Abstract
Purpose
To develop and evaluate a novel processing framework for the relative quantification of myelin content in cerebral white matter (WM) regions from brain MRI data via a computed ratio of T1 to T2 weighted intensity values.
Data
We employed high resolution (1mm3 isotropic) T1 and T2 weighted MRI from 46 (28 male, 18 female) neonate subjects (typically developing controls) scanned on a Siemens Tim Trio 3T at UC Irvine.
Methods
We developed a novel, yet relatively straightforward image processing framework for WM myelin content estimation based on earlier work by Glasser et al. We first co-register the structural MRI data to correct for motion. Then, background areas are masked out via a joint T1w and T2 foreground mask computed. Raw T1w/T2w-ratios images are computed next. For purpose of calibration across subjects, we first coarsely segment the fat-rich facial regions via an atlas co-registration. Linear intensity rescaling based on median T1w/T2w-ratio values in those facial regions yields calibrated T1w/T2w-ratio images. Mean values in lobar regions are evaluated using standard statistical analysis to investigate their interaction with age at scan.
Results
Several lobes have strongly positive significant interactions of age at scan with the computed T1w/T2w-ratio. Most regions do not show sex effects. A few regions show no measurable effects of change in myelin content change within the first few weeks of postnatal development, such as cingulate and CC areas, which we attribute to sample size and measurement variability.
Conclusions
We developed and evaluated a novel way to estimate white matter myelin content for use in studies of brain white matter development.
Topic areas: Image Processing, White matter, Myelin Content, Magnetic Resonance Imaging
Introduction
The human brain develops from a mostly non-myelinated state for the cerebral white matter to a nearly fully mature white matter myelination within the first few years of life. Quantification of the myelination process in the white matter brain is the goal of a number of brain development studies, especially also of interest with application to neurodevelopmental disabilities [1].
Diffusion tensor imaging (DTI) has emerged as one of the major ways to analyze this myelination process (e.g. [1]), mainly through the use of the DTI property maps Fractional Anisotropy (FA) and Radial Diffusivity (RD). Both of the measures are sensitive to myelination, but they are also sensitive to other brain maturation or pathologic processes, such as fiber organization/wiring, axonal size, fiber packing/density, changes in brain water content, synaptogenesis, axonal pruning and neuronal death. Thus, care should be taken when ascribing observed changes in diffusion measure to a specific microstructural attribute such as myelination. More refined measures such as axonal dispersion and density can be estimated from higher order diffusion information [8], though these additional measures do not completely untangle this issue. More specific measures of myelin maturation may be obtained via multi-component analysis of T1 and T2 relaxation, also called multi- component relaxometry [5]. By quantifying the myelin-bound water signal within myelin folds, the so-called myelin water fraction (MWF), a myelin biomarker can be obtained that correlates with histological assessments [6]. A limitation of traditional MWF imaging is the acquisition time of the MR protocol, which is prohibitive for applications in pediatric studies that necessitate fast scan acquisitions. Deoni et al [4] proposed a rapid MWF acquisition protocol and corresponding signal fitting procedure dubbed McDESPOT. MWF maps from McDESPOT are founded on strong local tissue assumptions to make up for the reduced signal information when compared to traditional MWF approaches and have been criticized for their potential lack of non-ambigious estimation capability [7]. We aimed to find a simpler, alternative way to quantify the myelination process that would help complement findings as measured via standard DTI and higher order diffusion property maps without the need for higher scan time. In this work, we present one such complementary method to do exactly that.
Our work is founded on the research recently proposed by Glasser et al [2,12]. Here, the use of high resolution T1 weighted (T1w) and T2 weighted (T2w) MR images, which are commonly acquired in many neuroimaging studies, is proposed to provide an estimate of myelin content via the ratio of these two images. While other sources also contribute to the signal in the T1w/T2w ratio values, the major contribution within the cerebrum originates from the local myelin content. They nicely show how these ratio images can be employed to show variations in myelin between cortical gray matter areas. Others have shown how that pattern of cortical myelin variation leads to improved cortical correspondence when incorporated into cortical surface analysis frameworks [13]. While the pattern of (relative) myelin variation is certainly also of interest in white matter, we want to go one step beyond here and propose a quantitative estimate of myelin content that can be compared across subjects at different ages.
