Abstract
The exocrine pancreas can give rise to endocrine insulin-producing cells upon ectopic expression of key transcription factors. However, the need for genetic manipulation remains a translational hurdle for diabetes therapy. Here we report the conversion of adult human nonendocrine pancreatic tissue into endocrine cell types by exposure to bone morphogenetic protein 7. The use of this U.S. Food and Drug Administration–approved agent, without any genetic manipulation, results in the neogenesis of clusters that exhibit high insulin content and glucose responsiveness both in vitro and in vivo. In vitro lineage tracing confirmed that BMP-7–induced insulin-expressing cells arise mainly from extrainsular PDX-1+, carbonic anhydrase II− (mature ductal), elastase 3a (acinar)−, and insulin− subpopulations. The nongenetic conversion of human pancreatic exocrine cells to endocrine cells is novel and represents a safer and simpler alternative to genetic reprogramming.
Introduction
Several approaches are presently under study to restore β-cell mass after the onset of type 1 diabetes. Islet transplantation has proven successful, but the scarcity of donors limits its implementation. Converting the nonendocrine cells of the pancreas (∼98% of the organ) into β-cells is one of the proposed alternatives. Proof of concept has been generated by reprogramming, which normally requires the ectopic expression of β-cell “master” genes (1–3) and, in the case of human exocrine cells, either lentiviral transduction of mitogen-activated protein kinase and signal transducer and activator of transcription 3 (4), or genome-wide chromatin-altering agents and adenoviral transduction of four reprogramming factors (3). These studies suggest the existence of cells in the exocrine (acinar and ductal) compartment with the ability to give rise to β-cells through reprogramming. Alternatively, reprogramming regimens may work on undifferentiated cell subpopulations potentially more amenable to switch fates, as reported in liver-to-pancreas settings (5,6). For practical purposes, any such undifferentiated cell capable of becoming a β-cell could be considered “progenitor like.” The widespread consensus is that putative progenitors in the pancreas should express PDX-1 (7–9). During pancreatic development, PDX-1 is expressed in progenitors at different stages (10), and it remains an insulin transcription regulator in adult β-cells (11). While Pdx1 has been reported to be mainly restricted to islet β-cells in adult mice (10), the human extrainsular tissue teems with PDX-1+/insulin− cells. Our team has reported that adult PDX-1–expressing progenitor-like cells mature into insulin-producing cells following in vitro induction with specific growth factors and extracellular matrix components (9).
Progenitor pool activation often depends on the simultaneous inhibition of transforming growth factor-β (TGF-β) signaling (which generally acts as a brake upon progenitor cell stimulation) (12–14) and the activation of the bone morphogenetic protein (BMP) pathway (14–17). BMP-7 is a U.S. Food and Drug Administration–approved homodimeric protein from the TGF-β superfamily with dual TGF-β inhibition/BMP activation abilities (12,17). This led us to further hypothesize that PDX-1–expressing putative β-cell progenitors may respond to BMP-7 stimulation. Here we describe the BMP-7–mediated conversion of cells within human nonendocrine pancreatic tissue (hNEPT) into endocrine cells that secrete insulin in response to glucose in vitro and in vivo at levels within the published range of islets isolated for research (18). In vitro lineage tracing suggests that BMP-7–responsive cells arise preferentially from a PDX-1+/hormone-negative subpopulation within hNEPT, rather than from carbonic anhydrase II (CAII)–expressing ductal cells, elas3a-expressing acinar cells, or pre-existing β-cells. Our findings offer new insights on β-cell regeneration and present a distinct translational potential.
Research Design and Methods
hNEPT Culture
Human islets were isolated at the Diabetes Research Institute, as in the study by Ricordi et al. (19), and hNEPT samples (2–4 mL) were obtained as an isolation by-product. Cells were washed and seeded on tissue culture–treated plates in FBS-supplemented and trypsin inhibitor–supplemented RPMI 1640 medium (Life Technologies, Grand Island, NY). After 48 h, floating cells were removed and cultures were treated with 100 ng/mL BMP-7 (ProSpec-Tany TechnoGene, Ness Ziona, Israel) or maintained in the starting medium as controls. Cells were allowed to grow for 4–6 days. Serum-containing medium was then replaced by serum-free Advanced RPMI 1640 (Life Technologies) without BMP-7. Three to four days later, cells either were subjected to static incubation/perifusion or were collected for further assessments/transplantation.
Immunofluorescence and Imaging Analysis
Immunofluorescence was performed as reported in the study by Vargas et al. (20). See Supplementary Table 2 for the specific antibodies used. For fluorescence imaging, Zeiss ApoTome Axiovert 200M and Zeiss LSM510 confocal microscopes were used. For quantification purposes (e.g., the percentage of C-peptide+ cells), we used ImageJ and the FIJI Analyze particles feature. For colocalization studies (e.g., lineage tracing), after background subtraction and binarization, the area overlap between EGFP and C-peptide channels was calculated by the AND operator in FIJI ImageJ. We subsequently calculated the ratio of overlap over the EGFP area and expressed it as a percentage.
Flow Cytometry
Flow cytometry was performed as previously reported (21).
Quantitative Real-Time PCR
Samples were washed in PBS and resuspended in RNAlater (Life Technologies). Quantitative real-time PCR (qRT-PCR) was conducted as in the study by Nieto et al. (22).
Glucose-Stimulated Insulin Release, Perifusion, and Total Insulin Determination
Glucose-stimulated insulin secretion (GSIS) and perifusion were conducted as previously reported in the studies by Fraker et al. (23) and Cabrera et al. (24), respectively. C-peptide/insulin content was determined by ELISA (Mercodia, Uppsala, Sweden). DNA content was used for normalization (Pico-Green dsDNA Assay Kit; Life Technologies).
Lineage Tracing
Lineage tracing was performed using the reporter lentiviral construct cytomegalovirus (CMV)-LoxP-dsRED-STOP-LoxP-EGFP (provided by Dr. P. Ravassard, Hôpital Pitié-Salpétrière-Paris, Paris, France) with CAII-Cre lentivirus, Elas3a-Cre lentivirus (both on second-generation vector pLenti-MP2), Adeno-RIP (rat insulin promoter)-Cre, and Adeno-Pdx1-Cre. CAII and ELA3A promoters were amplified by PCR from the plasmids pLightSwitch S709333 and S703278 (Switchgear Genomics, Carlsbad, CA), respectively. The 1614–4531 region of the mouse Pdx1 promoter (AF192495), containing the regulatory sequences directing β-cell expression (25) (active in human β-cells [26]), was PCR cloned from liver-isolated DNA. Cre was PCR amplified from pGD89 (Addgene). Recombinant lentiviruses were produced by the University of Miami Viral Vector Core Facility. Adeno-RIP-Cre and Adeno-Pdx1-Cre were produced in an adenovirus serotype 5 with E1/E3 deletion (Vector Biolabs, Malvern, PA). hNEPT was transduced with equal amounts of reporter and Cre constructs.
