Abstract
Previous studies show that circulating endothelial progenitor cells (EPCs) promote angiogenesis, which is a process associated with improved recovery in animal models of traumatic brain injury (TBI), and that recombinant human erythropoietin (rhEPO) plays a protective role following stroke. Thus, it was hypothesized that rhEPO would enhance recovery following brain injury in a rat model of TBI via an increase in the mobilization of EPCs and, subsequently, in angiogenesis. Flow cytometry assays using CD34− and CD133-specific antibodies were utilized to identify alterations in EPC levels, CD31 and CD34 antibody-stained brain tissue sections were used to quantify angiogenesis, and the Morris water maze (MWM) test and the modified Neurological Severity Score (mNSS) test were used to evaluate behavioral recovery. Compared with saline treatment, treatment with rhEPO significantly increased the number of circulating EPCs on days 1, 4, 7, and 14 (P<0.05), improved spatial learning ability on days 24 and 25 (P<0.05), and enhanced memory recovery on day 26 (P<0.05). Moreover, rhEPO treatment decreased mNSS assessment scores on days 14, 21, and 25 (P<0.05). There was a strong correlation between levels of circulating EPCs and CD34− and CD31-positive cells within the injured boundary zone (CD34+ r=0.910, P<0.01; CD31+ r=0.894, P<0.01) and the ipsilateral hippocampus (CD34+ r=0.841, P<0.01; CD31+ r=0.835, P<0.01). The present data demonstrate that rhEPO treatment improved functional outcomes in rats following TBI via an increase in the mobilization of EPCs and in subsequent angiogenesis.
Keywords: Traumatic brain injury, Erythropoietin, Endothelial progenitor cells, Angiogenesis
Introduction
Traumatic brain injury (TBI) is currently one of the leading causes of mortality and morbidity worldwide, particularly from childhood to the age of 44 years [1, 2]. According to the World Health Organization (WHO), TBI will be a major health issue and the leading cause of disability by the year 2020 [3]. Following TBI, the main causes of mortality are neuronal death and the breakdown of blood vessels, but neurogenesis and angiogenesis play key roles in the mediation of functional recovery [4–6]. In fact, angiogenesis in the brain may provide a critical neurovascular niche that allows for neuronal remodeling.
Circulating endothelial progenitor cells (EPCs) participate in angiogenesis and vasculogenesis [7, 8] and have been shown to decrease infarct volume, increase capillary density, and improve blood perfusion in myocardial and hind-limb ischemia animal models [9, 10]. Erythropoietin (EPO) promotes erythrocyte proliferation and differentiation and has been used clinically to treat anemia. EPO is protective against spinal cord trauma [11], retina ischemia [12], skeletal muscle ischemia [10], pulmonary hypertension [13], myocardium ischemia-reperfusion injury [14, 15], and brain trauma [16] via enhanced anti-apoptotic [1, 11], anti-inflammatory [12], and neuroprotective effects [17, 18]. EPO also enhances the mobilization of EPCs to promote angiogenesis, decrease neuronal cell death, and improve functional outcomes following stroke [17, 19, 20].
However, the underlying mechanisms that support the neuroprotective effects of EPO following TBI remain unclear. Thus, the present study used a rat model of TBI to investigate the role of recombinant human erythropoietin (rhEPO) in enhanced neurological recovery, which is thought to involve increased mobilization of EPCs and subsequent promotion of angiogenesis.
Methods
TBI Model
This study included adult male Wistar rats (300–350 g) supplied by the Military Medical Academy of China [Beijing, China]. All rats were housed in temperature- (22 °C) and humidity-controlled (60 %) rooms with a 12-h light/dark cycle and sufficient food and water. All experimental procedures were performed in accordance with the guidelines of the Chinese Small Animal Protection Association.
To study the effects of brain injury, the present study employed the conventional fluid percussion injury (FPI) model [21, 22]. Briefly, the rats were anesthetized intraperitoneally with chloride hydrate (3 ml/kg), while maintaining a normal body temperature using a heating pad. The head of each rat was then fixed in a stereotactic frame, and a 10-mm incision was made along the midline to expose the cranium. Then, while keeping the dura mater intact, a right craniotomy (diameter 4 mm) was performed 3.0 mm posterior to the coronal suture and 2.0 mm lateral to the sagittal suture. A percussion pressure of 1.8–2.0 atm (atm) was applied to produce TBI, as described previously [23]. The incision was then closed with sutures, and the rats were allowed to recover from the anesthesia. A sham group of rats was subjected to the craniotomy procedure only.
Administration of rhEPO
A total of 66 rats were randomly divided into three groups (n=22). Group 1 (TBI+rhEPO) consisted of rats that had undergone the TBI procedure and were injected intraperitoneally with rhEPO (5,000 u/kg, 3,000 u in 0.5 ml; ESPO; Tokyo, Japan) immediately following the TBI procedure and then daily for 7 consecutive days. The rhEPO dose selected was that most effective according to evidence from previous studies [24]. Group 2 (TBI+saline) consisted of rats that had undergone the TBI procedure and were injected intraperitoneally with saline (0.2 ml) immediately following the TBI procedure and then daily for 7 consecutive days (Fig. 1). Group 3 (sham) consisted of rats that had undergone a craniotomy but did not receive any injections.
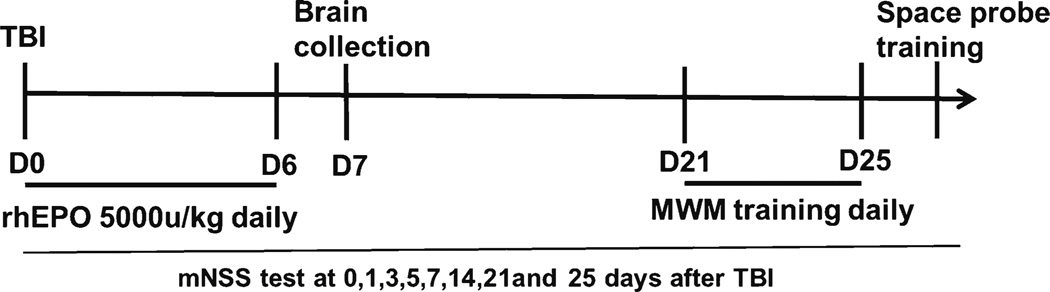
Fig. 1.
