Abstract
Gluconeogenesis is a complex metabolic process that involves multiple enzymatic steps regulated by myriad factors, including substrate concentrations, the redox state, activation and inhibition of specific enzyme steps, and hormonal modulation. At present, the most widely accepted technique to determine gluconeogenesis is by measuring the incorporation of deuterium from the body water pool into newly formed glucose. However, several techniques using radioactive and stable-labeled isotopes have been used to quantitate the contribution and regulation of gluconeogenesis in humans. Each method has its advantages, methodological assumptions, and set of propagated errors. In this review, we examine the strengths and weaknesses of the most commonly used stable isotopes methods to measure gluconeogenesis in vivo. We discuss the advantages and limitations of each method and summarize the applicability of these measurements in understanding normal and pathophysiological conditions.
Introduction
Glucose homeostasis is tightly regulated by an intricate and complex network of intracellular pathways and hormonal feedback mechanisms. To maintain plasma glucose concentrations within a narrow range (70–140 mg/dL) throughout the day, postprandial glucose absorption is balanced by regulation of peripheral glucose utilization and hepatic glucose production. During fasting, glucose production gradually decreases primarily due to a progressive decrease in glycogenolysis, and as glycogen stores are progressively depleted, there is a relative increase in the contribution from gluconeogenesis in the liver (1). Gluconeogenesis in the kidneys is minimal, except under acidotic conditions when the kidneys may account for up to 20% of total glucose production (2). Over the past three decades, gluconeogenesis has been the topic of intense investigation, as researchers sought to find the best method to quantitate its contribution to glucose production and understand its regulation and the role it plays in disorders associated with hypoglycemia and diabetes.
Understanding in vivo contributions of gluconeogenesis and glycogenolysis to glucose production has helped map the myriad pathways leading to glucose formation and has been invaluable in elucidating the redundancy and efficiency of the gluconeogenic process. Several techniques, using both radioactive and stable-labeled isotopes, have been used to trace intracellular events involved in gluconeogenesis. More recent isotopic techniques independently measure glucose production and fractional gluconeogenesis, and the rate of absolute gluconeogenesis is calculated by multiplying these two parameters. The rate of glycogenolysis is subsequently calculated as the difference between the rates of glucose production and absolute gluconeogenesis.
Alternatively, markers of glycogen content have been measured using nuclear magnetic resonance (NMR) spectroscopy to quantify glycogenolysis and subsequently derive rates of gluconeogenesis as the difference between glucose production and glycogenolysis. Every method has its own set of model assumptions and propagated errors, which should be considered when using the technique. In this review, we will provide a brief historical perspective and current views of the in vivo methodologies used to measure gluconeogenesis and glycogenolysis, discuss their advantages and limitations, and summarize the applicability of these measurements in understanding normal and pathophysiological conditions. Table 1 lists the tracers that have been used in the in vivo measurement of gluconeogenesis in humans over time.
Table 1.
Isotope techniques used to measure gluconeogenesis in humans
| Techniques | Isotope | References |
|---|---|---|
| Labeled metabolites and precursors | [2-14C]acetate | (37,38) |
| [3-13C]lactate | (48) | |
| [3-14C]lactate | (42,44) | |
| [U-14C]lactate | (43) | |
| [3-13C]alanine | (42) | |
| [U-14C]alanine | (43,45,46) | |
| [U-14C]glycerol | (120,121) | |
| [U-13C]glycerol | (41) | |
| Mass isotopomer analysis | [2-13C]glycerol | (12,35,47,48) |
| [U-13C]glucose | (12,26,31,35,49,50) | |
| 13C-NMR spectroscopy | None | (64–68,76) |
| Deuterium oxide | ||
| C5-HMT, GCMS | 2H2O | (36,75,82,84–87) |
| Average deuterium method, GCMS | 2H2O | (2,36,108) |
| 2H-NMR spectroscopy | 2H2O | (74,76,96,122) |
Measurements of Gluconeogenesis From Gene Expression
Many basic scientists have extrapolated changes in gluconeogenesis from the mRNA expression of key gluconeogenic enzymes such as glucose-6-phosphatase and PEPCK in ex vivo experiments using murine and human liver biopsy specimens (3). However, the process of converting potential gluconeogenic substrates to glucose is far more complex. In vivo measurements in mice and dogs show no correlations between gene expression of PEPCK and gluconeogenic flux (4–6). mRNA expression data of gluconeogenic enzymes are particularly useful for understanding experimental effects on key enzymes but do not provide a definitive picture of the complexities and interplay among substrate, hormone, and enzyme regulation of the gluconeogenic pathway. Any conclusions drawn about gluconeogenesis from mRNA expression data must be validated with specific in vivo kinetic measures of gluconeogenesis under similar experimental conditions. Therefore, mRNA measurements of gluconeogenic enzymes may be useful for estimating gene expression, but only in vivo isotopic measurements are capable of assessing the integrity of the pathways and rates of gluconeogenesis and glycogenolysis.