Animal Procedures
All procedures were approved by the University of Miami Institutional Animal Care and Use Committee. Male nu/nu mice (Taconic) were rendered diabetic with a single injection of streptozotocin (STZ) (200 mg/kg) and were considered to be diabetic when three consecutive glucose readings were >250 mg/dL. Insulin pellets (Linplant; LinShin Canada Inc., Toronto, ON, Canada) were placed subcutaneously for glucose homeostasis support. Under general anesthesia, hNEPT (1–2 × 107 cells) were injected under the kidney capsule of mice with sustained hyperglycemia (>300 mg/dL) in a minimal volume of saline. Buprenorphine was administered subcutaneously for pain management twice daily for 3 days. Blood glucose levels were checked daily for 10 days and then twice weekly.
For C-peptide detection, animals were fasted (∼2-4 h), and an intraperitoneal glucose bolus (2 g/kg body wt) was administered. Blood from the retro-orbital plexus was obtained under general anesthesia first after fasting and then 1 h after the bolus was administered. Human C-peptide levels in plasma were measured with the Ultrasensitive Human C-peptide ELISA Kit (Mercodia).
Statistics
GraphPad Prism version 5 was used for statistical analysis. Following the Shapiro-Wilk normality test, statistical differences between groups were calculated by using a two-tailed paired t test or Wilcoxon signed rank test. A P value of ≤0.05 was considered to be significant. Results are expressed as mean ± SD.
Results
BMP-7 Induces Phenotypic Changes Consistent With the Formation of New Endocrine Cells
hNEPT was obtained from our cGMP facility as a by-product of human islet isolation. While there is substantial variation based on donor variables, the flow cytometry analysis of five fresh hNEPT preparations yielded an average of 68.9 ± 25.8% PDX-1+ cells, 50.8 ± 24.6% CA19.9+ (ductal) cells, 35.9 ± 28.9% amylase+ (acinar) cells, 6.3 ± 2.9% CD105+ (mesenchymal) cells, and 1.7 ± 1.3% insulin+ cells. Of note, because of the rigors of isolation (which seemingly favors the preservation of specific cell types), the composition of fresh hNEPT, as determined by FACS, does not fully correlate with that observed by immunofluorescence analysis of pancreatic sections.
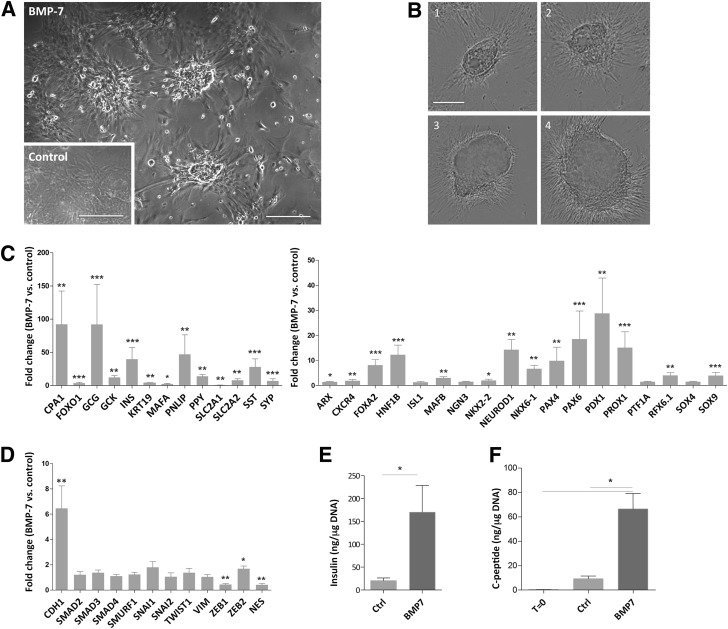
hNEPT was allowed to attach to tissue culture–treated plates for 48 h (phase 1) and subsequently was exposed to BMP-7 in the presence of serum for 4–6 days (phase 2). In a third phase, cells were cultured for 3–4 days in serum-free medium without BMP-7. Controls were cultured as above without BMP-7 in phase 2. Throughout the course of the experiment, BMP-7 induced the formation of abundant clusters (up to ∼2,000 colonies/mL, with a typical pellet of 30–40 mL from each organ). Clusters were much less abundant in control cultures, which became mainly mesenchymal like (Fig. 1A). Colony growth in the BMP-7 group was followed by in situ live cell imaging (Fig. 1B and Supplementary Fig. 1). Size increase was paralleled by an average rise of 4.8-fold in DNA content from the time of attachment to t = 12 days (n = 2). In contrast, pure islet preparations (n = 3) treated with BMP-7 failed to give rise to colonies.
Figure 1.
BMP-7 induces endocrine-like colonies in hNEPT cultures. A: The administration of BMP-7 to hNEPT results in the formation of cellular clusters, in contrast to the mostly monolayer pattern that is observed in untreated controls (inset) at the same time point (10–12 days from the beginning of culture). Scale bars, 100 μm. B: An Incucyte Zoom instrument was used to capture still images of the same colony at four time points throughout the 10 days after BMP-7 addition. Scale bar, 50 μm. Representative markers of adult pancreatic cells (C, left), pancreatic development (C, right), and EMT (D) were analyzed by TaqMan Low Density Array qRT-PCR in BMP-7–treated and untreated hNEPT at the same time point after completion of the protocol. Values represent fold change (RQ ratios) vs. the untreated control. Following the Shapiro-Wilk normality test, statistical differences between RQ ratios were calculated by a two-tailed paired t test for normal distributions or a Wilcoxon signed rank test for nonparametric distributions. ***P < 0.005; **P < 0.01; *P < 0.05. n.s., no significance (P > 0.05). E: Average insulin content (in nanograms per microgram DNA) of 10 independent hNEPT preparations after 12 days (untreated control [CTRL] and BMP-7 treated). *P = 0.019. F: Average C-peptide content (in nanograms per microgram DNA) of four independent hNEPT preparations at day 0 (t = 0) and after 12 days (CTRL and BMP-7 treated). *P = 0.02.
We studied the expression of 43 genes in hNEPT preparations (n = 8) treated with BMP-7 and untreated controls at the same time point. As shown in Fig. 1C, BMP-7 induced changes consistent with robust endocrine cell conversion, evidenced by average increases of 40-fold in insulin, 92-fold in glucagon, 14-fold in pancreatic polypeptide (PPY), 28-fold in somatostatin (SST), and 29-fold in PDX-1. GCK (glucokinase gene), MAFA, and NKX6.1, as well as islet development markers HNF1B and NEUROD1, were also elevated. The upregulation of cytokeratin-19, carboxypeptidase A, and pancreatic lipase indicates that BMP-7 induced the expression not only of endocrine genes, but also of ductal and acinar genes.
Interestingly, despite its well-documented role in preventing epithelial-to-mesenchymal transition (EMT) (12), BMP-7 had only a marginal effect on reducing the mesenchymalization of hNEPT, as only the EMT marker ZEB1 was significantly downregulated (0.4-fold, P < 0.01), while ZEB2 was even slightly upregulated (1.6-fold, P < 0.05). The epithelial marker E-cadherin (CDH1) was upregulated (sixfold, P < 0.01), but there were no substantial changes in other EMT genes (Fig. 1D). This is significant because EMT has been reported in human pancreatic exocrine cultures with long-term culture (as confirmed by our team; see Supplementary Fig. 2), and its prevention has been posited to be critical for their β-cell reprogrammability with PDX-1, MAFA, NGN3, and PAX4 (3).