Experimental design. The TBI procedure was performed on day 0, and either rhEPO or saline was injected intraperitoneally immediately following the procedure and then daily for 7 days. Brain tissue samples were collected from six rats from each group on days 0 and 7 and used to analyze CD34− and CD31-positive cells. MWM training was conducted from days 21 to 25 after the TBI procedure, and a space probe experiment was conducted on day 26. mNSS test assessments were conducted on days 0, 1, 3, 5, 7, 14, 21, and 25. Blood samples were collected prior to, 3 h, 6 h, and 1, 4, 7, 14, and 25 days following the TBI procedure for the quantification of EPCs and the HCT test. TBI traumatic brain injury; rhEPO recombinant human erythropoietin; MWM Morris water maze; mNSS Modified Neurological Severity Score
Monocyte Isolation and the Measurement of EPCs
To measure the number of circulating EPCs, 0.5 ml blood was collected from the retro-orbital venous plexus of each rat using a glass capillary and stored in ethylenediaminetetraacetic acid (EDTA)-treated tubes. These samples were then diluted with 0.5 ml phosphate-buffered saline (PBS; pH 7. 4). Monocytes were isolated by density gradient centrifugation using the Ficoll-Paque PLUS method (Amersham Pharmacia Biotech AB; Uppsala, Sweden), washed twice, and then incubated with a PE-conjugated CD34 antibody (Santa Cruz Biotechnology; Santa Cruz, CA, USA) and a FITC-conjugated CD133 antibody (goat polyclonal antibody; Santa Cruz Biotechnology) in PBS, bovine serum albumin (BSA), and 2 mM EDTA for 10 min at room temperature. The isolated monocytes were then analyzed using flow cytometry (Beckman Dickinson FACC Calibur system, BD Bioscience; San Jose, CA, USA).
Measurement of Hematocrit
To determine the effects of rhEPO on the hematocrit (HCT) test, a 200-µl blood sample was collected retro-orbitally from five rats from each group prior to and on days 4, 7, 14, and 25 following the TBI or sham procedures, using EDTA-treated tubes. The blood samples were analyzed using a blood analysis machine (xs-800i, Sysmex; Kobe City, Japan).
Neurobehavioral Tests
Modified Neurological Severity Score Test
The modified Neurological Severity Score (mNSS) test [21, 25] includes motor, sensory, reflex, and balance assessments (Table 1) with the highest possible score being 18. A score of 13–18 indicates severe injury, 7–12 indicates moderate injury, and 1–6 indicates mild injury. The mNSS test was performed in the saline- and rhEPO-treated rats prior to and on days 1, 4, 7, 14, and 25 after the TBI procedure.
Table 1.
Modified Neurological Severity Score points
| Motor tests | |
|---|---|
| Raising rat by tail | 3 |
| Flexion of forelimb | 1 |
| Flexion of hindlimb | 1 |
| Head moved >10° to vertical axis within 30 s | 1 |
| Placing rat on floor (normal=0, maximum=3) | 3 |
| Normal walk | 0 |
| Inability to walk straight | 1 |
| Circling toward paretic side | 2 |
| Falls down to paretic side | 3 |
| Sensory tests | 2 |
| Placing test (visual and tactile test) | 1 |
| Proprioceptive test (deep sensation, pushing paw against table edge to stimulate limb muscles) | 1 |
| Beam balance tests (normal=0; maximum=6) | 6 |
| Balances with steady posture | 0 |
| Grasps side of beam | 1 |
| Hugs beam and one limb fall down from beam | 2 |
| Hugs beam and two limbs fall down from beam, or spins on beam (>60 s) | 3 |
| Attempts to balance on beam but falls off (>40 s) | 4 |
| Attempts to balance on beam but falls off (>20 s) | 5 |
| Falls off; no attempt to balance or hang on to beam (<20 s) | 6 |
| Reflex absence and abnormal movements | 4 |
| Pinna reflex (head shake when auditory meatus is touched) | 1 |
| Corneal reflex (eye blink when cornea is lightly touched with cotton) | 1 |
| Startle reflex (motor response to a brief noise from snapping a clipboard paper) | 1 |
| Seizures, myoclonus, myodystony | 1 |
| Maximum points | 18 |
One point is awarded for inability to perform the task or for an absence the tested reflex: 13–18, severe injury; 7–12, moderate injury; and 1–6, mild injury
Morris Water Maze Test
A modified Morris water maze (MWM) test was used to assess memory and learning deficits in the rats following the surgical procedures [26]. The MWM apparatus consisted of a circular water tank (diameter 150 cm, height 50 cm) divided into four quadrants using imaginary lines and filled with water (19–22 °C), which was made opaque using India ink. There was an escape platform (diameter 10 cm) in the center of the southwest quadrant (target quadrant) that was submerged 2 cm under the surface of the water. The activity of the rats was monitored using a tracking system (Ethovision 3.0, Noldus Information Technology; Wageningen, Netherlands) that consisted of a video camera, computer, and software. The apparatus was placed in a spacious room with simple pictures attached to the curtain surrounding the tank that were visible to the rats.
All rats were trained on days 21–25 after the TBI procedure (Fig. 1) so that the speed of each rat could be recorded prior to the experiment in order to exclude disabled rats. On each day of training, the rats individually performed four random trials beginning from four different positions (north, east, southeast, and northwest), and the percentage of time spent swimming within the target quadrant was calculated relative to the total amount of time spent in the pool. The maximum swim time was 120 s per trial with a 5-min interval between each trial. If the rat could not find the platform within 120 s on a given trial, it was guided to the platform where it remained for 15 s before being returned to a holding cage. On day 26, a space probe experiment was conducted in which the platform was removed from the water, and the rat was placed into the tank at the farthest point from the position of the platform. The percentage of time spent in the target quadrant (which had previously held the platform) during 1 min of swimming was recorded.
