Abstract
Notch pathway activation in podocytes has been shown to play an important role in diabetic kidney disease (DKD) development; however, the receptors and ligands involved in the process have not been identified. Here, we report that conditional deletion of Notch1 in podocytes using NPHS2creNotch1flox/flox animals resulted in marked amelioration of DKD. On the contrary, podocyte-specific genetic deletion of Notch2 had no effect on albuminuria and mesangial expansion. Notch1-null podocytes were protected from apoptosis and dedifferentiation in vitro, likely explaining the protective phenotype in vivo. Deletion of Notch1 in podocytes also resulted in an increase in Notch2 expression, indicating an interaction between the receptors. At the same time, transgenic overexpression of Notch2 in podocytes did not induce phenotypic changes, while constitutive expression of Notch1 caused rapid development of albuminuria and glomerulosclerosis. In summary, our studies indicate that Notch1 plays a distinct (nonredundant) role in podocytes during DKD development.
Introduction
To date, approximately 9% of the U.S. population has diabetes. Diabetes is the leading cause of chronic kidney disease and end-stage renal failure. Diabetic kidney disease (DKD) is initiated in part because high level of blood glucose damages the glomerular filtration unit, resulting in protein leakage into the urine (1). The filtration unit is comprised of capillary endothelial cells, glomerular basement membrane, and specialized epithelial cells, podocytes. Once thought to be primarily quiescent and terminally differentiated cells, podocytes have been demonstrated to be the true culprit of DKD (1). Podocyte injury is characterized by pathological loss of regularity in foot branching and widening of the foot processes; changes termed “foot process effacement.” Foot process effacement is the typical mechanism of injury response of podocytes, and it is usually associated with a broader dedifferentiation process. Severe insult leads to podocyte loss by apoptosis or detachment (1). Reactivation of developmental pathways, including Wnt and Notch signaling, has been shown to play an important role in podocyte injury and DKD development by promoting dedifferentiation and apoptosis (2,3).
The Notch protein family is comprised of four receptors, Notch1–4, and five canonical ligands, Jagged1 and -2 (Jag1 and -2) and delta-like ligands (Dll)1, -3, and -4 (4). Canonical Notch signaling is typically transcellular; the ligand(s) expressed on one cell binds to receptors on neighboring cells and initiates cleavage of the receptor. Notch cleavage results in the release of the Notch intracellular domain (NICD or ICNotch), which translocates to the nucleus to become a transcriptional coregulator. Some of the transcriptional binding partners that engage NICD in the nucleus are common to all Notch receptors; including mastermind-like 1 (MAML1) and recombination signal binding protein for immunoglobulin kappa J (Rbpjκ) (4). Despite the common use of activation and signaling partners, Notch receptor functions are often nonredundant (5).
Notch1 and Notch2 show high structural similarities and an almost overlapping expression pattern in the developing and adult mammalian kidney. Despite their intersecting expression, Notch1 and -2 are functionally distinct. Mutations of NOTCH2 in patients cause Alagille syndrome, which is associated with renal developmental abnormalities (6,7). Similarly, genetic studies performed in mice indicated an absence of podocytes and proximal tubule development in Notch2 knockout animals (5,8). On the other hand, mice with kidney-specific deletion of Notch1 do not show renal developmental defects, highlighting that Notch1 and Notch2 play specific (nonredundant) roles during development. To understand this specificity, the Kopan group recently performed experiments swapping the intracellular and extracellular domains of Notch1 and Notch2 in the developing kidney (9). They propose that signal strength alterations might be responsible for the functional differences between Notch1 and Notch2 during kidney development.
Expressions of Notch pathway proteins are much lower in adult mouse and human kidneys. Increased expression of both Notch1 and Notch2 has been reported in kidney samples of patients with DKD (10), focal segmental glomerulosclerosis (10,11), HIV-associated nephropathy (12), and tubular interstitial fibrosis (13). Our studies also indicate that Notch plays a functional role in podocytes, as inducible expression of the Notch1 intracellular domain in mature podocytes causes severe albuminuria and glomerulosclerosis (2). Functional studies performed in cultured podocytes indicated that increased Notch1 expression induces apoptosis via upregulation of p53 (2). To prove that Notch signaling plays a functional role in podocytes, we generated mice with podocyte-specific deletion of Rbpjκ. Rbpjκ is a common transcriptional binding partner of all Notch isoforms. Podocyte-specific Rbpjk deletion resulted in significant (50%) reduction of proteinuria in the setting of diabetes (2). Lin et al. (14) also demonstrated that inhibition of Notch signaling by blocking release of the NICD with γ-secretase inhibitor treatment in a rat model ameliorated albuminuria and glomerulosclerosis in the setting of diabetes. Collectively, these results strongly indicate that Notch signaling plays an important role in DKD development. Unfortunately, all prior studies relied on simultaneous inhibition of all Notch isoforms; thus, the receptor-specific signaling architecture remains unknown. This is a critical question for potential therapeutics development, as broad inhibition of Notch signaling is associated with severe systemic side effects, while receptor- or ligand-specific targeting shows better profiles. An additional concern is that both the γ-secretase complex and Rbpjk are involved in multiple signaling cascades. A recent report even suggested that Notch2-activating antibodies could be protective in the context of nephrotic syndrome, indicating that different Notch receptors play dissimilar roles (15).
Here, we examined the role of Notch1 and Notch2 in podocytes using genetic deletion and overexpression systems in vivo and in vitro. We found that podocyte-specific loss of Notch1 protected from DKD development by ameliorating podocyte dedifferentiation and loss.
Research Design and Methods
Antibodies and Reagents
The following antibodies were used: cleaved Notch1 (Val1744, rabbit; Cell Signaling) 1:100 immunohistochemistry (IHC), 1:150 immunofluorescence (IF); Notch1 (D1E11, Cell Signaling) 1:1,000 Western blot (WB); cleaved Notch2 (cleaved-Ala1734, rabbit; Sigma) 1:150 IHC; cleaved Notch2 (cat. no. 07-1234, rabbit; Millipore) 1:200 IF, 1:1,000 WB; Snail1 (NBP1-19529, rabbit; Novus Biologicals) 1:200 IHC, 1:200 IF, 1:1,000 WB; Nephrin (guinea pig; Fitzgerald Laboratories) 1:250 IF; podocin (H-130, rabbit; Santa Cruz Biotechnology) 1:1,000 WB; Wilm's tumor suppressor gene 1 (WT-1) (C-19, mouse; Santa Cruz Biotechnology) 1:150 IF; GAPDH (clone GAPDH-71.1, mouse; Sigma) 1:5,000 WB; β-actin (clone AC-15, mouse; Sigma) 1:10,000 WB; and γ-tubulin (ab11316, mouse; Abcam) 1:1,000 WB. The following secondary antibodies were used: rhodamine-conjugated goat α guinea pig (Fitzgerald Laboratories) and fluorescein isothiocyanate–conjugated immunoabsorbed donkey α rabbit (The Jackson Laboratory). Nuclei were visualized with either Hoechst 33342 or NucBlue Fixed Cell stain (Life Technologies).
Animals
The Notch1flox/flox mice were on a 129SvJ background and purchased from The Jackson Laboratory (cat. no. 006951), the Notch2flox/flox mice on a C57BL/6J background were a kind gift of Ursula Zimber-Strobl (Helmholtz German Research Center for Environmental Health, Munich, Germany), and the podocin Cre NPHS2cre line was a kind gift of Lawrence Holzman (University of Pennsylvania). For generation of mice with Notch1 or Notch2 deletion specifically in podocytes, Notch1flox/flox mice or (16) Notch2flox/flox mice were crossed with podocin Cre mice (17). Transgenic animals were identified by genomic PCR using transgene specific primers. Nephrectomies were performed on male littermates of the NPHS2cre/Notchflox/flox × wild type Notchflox/flox matings at 4 weeks of age under sterile conditions. For induction of diabetes, the uninephrectomized mice were injected with streptozotocin (STZ) (50 mg/kg i.p. daily ×5 [low-dose protocol]) as described at www.diacomp.org. Mice were killed at 20 weeks of age. NICD2-overexpressing floxed mice (R26Notch2) were generated by R.N., and were previously described in detail (18). R26Notch2 mice were crossed with the NPHS2Cre mice to generate podocyte-specific NICD2-overexpressing animals. Animal studies were approved by Albert Einstein College of Medicine and at the University of Pennsylvania.
Phenotype Analysis
Urinary albumin and creatinine were determined using mouse albumin–specific ELISA and creatinine Companion kits (Exocell and Bethyl Laboratories) following the manufacturer’s protocol. Blood glucose was measured with OneTouch Glucometer Ultra2 (LifeScan). Formalin-fixed paraffin-embedded kidney sections were stained with periodic acid Schiff (PAS). Slides were examined and pictures were taken with Olympus BX43 microscope and DP73 camera.