Figure 1E shows the average total insulin content (in nanograms per microgram DNA) of 10 hNEPT preparations (BMP-7 treated and untreated) after 12 days in culture. The average insulin content for BMP-7–treated cells was 170 ng/μg DNA, with one preparation reaching 657 ng/μg DNA. This is within the range (75–454 ng/μg DNA) of the majority of 30 human islet preparations for research distributed through the Integrated Islet Distribution Program (18). To rule out false-positive results derived from the presence of insulin in the medium, we analyzed another four hNEPT preparations (BMP-7 treated and untreated) for C-peptide at day 12 (Fig. 1F). We also included C-peptide at t = 0, which was on average 0.29 ng/μg DNA (range 0.1–0.5 ng/μg DNA). The average C-peptide content of BMP-7–treated cells was 66.5 ng/μg DNA (range 46–103 ng/μg DNA). This represents an average ∼230-fold increase versus t = 0. Interestingly, control hNEPT cultured for the same length without BMP-7 also produced insulin and C-peptide (20.5 and 9.2 ng/μg DNA, respectively), albeit at significantly lower levels than BMP-7–treated cells (P = 0.01 and P = 0.02, respectively; Fig. 1E and F). As serum is known to contain BMPs (27), this observation suggests that serum could elicit per se an attenuated BMP-7–like response.
C-Peptide+ Cell Yield and Glucose-Responsive Insulin Secretion
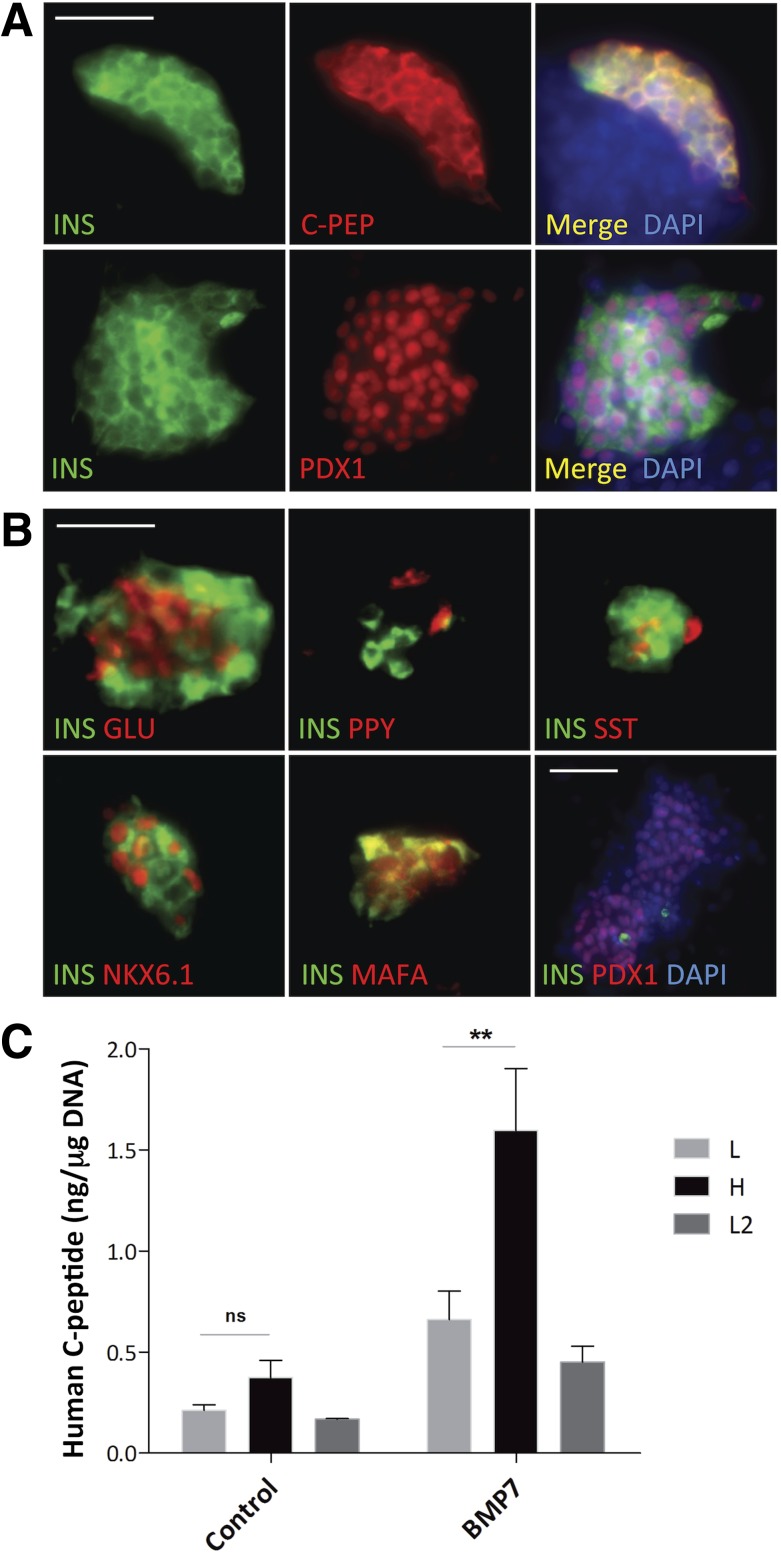
We next characterized insulin-expressing clusters by immunofluorescence. To rule out false-positive results, we also stained for C-peptide, as its detection indicates de novo insulin synthesis. Immunofluorescence was done in situ to preserve cluster cytoarchitecture. Most aggregates presented cytoplasmic insulin/C-peptide and nuclear PDX-1 (Fig. 2A). To quantify the number of C-peptide+ cells, we used the ImageJ/FIJI Analyze particles feature on an average of 12 fields/sample (n = 3). The percentage of C-peptide+ cells in hNEPT after BMP-7 treatment was 30.4 ± 4% vs. 8.1 ± 0.9% in controls (P < 0.0001).
Figure 2.
Immunofluorescence and functional in vitro analysis of BMP-7–treated hNEPT. A, top row: BMP-7–induced cell aggregates coexpress insulin (green) and C-PEP (C-peptide, red). Channel merge and DAPI nuclear staining (blue) are shown at the right. Bottom row: Nuclear PDX-1 (red)/cytoplasmic insulin (green). Channel merge and nuclear staining (blue) are shown at the right. Scale bar, 50 μm. B, top row, from left to right: INS (insulin, green)/GLU (glucagon, red); INS (insulin, green)/PPY (red); INS (insulin, green)/SST (red). Bottom row, from left to right: INS (insulin, green)/NKX6.1 (red); INS (green)/MAFA (red); and INS (green)/PDX-1 (red)/DAPI (blue) in a representative insulin−/PDX-1+ cluster. Scale bars, 50 μm for top row and pictures 1 and 2 on the bottom row. Picture 3, bottom row: 100 μm. C: GSIS of control and treated (BMP-7) hNEPT (n = 5 preparations). x-axis: L1, low glucose 1 (2.5 mmol/L); H, high glucose (20 mmol/L); L2, low glucose 2 (2.5 mmol/L). y-axis: human C-peptide (ng/μg of DNA). A second exposure to low glucose was performed to rule out false-positive results caused by “dumping” (the nonphysiological release of insulin). Data are presented as the mean ± SD (n = 5). n.s., no significance (P > 0.05, two-tailed paired t test). *Statistical significance (P < 0.05).