Immunohistochemistry
Brain tissue samples were collected from six rats from each group on days 0 and 7 following the TBI procedure and used to assess the presence of CD34− and CD31-positive cells. All brain sections were frozen in liquid nitrogen, embedded in tissue OCT compound (Leica 0201 06926; Germany), and a series of 7-µm coronal brain sections was cut using a cryostat (Leica; Germany) at −20 °C. Every tenth section from each injured brain block (a total of five sections) was used for immunohistochemical staining. The sections were fixed in methanol for 15 min, washed in PBS, treated with 3 % H2O2 at room temperature for 10 min, blocked with rabbit serum at 37 °C for 30 min, and then incubated overnight at 4 °C with antibodies against either CD34 (1/100 dilution; Santa Cruz Biotechnology Inc.) or CD31 (1/100 dilution; Santa Cruz Biotechnology Inc.), which is a platelet endothelial cell adhesion molecule (PECAM). Following washing, the sections were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody at 37 °C for 30 min, and positive signals were visualized using diaminobenzidine (DAB). The sections were counterstained using hematoxylin and eosin (HE), and negative controls were conducted by omitting either the primary or secondary antibodies.
Quantification of Angiogenesis
The quantification of CD31− and CD34-positive cells was conducted as previously described [21, 27]. Five sections (at 50-mm intervals) from each brain were obtained such that three non-overlapping fields in the injury boundary zone or the hippocampus were acquired randomly. Each section was analyzed using a light microscope (Leica) with a 20× objective lens, and all assessments were performed by two independent observers blinded to the experimental procedure.
Statistical Analysis
All analyses in this study were performed using SPSS 16.0 software [SPSS Inc, Chicago, IL]. Differences in the levels of circulating EPCs and CD31− and CD34-positive cell counts in the immunostained sections were analyzed using analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) test. Between-group comparisons of mNSS scores were conducted using Student’s t test, and the levels of circulating EPCs and CD31− and CD34-positive cells in the injured brain were evaluated by bivariate correlation analyses. All data were expressed as means±standard deviations (SD), and a P value less than 0.05 was considered statistically significant.
Results
Treatment with EPO Increased Circulating EPCs
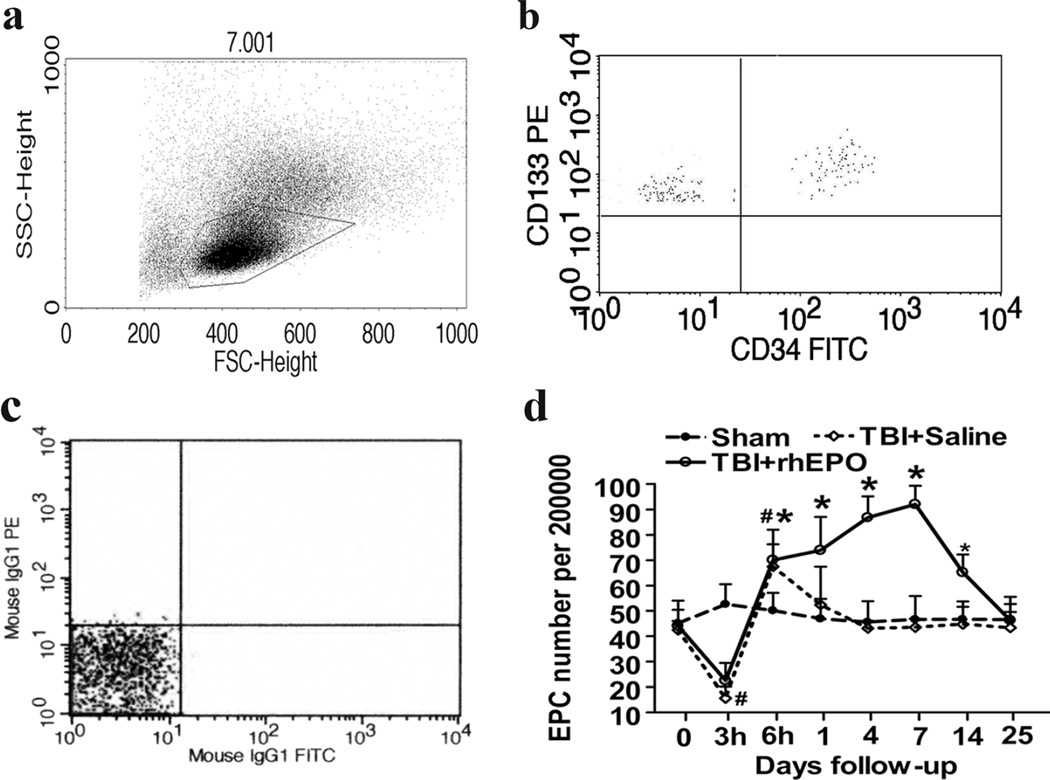
The exact definition of an EPC is an ongoing and controversial issue. However, when investigating EPCs using flow cytometry, the definition entails positivity of the CD34 marker in conjunction with co-expression of either CD133 or vascular endothelial growth factor (VEGF) receptor-2 (VEGFR-2) [28, 29]. Accordingly, previous studies from our lab [21, 30, 31] defined CD34− and CD133-double-positive cells as EPCs, and in the present study, which used flow cytometry, the number of circulating EPCs among 2 × 105 mononuclear cells (Fig. 2a, b) was quantified using the same definition. There were no significant differences among the three groups at baseline, but subsequent to the TBI procedure, the number of circulating EPCs in TBI rats decreased after 3 h (15.63±4.62 versus 52.59±7.86; P<0.001) and increased after 6 h (67.45±14.62 versus 50.08±7.05; P<0.01; Fig. 2c) compared with sham rats. The level of circulating EPCs in the saline group (TBI+saline) returned to the level of the sham group 1 day following the TBI procedure, but treatment with rhEPO (TBI+rhEPO) resulted in a continuous increase of circulating EPCs. ANOVA revealed a greater number of circulating EPCs in the rhEPO group than the saline group following the TBI procedure on days 1 (73.92±13.15 versus 52.41±15.08, respectively; P=0.001), 4 (86.83±8.43 versus 43.10±2.91, respectively; P<0.001), 7 (92.12±7.12 versus 43.47±3.37, respectively; P<0.001), and 14 (65.18±7.14 versus 44.68±6.89, respectively; P<0.001; Fig. 2c).