Immunostaining
Formalin-fixed, paraffin-embedded sections were blocked with fish skin gelatin, BSA, secondary antibody host serum (donkey or goat), and Triton X-100. Endogenous biotin was blocked with avidin/biotin-blocking kit (Vector Laboratories). Peroxidase-conjugated secondary antibodies were provided with the Vectastain Elite kit, and peroxidase development was achieved with the ImmPACT DAB peroxidase substrate kit (Vector Laboratories). O.C.T. Compound (Sakura) embedded cryosections were fixed in zinc-buffered formalin and probed with primary antibodies as described above.
Primary Glomerular Harvest and Podocyte Culture
Glomeruli for transcript, protein, and cell culture analysis were harvested using magnetic beads, and for immunofluorescence studies they were isolated using differential sieving. Magnetic Dynabeads (Life Technologies) isolation was performed as previously described (19). A 10-min red blood cell lysis was added after quenching of the collagenase digest (RBC Lysis buffer, ZenBio; Collagenase, Worthington Biochemical). Isolation by sieving first used a 30-min 1 mg/mL Collagenase A digest of minced kidneys at 37°C, followed by collagenase inhibition with 30% volume FBS addition. Isolates were pushed and washed through successive cell strainers of 100 and then 70 μm pore size. Glomeruli were collected on the top of a 40-μm filter.
For primary podocyte culture, harvested glomeruli were placed onto culture dishes coated with 0.1 mg/mL rat tail collagen type I as previously described (20). Culture medium from days 1–3 was RPMI 1640 containing 15% FBS (Atlanta Bio), penicillin streptomycin, and amphotericin B. On day 3 of culture, unattached glomeruli were washed away and medium was changed to 10% FBS. Podocytes were examined on day 6 of culture after harvest. Twelve hours prior to transforming growth factor (TGF)-β1 stimulation, medium was replaced with RPMI containing only 0.2% FBS.
Immortalized Podocytes
Immortalized mouse podocytes were cultured as previously reported (20,21). Briefly, differentiation was induced when cells were ∼60% of confluence by thermoshifting to 37°C in medium without interferon-γ for 14 days. Twenty-four hours prior to treatment, medium FBS was changed to 0.2%. Cells were treated with 1 μmol/L γ-secretase inhibitor XX (GSIXX) (dibenzazepine, Calbiochem) or vehicle for 1 h and then recombinant mouse TGF-β1 (Preprotech) or vehicle in a reverse time course as indicated.
Apoptosis Assays
Collagen-coated Lumox gas-permeable bottom plates (Sarsted) were used to culture glomeruli for all apoptosis assays. Kidneys from two mice of the same genotype were pooled and counted as one sample. After 48 h, unattached glomeruli were washed away with Dulbecco PBS and medium was replaced with RPMI with 0.2% FBS. After 18 h, NucBlue Live Cell Stain to label live nuclei and CellEvent Caspase-3/7 Green (Life Technologies) to measure apoptosis were added to each plate per the manufacturers’ instructions. Only cells that had migrated away from a glomerulus were quantified by summing the total number in three to five 10× fields per plate. Each plate was then treated with 5 ng/mL activated TGF-β1 and requantified 24 h later.
Quantitative RT-PCR
RNA was isolated using the RNeasy Mini kit (Qiagen) following the manufacturer’s instructions. RNA was reverse transcribed using the cDNA Archival kit (Life Technologies), and quantitative RT-PCR analysis was performed using SYBR Green Master Mix, gene-specific primers, and the ViiA 7 System (Life Technologies). The data were normalized to β-actin gene (ACTB) levels within each sample and analyzed using the ΔΔCt method. Primer sequences are as follows; Nphs2, forward 5′ ccgagaggcccacgggagaa, reverse 5′ ccgctctgctccagcatccg; Nphs1, forward 5′ agcgtgagccctcactcggt, reverse 5′ ctccgccagaggagtccccc; Notch1, forward 5′ acagtgcaaccccctgtatg, reverse 5′ tctaggccatcccactcaca; Notch2, forward 5′ ggcatgttggggaaagctac, reverse 5′ ggacacaaagcaggggtgag; Snai1, forward 5′ tggaaaggccttctctaggc, reverse 5′ ggagaatggcttctcaccag; Actb, forward 5′ accgtgaaaagatgacccag, reverse 5′agcctggatggctacgtaca.
Western Blotting
Protein was isolated using radioimmunoprecipitation assay buffer supplemented with phosphatase and protease inhibitors. Lysis was completed by shearing through a 26-gauge syringe needle. Samples were denatured with 10% β-mercaptoethanol (Sigma) in 2× Laemmli sample buffer at 95°C for 10 min. Primary podocytes were run on 4–15% SDS-PAGE gradient gels (Bio-Rad Laboratories). Immortalized podocytes were run on 10% SDS-PAGE gels. All gels were transferred onto polyvinylidene fluoride, blocked in 5% nonfat milk in PBS before being probed with primary antibodies and secondary antibodies. Blots were developed with Pierce ECL Western Blotting Substrate (Thermo Scientific).
Statistics
We used the two-tailed Student t test assuming unequal variance to compare two groups. Bonferroni correction was used for repeated analysis. For multiple group comparisons we used ANOVA. Correlations and R2 values were calculated using GraphPad Prism, version 6.0, for Mac OS X (GraphPad Software). A P value <0.05 was considered significant.
Results
Mice With Podocyte-Specific Deletion of Notch1 Were Protected From DKD
To address the role of Notch1 and Notch2 in podocytes in health and disease, we generated mice with podocyte-specific deletion of Notch1 by crossing mice carrying a Notch1 floxed allele (Notch1F/F) (16) with podocin Cre animals (NPHS2cre) (17). NPHS2creNotch1F/F mice were born at the expected Mendelian frequency and had no notable gross renal alterations even at 20 weeks of age. Successful deletion of Notch1 was confirmed by quantitative PCR–based transcript analysis of isolated glomeruli (Fig. 1A).
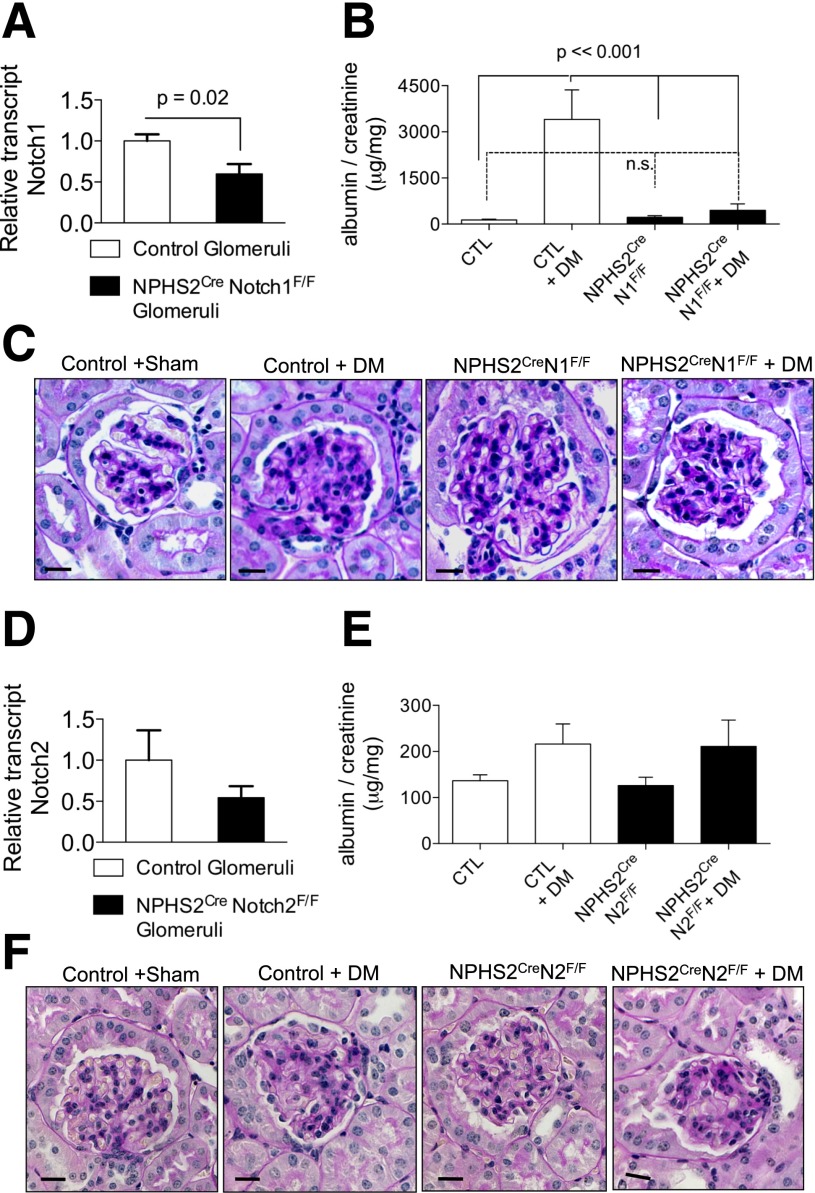
Figure 1.