Glucagon, SST, and PPY were also observed (Fig. 2B), but none of the islet hormones colocalized within the same cell. NKX6.1 and MAFA, two β-cell markers, were also detected in insulin+ cells. Most clusters exhibited these and other features of endocrine maturity, such as monohormonal expression in single cells. However, other clusters appeared more immature, with nuclear and cytoplasmic MAFA in insulin-expressing cells or PDX-1+/insulin− cells (Fig. 2B).
GSIS assays conducted at day 12 showed that BMP-7–induced clusters were glucose responsive in a statistically significant manner (Fig. 2C). The average C-peptide release in the experimental group was 1.6 ng/μg DNA (stimulation index [SI] 2.4), which is within the range reported for research-grade islets (18). In contrast, controls released 0.37 ng/μg DNA, with an average SI of 1.7. Although this response was not statistically significant in controls, it could be argued that there is a trend toward glucose responsiveness, which potentially could be explained by the presence of BMPs in the serum (27). Additional perifusion assays showed that BMP-7–treated hNEPT responded dynamically to glucose, although with a wider than typical first phase (data not shown).
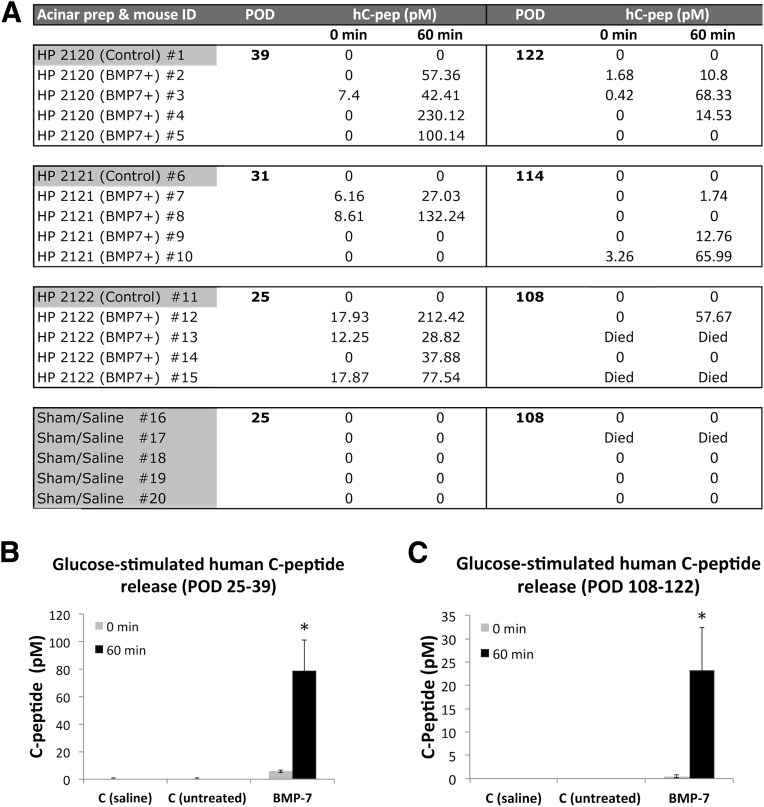
To assess in vivo function, we transplanted BMP-7–treated and control hNEPT under the kidney capsule of nu/nu STZ-diabetic mice. Three preparations were used (n = 5 animals/preparation). Five additional mice received saline (sham). For each experiment, four animals were transplanted with BMP-7–treated cells and one was transplanted with control (untreated, same time point) hNEPT. Intraperitoneal glucose tolerance tests were performed between postoperative days (PODs) 25–39 and subsequently at PODs 108–122. To this end, mice were fasted for 2–4 h before the administration of the glucose bolus. No human C-peptide could be detected in the plasma of sham-operated mice or those receiving untreated hNEPT, either prior to or after glucose stimulation. In contrast, mice transplanted with BMP-7–treated hNEPT had up to 230 pmol/L (700 pg/mL) of C-peptide upon glucose stimulation (Fig. 3A). The average glucose SI was 15.6 (P = 0.0064) at PODs 25–39 (Fig. 3B) and 43.4 (P = 0.034) at PODs 108–122 (Fig. 3C), although at the latter time point C-peptide values were generally lower than those measured at PODs 25–39. At any rate, transplanted hNEPT did not correct hyperglycemia (data not shown). In fact, insulin pellets attenuated, but did not correct, hyperglycemia in this experimental model either. Several mice died of causes associated with their background, and the remaining mice were humanely euthanized thereafter. Immunofluorescence analysis of the grafts showed insulin+ cells in close proximity to exocrine cells (Supplementary Fig. 3).
Figure 3.
Functional in vivo characterization of BMP-7–treated hNEPT. A: Human C-peptide determinations in nu/nu, STZ-treated mice transplanted with BMP-7–treated hNEPT, untreated hNEPT, or saline (sham) following the intraperitoneal glucose tolerance test. Left column: hNEPT/mouse recipient identifiers. Second/fifth columns: POD of serum human C-peptide (hC-pep determination). Third/fourth columns: human C-peptide concentrations (pmol/L) obtained prior to (0 min) and 60 min after glucose bolus injection (2.0 g/kg body wt) at PODs 25–39. Sixth/seventh columns: human C-peptide values obtained at PODs 108–122. Average glucose-stimulated human C-peptide release (0 and 60 min, represented by light gray and black columns, respectively) at PODs 25–39 (B) and PODs 108–122 (C). x-axis: C (saline), sham controls; C (untreated), control mice transplanted with untreated hNEPT; BMP-7, mice transplanted with BMP-7–treated NEPT. y-axis: C-peptide (pmol/L). Data are presented as the mean ± SD (n = 12). *P < 0.05. HP, human pancreas.
Newly Formed β-Like Cells Arise Mostly From PDX-1+ Cells
To determine the origin of β-like cells arising upon BMP-7 stimulation, we performed lineage tracing. The strategy entails transducing fresh hNEPT with a lentiviral reporter for constitutive, inheritable expression of a dsRed fluorescent marker flanked by loxP sites. The expression of a second viral construct in which Cre recombinase is placed under the control of a lineage-specific promoter results in the excision of the dsRed/STOP sequence and the subsequent expression of a second EGFP marker (Fig. 4A). In vitro lineage tracing of primary pancreatic tissues has potential limitations, such as the possibility that specific cell types are differentially transduced or that the promoters used are downregulated by the time the cells have attached and have been transduced. Still, lineage tracing is useful to provide a general indication about the most likely origin of any given cell type, and remains a powerful technique used prolifically in vivo and in vitro (3,28).
Figure 4.