Fig. 2.
rhEPO significantly increased levels of circulating EPCs in TBI rats. a Mononuclear cells were isolated from blood samples and selected based on size using flow cytometry; the gated dot plots represent the mononuclear cells. b The EPCs were identified using CD133 and CD34 monoclonal antibodies; the upper right dot plots represent the EPCs. c Negative controls: mouse IgG1-PE and mouse IgG1-FITC. d The quantification of EPCs. The rhEPO-treated group exhibited a higher number of circulating EPCs compared with saline-treated and sham rats on days 1, 4, 7, and 14 following the TBI procedure. Data are presented as means±SD. *P<0.05, rhEPO- versus saline-treated groups; #P<0.05 saline-treated versus sham group. EPCs endothelial progenitor cells; rhEPO recombinant human erythropoietin; TBI traumatic brain injury
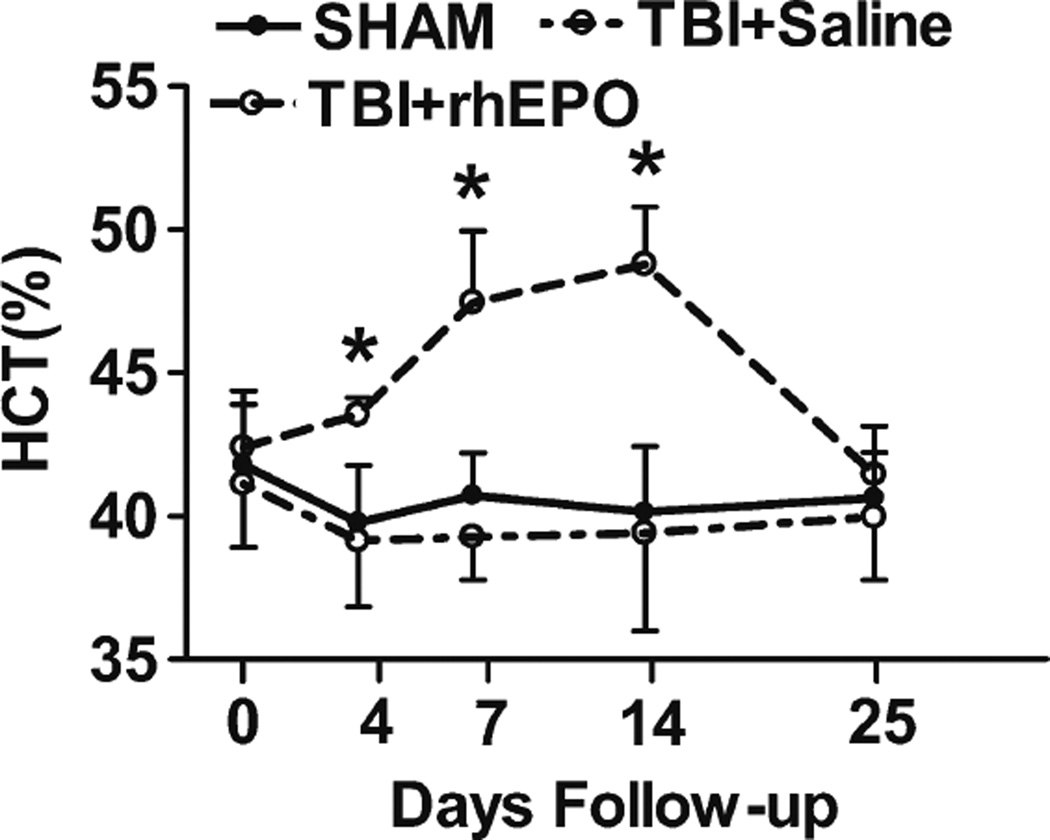
HCT Levels
Prior to TBI, HCT values were similar among the three groups (Fig. 3). However, ANOVA revealed higher HCT values in the rhEPO-treated group than in the sham and saline groups following the TBI procedure on days 4 (P<0.01), 7 (P<0.01), and 14 (P<0.01; Fig. 3).
Fig. 3.