The Cori Cycle and the Complexity of Problems When Measuring Gluconeogenesis In Vivo
The mechanism by which lactate is produced in muscle, taken up by the liver, and converted into glucose, the Cori cycle, was originally proposed in 1929 by Carl and Gerty Cori (7) and was widely accepted despite the lack of in vivo evidence of its existence in humans. Over the next 30 years, Cori and Cori elucidated much of the enzymatic, substrate, and hormonal regulation of glycogen metabolism in both liver and muscle. In 1947, Carl and Gerty Cori shared the Nobel Prize “for their discovery of the course of the catalytic conversion of glycogen” and identified the first inborn error of metabolism, glucose-6 phosphatase deficiency, now known as glycogen storage disease type 1a (8). However, it was not until 1961, when Reichard et al. (9) performed the first in vivo experiment using [U-14C]glucose proving the existence of the Cori cycle in humans. Subsequently, Cahill and colleagues (10) and Atwell and Waterhouse (11) used bolus isotope injections and various models of product precursor relationships to estimate the contribution of gluconeogenesis to glucose production in humans. Yet, there remained no consensus at that time as to the contribution of gluconeogenesis to glucose production because of significant intra- and interstudy and -subject variability. This is not surprising since we now recognize that to obtain a reasonable reflection of the contribution of a precursor to the glucose pool, the entire glucose pool must turnover, which is usually three to five biological half-lives of glucose or under basal conditions, 4–5 h (12).
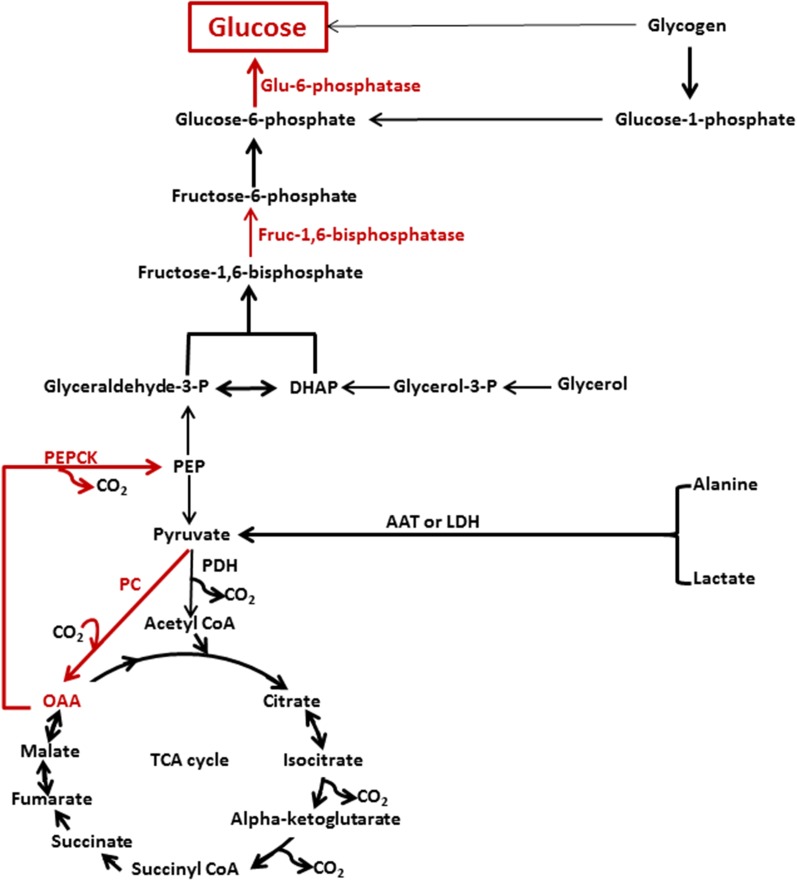
Cori and Cori’s original description, although brilliant, was restricted to a cycle involving glucose production during exercise or periods of relative hypoxia when lactate represents a major substrate for gluconeogenesis. However, the process of gluconeogenesis is not limited to lactate and involves the entry of a variety of carbon sources, including amino acids and glycerol as well as lactate and pyruvate (Fig. 1). As a result of substrate interconversions in the tricarboxylic acid (TCA) cycle, as well as the gluconeogenic and hexomonophosphate pathways, it is difficult, if not impossible, to quantitate exactly the relative contribution of one substrate from that of another. Early experimental designs also involved quantifying plasma alanine concentrations as a surrogate measure of gluconeogenesis (13,14). These studies helped lay the foundational framework of understanding the body’s response to fasting and hypoglycemia but only examined the contribution of one substrate to the gluconeogeneic pathway.
Figure 1.
Major enzymes and substrates involved in the regulation of gluconeogenesis. Red arrows and text represent the major enzymes and pathways involved in the regulation of gluconeogenesis. Direct glucose release from glycogen via the debranching enzyme accounts for <10% of the total glucose made via gluconeogenesis. AAT, alanine aminotransferase; fruc-1,6-bisphosphatase, fructose-1,6-bisphosphatase; glu-6-phosphatase, glucose-6-phosphatase; glyceraldehyde-3-P, glyceraldehyde-3-phosphate; glycerol-3-P, glycerol-3-phosphate; LDH, lactate dehydrogenase; OAA, oxaloacetate; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase.
