Abstract
A number of events must occur to preserve the integrity of the chromatin template during gene transcription. A study in this issue reveals a novel mechanism whereby chromatin remodelers are recruited to histone modifications within gene bodies to prevent aberrant histone exchange during transcriptional elongation.
The process of gene transcription involves a series of highly orchestrated events, each of which provides a potential node of regulation to assure that transcription occurs only when and where it should. For many years, regulation of gene expression was thought to occur primarily at the level of transcript initiation. However, transcript elongation has emerged more recently as another important point of gene regulation, and two recent papers by the Workman group published in Nature (Venkatesh et al.) and in this issue of Nature Structural and Molecular Biology (Smolle et al.) provide important new insights into this process.
Nucleosomes generally act as barriers to transcription, largely by hampering recruitment or movement of RNA polymerase II (RNAPII) and the associated transcription machinery. At least two mechanisms help to overcome such challenges during the process of transcript elongation. First, nucleosomes can be evicted or repositioned by ATP-dependent chromatin remodelers to allow passage of RNAPII. Alternatively, or in conjunction with active remodeling, post-translational modifications of histone proteins can directly alter chromatin compaction and can regulate recruitment of additional factors that facilitate transcription. Importantly, chromatin must also be ‘reset’ in the wake of the polymerase in order to prevent production of cryptic transcripts from within the gene body that might interfere with expression of the full-length gene product. Nucleosome stability and density must be preserved to prevent inappropriate engagement of the polymerase at sites other than the gene promoter and to preserve the overall integrity of the transcription template for the next round of initiation.
The steps that mediate chromatin disruption, transcription elongation, and chromatin resetting are turning out to be every bit as orchestrated as events in transcription initiation, requiring cooperation of several different histone and chromatin modifying activities. Workman and colleagues made beautiful use of the S. cerevisiae model system to define mechanisms that guide these steps, particularly the enzymes and proteins involved in modifying chromatin structure before, during, and after RNAPII passage. One of these enzymes, Set2, is a histone H3K36 methyltransferase. Interestingly, this histone modification is enriched in the gene bodies of actively transcribed genes, but Set2 and K36 methylation are repressive to transcription1. Studies carried out by Workman's group and others previously identified an interaction between Set2 and the phosphorylated C-terminal domain of elongating RNAPII, which elucidated how Set2 deposits H3K36 methylation co-transcriptionally throughout gene coding regions2-5 (Fig. 1a). The puzzling link between Set2-mediated methylation and transcriptional repression was subsequently clarified by elegant biochemical experiments demonstrating that the chromodomain of Eaf3, a component of the Rpd3C(S) histone deacetylase (HDAC) complex, binds to methylated H3K366-8. Histone deacetylation has long been associated with inhibition of transcription by promoting chromatin compaction. The Workman group, and others, went on to show that Set2-dependent interaction of Eaf3 and H3K36 inhibits aberrant transcription initiation from cryptic, intragenic promoters6-10 (Fig. 1b and c). Interestingly, Eaf3 is also a component of the NuA4 histone acetyltransferase complex, which is recruited to promoters by H3K4 methyltransferases11, suggesting that a balance of HAT/HDAC activity at transcribed genes is likely achieved through regulation of histone H3K4 and H3K36 methylation6,7.
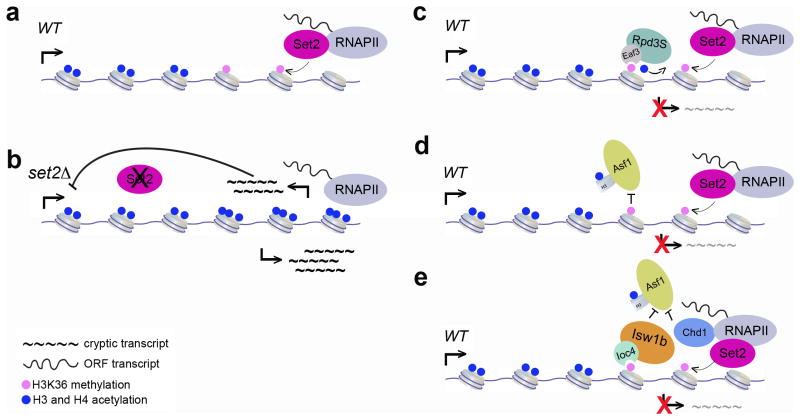
Figure 1. Set2 and histone H3K36 methylation safeguard transcription elongation.