Thus in this work, we propose an intensity calibration applicable to the T1w/T2w-ratio maps proposed by Glasser in order to be used in white matter studies in early postnatal development. We have applied this proposed method to brain MRI scans acquired in newborns and evaluated the statistical correlation between the calibrated T1w/T2w-ratio maps and the subject’s sex and postconceptual age at scan.
Data
Neonate brain MRI data
We applied the T1w/T2w-ratio map computation as estimates of myelin content to neonate datasets acquired on a Siemens Tim Trio 3T scanner at the University of Irvine (UCI). Children were scanned unsedated while asleep, fitted with ear protection and with their heads secured in a vacuum-fixation device. High-resolution T1-weighted images were acquired with a 3D magnetization prepared rapid gradient echo (MP-RAGE TR = 1820 ms, inversion time = 1100 ms, echo time = 4.38 ms, flip angle = 7°, resolution = 1 × 1 × 1 mm3, 6.18 mins). A high-resolution 3D T2-weighted sequence was also acquired at the same FOV and resolution as the T1w image (TR = 32000 ms, echo time = 255 ms, 4.18 mins). A total of 46 subjects (28 male, 18 female) were selected for the evaluation here, ranging in postconceptual age at scan from 40 to 48 weeks.
Reliability
For the purpose of testing reliability and stability of our calibrated T1/T2 ratio measures, we applied our method to a small set of 6 subjects accumulated from different studies of neonates scanned at Biomedical Image Research Center at the University of North Carolina (UNC), as well as at UCI. Scans originated from whole-body 3T Siemens Tim Trio scanners at UNC (4 cases) and UCI (2 cases). For these subjects a second set of T1weighted and T2weighted scans were acquired in the same scanning session, as the first set was considered of borderline quality by the scanning technicians. In all these cases, subsequent quality control procedures by trained image analysis experts showed all scans to pass quality assessment for structural morphometric analysis. This small size neonate scan-rescan database captures the low signal-to-noise and presence of motion (see Figure 2) very common in early postnatal scans and thus is well suited to estimate reliability of image processing procedures. In this study, we assessed reliability via coefficient of variation (COV = standard deviation/mean value) computation. It is noteworthy, that as at least 50% of these images were assessed to be of borderline quality by the scanner personnel, this scan-rescan database is likely to overestimate the expected variability in the average setting.
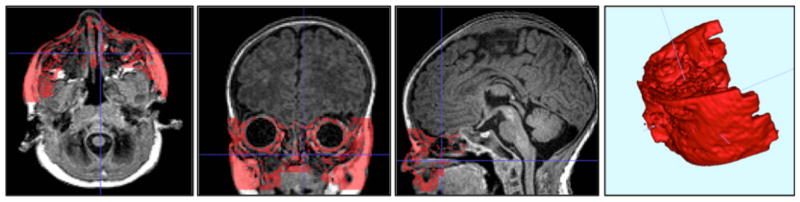
Figure 2.
Coarse segmentation of fat-rich face regions, which are dominated by the fat in the cheek regions. Segmentation as red overlay on T1-weighted image in an example neonate scan: axial (left), coronal (middle) and sagittal slicer (right) as well as the 3D reconstruction of the segmentation. As is common in neonate scan, mild motion artifacts are present in the scan.
Methods
We propose here the following processing framework for the quantitative analysis of T1w/T2w ratio measures within cerebral white matter.
Rigid registration of the T1w image into standard MNI space
Rigid registration of the T2w image to its aligned T1w image (1)
Computation of the raw T1w/T2w ratio maps from (1) and (2)
Intensity calibration of T1w/T2w maps via atlas based region statistics using deformable atlas registration.
Extraction of regional statistics from structural segmentations performed separately on the T1w and T2w data
Additionally, corresponding diffusion maps can be registered into the same MNI space for joint regional analysis, or for fiber tract based analysis via the NA-MIC atlas based fiber tract analysis framework [9]. Such an analysis is not performed in the studies presented here.
Details of the intensity calibration (step 4) and regional statistics extraction (step 5) are presented in the next subsections.