NPHS2CreNotch1F/F, but not NPHS2CreNotch2F/F, mice were protected from diabetic glomerulosclerosis. A: Notch1 transcript levels in control and NPHS2CreNotch1F/F glomeruli. Error bars represent SD and P values determined by t test. B: Urine albumin-to-creatinine ratios in nondiabetic and diabetic (DM) NPHS2CreNotch1F/F and control (CTL) mice. Error bars represent SD and P values determined by two-way ANOVA. n.s., no significant differences. C: Representative images of PAS-stained kidney samples from control and NPHS2CreNotch1F/F mice in the presence or absence of diabetes at 20 weeks of age. D: Notch2 transcript levels in control and NPHS2CreNotch2F/F glomeruli. E: Urine albumin-to-creatinine ratios in nondiabetic and diabetic NPHS2CreNotch2F/F and control mice. F: Representative images of PAS-stained kidney samples from control and NPHS2CreNotch2F/F mice in the presence or absence of diabetes at 20 weeks of age.
Male mice underwent uninephrectomy at 4 weeks of age, and type 1 diabetes was induced by low-dose STZ injection at 6 weeks of age. Littermates carrying just the Notch1 floxed allele were used as controls. Vehicle-treated uninephrectomized animals served as nondiabetic controls. At 20 weeks of age, urine was collected and animals were sacrificed. The degree of hyperglycemia was similar in the Notch1 knockout and in the control animals (Supplementary Table 1). As the colony was kept on a 129 SvJ background, they developed significant albuminuria after uninephrectomy and STZ-induced diabetes (mean albumin-to-creatinine ratio 3,406 μg/mg). Albuminuria was significantly lower in mice with podocyte-specific Notch1 deletion (448.4 μg/mg albumin/creatinine) (Fig. 1B). Histological changes as seen by PAS stain (Fig. 1C) and Masson trichrome stain (Supplementary Fig. 1), including mesangial expansion, were also ameliorated in podocyte-specific Notch1 knockout animals.
Mice With Podocyte-Specific Deletion of Notch2 Were Not Protected From DKD Development
Next, we generated mice with podocyte-specific deletion of Notch2 by intercrossing NPHS2cre and Notch2F/F animals. Reduced expression of Notch2 was confirmed by quantitative PCR-based transcript analysis (Fig. 1D). Podocyte-specific Notch2 knockout animals showed no phenotypic abnormalities, as the podocin Cre–mediated genetic deletion occurs late during development.
Diabetes was induced by low-dose STZ injection after uninephrectomy (Supplementary Table 1). These animals were on a C57BL/6J background, and the degree of proteinuria was much lower than in the 129 SvJ mice carrying the Notch1 deletion (Fig. 1E). We observed no significant differences in proteinuria or mesangial expansion when diabetic podocyte-specific Notch2 knockout animals were compared with control diabetic animals (Fig. 1E and F).
Lack of Notch1 Prevents Podocyte Dedifferentiation In Vivo
Nphs1 (Nephrin) is the most widely accepted marker of podocyte differentiation and is required for maintenance of the slit diaphragm (22). Loss of Nphs1 expression is an early hallmark of glomerular disease; therefore, we next analyzed Nphs1 expression. In healthy animals, Nphs1 expression was similar regardless of the genotype (Fig. 2A). As previously reported, Nphs1 expression was markedly reduced in diabetic mice (23). In diabetic podocyte-specific Notch2 knockout animals, the decrease was similar to controls, whereas Nphs1 expression was largely preserved in podocyte-specific Notch1 knockout animals (Fig. 2A).
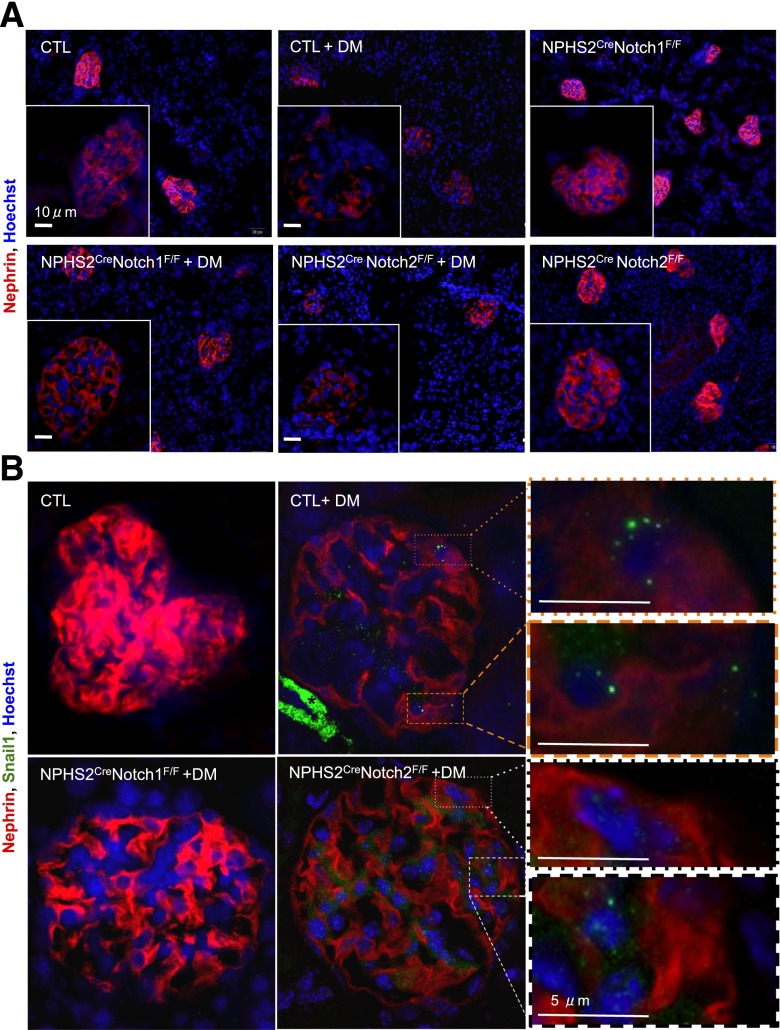
Figure 2.
Notch1 regulated Nphs1 and Snai1 expression in podocytes in diabetic animals. A: Representative images of Nphs1 (Nephrin) immunofluorescence (red) in nondiabetic and diabetic (DM) NPHS2creNotch1F/F and NPHS2creNotch2F/F mice. Images were obtained with identical capture parameters. B: Representative immunofluorescence images of Nphs1 (red), Snai1 (green), and nuclei (Hoechst 33342, blue) of kidney sections of control (CTL), NPHS2creNotch1F/F, and NPHS2creNotch2F/F mice at 20 weeks of age. Insets show examples of punctate nuclear Snail1 staining in podocytes from control diabetic mouse (top two) and NPHS2creNotch2F/F mouse (bottom two). *Positive Snail1 staining in the smooth muscle layer. Images were obtained with identical capture parameters.
To further probe the issue of dedifferentiation, we analyzed the expression of Snai1 (Snail). Snai1 is a known upstream regulator of podocyte differentiation by negatively regulating Nephrin expression (24). Snai1 expression, as analyzed by immunostaining was markedly increased in control diabetic glomeruli (Fig. 2B). In all sections, we saw strong positive staining for Snai1 in the smooth muscle layer of vasculature consistent with a known expression pattern. Double immunofluorescence staining with Nephrin indicated increased Snai1 expression in podocytes. Snai1 expression on the other hand was not changed in podocyte-specific Notch1 knockout animals (Fig. 2B).
