2.8 (6.1) | 1.3 (2.5) | 0.21 |
| Month 32 | 1.1 (2.3) | 197.3 (150.5) | 0.00 |
| Month 63 | 10.6 (37.8) | 206.6 (156.8) | 0.00 |
| Equol ng/mL (SD) | |||
| Baseline1 | 0.3 (1.5) | 0.1 (0.5) | 0.55 |
| Month 32 | 0.2 (0.5) | 20.3 (50.7) | 0.03 |
| Month 63 | 0.1 (0.6) | 19.6 (40.5) | 0.01 |
Baseline assay data unavailable for three subjects: two placebo- and one isoflavone-treated subject
Month 3 assay data unavailable for two subjects: one from each group
Month 6 assay data unavailable for four subjects: one placebo- and three isoflavone-treated Subjects
Despite subjects’ compliance with treatment, there was high intersubject variability in the plasma concentrations within the isoflavone-treated subjects. This is illustrated in Supplementary Figure 1, which portrays individual subjects’ change from baseline for specific isoflavones for participants randomized to the active treatment condition. Only a quarter of subjects demonstrated a measurable increase in equol levels after 6 months of treatment (7/27 subjects randomized to receive soy isoflavones, from whom equol levels were obtained at their final visit).
3.3 Effect of treatment with soy isoflavones on cognition
As anticipated there was no significant change in global cognition with 6 months of treatment with soy isoflavones or placebo. Both treatment groups appeared to decline on the Mini Mental State Examination over the course of treatment (see Table 4).
Table 4.
Estimated Marginal Means for Cognitive Tests after 6 months of treatment with Soy Isoflavones or Placebo.
| Domain | Test | Placebo Treated MEAN VALUE (SE) |
Isoflavone Treated MEAN VALUE (SE) |
P-value | |
|---|---|---|---|---|---|
| Cognitive Outcomes | Global Cognition | Mini-Mental State Examination (MMSE) (N=59) | 21.3 (1.0) | 23.4 (1.0) | 0.15 |
| Verbal Memory | List Learning Delayed Free Recall; Number of words recalled (N=57) | 1.8 (0.50) | 2.2 (0.50) | 0.56 | |
| Logical Memory Delayed Recall; Number of story elements recalled (n=57) | 4.6 (0.95) | 5.7 (0.9) | 0.40 | ||
| Executive Function | Mazes; Time to complete (N=53) | 32.2 (5.3) | 22.6 (5.4) | 0.22 | |
| Trail Making Test B; Time to complete (N=52) | 95.5 (16.7) | 95.7 (16.0) | 0.99 | ||
| Stroop Color Word Test; Time to complete (N=54) | 105.0 (12.0) | 91.8 (12.0) | 0.44 | ||
| Language Executive Function |
Category Fluency; Number of words generated (N=55) | 21.5 (1.9) | 25.7 (2.0) | 0.13 | |
| Phonemic Fluency; Number of words generated (N=56) | 25.9 (2.4) | 35.2 (2.4) | 0.04* | ||
| Visual Memory | Complex Figure Delayed Recall; Number of points (N=57) | 10.6 (1.8) | 13.4 (1.8) | 0.27 | |
| Benton Visual Retention Test; Number of correct figures (N=56) | 2.7 (0.4) | 2.8 (0.4) | 0.78 | ||
| Benton Visual Retention; Number of errors (N=56) | 14.5 (1.2) | 13.1 (1.2) | 0.42 | ||
| Visual Motor | Complex Figure Copy; Number of points (N=57) | 27.6 (1.1) | 29.0 (1.1) | 0.35 | |
| Grooved Pegboard Dominant Hand; Time to complete (N= (N=51) | 134.3 (17.6) | 149.8 (16.6) | 0.53 | ||
| Grooved Pegboard Non-Dominant Hand; Time to complete (N=48) | 161.8 (24.4) | 156.0 (22.4) | 0.87 | ||
| Mood Outcomes | Depression | Geriatric Depression Scale – Subject Report (N=57) | 2.1 (0.6) | 3.3 (0.6) | 0.41 |
| Geriatric Depression Scale – Study Partner Report (N=57) | 5.2 (0.7) | 6.3 (0.7) | 0.25 | ||
| Multiple- Mood States |
Profile of Mood States (POMS) – Tension Scale (N=56) | 8.2 (1.3) | 9.3 (1.3) | 0.55 | |
| POMS – Depression Scale (N=56) | 9.0 (1.8) | 11.4 (1.7) | 0.34 | ||
| POMS – Anger Scale (N=56) | 6.8 (1.4) | 8.3 (1.4) | 0.45 | ||
| POMS – Fatigue Scale (N=56) | 10.3 (1.2) | 10.3 (1.2) | 0.97 | ||
| POMS – Vigor Scale (N=56) | 12.4 (1.1) | 12.0 (1.1) | 0.79 | ||
| POMS – Confusion Scale (N=56) | 15.5 (1.1) | 14.0 (1.1) | 0.35 |
All means were adjusted for covariates including age at baseline (76.4 years) and education (14.5 years). Subjects performed similarly on tests at baseline with the exception of Phonemic Fluency. Individuals randomized to receive Soy Isoflavones out-performed those on Placebo in Baseline Phonemic Fluency.