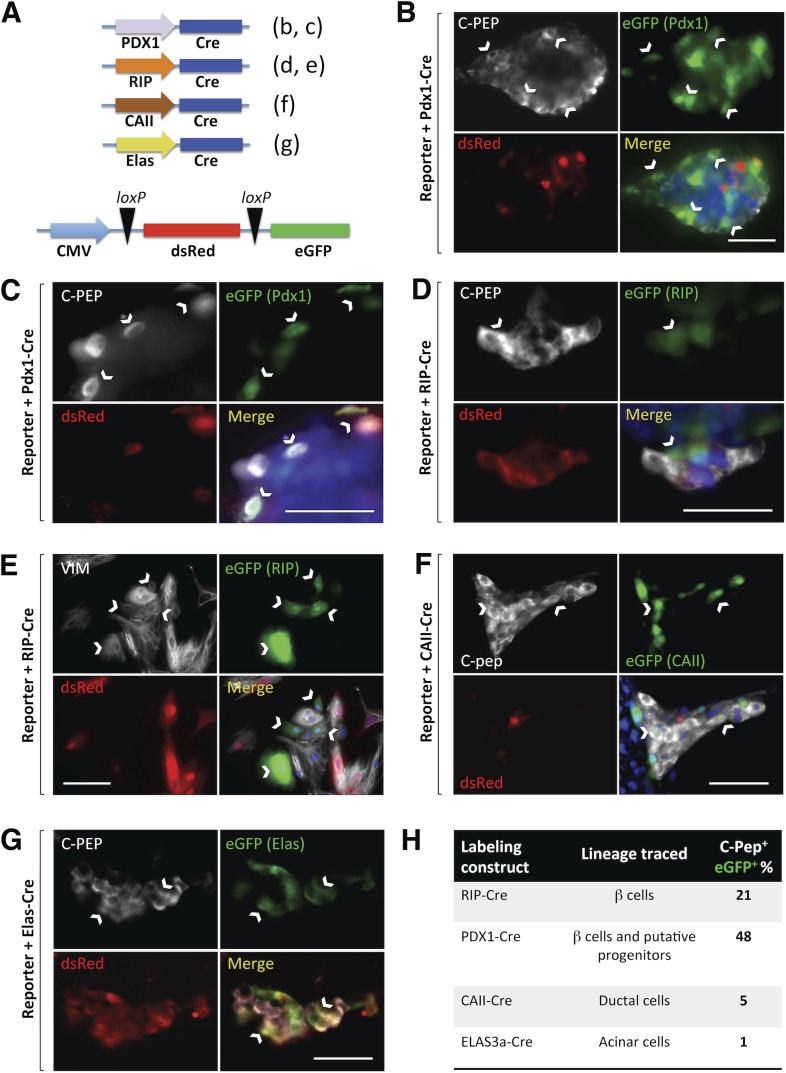
Lineage-tracing studies. A: Tissue-specific promoters (PDX-1: β-cells and putative progenitors; β-cells; CAII, ductal; ELAS: elastase 3a, acinar) drive Cre expression. The reporter expresses dsRed (red) or EGFP (green) upon Cre-mediated loxP excision. Panels corresponding to each experiment are in parentheses. C-PEP, C-peptide (white); EGFP (green); dsRed (red); and channel merge (DAPI, blue) are shown for all experiments. B: PDX-1–Cre plus reporter. Abundant C-peptide+ cells expressed EGFP (white arrows), suggesting a significant participation of PDX-1+ cells in BMP-7–induced C-peptide+ cells. Another representative field is shown in higher magnification in C. D: RIP-Cre plus reporter. A smaller percentage of RIP-expressing cells contributed to the C-peptide+ population. One such EGFP+/C-peptide+ cell (white arrow) is shown among several other dsRed+/EGFP−/C-peptide+ cells. E: RIP-Cre plus reporter. VIM, vimentin (white). Most green cells (white arrows) expressed vimentin, suggesting that residual β-cells typically undergo EMT. F: CAII-Cre plus reporter. Approximately 5% of C-peptide–expressing cells were EGFP tagged (white arrows). G: ELAS-Cre plus reporter. Several dsRed+/EGFP−/C-peptide+ cells and two EGFP+/C-peptide+ cells (white arrows) are shown. ImageJ-aided quantification of double positives indicated only a marginal acinar contribution to C-peptide+ clusters. H: Table showing the relative estimated contribution (%) of each population. Scale bars for all panels, 50 μm.
We generated constructs with RIPs, CAII, elastase 3a, and PDX-1 to tag pre-existing β-cells, mature ductal cells, acinar cells, and putative progenitors/β-cells, respectively. The reasoning behind the use of the latter is that PDX-1 expression in non–β-cells has been proposed to be a hallmark of adult pancreatic β-cell progenitors (8,29). The specificity of RIP, PDX-1, and CAII has been confirmed in multiple studies (3,30–32). Although the rat elastase 1 promoter has been used to express genes in pancreatic acinar tissues (33), human elastase 1 is evolutionarily silent, owing to promoter mutations (34). We thus chose the elastase 3a promoter, which is highly expressed in human acinar tissues (35). Lineage-specific constructs for ductal and acinar cells (CAII-Cre and Elas3a-Cre) were shuttled within lentiviral particles. However, PDX-1–Cre and RIP-Cre were transduced using adenoviral vehicles for transient expression. This was done because, if constitutively expressed throughout the experiment, the PDX-1 and insulin promoters could be reactivated in de novo–generated β-like cells (thus engaging the reporter and tagging them at that time regardless of their origin). Three independent experiments (n = 3) were conducted for these determinations. A minimum of 25 C-peptide+ cells were counted in each field (n = 12 fields/lineage-specific marker/experiment).
We first used the reporter lenti-CMV-LoxP-dsRED-STOP-LoxP-EGFP with adeno-PDX-1-Cre. At the end of the experiment (day 12), 47.7 ± 5.1% of C-peptide+ cells were EGFP tagged, thus confirming that a large proportion of newly formed C-peptide+ cells derived from cells that were PDX-1+ at the beginning of culture (Fig. 4B and C).
If new insulin+ cells arose from pre-existing β-cells (which also express PDX-1), the prediction was that the cotransduction of reporter plus RIP-Cre should yield a percentage of tagged C-peptide+ cells similar to that obtained with PDX-1-Cre. However, when we conducted this experiment, only 21.1 ± 9.2% of C-peptide+ cells were tagged (Fig. 4D), indicating that there is a smaller percentage of insulin-expressing cells deriving from cells with active RIP at the time of transfection. Interestingly, 78.6 ± 23.6% of the EGFP+ tagged cells that expressed insulin at the beginning of the experiment had become vimentin+ at day 12 (Fig. 4E), suggesting the occurrence of EMT, as previously reported in the study by Russ et al. (28). Similarly arguing against a selective preservation of β-cells as a result of BMP-7 treatment was our finding that several pancreatic epithelial markers (including insulin) were drastically downregulated during stage 2 (BMP-7 exposure) compared with their expression at the beginning of culture (stage 1), only to increase many fold after the removal of BMP-7 in stage 3 (Supplementary Fig. 4).
To further determine whether BMP-7–responsive cells also resided in the ductal tree, we conducted lineage tracing with CAII-driven Cre. As shown in Fig. 4F, only 5.4% of C-peptide+ cells had the green tag at day 12. This result confirms that CAII+ cells have the potential to mature into C-peptide+ cells, but the occurrence of such conversion is rare in this setting.