Notch1-Null Podocytes Were Protected From TGF-β1–Induced Dedifferentiation
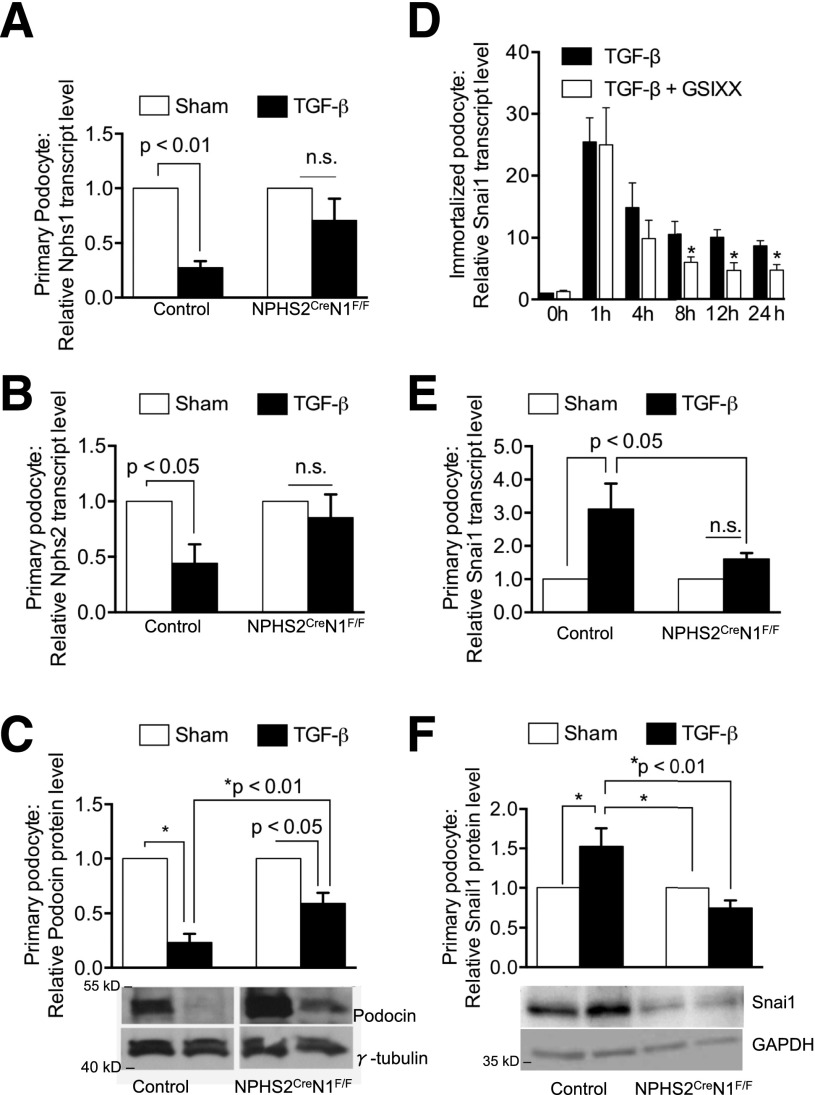
Next, we wanted to examine whether Notch1 directly regulated Nphs1 expression and podocyte dedifferentiation in vitro. We cultured primary podocytes from control and NPHS2creNotch1F/F mice and treated them with TGF-β1. TGF-β1 is a growth factor that is strongly increased in diabetes and known to play an important role in DKD development (2,25–28). We confirmed that TGF-β1 activity was increased in our diabetic models, regardless of genotype, by staining for nuclear localization of phosphorylated Smad 2/3, an indicator of active TGF-β signaling (Supplementary Fig. 2). Previous studies indicated that TGF-β1 increased cleaved Notch1 levels in podocytes via upregulating Jag1 (ligand) expression (2). We confirmed TGF-β–mediated Jag1 expression and Notch1 cleavage in primary control podocytes (Supplementary Fig. 3D). As expected, in the Notch1 knockout animals, Jag1 expression was still increased after TGF-β1 treatment but cleaved Notch1 was absent (Supplementary Fig. 3D). After 24 h of TGF-β1 treatment, Nphs1 transcript levels were significantly reduced in control podocytes, reflecting the dedifferentiation process that took place after TGF-β1 treatment. On the other hand, Nphs1 levels were maintained in Notch1-null podocytes even after TGF-β1 stimulation (Fig. 3A). Next, we examined whether the effect of Notch1 was specific for Nphs1 or if other podocyte-specific differentiation markers were also regulated. Both transcript and protein expression of Nphs2 (podocin) were significantly reduced 24 h after TGF-β1 treatment in control cells. In Notch1-null podocytes, however, Nphs2 transcript expression was maintained after TGF-β1 stimulation. Podocin protein levels were significantly reduced in Notch1-null cells but not with the severity seen in the control cells, indicating a broader effect on podocyte differentiation (Fig. 3B and C).
Figure 3.
Notch1-mediated TGF-β1–induced podocyte dedifferentiation. A: Transcript levels of Nphs1 (Nephrin) in primary cultured podocytes obtained from control (n = 5) and NPHS2creNotch1F/F (n = 4) mice and treated with or without TGF-β1 for 24 h. B: Transcript levels of Nphs2 (podocin) in primary cultured podocytes obtained from control (n = 5) and NPHS2creNotch1F/F (n = 4) mice and treated with or without TGF-β1 for 24 h. C: Representative Western blots and densitometry-based quantification of Nphs2 protein levels in cultured glomeruli. Each sample was normalized to γ-tubulin expression and expressed as a fold change between control and TGF-β1–treated samples (control, n = 6; NPHS2creNotch1F/F, n = 6). D: Relative Snai1 transcript levels of immortalized mouse podocytes treated with TGFβ1 in the presence or absence of GSIXX (n = 5). E: Relative transcript levels of Snai1 in control and Notch1 podocytes with sham or TGFβ1 treatment. F: Representative Western blots and densitometry-based quantification of Snai1 protein expression in cultured primary podocytes. Each sample was normalized to GAPDH expression and expressed as a fold change between control and TGF-β1–treated samples (control, n = 4; NPHS2creNotch1F/F, n = 4). Data information: *Statistically significantly differences by Student t test, P value <0.05. n.s., indicates no significant difference.
Snai1 and -2 are well-known direct, transcriptional targets of Notch (29), and as we had already observed differences in Nephrin and Snai1 expression in vivo, we next analyzed Snai1 and -2 regulations in cultured cells. In immortalized mouse podocytes, TGF-β1 treatment resulted in an increase in Snai1 transcript levels (Fig. 3D). Treatment of podocytes with GSIXX to block Notch cleavage did not alter Snai1 expression at the early time points (Fig. 3D) but reduced the expression of Snai1 at later time points (after 8 h). These results indicate that the later, sustained effect of TGFβ1 on Snai1 expression is Notch dependent. To determine whether the sustained expression of Snai1 was indeed Notch1 dependent, we tested the response of primary podocytes from NPHS2creNotch1F/F and control mice to 24 h of TGF-β1 stimulation. Similar to treatment in immortalized podocytes, TGF-β1 treatment also led to an increase of Snai1 expression in control primary podocytes. Snai1 expression (both transcript and protein level) was significantly lower in Notch1-null primary podocytes after TGF-β1 treatment (Fig. 3E and F) indicating that sustained expression of Snai1 in podocytes depends on Notch1.
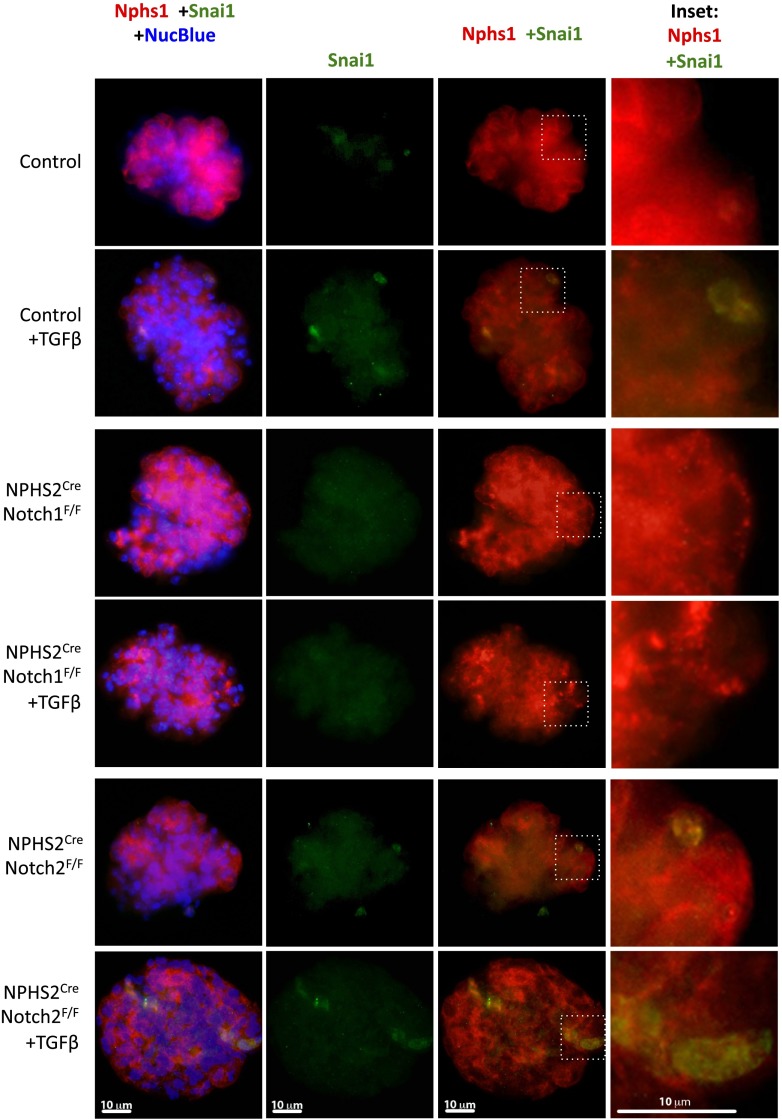
Notch signaling is highly context dependent; therefore, we next analyzed podocyte dedifferentiation in primary glomeruli without significant in vitro culturing. In addition, this method gave us the opportunity to analyze Notch2-null glomeruli, as these cells did not tolerate in vitro culturing. Snai1 expression was increased after TGF-β1 treatment in podocytes of glomeruli isolated from control mice (Fig. 4). Nuclear-specific Snai1 staining in NPHS2creNotch1F/F glomeruli was notably absent after TGF-β1 treatment, consistent with the primary podocyte data. In contrast, Snai1 expression in podocytes of glomeruli isolated from NPHS2creNotch2F/F animals was upregulated similarly to control glomeruli (Fig. 4). Nphs1 expression followed the pattern that we previously observed in the diabetic mice and the TGF-β1–treated podocytes. Notch1-null podocytes showed protection from dedifferentiation, while it appeared that Nphs1 expression in NPHS2CreNotch2F/F glomeruli was similar to control, indicating a smaller role for Notch2 in regulating Nphs1 expression (Fig. 4). In summary, these in vitro results suggest that Notch1, but not Notch2, plays an important role in podocyte dedifferentiation by regulating Nphs1, Nphs2, and Snai1 expressions.