The net production of glucose via gluconeogenesis involves the orchestrated activities of several key enzymes (pyruvate carboxylase, PEPCK, aldolase, fructose-1,6-diphosphatase, and glucose-6-phosphatase) in conjunction with dynamic changes of the rates of glycogen synthesis and glycogenolysis (15) (Fig. 1). In addition, each of these enzymatic steps may be influenced by 1) substrate concentrations, 2) cofactor requirements, 3) substrate inhibitions and activation, 4) the redox state of the cell, and 5) hormonal modulation, which results in additional layers of complexity under normal circumstances.
Cherrington et al. (16) developed and continue to exploit an elegant multiple catheterized model in the conscious dog. By measuring blood flow through the liver, plasma concentrations of glucose and substrate concentrations across the liver, and glucose turnover, they have carefully dissected the roles of hormones and substrates in the regulation of both glycogenolysis and gluconeogenesis (6,16,17). Yet, because of differences in the physiologic regulation between dogs and humans, as well as the absence of our ability to perform comparable experiments in humans, the veracity of their observations remains to be determined in the human condition.
Considerations of Measuring Gluconeogenesis In Vivo
Several theoretical and technical factors should be considered when estimating in vivo rates of gluconeogenesis.
Theoretical Considerations
All methods used to measure gluconeogenesis quantify fractional gluconeogenesis or the fraction of new glucose made as a function of total glucose turnover. Therefore, a fundamental factor in quantifying total gluconeogenesis is the measurement of total glucose turnover or glucose flux (rate of appearance = utilization). Under noninvestigative conditions, glucose rate of appearance into the plasma space has three sources: 1) exogenous glucose resulting from the digestion and absorption of dietary carbohydrates, 2) hepatic glycogenolysis, and 3) hepatic (±renal) gluconeogenesis. Under near steady-state conditions, glucose production is equivalent to total glucose turnover minus the exogenously infused natural and tracer glucose. To measure glucose turnover, a variety of recyclable and nonrecyclable tracers have been used (18). From our perspective, using the nonrecyclable tracer [6,6-2H2]glucose is ideal because there is no real potential for this tracer to be released into the circulation via gluconeogenesis (indirect pathway) and very little, if any, for it to be incorporated and subsequently released from glycogen into the systemic circulation except by the potential of glycogen cycling (12,19–21). However, at the enrichments generally used and the rates of potential glycogen cycling reported under both insulin clamp and postprandial states, this may be below the levels of detection by existing instrumentation, if it occurs at all.