When model is corrected for baseline differences, difference between groups is no longer significant.
Wilcoxon general linear models, examining treatment effects by time revealed no significant differences between treatment groups. Planned subgroup analyses explored the effect of gender and APOE e4 genotype and on treatment response. Neither men nor women with AD demonstrated enhanced cognitive performance with isoflavone treatment compared to placebo. Likewise, APOE e4 genotype (positive or negative for the risk factor) did not influence response to treatment with isoflavones.
A final planned analysis explored the relationship between plasma levels of isoflavones and cognitive changes. Specifically, the associations between changes in plasma isoflavone levels and changes in cognitive scores were tested for four domains identified in our previous study as favorably influenced by isoflavone treatment; specifically, the cognitive domains included visual spatial memory, visual-motor, verbal fluency, and speeded dexterity. Ten sub-test scores were derived from the tests measuring the four domains. Three of the ten comparisons (e.g., two of four measures selected, speeded dexterity and verbal fluency) showed significant correlations.Table 5 lists all correlations. Specifically, total plasma equol was significantly correlated with performance on a measure of speeded dexterity (Grooved Pegboard, non-dominant hand: r = −0.33, p = 0.02; dominant hand r = −0.29, p = 0.05). A positive association between total plasma equol and performance on a verbal fluency test (Phonemic Fluency: r = 0.29, p = 0.04). Importantly, given the small sample size and multiple outcomes, these analyses must be considered merely exploratory.
Table 5.
Estimated Marginal Means for Cognitive Tests after 6 months of treatment with Soy Isoflavones or Placebo.
| Domain | Test | Spearman’s rho Correlation of test score with 6 month Change in Plasma Isoflavones (ng/mL) |
|||||
|---|---|---|---|---|---|---|---|
| ΔGenistein | ΔDiadzein | ΔEquol | |||||
| r | p | r | p | r | p | ||
| Verbal Fluency | Animal Fluency; Number of words generated | 0.11 | 0.45 | 0.19 | 0.20 | 0.23 | 0.12 |
| Vegetable Fluency; Number of words generated | 0.17 | 0.26 | 0.20 | 0.17 | 0.09 | 0.55 | |
| Fruit Fluency; Number of words generated | 0.19 | 0.19 | 0.25 | 0.08 | 0.22 | 0.13 | |
| Phonemic Fluency; Number of words generated | 0.19 | 0.18 | 0.28 | 0.11 | 0.29 | 0.04 | |
| Visual Spatial Memory | Complex Figure Delayed Recall; Number of points | 0.04 | 0.80 | 0.03 | 0.85 | 0.14 | 0.34 |
| Benton Visual Retention Test; Number of correct figures | 0.24 | 0.10 | 0.20 | 0.17 | 0.20 | 0.17 | |
| Benton Visual Retention Test; Number of errors | −0.27 | 0.06 | −0.24 | 0.10 | −0.21 | 0.15 | |
| Visual-Motor Construction | Complex Figure Copy; Number of points | 0.13 | 0.37 | 0.10 | 0.51 | 0.22 | 0.13 |
| Speeded Dexterity | Grooved Pegboard Dominant Hand; Time to complete | −0.07 | 0.64 | −0.04 | 0.77 | −0.29 | 0.05 |
| Grooved Pegboard Non-Dominant Hand; Time to complete | −0.11 | 0.45 | −0.13 | 0.40 | −0.33 | 0.02 | |
4. DISCUSSION
This randomized, controlled, clinical trial investigated the cognitive effects of soy isoflavones in older adults with Alzheimer’s disease (AD). The results show no cognitive benefits over placebo after 6 months of therapy with 100mg/day soy isoflavone tablets, and global cognition declined at similar rates in both treatment and control groups. Among individuals who were effectively able to metabolize the soy isoflavone daidzein to equol however, data suggested an association between plasma levels of equol and performance on two measures of neuropsychological function: verbal fluency and speeded manual dexterity. While preliminary, these results suggest a need to further clarify how soy isoflavone metabolism influences efficacy.. To our knowledge, these findings are the first to report on the function of soy isoflavones among older adults with cognitive impairment.