Figure 4.
Podocyte Snai1 expression is regulated by Notch1. Representative immunofluorescent images of Nephrin (red) and Snai1 (green) in glomeruli obtained from control, NPHS2creNotch1F/F, and NPHS2creNotch2F/F animals with or without 10 ng/mL TGF-β1. Nuclei were visualized using NucBlue Fixed. Insets show higher magnification of Snai1 + Nephrin–positive podocytes.
Notch1 Plays an Important Role in Podocyte Apoptosis
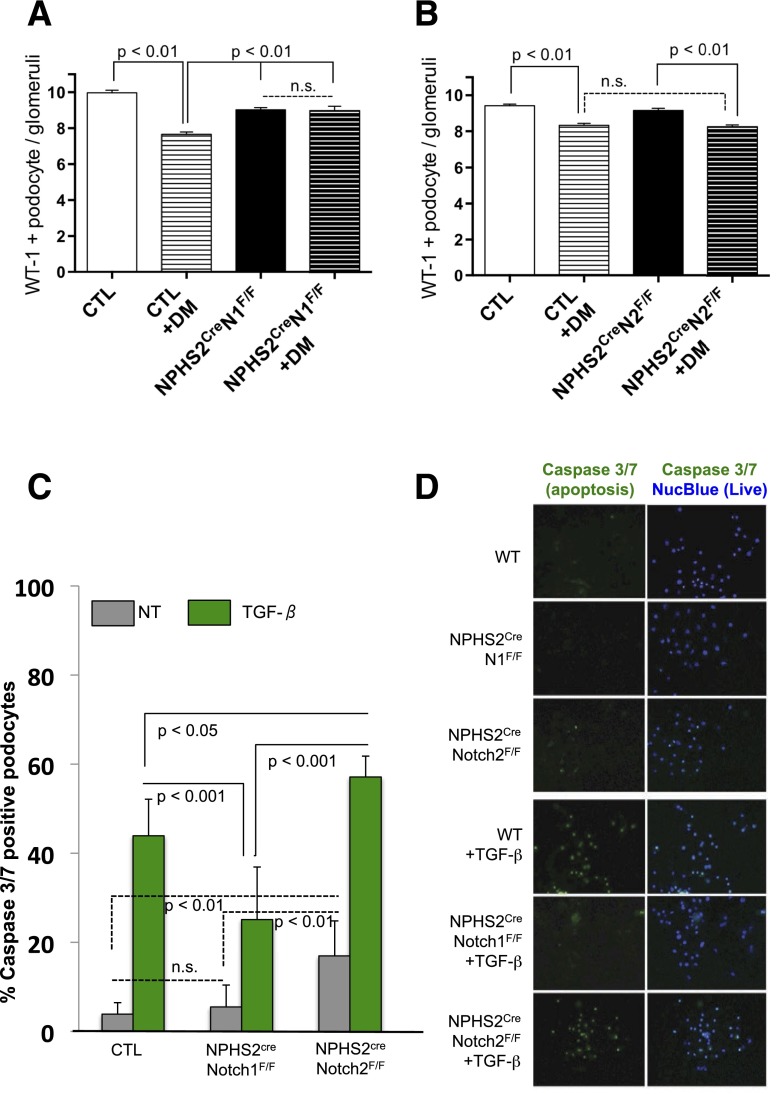
After severe podocyte injury, podocytes are lost by detachment and apoptosis, which is considered an irreversible step leading to glomerulosclerosis (30). Therefore, we next counted glomerular podocyte number by quantifying WT-1–positive podocytes in the healthy, diabetic, and podocyte-specific knockout animals. As previously reported (1,31), glomerular podocyte number was significantly lower in diabetic control animals compared with their healthy littermates (Fig. 5A and B). We found no statistically significant differences in glomerular podocyte number when diabetic NPHS2creNotch2F/F animals were compared with diabetic control animals (Fig. 5B). In contrast, podocyte number was preserved in diabetic NPHS2creNotch1F/F mice (Fig. 5A). Together, these results indicated that podocyte-specific Notch1 deletion protected kidneys from diabetes-induced podocyte loss.
Figure 5.
Loss of Notch1 prevented, whereas loss of Notch2 exacerbates, podocyte apoptosis. A: Quantification of WT-1–positive podocytes per glomerular cross-section in control (CTL) and diabetic (DM) NPHS2CreNotch1F/F mice. Error bars represent SD and P values determined by two-way ANOVA. B: Quantification of WT-1–positive podocytes per glomerular cross-section in control and diabetic NPHS2CreNotch2F/F mice. Error bars represent SD and P values determined by two-way ANOVA. n.s., indicates no significant differences. C: Mean percent of apoptotic (caspase 3/7 positive) podocytes isolated from control, NPHS2CreNotch1F/F, or NPHS2CreNotch2F/F mice and quantified before (NT) or after 24 h of TGF-β1 stimulation (TGF-β). Error bars represent SD and P values determined by Student t test. D: Representative images of caspase 3/7 reporter (green nuclei) and nuclear blue (live cells) in primary podocytes.
Next, we asked whether the protection from podocyte loss in diabetic NPHS2creNotch1F/F mice could be related to the effect of Notch1 on podocyte apoptosis. We tested this by quantifying caspase 3/7 positive primary podocytes isolated from glomeruli of control, NPHS2creNotch1F/F, and NPHS2creNotch2F/F mice. Caspase 3/7 is a sensitive marker of apoptosis. As previously described, podocyte apoptosis was significantly increased 24 h after TGF-β1 stimulation (32). We found that the number of apoptotic nuclei was significantly lower in NPHS2creNotch1F/F podocytes compared with control TGF-β1–treated cells (25% vs. 44%, respectively), indicating the important role of Notch1 in podocyte apoptosis. In contrast, we found that Notch2 deletion did not ameliorate podocyte apoptosis induced by TGF-β1. In fact, podocyte apoptosis was significantly increased in podocytes isolated from NPHS2creNotch2F/F mice compared with control cells (Fig. 5C and D). These results indicate that Notch1 plays an important role in podocyte apoptosis in vitro and may explain the protective effect of Notch1 inhibition in the context of diabetes.
Notch2 Expression Was Notch1 Dependent in Podocytes
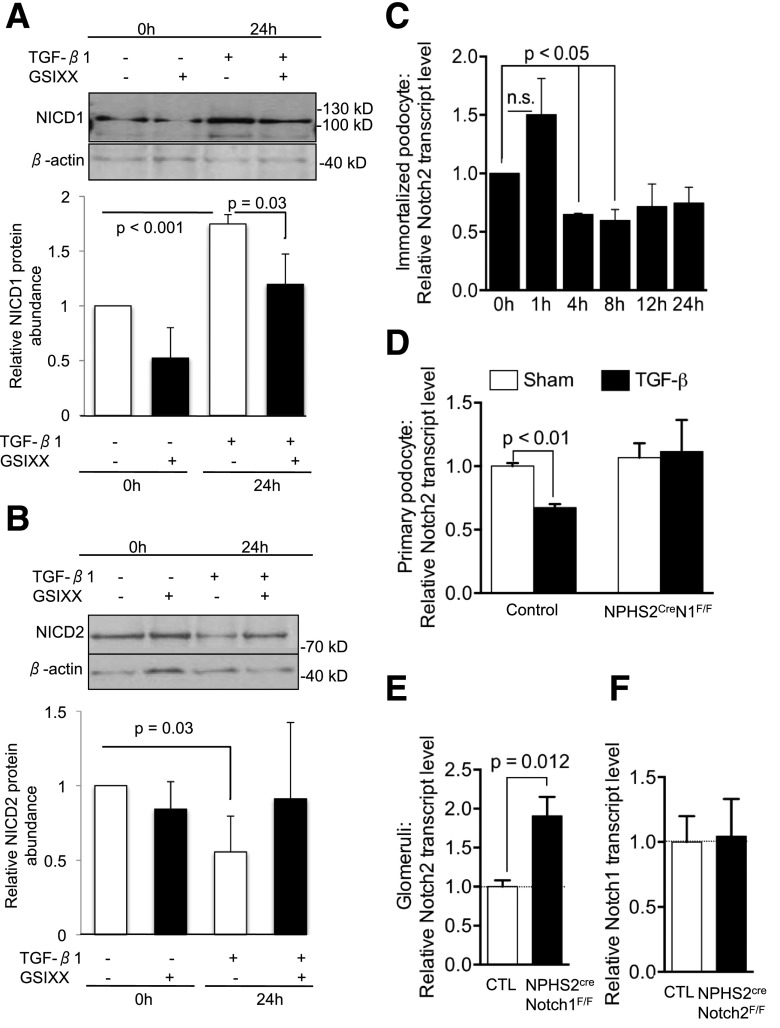
To further understand functional differences between Notch1 and Notch2 receptors and the potential interplay between them, we examined whether there is a direct regulation or compensation between these two receptors. As previously described, we found that the level of cleaved Notch1 was increased after TGF-β1 treatment (Fig. 6A and B). Treatment of cells with GSIXX ameliorated the TGF-β1–induced cleaved Notch1 expression, consistent with the previously reported ligand-mediated (Jag1) cleavage and activation of Notch1. However, unexpectedly, we found that protein level of cleaved Notch2 was decreased after TGF-β1 treatment (Fig. 6A and B). To see if this regulation occurred at the transcriptional level, we analyzed Notch mRNA levels in immortalized podocytes. We found that 4 h after TGF-β1 stimulation, Notch2 transcript levels were lower (Fig. 6C). This effect was not limited to cultured podocytes, as Notch2 levels were also decreased in primary podocytes after TGF-β1 treatment (Fig. 6D). These results indicate that while Notch1 increased after TGF-β1 treatment, Notch2 expression was decreased.