It is equally important to ensure that assessments of glucose turnover and fractional gluconeogenesis are collected during near isotopic and substrate steady-state period. For the primary measurements of total glucose turnover, the isotopic tracer infusion should be sufficiently long to achieve near steady-state conditions. Since no real steady state exists (i.e., the body is continuously in metabolic adjustment), the near steady-state period is defined as the time required for the entire glucose pool to be totally replaced, which requires approximately three to five times the biological half-life of plasma glucose or ∼4–5 h in humans under study conditions (22). Under study conditions using the hyperinsulinemic-euglycemic clamp technique, this time will be significantly shorter. Plasma samples for isotopic enrichment should be collected during this near steady-state period because shorter infusion times of the labeled glucose result in higher estimated rates of glucose production (12). Since the timing of the glucose tracer infusion is independent of measurements of fractional gluconeogenesis, this artifact could result in overestimates of the rate of absolute gluconeogenesis.
Similarly, when quantifying fractional gluconeogenesis, the isotopic label from a potential gluconeogenic precursor initially enters a glucose pool without carbon or hydrogen labeling, and the enrichment or specific radioactivity will progressively rise over time until the rate of entry and removal of the carbon-labeled glucose are equal. This again takes generally three to five biologic half-lives of glucose. Therefore, soon after the start of the infusion of the labeled gluconeogenic precursor (alanine, lactate, or glycerol), the product-precursor relationship reflects only the small amount of product initially made with the labeled tracer. As near steady-state enrichment of the product pool is attained (with the entire turnover of the circulating glucose pool), the product-precursor relationship more accurately reflects the contribution of the precursor to glucose production via gluconeogenesis.
During hyperinsulinemic-euglycemic or hyperglycemic clamps, glucose isotopes are infused with the exogenous glucose to approximate the near steady-state enrichment or specific activity under the baseline conditions (prior to the clamp). This is known as the “hot GIF” method and avoids wide swings in the plasma glucose enrichment (or specific activity) and minimizes the need to make assumptions about changes in pool size over the course of an experiment. Under non–steady-state conditions, it is difficult to estimate (and thus interpret) rates of gluconeogenesis unless complex mathematical models are used to simulate glucose turnover (23,24), changes in precursor concentrations, and enrichment (25). Thus, although such calculations can be made, in our opinion, these models are impossible to validate. As a result of this factor alone, we have always tried to use protocol designs that attempt to achieve both isotopic and substrate steady state over a time period sufficiently extending beyond three to five biological half-lives of the glucose pool. We acknowledge this experimental condition is nonphysiological, as we as humans are rarely at steady state. We have used a similar approach during entral absorption. By using small frequent feeding over a 4–5-h period to achieve near steady-state enrichment, we believe we have been able to obtain reasonable estimates of gluconeogenesis that are reproducible and mimic the glucose homeostasis achieved after a bolus meal (26,27).
Last, it is important to consider that both the liver and kidney may produce glucose (1). Glucose production by the kidney, under nonacidotic conditions, is offset by the uptake of glucose by the kidney, which results in a net zero contribution to systemic glucose production (28). Metabolic acidosis results in the catabolism of glutamine by the kidney and the production and excretion of ammonium as a compensatory mechanism. This facilitates the excretion of hydrogen ions and provides the carbon skeleton of glutamine to be used for gluconeogenesis and the return of this glucose to the systemic circulation (2,29,30).