Treatment with rhEPO increased hematocrit in TBI rats. Quantification of hematocrit. *P<0.05
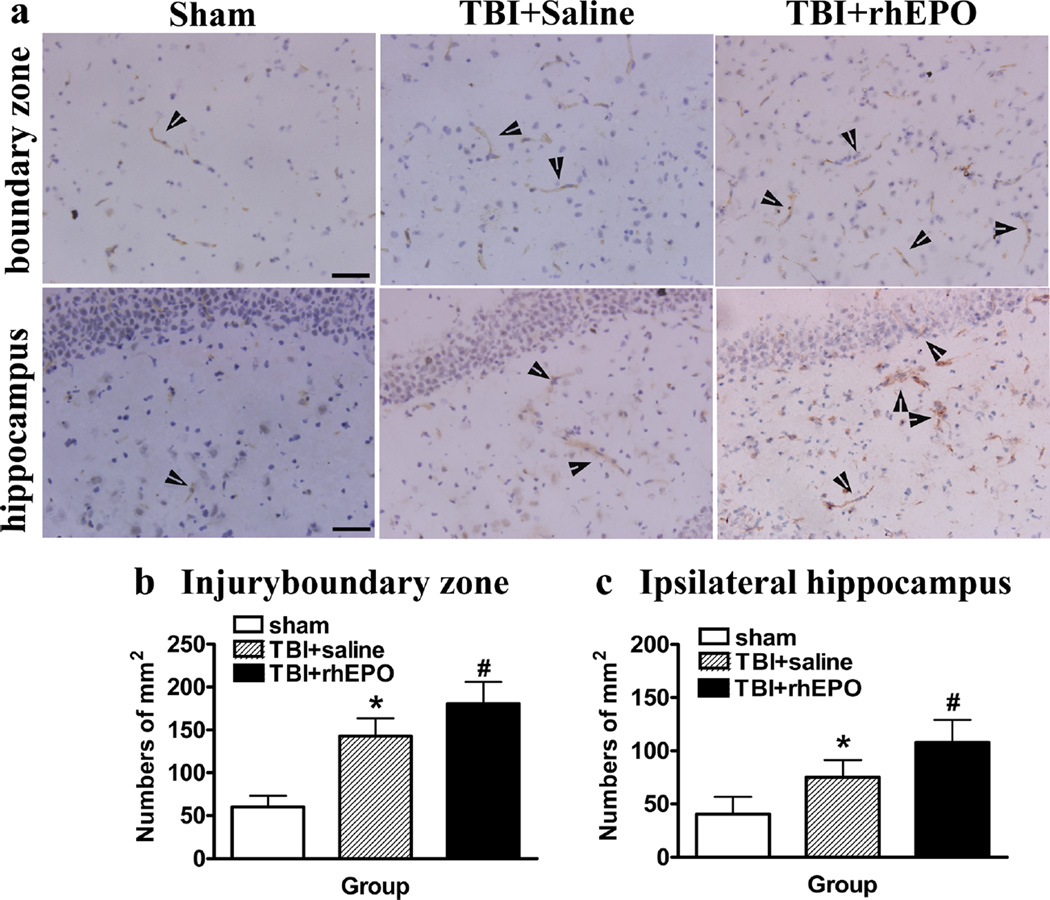
rhEPO Increased the Numbers of CD34− and CD31-Positive Cells in the Injured Boundary Zone and the Ipsilateral Hippocampus
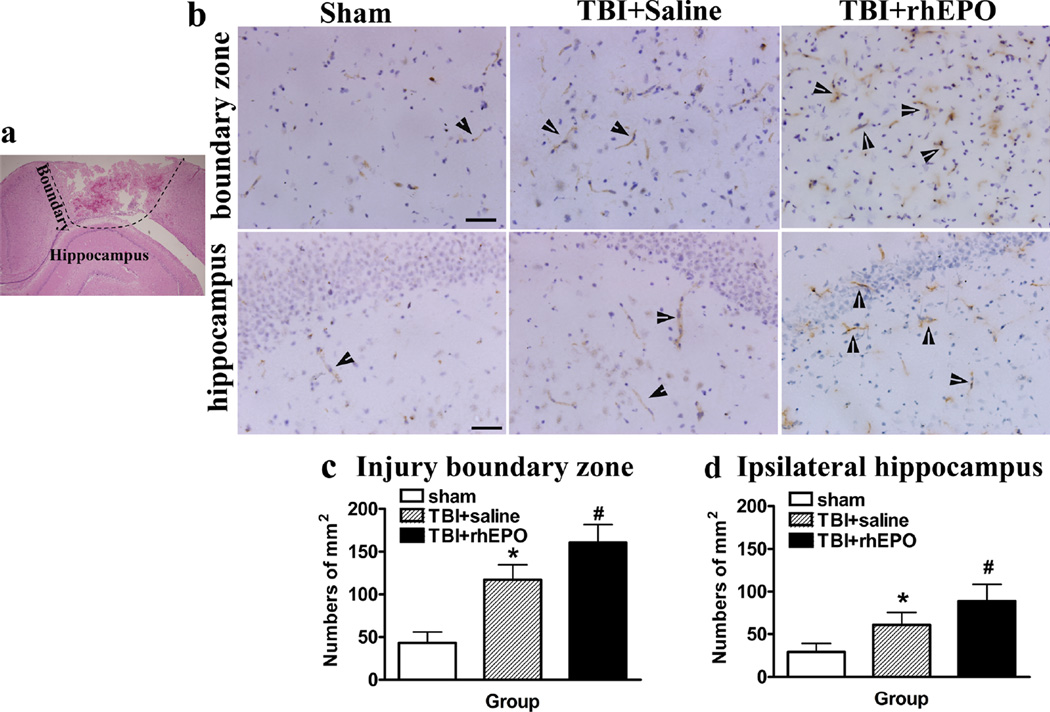
This study utilized CD34 as a marker of microvascular endothelial cells in injured brain tissue. ANOVA revealed a significant increase in CD34 expression in the injured boundary zone (160.78±20.67 versus 117±17.68; P=0.001; Fig. 4a) and ipsilateral hippocampus (89±19.50 versus 61.11±14.50; P<0.01; Fig. 4a) of rhEPO-treated rats compared with saline-treated rats (Fig. 4b–d). CD31 is an endothelial marker used to identify angiogenesis in the brain following TBI and hypoxia-ischemia [21, 32–34]. There was a significant increase in CD31-positive cells in the injured boundary zone (180.59±25.36 versus 143.11±20.78, P<0.01) and ipsilateral hippocampus (108±21.3 versus 75.22±16.27, P<0.01) of rhEPO-treated rats compared with saline-treated rats (Fig. 5a–c). Additionally, a strong association was observed among the levels of circulating EPCs and CD34− and CD31-positive cells in the injured boundary zone (CD34+ r=0.910; CD31+ r= 0.894) and ipsilateral hippocampus (CD34+ r=0.841; CD31+ r=0.835; Table 2).