Intensity calibration
We propose here to apply a relatively straightforward intensity calibration that allows the use of T1w/T2w maps for inter-subject quantitative analysis. This calibration is only applied to the T1w/T2w ratio maps, rather than the individual T1w and T2w maps, as the ratio maps do not suffer from major intensity inhomogeneity artifacts/bias. T1w and T2w images exhibit the same receive field bias, which is effectively removed when computing the ratio. On the other hand, the T1w/T2w-ratio map is not completely corrected for transmit field biases in intensity and contrast, since they are only correlated but not identical. In the presence of large motion, or if the T1w and T2w images were acquired in different sessions, the two images do not experience the same receive field bias and the proposed processing would likely create incorrect measurements in such cases.
While the computation of the raw T1w/T2w ratio is straightforward, the calibration proposed here necessitates a population atlas for the purpose of segmenting image regions whose intensity statistics can be employed in a calibration process that does not bias the calibrated intensity values within the brain for subsequent analysis. Here, we opted for a coarse segmentation of fat-rich regions in the face region, focusing on cheek and nose regions (see Figure 2 for an example segmentation). This processing assumes that the median voxel-wise fat content within the face region is consistent across subjects. This assumption could be inappropriate in pathologies that affect the general fat content of soft tissue and muscle regions, such as muscular dystrophies, and caution should be taken using the proposed calibration procedure.
In detail, the following processing was applied for the calibration procedure:
Foreground mask computation by combination of foreground masks computed on each T1w and T2w separately via standard Otsu threshold computation. Statistics are only computed on this foreground masks.
Combination of a prior, simple mask of facial regions in MNI space with the foreground mask resulting in the coarse face region segmentation mask (see Fig 2).
Computation of the minimum Rmin and median Rmed raw T1w/T2w ratio values within the coarse face region.
Calibration via linear rescaling of the raw T1w/T2w ratio image such that the Rmin and Rmed values of the rescaled ratio images are 0 and 1000, respectively.
Regional statistics
For the regional analysis of myelin content estimate, we performe first a multi-atlas based expectation maximization based tissue segmentation [11] specialized for the neonate setting [10]. We fuse the unmyelinated and early-myelinated tissue classes computed in the prior step. This yields a full cerebral white matter segmentation, which is then subdivided into lobar regions (left and right hemispheric pre-frontal, frontal, parietal, occipital, temporal, callosal, cerebellar and subcortical structure regions) via an atlas based parcellation[3]. Finally, we compute lobar region histograms of calibrated T1w/T2w ratio measures and compute mean ratio values for each region.
Statistical analysis in the neonate study was performed via R, using a standard linear regression analysis with a linear model using sex and gestational age at scan as covariates (no cross-interactions) for all white matter regions except for the subcortical structure region (which is largely composed of gray matter). Reliability assessment was performed by computing average relative differences in T1w/T2w ratio measures between the scan/rescan data.
Results
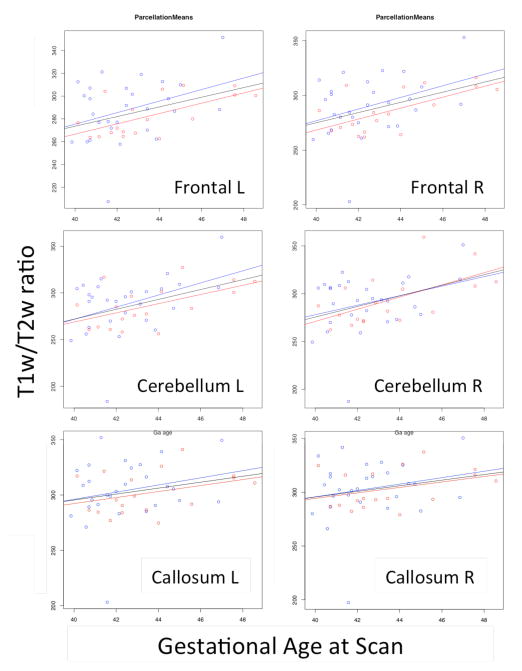
Figure 1 shows an example of the calibrated T1w/T2w maps computed with the proposed approach. Figure 3 shows plots of the lobar mean T1w/T2w-ratio values versus the postconceptual age at scan (from 40 to 48 weeks in our sample) within selected lobar regions. A positive correlation between the two measures is visible in the plots. The corresponding statistical results are displayed in Table 1. Most lobes show highly significant interactions between postconceptual age at scan and the proposed myelin content estimate, as would be expected, with R coefficients above 0.4. Only the cingulate and callosal regions do not show significant interactions with postconceptual age. It is noteworthy that these two regions are expected to show very low myelination at the studied age [4]. In addition, prefrontal and temporal lobes showed significant correlation between postconceptual age and sex.