(a) Set2 interacts with RNAPII to methylate H3K36 across gene bodies.
(b) In the absence of Set2, increased histone acetylation is detected across ORFs along with an accumulation of transcripts that arise from intragenic cryptic promoters.
(c) The chromodomain of Eaf3 binds to methylated H3K36 and recruits the Rpd3C(S) HDAC complex to deacetylate histones in the 3′ ends of ORFs, which effectively suppresses the expression of transcripts from cryptic promoters.
(d) Set2-mediated methylation of H3K36 blocks association of the Asf1 chaperone to prevent the exchange of pre-acetylated histones over the 3′ ends of ORFs, thereby suppressing cryptic transcription.
(e) The PWWP domain within the Ioc4 subunit of Isw1b chromatin remodeling complex binds to methylated H3K36, and recruits the complex to the coding regions of genes undergoing transcription. Isw1b, along with Chd1, prevent the exchange of pre-acetylated histones over ORFs thereby suppressing the expression of transcripts from cryptic promoters.
The above studies raise at least two interesting questions: How important is histone deacetylation by Rpd3 in preventing initiation of cryptic transcripts, and what histone acetyltransferases are responsible for laying down acetylation marks within the bodies of transcribed genes? In the course of addressing these questions, the Workman group has now discovered yet another level of Set2-mediated transcriptional regulation that prevents inappropriate histone exchange over transcribed genes. Histones are deposited onto DNA mostly at the time of replication. However, replication independent histone exchange can occur at actively transcribed genes, and this process has been suggested to provide a mechanism by which collections of pre-existing histone modifications might be simultaneously erased. Using an established in vivo system to monitor histone exchange12, Venkatesh et al. have now demonstrated that incorporation of new histones occurs more readily over open reading frames (ORFs) in the absence of Set2. Furthermore, acetylation events specific to newly incorporated histones increase towards the 3′ ends of ORFs, suggesting that histone exchange provides a mechanism for introducing acetylation into actively transcribed gene bodies.
Several factors regulate the eviction and replacement of nucleosomal histones during transcription elongation, including histone chaperones, differential acetylation of newly incorporated and pre-existing histones, and the rate of transcription (reviewed in 13-15). The findings by Venkatesh et al. link these modes of regulation as they demonstrate that H3K36 methylation, mediated by Set2, inhibits the association of the Asf1 histone chaperone with H3, thereby blocking histone exchange and reducing incorporation of histone H3 acetylated at K56 over ORFs (Fig. 1d). Set2 and H3K36 methylation, then, safeguard the transcription process both by limiting inappropriate incorporation of acetylated histones and by recruiting the Rpd3 HDAC to remove any acetylation that does occur. Increased ASF1 expression and H3K56 acetylation levels are observed in several different tumor types suggesting that the propagation of this mark is tightly controlled to ensure appropriate cell division in higher eukaryotes16. The findings presented by Venkatesh and colleagues reaffirm the notion that failure to maintain appropriate levels of histone H3K56 acetylation results in disruption of cellular processes, particularly transcriptional elongation.
Workman and colleagues went on to further define the mechanism by which Set2 and histone H3K36 methylation inhibit the incorporation of new histones in the wake of RNAPII passage. Smolle et al. report on page XXX in this issue that two chromatin remodelers, Isw1 and Chd1, function in the same pathway as Set2 to suppress the production of intragenic cryptic transcripts (Fig. 1e). Methylated H3K36 serves as a docking site for a PWWP domain within the Ioc4 subunit of the Isw1b complex and recruits the complex to coding regions. Even though Chd1 does not preferentially bind nucleosomes methylated at H3K3610, this remodeler is recruited to gene bodies through interactions with RNAPII and other components of the elongation machinery17,18. Subsequently, Isw1b and Chd1 function together to inhibit trans-histone exchange and prevent the incorporation of acetylated histones over ORFs, thereby effectively suppressing cryptic transcription from intragenic sites. Furthermore, Smolle et al. propose that the localization of Isw1b and Chd1 to actively transcribed coding regions likely promotes the reassembly and proper spacing of nucleosomes following the passage of RNAPII.