Figure 6.
Notch1-dependent regulation of Notch2 in podocytes. A: Representative immunoblot of cleaved Notch1 in immortalized mouse podocytes treated with TGF-β in absence or presence of GSIXX. Graph shows densitometry-based quantification of protein levels of cleaved Notch1 (n = 4) normalized to the average no treatment at 0 h and β-actin for loading control. P values determined by Student t test. n.s., indicates no significant differences. B: Representative immunoblot of cleaved Notch2 in immortalized mouse podocytes treated with TGF-β in absence or presence of GSIXX. Graph shows densitometry-based quantification of protein levels of cleaved Notch2 (n = 3) normalized to the average no treatment at 0 h and β-actin for loading control. P values determined by Student t test. C: Relative transcript levels of Notch2 in immortalized mouse podocytes treated with TGF-β for 0–24 h (D). Relative Notch2 transcript levels of control and NPHS2creNotch1F/F primary podocytes at baseline and after TGF-β treatment. P values determined by Student t test. E: Relative Notch2 transcript levels in control and NPHS2creNotch1F/F glomeruli (n = 5; P values determined by two-way ANOVA). F: Relative Notch1 transcript levels in control and NPHS2creNotch2F/F glomeruli (n = 4; P values determined by two-way ANOVA). Data information: error bars indicate SEM.
Next, we examined whether Notch2 expression and cleavage were directly regulated by Notch1 levels. We found that Notch2 expression was unchanged in NPHS2creNotch1F/F podocytes after TGF-β1 treatment (Fig. 6D), indicating that the decrease in Notch2 expression depends on the presence of Notch1. We also examined whether baseline (nonstimulated) Notch2 levels were regulated by Notch1. We found that Notch2 expression was slightly, but significantly (1.9-fold, P = 0.01), increased in the absence of Notch1 in NPHS2creNotch1F/F glomeruli (Fig. 6E). On the other hand, Notch1 did not compensate for the Notch2 loss in the NPHS2creNotch2F/Fmice and had levels of Notch1 identical to control glomeruli (Fig. 6F). These results indicate that the regulation of Notch2 in podocytes, both at baseline and after TGF-β1 treatment, is Notch1 dependent.
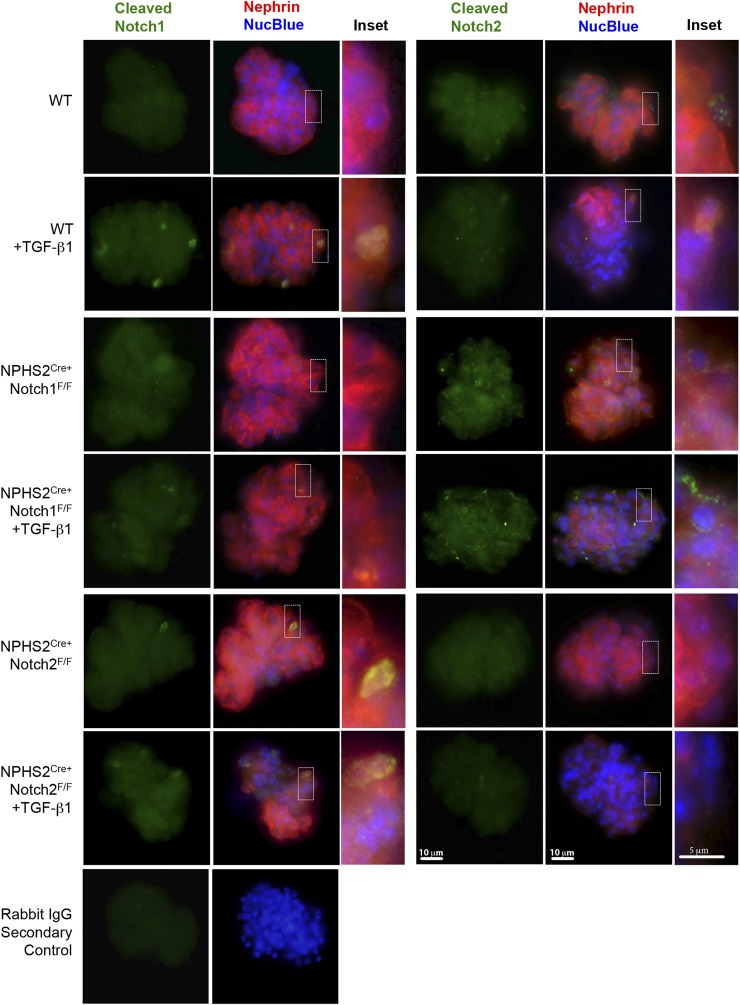
Because we did not detect increased Notch2 levels in the primary cultured Notch1 knockout podocytes but did detect this increase in the whole glomeruli, we further probed the Notch1-dependent Notch2 podocyte regulation in isolated glomeruli. We found increased cleaved Notch1 expression in the nuclei of podocytes from control glomeruli after TGF-β1 stimulation (Fig. 7). Glomeruli from NPHS2creNotch2F/F animals occasionally showed cleaved Notch1 expression in podocytes prior to stimulation, but after TGF-β1 treatment cleaved Notch1 expression was increased to a similar degree as in control glomeruli (Fig. 7). Unlike cleaved Notch1, we detected a low level of cleaved Notch2 in wild-type glomeruli at baseline in both Nphs1-positive and -negative cells. After TGF-β stimulation, the overall expression of Notch2 decreased (Fig. 7), results congruent with the TGF-β–induced decrease of Notch2 protein and mRNA in the immortalized mouse podocytes (Fig. 6B–D). There was no change in cleaved Notch2 levels in NPHS2CreNotch1F/F glomeruli after TGF-β1 treatment (Fig. 6D). These results indicate that Notch2 levels are regulated by Notch1 both at baseline and after TGF-β1 stimulation in podocytes but this compensation is not reciprocal.
Figure 7.
Increased Notch2 expression in NPHS2creNotch1F/F glomeruli. Representative immunofluorescence images of cleaved Notch1 (green), cleaved Notch2 (green), and Nephrin (red) of glomeruli isolated from wild-type (WT), NPHS2creNotch1F/F, and NPHS2creNotch2F/F animals after TGF-β1 or vehicle treatment for 3 h. Negative rabbit IgG control is shown in bottom-most row.
We also examined the expression of Notch3 and -4 receptors in our system. Notch3 (33,34) has been proposed to play a role in tubulointersitial injury and nephrotoxin-induced glomerulonephritis, respectively. Upregulation of Notch4 has been described in HIV-associated nephropathy (12). In our hands, Notch3 and Notch4 transcripts were not increased in immortalized podocytes after TGF-β1 stimulation (Supplementary Fig. 6A and D). Similarly, we did not observe differences in Notch3 transcript and protein expression after TGF-β1 stimulation of primary podocytes (Supplementary Fig. 6B and C).