Finally, as acinar cells have also been linked to endocrine fate reassignment in reprogramming studies (1,36), we set out to test their contribution using an Elas3a-Cre cassette (Fig. 4G). In this case, only 1.37% of EGFP+ cells turned out to be C-peptide+ (Fig. 4H).
In summary, our results suggest that most BMP-7–induced β-like cells arise from PDX-1+ cells. There was a small contribution of cells that expressed ductal or acinar markers. A substantial percentage of C-peptide+ cells derived from cells that expressed insulin at the beginning of the experiment, but such contribution was still nearly 2.5-fold lower than that observed when tagging PDX-1+ cells in general.
BMP-7 Induces the Formation of C-Peptide+ Cells in Part Through the Activin-Like Kinase 3/SMAD Pathway
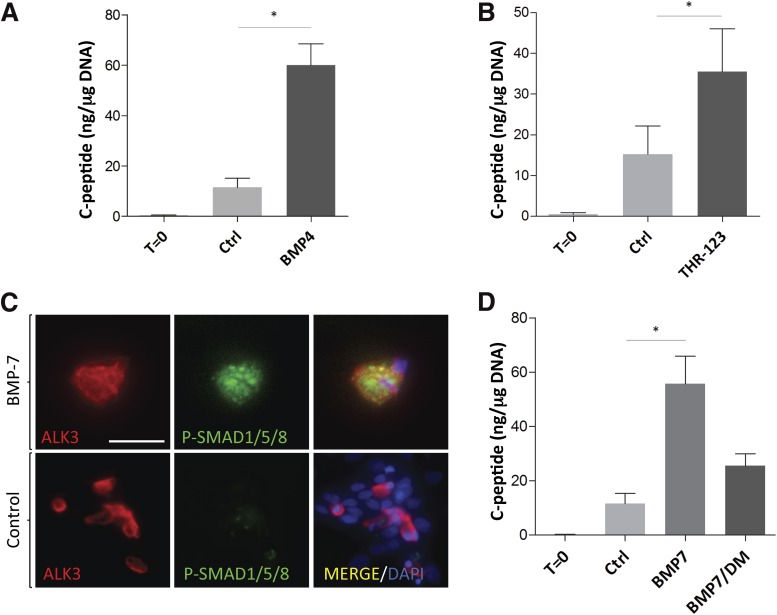
BMP-7 binds to heteromeric complexes formed by BMPR2 (bone morphogenetic receptor type II) and the activin-like kinase (ALK) 3, ALK6, or ALK2 type I serine/threonine kinase receptors (37). We found that BMP-4, which signals through ALK3 and ALK6, but not ALK2 (38), induced C-peptide production on hNEPT (150-fold vs. t = 0 [P < 0.01] and 5.4-fold (P = 0.05) vs. untreated control; n = 3) (Fig. 5A and Supplementary Table 1). These values are in line with the induction observed with BMP-7 (229-fold vs. t = 0 and 7.2-fold vs. control; see Fig. 1F), thus suggesting the involvement of ALK3, ALK6, or both. Furthermore, we observed that THR-123, an ALK3-specific agonist peptide that does not recognize ALK6 (39), also exhibited similar C-peptide induction potential (376-fold vs. t = 0 [P < 0.01] and 4.3-fold vs. control [P < 0.01], n = 3) (Fig. 5B and Supplementary Table 1). These experiments suggest that BMP-7 acts through ALK3 in this setting. Our findings are consistent with numerous reports that identify ALK3 as the mediator of BMP-7 function in several models of regeneration, including adult liver regrowth (40), epidermal Langerhans cell differentiation (41), and kidney regeneration/fibrosis reversal (39). Canonical BMP activation entails the phosphorylation of SMAD1/5/8 upon ALK3 engagement. As determined by immunofluorescence, ALK3-expressing cells presented SMAD1/5/8 phosphorylation 2 h after the addition of BMP-7 to hNEPT (Fig. 5B). To further confirm these findings, we used dorsomorphin (DM), a SMAD1/5/8 phosphorylation inhibitor, alongside BMP-7 (n = 3). We observed an ∼50% reduction in C-peptide expression compared with BMP-7 alone (Fig. 5D and Supplementary Table 1). Although this effect was not statistically significant (P = 0.13), there was a strong trend in this direction. The observation that DM does not completely decrease the BMP-7–mediated induction of C-peptide to control levels may be due to either incomplete inhibition of BMP activity by DM or the partially redundant induction of SMAD-independent pathways (including mitogen-activated protein kinase) (42) through BMP-7. Taken together, our results suggest that the effect of BMP-7 on hNEPT is at least partly mediated through the ALK3-SMAD1/5/8 BMP pathway.
Figure 5.
BMP-7 acts partially through the SMAD pathway. A: C-peptide (ng/μg DNA) following hNEPT treatment with BMP-4. *P < 0.05. B: C-peptide (ng/μg DNA) following hNEPT treatment with 100 ng/mL THR-123, an ALK3 agonist. C: Immunofluorescence detection of phosphorylated SMAD1/5/8 (green) 2 h after BMP-7 addition to hNEPT cultures (top row). ALK3 (red) and merged channels are also shown. Controls (BMP-7–untreated hNEPT, bottom row) do not exhibit phospho (P)-SMAD1/5/8 staining. Scale bar, 50 μmol/L. D: C-peptide (ng/μg DNA) following hNEPT treatment with BMP-7 and BMP-7 plus dorsomorphin, a BMP-SMAD phosphorylation inhibitor. P = 0.13. Ctrl, control.
Discussion
We show for the first time efficient conversion of primary human pancreatic exocrine tissue into functional islet endocrine cells using a simple nongenetic method. Exposure to BMP-7 (already in clinical use for unrelated conditions [43]) was sufficient to elicit this conversion, yielding abundant clusters that secreted insulin at higher levels than any exocrine (ductal or acinar) conversion method reported thus far (3,44) and exhibiting glucose responsiveness in vitro and in vivo. The rationale for these experiments stems from an extensive body of work (including our own [9]) supporting the hypothesis that the pancreatic exocrine compartment harbors β-cell progenitors.