Fig. 4.
rhEPO increased CD34-positive cells in the injury boundary zones and hippocampal areas of TBI rats. a HE-stained sections revealed the injury areas, boundary zones, and hippocampal areas of TBI rats. b Representative images of sections stained with the CD34 antibody. c Quantification of CD34-positive cells in the injury boundary zone. d Quantification of CD34-positive cells in the ipsilateral hippocampus; rhEPO treatment increased CD34-positive cells in the injury boundary zone and hippocampus compared with the saline-treated group. *P<0.05 versus the sham group; #P<0.05 versus the saline-treated group. Data are presented as means±SD. Scale bars 50 µm; arrows show the CD34-positive cells. HE hematoxylin and eosin; TBI traumatic brain injury; rhEPO recombinant human erythropoietin
Fig. 5.
rhEPO treatment increased CD31-positive cells in the injury boundary zones and hippocampal areas of TBI rats. a Representative images of sections stained with a CD31 antibody. b Quantification of CD31-positive cells in the injury boundary zone. c Quantification of CD31-positive cells in the ipsilateral hippocampus; rhEPO treatment increased CD31-positive endothelial cells in the injury boundary zone and hippocampus compared with the saline-treated group. *P<0.05 versus the sham group; #P<0.05 versus the saline-treated group. Data are presented as means±SD. Scale bars 50 µm; arrows show the CD31-positive cells. rhEPO recombinant human erythropoietin
Table 2.
Correlation between the circulating EPCs and CD31+ or CD34+ cells in injured rat brain
| Subside cells | Pearson correlation with EPCs |
P value |
|---|---|---|
| CD34+cells (injured boundary zone) | 0.910 | 0.000 |
| CD34+cells (ipsilateral hippocampus) | 0.841 | 0.001 |
| CD31+cells (injured boundary zone) | 0.894 | 0.000 |
| CD31+cells (ipsilateral hippocampus) | 0.835 | 0.001 |
rhEPO Treatment Improved Spatial Learning Ability and Neurological Recovery in TBI Rats
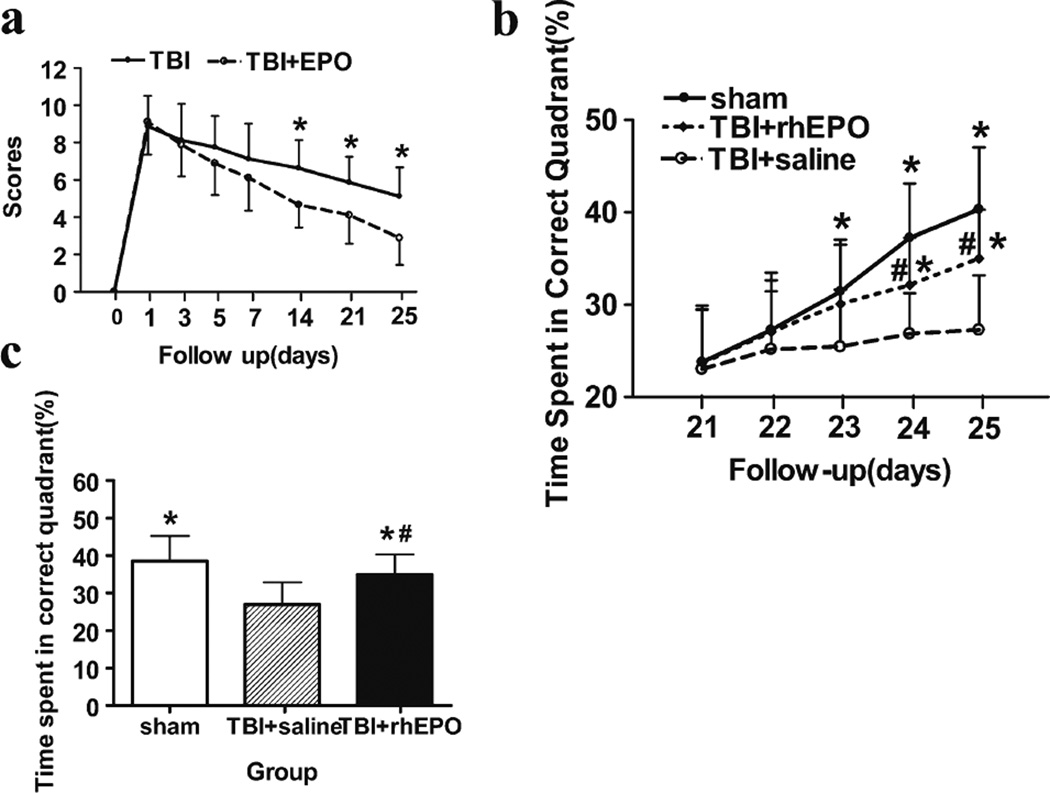
The mNSS and MWM tests were used to determine whether rhEPO treatment improves neurological function in TBI rats. The motor, sensory, and beam balance assessments of the mNSS test were conducted prior to and 1, 3, 5, 7, 14, 21, and 25 days following the TBI procedure in both rhEPO- and saline-treated rats. There were no differences between the groups 1 day after the TBI procedure, but the mNSS scores of the rhEPO group were lower than those of the saline group on days 14 (4.7±1.2 versus 6.6±1.5, respectively; P=0.01), 21 (4.1±1.5 versus 5.9±14, respectively; P<0.05), and 25 (2.9±1.5 versus 5.1±1.6, respectively; P<0.01; Fig. 6a) after the TBI procedure.
Fig. 6.
rhEPO treatment improved neurofunctional outcomes. a Quantification of mNSS test results. b Quantification of the time spent in the target quadrant during training on the MWM test on days 21–25 after TBI. c Quantification of the percentage of time spent in the target quadrant during the probe test. *P<0.05 versus the TBI+saline group; #P<0.05 versus the sham group. Data are presented as means±SD
The saline group demonstrated impaired learning on days 23, 24, and 25 following the TBI procedure. However, TBI rats treated with rhEPO spent more time in the target quadrant than saline-treated rats on days 24 (32.2±5.1 % versus 26.8±4.4 %, respectively; P<0.05) and 25 (35.0±5.3 %versus 27.3±5.9 %, respectively; P<0.05; Fig. 6b), indicating an improved learning ability. A space probe experiment was used to evaluate memory recovery in TBI rats on day 26 following the TBI procedure. Treatment with rhEPO significantly increased the time spent in the target quadrant compared with saline-treated rats (32.86±5.3 % versus 27.0±5.9 %, respectively; P<0.05; Fig. 6c).