The present data suggest that soy isoflavones may exert a positive, albeit modest effect on cognition. However, differences were observed only for individuals able to metabolize isoflavones, as demonstrated by circulating plasma equol levels. These data confirm in part our previous results in cognitively healthy older adults,[11] and extend findings across a wider spectrum of cognitive ability, to individuals with AD. As noted previously, the few intervention studies conducted so far enrolled primarily younger, cognitively healthy, postmenopausal women. A large randomized, controlled trial enrolling postmenopausal women age 45 to 92 revealed a decline on general intelligence with soy treatment, but a beneficial effect for visual memory.[18] The conflicting results may explain why findings from small trials are highly mixed.[7–10][11–14][15–17]
While the findings of the present study were neutral overall, the findings related to equol production are encouraging given that research on AD therapies is most notable for a lack of therapeutic effect. Moreover, the potential for achieving positive effects with a relatively benign, natural agent is promising. Correlations between improved performance and plasma equol levels were found in two domains of cognitive function; specifically, on measures of verbal fluency and speeded dexterity. As both tasks are timed, successful performance in each relies on cognitive speed. The present results suggest the possibility that soy isoflavones may function to facilitate cognitive processing speed, either directly or indirectly. Conversely, our results did not show any group differences on measures of memory or other, more complex speeded tasks of visuomotor processing. Moreover, our sample sizes are small, particularly considering the limited number of participants who demonstrated a measurable change in equol levels following administration of soy isoflavone tablets. Additional research is necessary to replicate the findings, delineate the cognitive domains potentially affected by isoflavones, and the mechanisms or conditions in which soy isoflavones may optimally influence cognition, e.g., equol production.
This study used an isoflavone supplement that consisted mainly of isoflavone glycosides, which are not the biologically active forms of isoflavones.[39, 40] Even though hydrolysis of the glycoside is efficient,[39, 41, 42] there are significant differences in the pharmacokinetics of glycosides when compared with aglycons, with the latter being absorbed much faster and reaching higher peak plasma concentrations.[43] While some controversy exists,[44, 45] numerous lines of evidence now suggest that biologically active aglycons are likely to have increased bioavailability relative to glycosides [46] and better efficacy.[36] These data can be contrasted to the findings from St. John et al (CITE) that revealed that change in urinary excretion of isoflavones, excluding equol, was inversely correlated with change in a general intelligence factor. In other words, genistein and diadzein production was associated with a potential harmful cognitive effect.
However, production the aglycons, genistein and diadzein is not necessarily aligned with equol production. Research on the pharmacokinetics of isoflavones has established a high level of individual variability in metabolism of isoflavones, such that not all adults are “equol producers,” that is, able to convert daidzen to equol.[36, 37] Possible contributing factors to successful conversion include content and availability of isoflavones from food, variability in lactase persistence status, presence of specific gastrointestinal microflora, gastric transit times, and systemic metabolic rates.[38] Data from subjects younger and healthier than the participants included in the present study indicate that approximately one one-third of non-vegetarians are equol producers. (Setchell 2006; Newton et al. 2015). In the present study, only for individuals able to make this conversion did a pattern emerge suggesting a beneficial effect of soy.