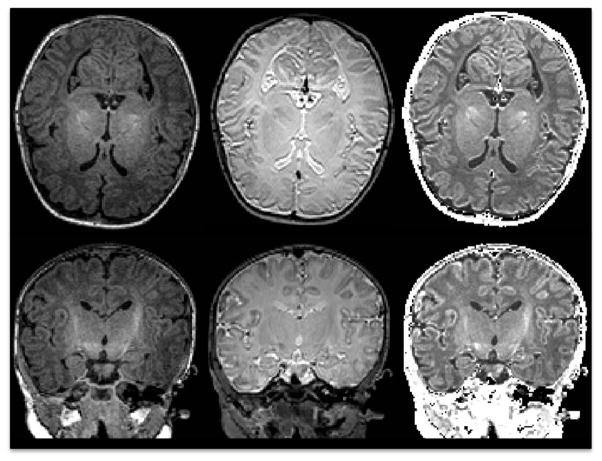
Figure 1.
Raw T1w(left), T2w (middle) images, as well as the corresponding calibrated T1w/T2w ratio image (right).
Figure 3.
Plots of gestational age at scan versus the regional mean T1w-T2w ratio with corresponding linear fits over male (blue), female data (red) and both (black).
Table 1.
Results of interaction tests (significant interactions shown in bold): most lobes show significant association with postconceptual age at scan, whereas only the prefrontal and temporal lobes show also interactions with sex. The corpus callosum and cingulate regions did not show significant
| Callosum L | Callosum R | Occipital L | Occipital R | Prefrontal L | Prefrontal R | |
|---|---|---|---|---|---|---|
|
|
||||||
| P Sex | 0.486 | 0.721 | 0.192 | 0.425 | 0.049 | 0.064 |
| P Age at Scan | 0.093 | 0.113 | 0.001 | 0.001 | 0.004 | 0.002 |
| R coef Age | 0.257 | 0.240 | 0.474 | 0.466 | 0.452 | 0.470 |
| Similar stats: | Prefrontal Cingulate | Parietal | Cerebellum | Temporal | ||
| Frontal Cingulate | Insula | Frontal | ||||
| Parietal Cingulate | ||||||
In order to perform an evaluation of the stability of the main parameters of the proposed calibration, we repeated the analysis with slightly modified rescaling parameters. In particular, we varied the calibration quantile target of the raw T1w/T2w ratio histogram in the face region from the median to the 40th and 60th percentile value. The resulting calibrated T1w/T2w-ratio values highly correlated (p < 0.0001) in both cases with those for the proposed (median) parameter, as well as the lobar statistics showed the a similar pattern of statistical results (though p-values obviously were slightly different).
We performed the proposed scan-rescan reliability evaluation and found that for whole white matter a COV of 3.15% is present. The COV for the individual lobar regions varied between 2.6% (left cerebellum) and 4.2% (right prefrontal cingulate), with an average of 3.4% over all regions. Whole WM and lobar measures can thus be considered reliable, especially as we expect the computed COV to be a conservative estimate. These COV values are significantly smaller than the fitted linear regression in myelin content in the studied population (e.g. left cerebellum shows a regressed change of 10.5% from postconceptual age 40 to 48 weeks, at a COV of 2.5%).
Discussion and Conclusions
In this paper we presented a novel intensity calibration method to be used for T1w/T2w-ratio maps. The resulting calibrated maps were applied in a study of cerebral white matter development. The results show that several brain regions display significant positive correlation between postconceptual age and the T1w/T2w-ratio even in a relatively short age window of 8 weeks within the early postnatal phase.