Discussion
Angiogenesis is critical for the repair of neural cells and functional recovery following injury in the brain, and a number of studies have shown that EPCs play a crucial protective role in vascular-related diseases such as stroke and TBI [35–37]. Similarly, the present study demonstrated that treatment with rhEPO increased the number of circulating EPCs and CD34− and CD31-positive cells in the injured boundary zones and ipsilateral hippocampi of TBI rats.
In previous studies, EPCs have primarily been evaluated following myocardial ischemic, tumor, hind-limb ischemic, or stroke damage [27, 31, 38]. TBI results in vascular breakdown within the injured area as well as in induction of angiogenesis or vasculogenesis, which are both important for recovery. Therefore, EPCs may play an important role in TBI injury and recovery. We previously [30] found that the number of circulating EPCs is lower than normal during the early stages of TBI but that these levels subsequently increase and peak 7 days after the injury, before returning to normal levels after 21 days. The level of circulating EPCs has been strongly correlated with the severity of TBI in clinical research [34], and studies in TBI rats show that atorvastatin and progesterone increase the level of circulating EPCs and enhance angiogenesis [21, 35]. Likewise, the present study found that rhEPO treatment increased circulating EPCs in conjunction with mobilization of the HCT in TBI rats.
EPCs are present in bone marrow and peripheral blood. Increased levels of circulating EPCs are important for angiogenesis and vascular remodeling [39, 40], and accordingly, the transfusion of EPCs increases angiogenesis in animal models of stroke and TBI [7, 41]. Importantly, enhanced neovascular regeneration is correlated with improved functional results following TBI or stroke [17, 18]. In the present study, treatment with rhEPO increased circulating EPCs (CD34− and CD133-double-positive cells) and enhanced angiogenesis (CD34− or CD31-positive cells) in injured brain regions. EPCs promote the remodeling of denude vascular walls and the renewal of endothelial cells [36], and bone marrow-derived EPCs can reduce capillary breakdown following midline TBI in rats [18]. In the present study, levels of CD34− or CD31-positive cells in the injured brain were correlated with changes in circulating EPCs. Thus, circulating EPCs likely play an important role in the enhanced angiogenesis that occurs subsequent to brain injury, and the presence of vasculogenesis or angiogenesis may contribute to the improvement of functional outcomes as well.
rhEPO has been used clinically to treat anemia for more than 10 years and is generally considered safe. Many studies have demonstrated that rhEPO not only has anti-apoptotic, anti-inflammatory, and neuroprotective effects in many organs [3, 12, 42] but that it is an important mediator of angiogenesis, although the underlying mechanisms of this process remain unclear. The EPO receptor is thought to play a key role in neuroprotection and neurorestoration [43], but a recent study found that rhEPO improves functional outcomes and enhances angiogenesis in the injured mouse brain following TBI in the absence of neural EPO receptors [44]. Experimental [45] and clinical studies [46] have shown that EPO enhances angiogenesis and protects against ischemia-related damage via increases in levels of circulating EPCs following myocardial ischemia and stroke. Similarly, the present study found that rhEPO significantly increased the level of circulating EPCs and improved angiogenesis following TBI in rats.
Because the amount of EPCs in peripheral blood is very low, the mobilization of EPCs from bone marrow to peripheral blood using rhEPO is a safer and more feasible option than EPC transplantation. EPO therapy has been used to treat clinical stroke patients and was shown to increase levels of circulating EPCs and enhance functional recovery 90 days after injury [46]. EPO has also been evaluated using experimental TBI animal models for a number of years. For example, in a model of diffuse TBI, rhEPO treatment significantly reduces the development of brain edema [47]. Additionally, in a mouse model of TBI, EPO exerted neuroprotective effects in the ipsilateral hippocampus and cortex of the injured brain via enhancement of anti-apoptotic proteins, such as p-Akt and Bcl-XL [44], and of VEGF and VEGFR expression [48]. The VEGF system and anti-apoptotic proteins are known to support neurovascular remodeling in EPO-treated rats following TBI, and EPO is also used as a multifunctional pharmacotherapy with a promising future for the treatment of clinical TBI patients.
Conclusion
TBI is one of the leading causes of mortality and morbidity in people under the age of 44 years. The primary pathological issue associated with TBI is damage to blood vessels in the brain, but very little research concerning the relationship between the level of circulating EPCs and angiogenesis in the brain following TBI has been conducted. EPO has been shown to improve functional recovery and neurogenesis in animal models of TBI. The present data demonstrate that a novel mechanism, the EPO-induced mobilization of circulating EPCs, may play an important role in the enhancement of angiogenesis and contribute to the improvement of functional outcomes following TBI.
Acknowledgments
We thank Li Liu, Weiyun Cui, Fanglian Chen, and Lei Zhang for their excellent technical support. The authors thank Voltaire Gungab for the editorial assistance. This work was supported by grants from the Emerging Project Committee of Science and Technology of Tanggu District of Tianjin (2012XQ15-07), China; the National Natural Science Foundation of China (grants 81100920 and 81200907); and Tianjin Research Program of Application Foundation and Advanced Technology (grants12JCQNJC6800).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Liang Wang, Department of Neurosurgery, Tianjin 5th Center Hospital, Number 41 Zhejiang Road, Tanggu District, Tianjin 300450, China.
Xiaonan Wang, Department of Neurosurgery, Nankai Hospital, Number 6, Changjiang Road, Nankai District, Tianjin 300102, China.
Hua Su, Center for Cerebrovascular Research, Department of Anesthesia and Perioperative Care, University of California, San Francisco, 1001 Potreto Avenue, Box 1363, San Francisco, CA 94110, USA.
Zhenying Han, Department of Neurosurgery, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin 300052, China.
Huijie Yu, Department of Neurosurgery, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin 300052, China.
Dong Wang, Department of Neurosurgery, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin 300052, China.
Rongcai Jiang, Department of Neurosurgery, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin 300052, China.