The mechanisms by which soy isoflavones appear to exert beneficial effects on cognition may be multifactorial given their wide range of biological actions. Isoflavones genistein and the dadizein metabolite, equol are selective estrogen receptor modulators, in having greater affinity for ERβ, a receptor that is widely distributed in brain tissue, than for ERα.[47, 48] Soy isoflavones may work to confer a neuroprotective effect on brain tissue via estrogenic receptor pathways [35] and thereby defend against cell apoptosis and neurotoxicity.[49] Additionally, isoflavones are very effective antioxidants [50, 51] having greater in vitro antioxidant activity than vitamin C or E. Thus soy isoflavones may improve cognition via protection against oxidative stress. Studies indicate that amyloid β is associated with oxidative stress and mitochondrial dysfunction in the Alzheimer’s brain.[52, 53] Soy isoflavones may counteract this process by up-regulating serum and brain tissue antioxidant levels.[53] In addition, soy isoflavones may reduce reactive oxygen species (oxidants) and ameliorate β amyloid induced cellular apoptosis.[54] A further mechanism of action for soy isoflavones may involve reduction of amyloid fibril accumulation.[55]
The strengths of this study include its prospective design, and measurement of plasma isoflavones concentrations. Additionally, our study examined the potential application of isoflavones in a unique cohort with high need for effective treatment. The weaknesses include its small sample size and regional, socioeconomic and racial homogeneity of the sample. It is unknown to what extent cultural, ethnic, and dietary factors may influence the ability to metabolize soy isoflavones, and investigation of individuals from diverse cultural and ethnic backgrounds may yield different results. Identifying multiple outcomes increases the risk of spurious findings. Additionally, the study used a glycoside conjugate rather than aglycone forms of soy isoflavones, which may have yielded different results.
Conclusion
This small prospective study suggested no cognitive benefits over placebo from 6-months of therapy with 100mg/day a soy isoflavone supplement. However, there was evidence for mild to modest cognitive benefit with among older individuals with AD, who were able to metabolize the isoflavone supplement, specifically to equol. Given the lack of efficacy of current pharmacotherapy in AD, the possibility of any additional treatments is encouraging.
Supplementary Material
ACKNOWLEDGMENTS
SOURCES OF SUPPORT
Jodi Barnet, MS for statistical analysis support; Archer Daniel Midland for the supply of medication and matching placebo; and the participants and their study partners.
This research was supported by NIH grants K23 AG024302 and ADRC P50 AG033514.
REFERENCES
- 1.Woo J, Lynn H, Lau WY, Leung J, Lau E, Wong SY, Kwok T. Nutrient intake and psychological health in an elderly Chinese population. Int J Geriatr Psychiatry. 2006;21:1036–1043. doi: 10.1002/gps.1603. [DOI] [PubMed] [Google Scholar]
- 2.Hogervorst E, Mursjid F, Priandini D, Setyawan H, Ismael RI, Bandelow S, Rahardjo TB. Borobudur revisited: soy consumption may be associated with better recall in younger, but not in older, rural Indonesian elderly. Brain Res. 2011;1379:206–212. doi: 10.1016/j.brainres.2010.10.083. [DOI] [PubMed] [Google Scholar]
- 3.Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, van der Schouw YT. Dietary phytoestrogen intake and cognitive function in older women. J Gerontol A Biol Sci Med Sci. 2007;62:556–562. doi: 10.1093/gerona/62.5.556. [DOI] [PubMed] [Google Scholar]
- 4.Huang MH, Luetters C, Buckwalter GJ, Seeman TE, Gold EB, Sternfeld B, Greendale GA. Dietary genistein intake and cognitive performance in a multiethnic cohort of midlife women. Menopause. 2006;13:621–630. doi: 10.1097/01.gme.0000227336.35620.8f. [DOI] [PubMed] [Google Scholar]
- 5.Franco OH, Burger H, Lebrun CE, Peeters PH, Lamberts SW, Grobbee DE, Van Der Schouw YT. Higher dietary intake of lignans is associated with better cognitive performance in postmenopausal women. J Nutr. 2005;135:1190–1195. doi: 10.1093/jn/135.5.1190. [DOI] [PubMed] [Google Scholar]
- 6.White LR, Petrovitch H, Ross GW, Masaki K, Hardman J, Nelson J, Davis D, Markesbery W. Brain aging and midlife tofu consumption. J Am Coll Nutr. 2000;19:242–255. doi: 10.1080/07315724.2000.10718923. [DOI] [PubMed] [Google Scholar]