Podocyte-Specific Expression Notch1 Intracellular Domain Causes Glomerulosclerosis, but Notch2 Intracellular Domain Does Not
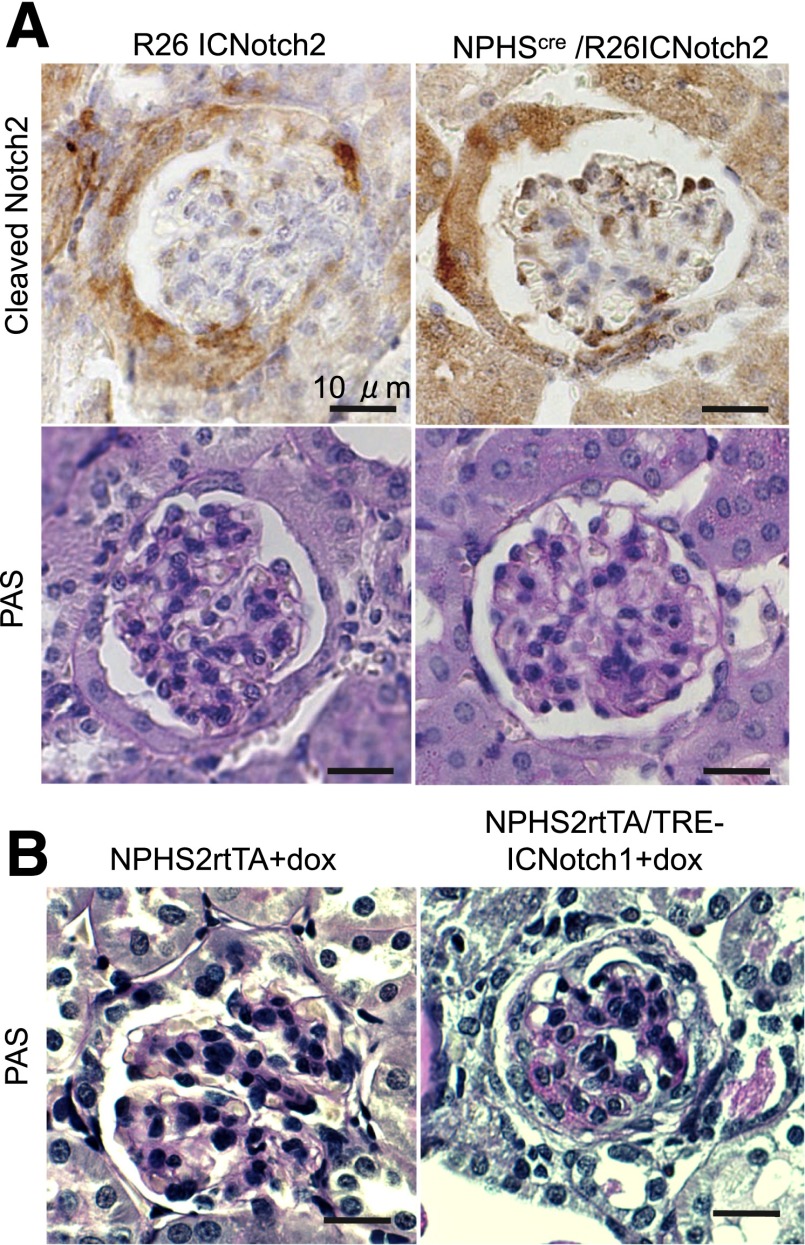
As we observed marked differences in phenotype development after podocyte-specific Notch1 and Notch2 deletion, we asked whether increased Notch1 and Notch2 are functionally equivalent. Previously, our group and the Piscione group found that overexpression of the Notch1 intracellular domain in podocytes resulted in rapid development of albuminuria and glomerulosclerosis (2,35). Therefore, we examined whether expression of the intracellular domain of Notch2 could have similar effects in podocytes. To address this, we developed a new mouse model by crossing the podocin Cre mouse (NPHS2Cre) with a transgenic mouse line expressing the intracellular domain of Notch2 (R26Notch2) (18). The R26Notch2 mouse line carries a stop codon flanked by a LoxP site just prior to the inserted ICNotch2 cassette. This mouse line has previously been shown to drive robust expression of Notch2 during development (18). We confirmed that the domain structure of the overexpressed proteins (aa1749–2293 for NICD1 and aa1701–2470 for NICD2) was structurally equivalent. In this model, the Cre-mediated recombination results in cell type–specific deletion of the stop codon, thus initiating expression of the Notch2 intracellular domain. Robust cleaved Notch2 expression in podocytes was confirmed using an antibody specific to amino acid alanine 1733 (the cleaved Notch2) (Fig. 8).
Figure 8.
Podocyte-specific constitutive expression of Notch2 intracellular domain does not induce glomerulosclerosis. A: Representative images of kidney section of control and NPHS2Cre/R26Notch2 animals, stained for cleaved Notch2 or PAS. B: Representative PAS-stained images of kidney sections of control and NPHS2rtTA/TRE-ICNotch1 animals treated with doxycycline for 3 weeks.
We did not observe significant structural abnormalities in mice with podocyte-specific NICD2 expression even at 20 weeks of age. This was in sharp contrast to mice with podocyte-specific Notch1 overexpression (Fig. 8), using the Nephrin rtTA/TRE ICNotch1. We concluded from these results that the Notch2 intracellular domain might have a different effect from the Notch1 intracellular domain, and while NICD1 overexpression causes severe glomerulosclerosis, the NICD2 overexpression did not.
Discussion
In this study, we show that Notch1 expression in podocytes plays a nonredundant role in DKD development. We used mouse models with genetic deletion of Notch1 or Notch2 specifically from mature podocytes and show that deletion of Notch1 protected mice from DKD, including albuminuria and glomerulosclerosis. On the contrary, deletion of Notch2 had no effect on disease course. We show that Notch1 plays an important role in regulating Nphs1, Nphs2, and Snai1 and thereby podocyte dedifferentiation. Snai1 is a critical transcription factor for dedifferentiation and transdifferentiation in epithelial cells and it is a well-known target of both Notch and TGF-β/Smad3 (24). Regulation of Snai1 in podocytes has been shown in vitro and in the puromycin aminonucleoside injury model in vivo (24). Here, we show that while the initial activation of Snai1 by TGF-β/Smad3 is independent of Notch signaling, Notch1 is required for its sustained expression. This sustained Snai1 activation might play an important role in glomerular disease, as we observed differences in Snai1 expression both in animal models and in patient samples with DKD (Figs. 2 and 3 and Supplementary Fig. 2). Our studies indicated that Notch regulated Snai1 and Nphs1 expression and the dedifferentiation program. Direct interaction between the Nephrin ortholog (Sticks and Stones and Hibris) and Notch has also been described in Drosophila (36). It has previously been shown that Notch regulates Nephrin levels via endocytosis (37). Further studies shall determine the contribution of each of these mechanisms to DKD development.
Dedifferentiation and apoptosis are two key alterations affecting podocytes during disease conditions. It is likely that early on, dedifferentiation is an important mechanism to protect cells from apoptosis; however, with severe damage podocytes eventually die or detach leading to permanent scarring of the glomerulus (38). Deletion of Notch1 was sufficient to protect podocytes from TGF-β–induced apoptosis. This is consistent with our prior observations showing that overexpression of the Notch1 intracellular domain lead to p53-mediated apoptosis (2). Putting all this together indicates that Notch1 is both sufficient and necessary for TGF-β–induced apoptosis. While Notch1 deletion protected from apoptosis, the rate of apoptosis was actually higher in Notch2-null cells. These results are also consistent with recent reports by Tanaka et al. (15) indicating that a Notch2 agonist antibody activates Akt and other prosurvival pathways in podocytes. In summary, it seems that Notch1 and Notch2 play different and, in the case of apoptosis, perhaps even antagonistic roles in podocytes.
Within the field of Notch biology, there are several examples for differential functions of Notch1 and Notch2. The closest example to our study is the differential effect of Notch1 and Notch2 deletion during kidney development. While genetic deletion of Notch2 in mouse models and genetic mutation of NOTCH2 in patients cause kidney developmental abnormalities, deletion of Notch1 does not have a similar effect. Opposite effects of Notch1 and Notch2 on cancer growth and survival are noted in embryonic brain tumors (39) and astrocytic gliomas (40). A study of >1,000 Chinese patients with colorectal cancer found that higher Notch1 expression was predictive of lower (∼25%) 5-year survival rates (41). Conversely, higher Notch2 tissue expression predicted better survival (∼60%) with the highest rates found in patients who had tumors that were both Notch1 negative but Notch2 positive (41).
Notch receptors show high degrees of similarities; in particular, the intracellular domains of Notch1 and Notch2 share >97% homology. Therefore, it is fascinating to learn about functional differences between Notch1 and Notch2 within the same tissue. The Kopan group generated chimeric proteins between the extracellular and intracellular domains of Notch1 and Notch2. Using these genetically engineered chimeric proteins, they demonstrated that during kidney development, Notch2 is able to reach the cell surface more efficiently than Notch1 due to differences in their extracellular domains (9). In this context, the same study demonstrated that the intracellular domains of Notch1 and -2 are functionally interchangeable and that only the difference in cell surface abundance and ligand affinity was enough to confer proximal tubule developmental dependence on Notch2 but not Notch1.
Furthermore, in this study we show that genetic overexpression of Notch1 or Notch2 intracellular domains are not equivalent in podocytes. Increased expression of Notch1 causes albuminuria and glomerulosclerosis, while mice with overexpression of the Notch2 intracellular domain does not show phenotypic alterations. This is surprising, as even during development, overexpression of the Notch1 intracellular domain is able to compensate for Notch2 to rescue the developmental defect (9). In our studies, we made sure to use proteins with comparable domain structures with the hope that they generate comparable signal strengths. At the moment, we entertain two different but nonexclusive possibilities for our observations in the podocyte NICD-overexpressing mice. The first possibility is that despite our efforts, the signal strengths generated by the Notch1 and Notch2 intracellular domains are different, leading to their different transcriptional outcome. The second possibility is that despite the similarities, Notch1 and Notch2 intracellular domains have different transcriptional binding partners. Detailed future experiments will be needed to differentiate between these possibilities.