The proposed coarse facial region segmentation is simple and needs to improved, which is an area of future research. Use of posterior scalp or neck fat would potentially further stabilize the computation. Furthermove, it has been reported [14] that buccal regions below the subcutaneous fat are changing in adipocyte size as it goes from brown adipose tissue like to white adipose tissue like. This indicates the need for segmentation that separates subcutaneous fat regions from buccal fat regions in order for the calibration to be free of effects of that fat change in the buccal region.
We expect that this T1w/T2w-ratio measure is likely only consistent and useful for developmental neuroimaging studies when exactly same scan protocol is employed on the same scanner type. Different scan protocol settings (resolution, echo times, flip angle, sequence type) will surely affect the T1w/T2w-ratio, and thus we advise against using this proposed measures with images acquired with different imaging protocols. Whether it can be used across different scanner vendors is unclear, and would need to be evaluated. If the same scanner type and protocol is employed within a multi-site study, we would expect that data from different sites (using the same scanner, e.g. a Siemens Tim Trio 3T scanner as used in the presented data) can be compared directly with the proposed technique.
It remains to be seen whether this technique is applicable to MRI data from subjects at older ages (older than 2–4 years of age), as the myelin content is considered to be relatively mature and stable. The inherent variability of our calibrated T1w/T2w-ratio measure is possibly too high to be of use in such a setting.
Acknowledgments
This work was supported by the National Institutes of Health (MH064065, MH070890, MH091351, MH091645, HD079124, HD03110).
References
- 1.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J, Network IBIS. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012 Jun;169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glasser MF, Goyal MS, Preuss TM, Raichle ME, Van Essen DC. Trends and properties of human cerebral cortex: Correlations with cortical myelin content. Neuro Image. 2013 Apr; doi: 10.1016/j.neuroimage.2013.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knickmeyer RC, Kang C, Woolson S, Smith JK, Hamer RM, Lin W, Gerig G, Styner M, Gilmore JH. Twin-singleton differences in neonatal brain structure. Twin Res Hum Genet. 2011 Jun;14(3):268–276. doi: 10.1375/twin.14.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deoni SCL, Dean DC, O’muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuro Image. 2012 Aug;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;7:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- 6.Laule C, Leung E, Lis DK, Traboulsee AL, Paty DW, MacKay AL, Moore GR. Myelin water imaging in multiple sclerosis: quantitative correlations with histopatholgy. Mult Scler. 2006;2:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- 7.Lankford CL, Does MD. On the inherent precision of mcDESPOT. Magn Reson Med. 2013 Jan;69(1):127–136. doi: 10.1002/mrm.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuro Image. 2012 Jul;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 9.Verde AR, Budin F, Berger J-B, Gupta A, Farzinfar M, Kaiser A, Ahn M, Johnson H, Matsui J, Hazlett HC, Sharma A, Goodlett CB, Shi Y, Gouttard S, Vachet C, Piven J, HUHZ, Gerig G, Styner M. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Front Neuroinform. 2014;7:51. doi: 10.3389/fninf.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prastawa M, Gilmore JH, Lin W, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 2005 Oct;9(5):457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Cherel M, Budin F, Prastawa M, Gerig G, Lee K, Lyall A, Consing KNZ, Styner M. Automatic Tissue Segmentation of Neonate Brain MR Images with Subject-specific Atlases. SPIE Medical Imaging. 2015 doi: 10.1117/12.2082209. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1-and T2-weighted MRI. The Journal of Neuroscience. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson EC, Jbabdi S, Andersson J, Smith S, Glasser MF, Van Essen DC, et al. Multimodal surface matching: fast and generalisable cortical registration using discrete optimisation. Information Processing in Medical Imaging. 2013;23:475–486. doi: 10.1007/978-3-642-38868-2_40. [DOI] [PubMed] [Google Scholar]
- 14.Ponrartana S, Patil S, Aggabao PC, Pavlova Z, Devaskar SU, Gilsanz V. Brown adipose tissue in the buccal fat pad during infancy. PLoS ONE. 2014;9(2):e89533. doi: 10.1371/journal.pone.0089533. [DOI] [PMC free article] [PubMed] [Google Scholar]