We first considered the alternative possibility that these effects were merely the result of a BMP-7–dependent selective preservation of pre-existing β-cells, given their residual presence in hNEPT and the reported beneficial effects of BMP signaling on their function (45). However, the following three lines of evidence argued against this hypothesis: 1) selective β-cell enrichment is inconsistent with the simultaneous overall increase in cell number from t = 0 to t = 12 and the augmentation of insulin/C-peptide production upon BMP-7 treatment (Fig. 1E and F)—the observation that the insulin+ cell percentage rose from 1.7% at t = 0% to 30.4% at t = 12 while the overall DNA content also increased by 4.8-fold argues against selective β-cell preservation, and this was confirmed by standardized qRT-PCR, which also shows increases in acinar/ductal marker expression; 2) notwithstanding the theoretical caveat that exocrine tissue might be necessary for islet-derived cells to form colonies, pure islet preparations treated with BMP-7 failed to form new colonies; and 3) gene and protein expression profiling of primary hNEPT over time (Supplementary Fig. 2A and B) indicates that endocrine, exocrine, and ductal epithelial markers are rapidly downregulated in vitro, while fibroblastic/mesenchymal markers are upregulated. Immunofluorescence analysis (Supplementary Fig. 1B) confirmed our finding that mesenchymal cell types take over the cultures at the expense of epithelial cell types, which would suggest a process of EMT. Despite its reported ability to suppress EMT, the addition of BMP-7 to hNEPT did not prevent this process compared with untreated controls in any meaningful manner, as confirmed by qRT-PCR analyses (Fig. 1D). In fact, lineage-tracing analyses further indicated that at least 78% of the pre-existing RIP-expressing cells (epithelial cell types) turned into vimentin+ cells after 12 days in culture despite the presence of BMP-7 in the medium. qRT-PCR analysis performed at the end of each stage shows that, compared with the initial content, the expression of insulin significantly decreases at the end of stage 2, increasing several fold by the end of stage 3 (Supplementary Fig. 4). Other pancreatic epithelial genes exhibit a similar pattern of expression. In contrast, the expression of vimentin increases continuously in the first 10 days of culture (data not shown) and is not affected by BMP-7. Taken together, the above data are inconsistent with the mere preservation of pre-existing epithelial cells by BMP-7, as we show that epithelial markers are downregulated after attachment and then upregulated in the last stage of the protocol. Instead, our results suggest a process in which BMP-7–mediated stimulation of progenitor-like cells is followed by differentiation. In fact, such explanation is aligned with earlier observations showing that BMP-4, a BMP ligand that also stimulates hNEPT, blocks the differentiation of endocrine progenitor cells, instead promoting their expansion (46).
Another potential explanation of our results is that BMP-7 could “redifferentiate” β-cells that may have reverted to an insulin-negative progenitor-like stage due to isolation stress, as described in mice (47). For this scenario to be consistent with our lineage-tracing data, dedifferentiated β-cells would have lost insulin but kept PDX-1 expression, which would be in contradiction with the above report, in which PDX-1 expression is also lost in the β-cell dedifferentiation process. We cannot rule out, however, the contribution of other potential dedifferentiation mechanisms in which PDX-1 expression is preserved.
Indeed, lineage tracing additionally suggested that new insulin-producing cells arose mainly from a PDX-1–expressing subpopulation within hNEPT. Parallel experiments in which we tagged cells that expressed insulin at the beginning of the culture (e.g., residual β-cells that may persist in hNEPT, which also express PDX-1) yielded nearly 2.5-fold less colocalization of C-peptide and EGFP. These results are therefore consistent with the hypothesis that extrainsular progenitor-like cells are major contributors to newly formed insulin-producing cells. Rare PDX-1+ progenitor-like cells have been described (29) within the islet, and their most salient feature was that they expressed low levels of insulin. If the PDX-1+ cells described here also expressed insulin (albeit at levels that rendered it undetectable by standard immunofluorescence), the observed RIP tagging could be explained not just as persisting β-cells, but also as the result of Cre activity in PDX-1+ progenitors where insulin expression goes above a certain threshold. Regarding the small participation of elastase+ and CAII+ cells, ductal and acinar tissues are developmentally labile (1,3,7,8,36). Although we cannot discard the possibility that BMP-7 may induce elastase+ and CAII+ cell conversion, our data indicate that this is uncommon.
Transplantation experiments demonstrate long-term engraftment and function, but diabetes was not reversed. Two hypotheses could explain this observation. First, owing to the limitations of in vitro settings, BMP-7 may have induced an impaired maturation state insufficient to maintain glucose homeostasis in vivo. This would be consistent with the occasional signs of immaturity detected in the BMP-7–induced clusters (e.g., the occurrence of nuclear/cytoplasmic MAFA and PDX-1+/insulin− cells). Similar limitations were encountered in the human embryonic stem (hES) cell field, where earlier differentiation attempts also yielded cells that had high insulin content but were unable to reverse diabetes (48). Even with the most recent advances, hES cell–derived β-like cells seemingly require maturation in the host to achieve functional competence (49). Of note, it has been shown recently that the BMP inhibition used in prevailing methods for hES-to-PDX-1+ progenitor specification originates dysfunctional/polyhormonal cells. Inhibitor removal eliminated this problem (50). These findings lend additional support to our hypothesis that BMP signaling sustains functional maturation of PDX-1+ progenitor-like cells within the adult pancreas.
The second hypothesis to explain in vivo suboptimal function is exocrine contamination. Graft analysis revealed exocrine cells adjacent to endocrine cells (Supplementary Fig. 3). Acinar proteases impair islet viability in vivo and in vitro, and antiproteases were shown to rescue function (51). Transplantation outcomes might therefore be improved by purification of the endocrine fraction prior to transplantation.
There was high variability in the ability of individual hNEPT preparations to form colonies and the extent to which they produced insulin. This is hardly surprising owing to the limitations inherent in the study of primary human pancreatic tissue, which include donor age, sex, and weight; ischemia time; tissue digestion length; and yield. The establishment of quality control parameters prior to treatment is a current priority in our laboratory.
The possibility that the effects herein described are mediated through a process of dedifferentiation and subsequent redifferentiation cannot be entirely ruled out, and any extrapolation of our findings to potential in vivo physiological regeneration phenomena would be speculative at this point. While our data have led us to hypothesize that BMP-7 induces a population with progenitor-like characteristics, any such population would need to be thoroughly characterized in order to validate the “progenitor cell hypothesis.” This characterization must include expansion and multilineage differentiation potential. While the focus of our preliminary data has been on β-cells, more comprehensive lineage-tracing studies are needed to determine the full differentiation potential of BMP-7–responsive cells. As shown in Fig. 1C and Supplementary Fig. 4, other pancreatic endocrine and exocrine markers are also elevated upon BMP-7 treatment. Based on this and our experience with biliary tree progenitors (which become either pancreatic or hepatic cell types, depending on specific extracellular matrix cues [9]), as well as the observation that other putative pancreatic progenitor-like cells described in the literature are also multipotent (29,52), we believe this hypothesis to be highly plausible. Our lineage-tracing setting is already in place and will be used to further ascertain the origin of other endocrine and exocrine cell types after BMP-7 treatment.
Further studies will help us determine whether these BMP-7–responsive cells remain intact in patients with type 1 diabetes, which may set the ground for potential therapeutic interventions to induce β-cell regeneration in vivo.
Supplementary Material
Article Information
Acknowledgments. The authors thank Kevin Johnson, Alberto Fachado, and Marta García Contreras, as well as the staff of the Preclinical Cell Processing and Translational Models Core and the Clinical Chemistry, Biomarkers and Immunoassay Laboratory (all at the Diabetes Research Institute) for their technical contribution and helpful discussions. The authors also thank Mar Pairó Delgado and Eduard Montanya (Bellvitge Biomedical Research Institute, IDIBELL, Barcelona, Spain), as well as Abelardo Montalvo, Arthur Soderberg, Runa de Negreiros Fernandes, and Ana Flavia Pacheco Dos Santos for their contributions while they were in training in the laboratory of J.D.-B.