- 7.Duffy R, Wiseman H, File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. 2003;75:721–729. doi: 10.1016/s0091-3057(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 8.File SE, Hartley DE, Elsabagh S, Duffy R, Wiseman H. Cognitive improvement after 6 weeks of soy supplements in postmenopausal women is limited to frontal lobe function. Menopause. 2005;12:193–201. doi: 10.1097/00042192-200512020-00014. [DOI] [PubMed] [Google Scholar]
- 9.File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H. Eating soya improves human memory. Psychopharmacology (Berl) 2001;157:430–436. doi: 10.1007/s002130100845. [DOI] [PubMed] [Google Scholar]
- 10.Kritz-Silverstein D, Von Muhlen D, Barrett-Connor E, Bressel MA. Isoflavones and cognitive function in older women: the SOy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause. 2003;10:196–202. doi: 10.1097/00042192-200310030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gleason CE, Carlsson CM, Barnet JH, Meade SA, Setchell KD, Atwood CS, Johnson SC, Ries ML, Asthana S. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing. 2009;38:86–93. doi: 10.1093/ageing/afn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howes JB, Bray K, Lorenz L, Smerdely P, Howes LG. The effects of dietary supplementation with isoflavones from red clover on cognitive function in postmenopausal women. Climacteric. 2004;7:70–77. doi: 10.1080/13697130310001651490. [DOI] [PubMed] [Google Scholar]
- 13.Islam F, Sparkes C, Roodenrys S, Astheimer L. Short-term changes in endogenous estrogen levels and consumption of soy isoflavones affect working and verbal memory in young adult females. Nutr Neurosci. 2008;11:251–262. doi: 10.1179/147683008X301612. [DOI] [PubMed] [Google Scholar]
- 14.Casini ML, Marelli G, Papaleo E, Ferrari A, D’Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85:972–978. doi: 10.1016/j.fertnstert.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Fournier LR, Ryan Borchers TA, Robison LM, Wiediger M, Park JS, Chew BP, McGuire MK, Sclar DA, Skaer TL, Beerman KA. The effects of soy milk and isoflavone supplements on cognitive performance in healthy, postmenopausal women. J Nutr Health Aging. 2007;11:155–164. [PubMed] [Google Scholar]
- 17.Pilsakova L, Riecansky I, Ostatnikova D, Jagla F. Missing evidence for the effect one-week phytoestrogen-rich diet on mental rotation in two dimensions. Neuro Endocrinol Lett. 2009;30:125–130. [PubMed] [Google Scholar]
- 18.Henderson VW, St John JA, Hodis HN, Kono N, McCleary CA, Franke AA, Mack WJ, Group WR. Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology. 2012;78:1841–1848. doi: 10.1212/WNL.0b013e318258f822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos-Galduroz RF, Galduroz JC, Facco RL, Hachul H, Tufik S. Effects of isoflavone on the learning and memory of women in menopause: a double-blind placebo-controlled study. Braz J Med Biol Res. 2010;43:1123–1126. doi: 10.1590/s0100-879x2010007500104. [DOI] [PubMed] [Google Scholar]
- 20.Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid beta(1–40) rat model of Alzheimer’s disease. Neurobiol Learn Mem. 2011;95:270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Ma WW, Xiang L, Yu HL, Yuan LH, Guo AM, Xiao YX, Li L, Xiao R. Neuroprotection of soyabean isoflavone co-administration with folic acid against beta-amyloid 1–40-induced neurotoxicity in rats. Br J Nutr. 2009;102:502–505. doi: 10.1017/S0007114509274757. [DOI] [PubMed] [Google Scholar]
- 22.Jefremov V, Rakitin A, Mahlapuu R, Zilmer K, Bogdanovic N, Zilmer M, Karelson E. 17beta-Oestradiol stimulation of G-proteins in aged and Alzheimer's human brain: comparison with phytoestrogens. J Neuroendocrinol. 2008;20:587–596. doi: 10.1111/j.1365-2826.2008.01696.x. [DOI] [PubMed] [Google Scholar]
- 23.Frankenfeld CL, Patterson RD, Kalhorn TF, Skor HE, Howald WN, Lampe J. Validation of a soy food frequency questionnaire with plasma concentrations of isoflavones in US adults. Journal of the American Dietetic Association. 2002;102:1407–1413. doi: 10.1016/s0002-8223(02)90313-5. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C–labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 25.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 26.Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 27.Wechsler D. Wechsler Memory Scales-3rd Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 28.Benton A. Revised Visual Retention Test Manual. New York: Psychological Corporation; 1974. [Google Scholar]
- 29.Golden C. The Stroop Color and Word Test: A manual for clinical and experimental uses. Chicago: Stoetling; 1978. [Google Scholar]