Zhenlin Liu, Email: wjzhenlin817@sina.com, Department of Neurosurgery, Tianjin 5th Center Hospital, Number 41 Zhejiang Road, Tanggu District, Tianjin 300450, China.
Jianning Zhang, Email: jianningzhang@hotmail.com, Department of Neurosurgery, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin 300052, China.
References
- 1.Potts MB, Koh SE, Whetstone WD, Walker BA, Yoneyama T, Claus CP, et al. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx: J Am Soc Exp NeuroTher. 2006;3:143–153. doi: 10.1016/j.nurx.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan Q, Liu H, Wu X, Sun Y, Yao H, Zhou L, et al. Characteristics of acute treatment costs of traumatic brain injury in Eastern China—a multi-centre prospective observational study. Injury. 2012;43:2094–2099. doi: 10.1016/j.injury.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neuro Rehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- 4.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, et al. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurother. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 9.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 10.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113:1442–1450. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 14.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsic E, van derMeer P, Henning RH, Suurmeijer AJ, Boddeus KM, van Veldhuisen DJ, et al. Timing of erythropoietin treatment for cardioprotection in ischemia/reperfusion. J Cardiovasc Pharmacol. 2004;44:473–479. doi: 10.1097/01.fjc.0000140209.04675.c3. [DOI] [PubMed] [Google Scholar]
- 16.Akdemir Ozisik P, Oruckaptan H, Ozdemir Geyik P, Misirlioglu M, Sargon MF, Kilinc K, et al. Effect of erythropoietin on brain tissue after experimental head trauma in rats. Surg Neurol. 2007;68:547–555. doi: 10.1016/j.surneu.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Sobrino T, Hurtado O, Moro MA, Rodriguez-Yanez M, Castellanos M, Brea D, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke: J cereb circ. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 18.Park KJ, Park E, Liu E, Baker AJ. Bone marrow-derived endothelial progenitor cells protect postischemic axons after traumatic brain injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2014;34:357–366. doi: 10.1038/jcbfm.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Zhang Z, Zhang R, Hafner MS, Wong HK, Jiao Z, et al. Erythropoietin up-regulates SOCS2 in neuronal progenitor cells derived from SVZ of adult rat. NeuroReport. 2004;15:1225–1229. doi: 10.1097/01.wnr.0000127636.15181.c1. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Wang B, Kan Z, Zhang B, Yang Z, Chen J, et al. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma. 2012;29:343–353. doi: 10.1089/neu.2011.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Sun L, Tian Y, Li Z, Wei H, Wang D, et al. Effects of atorvastatin in the regulation of circulating EPCs and angiogenesis in traumatic brain injury in rats. J Neurol Sci. 2012;319:117–123. doi: 10.1016/j.jns.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 24.Xiong Y, Zhang Y, Mahmood A, Meng Y, Qu C, Chopp M. Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl Stroke Res. 2011;2:619–632. doi: 10.1007/s12975-011-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke: J cereb cir. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 26.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, et al. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J Off Publ Fed Am Soc Exp Biol. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 28.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 29.Grisar JC, Haddad F, Gomari FA, Wu JC. Endothelial progenitor cells in cardiovascular disease and chronic inflammation: from biomarker to therapeutic agent. Biomark Med. 2011;5:731–744. doi: 10.2217/bmm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Liu H, Jiao J, Bergeron A, Dong JF, Zhang J. Changes in circulating human endothelial progenitor cells after brain injury. J Neurotrauma. 2007;24:936–943. doi: 10.1089/neu.2006.0250. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 32.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson AN, Liu H, Schiborra F, Shaw O, Sammut IA, Jackson DM, et al. Angiogenesis as a predictive marker of neurological outcome following hypoxia-ischemia. Brain Res. 2007;1171:111–121. doi: 10.1016/j.brainres.2007.06.100. [DOI] [PubMed] [Google Scholar]
- 34.Gong D, Hao M, Liu L, Liu C, Dong J, Cui Z, et al. Prognostic relevance of circulating endothelial progenitor cells for severe traumatic brain injury. Brain Injury [BI] 2012;26:291–297. doi: 10.3109/02699052.2011.648710. [DOI] [PubMed] [Google Scholar]
- 35.Resch T, Pircher A, Kahler CM, Pratschke J, Hilbe W. Endothelial progenitor cells: current issues on characterization and challenging clinical applications. Stem Cell Rev. 2012;8:926–939. doi: 10.1007/s12015-011-9332-9. [DOI] [PubMed] [Google Scholar]
- 36.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 37.Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, et al. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26:819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]
- 38.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 39.Ladhoff J, Fleischer B, Hara Y, Volk HD, Seifert M. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovas Res. 2010;88:121–129. doi: 10.1093/cvr/cvq109. [DOI] [PubMed] [Google Scholar]
- 40.Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, et al. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood. 2009;113:233–243. doi: 10.1182/blood-2008-06-162891. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Li Y, Wang S, Han Z, Huang X, Li S, et al. Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J Surg Res. 2013;185:441–449. doi: 10.1016/j.jss.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 42.Westenbrink BD, Lipsic E, van derMeer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 43.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong Y, Mahmood A, Qu C, Kazmi H, Zhang ZG, Noguchi CT, et al. Erythropoietin improves histological and functional outcomes after traumatic brain injury in mice in the absence of the neural erythropoietin receptor. J Neurotrauma. 2010;27:205–215. doi: 10.1089/neu.2009.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirata A, Minamino T, Asanuma H, Fujita M, Wakeno M, Myoishi M, et al. Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol. 2006;48:176–184. doi: 10.1016/j.jacc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Yip HK, Tsai TH, Lin HS, Chen SF, Sun CK, Leu S, et al. Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit Care. 2011;15:R40. doi: 10.1186/cc10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de Looij Y, et al. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- 48.Xiong Y, Zhang Y, Mahmood A, Meng Y, Qu C, Chopp M. Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl Stroke Res. 2011;2:619–632. doi: 10.1007/s12975-011-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]