Funding. This study was funded by the Diabetes Research Institute Foundation, the Foundation for Diabetes Research, the Scientific Awards Committee of the Miller School of Medicine at the University of Miami, the University of Miami Miller School of Medicine Dean's Bridge Award, the Fred and Mabel R. Parks Foundation, the Michael J. and Katherine E. Franco Foundation, the Frank Strick Foundation, and Mildred Graff. Islets were obtained through the Integrated Islet Distribution Program, which is coordinated by the City of Hope (Duarte, CA), and is supported in part by National Institutes of Health grants DK-70460 and U42-RR-016603.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.K., S.Á.-C., and G.L. contributed to the discussion; gave advice on the experimental design; and contributed to data collection, analysis, and interpretation. N.V. and K.R.P. contributed to data collection and analysis. M.B. contributed to the discussion; gave advice on the experimental design; and contributed to data collection and analysis. C.R. and L.I. contributed to the discussion and gave advice on the experimental design. R.L.P. and J.D.-B. contributed to the conception and design of the study and the data analysis and wrote the article. R.L.P. and J.D.-B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0688/-/DC1.
References
- 1.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J, Sugiyama T, Liu Y, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 2013;2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima MJ, Muir KR, Docherty HM, et al. Suppression of epithelial-to-mesenchymal transitioning enhances ex vivo reprogramming of human exocrine pancreatic tissue toward functional insulin-producing β-like cells. Diabetes 2013;62:2821–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemper M, Leuckx G, Heremans Y, et al. Reprogramming of human pancreatic exocrine cells to beta-like cells. Cell Death Differ 2015;22:1117–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yechoor V, Liu V, Espiritu C, et al. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell 2009;16:358–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banga A, Akinci E, Greder LV, Dutton JR, Slack JM. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A 2012;109:15336–15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner-Weir S, Inada A, Yatoh S, et al. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans 2008;36:353–356 [DOI] [PubMed] [Google Scholar]
- 8.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes 2004;5(Suppl. 2):16–22 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Lanzoni G, Carpino G, et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cells 2013;31:1966–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet 2002;3:524–532 [DOI] [PubMed] [Google Scholar]
- 11.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 1998;12:1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003;9:964–968 [DOI] [PubMed] [Google Scholar]
- 13.Cheifetz S, Li IW, McCulloch CA, Sampath K, Sodek J. Influence of osteogenic protein-1 (OP-1;BMP-7) and transforming growth factor-beta 1 on bone formation in vitro. Connect Tissue Res 1996;35:71–78 [DOI] [PubMed] [Google Scholar]
- 14.Jiang FX, Stanley EG, Gonez LJ, Harrison LC. Bone morphogenetic proteins promote development of fetal pancreas epithelial colonies containing insulin-positive cells. J Cell Sci 2002;115:753–760 [DOI] [PubMed] [Google Scholar]
- 15.Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science 2009;324:1707–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung WS, Andersson O, Row R, Kimelman D, Stainier DY. Suppression of Alk8-mediated Bmp signaling cell-autonomously induces pancreatic beta-cells in zebrafish. Proc Natl Acad Sci U S A 2010;107:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui L, Geens M, Sermon K, Bouwens L, Mfopou JK. Role of BMP signaling in pancreatic progenitor differentiation from human embryonic stem cells. Stem Cell Rev 2013;9:569–577 [DOI] [PubMed] [Google Scholar]
- 18.Kayton NS, Poffenberger G, Henske J, et al. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am J Physiol Endocrinol Metab 2015;308:E592–E602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988;37:413–420 [DOI] [PubMed] [Google Scholar]
- 20.Vargas N, Álvarez-Cubela S, Giraldo JA, et al. TAT-mediated transduction of MafA protein in utero results in enhanced pancreatic insulin expression and changes in islet morphology. PLoS One 2011;6:e22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein D, Misawa R, Bravo-Egana V, et al. MicroRNA expression in alpha and beta cells of human pancreatic islets. PLoS One 2013;8:e55064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieto M, Hevia P, Garcia E, et al. Antisense miR-7 impairs insulin expression in developing pancreas and in cultured pancreatic buds. Cell Transplant 2012;21:1761–1774 [DOI] [PubMed] [Google Scholar]
- 23.Fraker CA, Cechin S, Álvarez-Cubela S, et al. A physiological pattern of oxygenation using perfluorocarbon-based culture devices maximizes pancreatic islet viability and enhances β-cell function. Cell Transplant 2013;22:1723–1733 [DOI] [PubMed] [Google Scholar]
- 24.Cabrera O, Jacques-Silva MC, Berman DM, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant 2008;16:1039–1048 [PMC free article] [PubMed] [Google Scholar]
- 25.Gannon M, Gamer LW, Wright CV. Regulatory regions driving developmental and tissue-specific expression of the essential pancreatic gene pdx1. Dev Biol 2001;238:185–201 [DOI] [PubMed] [Google Scholar]
- 26.Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology 2009;150:1627–1635 [DOI] [PubMed] [Google Scholar]
- 27.Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol 2009;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russ HA, Ravassard P, Kerr-Conte J, Pattou F, Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One 2009;4:e6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 2011;8:281–293 [DOI] [PubMed] [Google Scholar]
- 30.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human β-cells revealed by cell-lineage tracing. Diabetes 2008;57:1575–1583 [DOI] [PubMed] [Google Scholar]
- 31.Inada A, Nienaber C, Fonseca S, Bonner-Weir S. Timing and expression pattern of carbonic anhydrase II in pancreas. Dev Dyn 2006;235:1571–1577 [DOI] [PubMed] [Google Scholar]
- 32.Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 2003;120:35–43 [DOI] [PubMed] [Google Scholar]
- 33.Hammer RE, Swift GH, Ornitz DM, et al. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol 1987;7:2956–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose SD, MacDonald RJ. Evolutionary silencing of the human elastase I gene (ELA1). Hum Mol Genet 1997;6:897–903 [DOI] [PubMed] [Google Scholar]
- 35.Kim MS, Pinto SM, Getnet D, et al. A draft map of the human proteome. Nature 2014;509:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeyens L, Lemper M, Leuckx G, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol 2014;32:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 2005;16:251–263 [DOI] [PubMed] [Google Scholar]
- 38.ten Dijke P, Yamashita H, Sampath TK, et al. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem 1994;269:16985–16988 [PubMed] [Google Scholar]
- 39.Sugimoto H, LeBleu VS, Bosukonda D, et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 2012;18:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimoto H, Yang C, LeBleu VS, et al. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J 2007;21:256–264 [DOI] [PubMed] [Google Scholar]
- 41.Yasmin N, Bauer T, Modak M, et al. Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J Exp Med 2013;210:2597–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584 [DOI] [PubMed] [Google Scholar]
- 43.Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 2010;11:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corritore E, Dugnani E, Pasquale V, et al. β-Cell differentiation of human pancreatic duct-derived cells after in vitro expansion. Cell Reprogram 2014;16:456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab 2007;5:207–219 [DOI] [PubMed] [Google Scholar]
- 46.Hua H, Zhang YQ, Dabernat S, et al. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem 2006;281:13574–13580 [DOI] [PubMed] [Google Scholar]
- 47.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 49.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014;32:1121–1133 [DOI] [PubMed] [Google Scholar]
- 50.Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015;34:1759–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loganathan G, Dawra RK, Pugazhenthi S, et al. Insulin degradation by acinar cell proteases creates a dysfunctional environment for human islets before/after transplantation: benefits of α-1 antitrypsin treatment. Transplantation 2011;92:1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 2004;22:1115–1124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.