- 30.Wechsler D. WAIS-R Wechsler adult intelligence scale-III. New York, N.Y: Psychological Corporation; 1991. [Google Scholar]
- 31.Kløve H. Clinical neuropsychology. New York: Saunders; 1963. [Google Scholar]
- 32.Lorr M, McNair D. Manual for the profile of mood states, Bi-Polar form. San Diego, CA: Educational and Industrial Testing Service; 1983. [Google Scholar]
- 33.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: A comparison with the 30-item form. Journal of Geriatric Psychiatry and Neurology. 1991;4:173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 34.Hettmansperger TP, McKean JW. Robust nonparametric statistical methods. London; New York, NY: Arnold; John Wiley; 1998. [Google Scholar]
- 35.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr Biochem. 2005;16:641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandrasekharan S, Aglin A. Pharmacokinetics of Dietary Isoflavones. Steroids and Hormonal Science. 2013;S12 [Google Scholar]
- 38.Chandrasekharan S, Aglin A. in Steroids and Hormonal Science. 2013 [Google Scholar]
- 39.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 40.Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8:89–98. doi: 10.1089/lrb.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Lima FS, Ida EI. Optimisation of soybean hydrothermal treatment for the conversion of beta-glucoside isoflavones to aglycones. Lwt-Food Science and Technology. 2014;56:232–239. [Google Scholar]
- 42.Yeom SJ, Kim BN, Kim YS, Oh DK. Hydrolysis of Isoflavone Glycosides by a Thermostable beta-Glucosidase from Pyrococcus furiosus. J Agric Food Chem. 2012;60:1535–1541. doi: 10.1021/jf204432g. [DOI] [PubMed] [Google Scholar]
- 43.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. Journal of Nutrition. 2000;130:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 44.Andrade JE, Twaddle NC, Helferich WG, Doerge DR. Absolute Bioavailability of Isoflavones from Soy Protein Isolate-Containing Food in Female Balb/c Mice. J Agric Food Chem. 2010;58:4529–4536. doi: 10.1021/jf9039843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rufer CE, Bub A, Moseneder J, Winterhalter P, Sturtz M, Kulling SE. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. American Journal of Clinical Nutrition. 2008;87:1314–1323. doi: 10.1093/ajcn/87.5.1314. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Hu M. Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perfused rat intestinal model. Drug Metabolism and Disposition. 2002;30:370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 47.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-Equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial floral. American Journal of Clinical Nutrition. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 48.Muthyala RS, Ju YH, Sheng SB, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R-and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorganic & Medicinal Chemistry. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 49.Kajta M, Rzemieniec J, Litwa E, Lason W, Lenartowicz M, Krzeptowski W, Wojtowicz AK. The key involvement of estrogen receptor beta and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience. 2013;238:345–360. doi: 10.1016/j.neuroscience.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Nadal-Serrano M, Pons DG, Sastre-Serra J, Blanquer-Rossello MD, Roca P, Oliver J. Genistein modulates oxidative stress in breast cancer cell lines according to ER alpha/ER beta ratio: Effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymes. International Journal of Biochemistry & Cell Biology. 2013;45:2045–2051. doi: 10.1016/j.biocel.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Chung MJ, Kang AY, Lee KM, Oh E, Jun HJ, Kim SY, Auh JH, Moon TW, Lee SJ, Park KH. Water-soluble genistin glycoside isoflavones up-regulate antioxidant metallothionein expression and scavenge free radicals. J Agric Food Chem. 2006;54:3819–3826. doi: 10.1021/jf060510y. [DOI] [PubMed] [Google Scholar]
- 52.Bowling AC, Beal MF. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56:1151–1171. doi: 10.1016/0024-3205(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 53.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25–35 in PC12 cells. Int J Dev Neurosci. 2010;28:289–295. doi: 10.1016/j.ijdevneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Henry-Vitrac C, Berbille H, Merillon JM, Vitrac X. Soy isoflavones as potential inhibitors of Alzheimer beta-amyloid fibril aggregation in vitro. Food Research International. 2010;43:2176–2178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.