In addition to identifying differences between Notch1 and Notch2 activation, we found that Notch1 regulated Notch2 expression in podocytes. Deletion of Notch1 resulted in an increase in Notch2 expression. Such compensatory increase is usually not the rule, but it has been shown previously in mesothelial cells (42) and mouse embryonic fibroblasts (43). The Nakayama group found that in fibroblasts, the loss of cell-cycle regulator Fbxw7 led to increased stabilization of the NICD1 and a decrease in the expression of NICD2. They concluded that the decrease in NICD2 is a direct result of an increase in NICD1, as NICD1 overexpression also lowered NICD2 levels. Findings in mesothelioma cells showed striking similarities to podocytes indicating a compensatory increase in Notch2 levels upon Notch1 deletion (42). These results indicate a potentially interesting circuit in Notch regulation that would need to be further explored; unfortunately, while in vitro studies have provided some interesting and important insight into this circuitry, in vivo studies will be important to define this mechanism.
In our view, the diabetic and/or TGF-β–driven podocyte injury is more likely a reflection of increased Notch1 expression, as we found little evidence that Notch2 was playing a role in pathology. In the context of podocyte injury, Notch1 was harmful and Notch2 had a protective effect on apoptosis, but only Notch1 induced podocyte dedifferentiation. This differential role of Notch1 and -2 may explain why loss of the Notch1 receptor, but preservation of the Notch2 receptor, in podocytes appears to be more effective in ameliorating glomerulosclerosis and proteinuria than was podocyte-specific loss of the pan-Notch cotranscriptional regulator, Rbpjk (2), or pharmacological inhibition of Notch signaling by γ-secretase inhibitors. These observations are in keeping with recent pharmacological studies indicating that Notch2 activation (by agonist antibodies) has a protective effect in the doxorubicin-induced nephrosis model (15). In that study, the authors show that a Notch2 agonist led to the activation of prosurvival pathways in podocytes. On the other hand, our genetic studies do not fully support the role of Notch2, as loss of Notch2 in vivo did not exacerbate diabetic nephropathy development. It is possible that while the short-term effect of increased Notch2 signaling is associated with a strong survival benefit, it is lost in long-term models such as diabetes.
Our studies indicate that the timing, duration, and strength of the Notch receptor and ligand expression patterns likely play important roles in negotiating the balance between physiologic healing and pathologic fibrosis. Prior studies by the Romagnani group support these conclusions (44). The coregulation of the Notch1 and -2 receptors in the podocyte suggests that global targeting of canonical Notch signaling might not be effective in preventing diabetic renal injury. Rather, our results demonstrate the need for a more directed therapeutic approach involving both the inhibition of Notch1 and, possibly, the maintenance of Notch2.
Supplementary Material
Article Information
Acknowledgments. The authors thank Frank Chinga from the University of Pennsylvania for technical assistance with animal breeding and care.
Funding. This work was supported by National Institutes of Health grants DK-076007S1 to M.T.S. and DK-076007 to K.S.
Duality of Interest. T.N. and K.S. hold a patent application for the use of Notch-based inhibitors in kidney disease. The laboratory of K.S. receives research support from Boehringer Ingelheim and Biogen for studies not related to this work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.T.S., A.G., T.N., and K.S. participated in experimental design and data analysis. M.T.S., T.N., and A.G. performed experiments and collected data. M.T.S. and K.S. wrote and edited the manuscript. R.N. and L.J.S. created some of the mouse models used in the studies and provided editorial insight. K.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0260/-/DC1.
References
- 1.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006;55:225–233 [PubMed] [Google Scholar]
- 2.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 2008;14:290–298 [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Gruenwald A, Suh JH, et al. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 2011;286:26003–26015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell 2007;128:1246. [DOI] [PubMed] [Google Scholar]
- 5.Cheng HT, Kim M, Valerius MT, et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 2007;134:801–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012;158A:85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath BM, Bauer RC, Loomes KM, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet 2012;49:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonegio R, Susztak K. Notch signaling in diabetic nephropathy. Exp Cell Res 2012;318:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Chen S, Boyle S, et al. The extracellular domain of Notch2 increases its cell-surface abundance and ligand responsiveness during kidney development. Dev Cell 2013;25:585–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murea M, Park JK, Sharma S, et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int 2010;78:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno T, Kobayashi N, Nakayama M, et al. Aberrant Notch1-dependent effects on glomerular parietal epithelial cells promotes collapsing focal segmental glomerulosclerosis with progressive podocyte loss. Kidney Int 2013;83:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma M, Callen S, Zhang D, Singhal PC, Vanden Heuvel GB, Buch S. Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS 2010;24:2161–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bielesz B, Sirin Y, Si H, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 2010;120:4040–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CL, Wang FS, Hsu YC, et al. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes 2010;59:1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka E, Asanuma K, Kim E, et al. Notch2 activation ameliorates nephrosis. Nat Commun 2014;5:3296. [DOI] [PubMed] [Google Scholar]
- 16.Surendran K, Selassie M, Liapis H, Krigman H, Kopan R. Reduced Notch signaling leads to renal cysts and papillary microadenomas. J Am Soc Nephrol 2010;21:819–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 2003;35:39–42 [DOI] [PubMed] [Google Scholar]
- 18.Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R. Notch2 activation in the embryonic kidney depletes nephron progenitors. J Am Soc Nephrol 2010;21:803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 2002;161:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krtil J, Pláteník J, Kazderová M, Tesar V, Zima T. Culture methods of glomerular podocytes. Kidney Blood Press Res 2007;30:162–174 [DOI] [PubMed] [Google Scholar]
- 21.Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int 2007;72:26–36 [DOI] [PubMed] [Google Scholar]
- 22.Saleem MA, Ni L, Witherden I, et al. Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol 2002;161:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen MP, Chen S, Ziyadeh FN, et al. Evidence linking glycated albumin to altered glomerular nephrin and VEGF expression, proteinuria, and diabetic nephropathy. Kidney Int 2005;68:1554–1561 [DOI] [PubMed] [Google Scholar]
- 24.Matsui I, Ito T, Kurihara H, Imai E, Ogihara T, Hori M. Snail, a transcriptional regulator, represses nephrin expression in glomerular epithelial cells of nephrotic rats. Lab Invest 2007;87:273–283 [DOI] [PubMed] [Google Scholar]
- 25.Ka SM, Yeh YC, Huang XR, et al. Kidney-targeting Smad7 gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear factor κB (NF-κB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia 2012;55:509–519 [DOI] [PubMed] [Google Scholar]
- 26.Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Böttinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int Suppl 2000;77:S45–S52 [DOI] [PubMed] [Google Scholar]
- 27.Kumar PA, Chitra PS, Lu C, Sobhanaditya J, Menon R. Growth hormone (GH) differentially regulates NF-kB activity in preadipocytes and macrophages: implications for GH’s role in adipose tissue homeostasis in obesity. J Physiol Biochem 2014;70:433–440 [DOI] [PubMed] [Google Scholar]
- 28.Lan HY. Transforming growth factor-β/Smad signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol 2012;39:731–738 [DOI] [PubMed] [Google Scholar]
- 29.Saad S, Stanners SR, Yong R, Tang O, Pollock CA. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol 2010;42:1115–1122 [DOI] [PubMed] [Google Scholar]
- 30.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest 2014;124:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabó C, Biser A, Benko R, Böttinger E, Suszták K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes 2006;55:3004–3012 [DOI] [PubMed] [Google Scholar]
- 32.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 2001;108:807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djudjaj S, Chatziantoniou C, Raffetseder U, et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol 2012;228:286–299 [DOI] [PubMed] [Google Scholar]
- 34.El Machhour F, Keuylian Z, Kavvadas P, Dussaule JC, Chatziantoniou C. Activation of Notch3 in Glomeruli Promotes the Development of Rapidly Progressive Renal Disease. J Am Soc Nephrol 2015;26:1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters AM, Wu MY, Onay T, et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol 2008;19:1139–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 2001;128:4251–4264 [DOI] [PubMed] [Google Scholar]
- 37.Waters AM, Wu MY, Huang YW, et al. Notch promotes dynamin-dependent endocytosis of nephrin. J Am Soc Nephrol 2012;23:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato H, Susztak K. Repair problems in podocytes: Wnt, Notch, and glomerulosclerosis. Semin Nephrol 2012;32:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res 2004;64:7787–7793 [DOI] [PubMed] [Google Scholar]
- 40.Xu P, Zhang A, Jiang R, et al. The different role of Notch1 and Notch2 in astrocytic gliomas. PLoS One 2013;8:e53654. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Chu D, Zhang Z, Zhou Y, et al. Notch1 and Notch2 have opposite prognostic effects on patients with colorectal cancer. Ann Oncol 2011;22:2440–2447 [DOI] [PubMed] [Google Scholar]
- 42.Graziani I, Eliasz S, De Marco MA, et al. Opposite effects of Notch-1 and Notch-2 on mesothelioma cell survival under hypoxia are exerted through the Akt pathway. Cancer Res 2008;68:9678–9685 [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa Y, Onoyama I, Nakayama KI, Nakayama K. Notch-dependent cell cycle arrest and apoptosis in mouse embryonic fibroblasts lacking Fbxw7. Oncogene 2008;27:6164–6174 [DOI] [PubMed] [Google Scholar]
- 44.Lasagni L, Ballerini L, Angelotti ML, et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 2010;28:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.