These results put forward by Workman and colleagues address one of the longest standing questions in the transcription field: how is RNAPII limited in action to bona fide promoters? RNAPII itself has very little, if any, sequence specificity. Findings here indicate that in addition to general transcription factors that help direct polymerase to genuine promoters, other factors harness the repressive power of chromatin to ‘hide’ cryptic promoters.
It has become clear that several distinct, yet potentially overlapping mechanisms are in place to direct chromatin ‘resetting’ during transcript elongation, ensuring transcription of a full-length gene product. Remarkably, the methylation status of a single histone residue, H3K36, is central to these mechanisms, as at least two distinct effector molecules are recruited to this mark to initiate individual programs that result in the same biological outcome – maintenance of low levels of histone acetylation over ORFs (Fig. 1c-e). The following questions remain to be answered: When are each of these mechanisms used? Do they occur independently or cooperatively? The major machinery and histone modifications driving these mechanisms are conserved from yeast to humans. It will be interesting to learn whether the interplay of the components defined by the Workman group in yeast is conserved in higher eukaryotes. The emergence of non-coding RNA molecules as significant regulators of the mammalian transcriptome (reviewed in19) highlight the importance of understanding how transcripts originating from within protein coding genes are regulated. Moreover, defining mechanisms that restore the integrity of the chromatin template through multiple rounds of transcription will be particularly important to delineating complex human conditions associated with genomic instability such as cancer and aging.
References
- 1.Strahl BD, et al. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol Cell Biol. 2002;22:1298–306. doi: 10.1128/mcb.22.5.1298-1306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krogan NJ, et al. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol. 2003;23:4207–18. doi: 10.1128/MCB.23.12.4207-4218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Howe L, Anderson S, Yates JR, 3rd, Workman JL. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem. 2003;278:8897–903. doi: 10.1074/jbc.M212134200. [DOI] [PubMed] [Google Scholar]
- 4.Schaft D, et al. The histone 3 lysine 36 methyltransferase, SET2, is involved in transcriptional elongation. Nucleic Acids Res. 2003;31:2475–82. doi: 10.1093/nar/gkg372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao T, et al. Phosphorylation of RNA polymerase II CTD regulates H3 methylation in yeast. Genes Dev. 2003;17:654–63. doi: 10.1101/gad.1055503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrozza MJ, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–92. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Keogh MC, et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell. 2005;123:593–605. doi: 10.1016/j.cell.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Li B, et al. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science. 2007;316:1050–4. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]
- 9.Li B, et al. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev. 2007;21:1422–30. doi: 10.1101/gad.1539307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, et al. Histone H3 lysine 36 dimethylation (H3K36me2) is sufficient to recruit the Rpd3s histone deacetylase complex and to repress spurious transcription. J Biol Chem. 2009;284:7970–6. doi: 10.1074/jbc.M808220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morillon A, Karabetsou N, Nair A, Mellor J. Dynamic lysine methylation on histone H3 defines the regulatory phase of gene transcription. Mol Cell. 2005;18:723–34. doi: 10.1016/j.molcel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Dion MF, et al. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–8. doi: 10.1126/science.1134053. [DOI] [PubMed] [Google Scholar]
- 13.Williams SK, Tyler JK. Transcriptional regulation by chromatin disassembly and reassembly. Curr Opin Genet Dev. 2007;17:88–93. doi: 10.1016/j.gde.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Das C, Tyler JK. Histone exchange and histone modifications during transcription and aging. Biochim Biophys Acta. 2012;1819:332–42. doi: 10.1016/j.bbagrm.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petesch SJ, Lis JT. Overcoming the nucleosome barrier during transcript elongation. Trends Genet. 2012;28:285–94. doi: 10.1016/j.tig.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–7. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krogan NJ, et al. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol. 2002;22:6979–92. doi: 10.1128/MCB.22.20.6979-6992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simic R, et al. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 2003;22:1846–56. doi: 10.1093/emboj/cdg179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berretta J, Morillon A. Pervasive transcription constitutes a new level of eukaryotic genome regulation. EMBO Rep. 2009;10:973–82. doi: 10.1038/embor.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]