Technical Considerations
Potential errors in experimental designs and analytical procedures should also be recognized prior to analyses. For example, when infusing trace and/or unlabeled glucose, the accuracy of the infusion rate must be ensured by calibrating the infusion pump(s) on a regular basis. Volume displacement pumps are far more accurate and precise than peristaltic pumps and are usually preferred by us. However, depending on the study design, this may not always be feasible. During the analytical phase, it is also important to measure the infusate isotopic enrichment and concentration given to each subject in order to confirm the rate of isotope delivery throughout the study protocol. Finally, when using gas chromatography–mass spectrometry (GCMS), appropriate and frequent standard solutions should be used to calibrate the machine and construct standard calibration curves to increase the reliability of the methodology. In addition, such standards should be scattered throughout the analysis (particularly long runs) to protect the accuracy by identifying instrument drift over the course of analysis. Due to these and other minor technical errors, if multiple methods are used (e.g., stable vs. radioisotopes to measure glucose production and fractional gluconeogenesis), one must be cautious of propagated errors from each method.
In the last 30 years, many experimental techniques using both radio-labeled and stable-labeled isotopes have been proposed to directly or indirectly quantify gluconeogenesis (Table 1). However, because of the factors mentioned above, there is no gold standard (and most likely will never be) by which to judge the relative value of one method over another. We have used and published studies using most of the isotopic methods outlined (12,26,27,31–36) and therefore hope we stand in a good position to provide a reasonable evaluation of each. We will acknowledge our potential bias as a result of our experience, but we attempt to present a fair and valid methodological evaluation. In addition, it is our desire that the interested reader will gain an understanding of the theoretical background of each method and the resources required to undertake such studies. Data interpretation and extrapolation relies heavily on the individual experimental study design and inclusion of the proper control subjects. Direct comparisons between centers using the same methods are also difficult, and if different methods are used, valid data comparisons can be problematic.
Stable Isotope Techniques for Measuring Gluconeogenesis
Labeled Acetate
Administering a labeled metabolite was one of the earliest methods used to trace gluconeogenesis in vivo (37,38). In these early experiments, [2-14C]acetate was given intravenously with the assumption that the carbon label would be incorporated into acetyl-CoA, phosphoenolpyruvate (PEP), and ultimately glucose. The acetate was an intermediate metabolite used to deliver the label to the intrahepatic acetyl-CoA pool. Gluconeogenesis was derived as the ratio of the labeled glucose (product) to the labeled precursor with the assumption that the majority of gluconeogenic flux occurs via the TCA cycle. The labeling of glucose from an acetate carbon tracer involves incorporation of the tracer into hepatic citric acid cycle intermediates, including oxaloacetate, followed by transfer into PEP via PEPCK and subsequently into glucose via gluconeogenesis (Fig. 1). Due to carbon exchanges and tracer dilutions in the citric acid cycle that are challenging to quantify, the level of glucose labeling from a given enrichment or specific activity of acetyl-CoA entering the hepatic citric acid cycle is difficult to predict.
We have personally labeled the glucose pool with labeled acetate, and other metabolites such as sodium bicarbonate and α-ketoisocaproate. However, when using these carbon tracers, the inability to determine accurately the precursor pool enrichment or specific activity limits the generalizability of these measurements. These measurements severely underestimated rates of gluconeogenesis because of flaws in three key assumptions. First, acetate may be extensively metabolized in tissues other than the liver, which may reflect overall acetate oxidation as well as intrahepatic Kreb cycle activity (39,40). Second, the true precursor pool of intrahepatic acetyl-CoA was not easily quantified and [14C]β-hydroxybutyrate (β-OHB)–specific activity was used to estimate intramitochondrial acetyl-CoA–specific activity (40). However, β-OHB–specific activity may not accurately reflect the mitochondrial acetyl-CoA precursor pool because of compartmentalization of acetyl-CoA within the liver mitochondria (39). Last, correction equations were developed to estimate the fraction of glucose originating from PEP, but these derivations did not include the contribution of the triose phosphate pool (i.e., via glycerol), which may contribute up to 15–20% of carbons to gluconeogenesis (41).
Labeled Alanine and Lactate
Other investigators estimated rates of gluconeogenesis by directly administering radioactive or stable-labeled gluconeogenic substrates, such as lactate or alanine using conventional isotope dilution techniques (42–46). Yet these measurements also resulted in underestimates of gluconeogenic flux. The labeled precursors lactate or alanine (a commonly shared pool of carbon) must enter the oxaloacetate pool as pyruvate via pyruvate carboxylase to be converted to PEP, and carbon label is lost via exchange within the TCA cycle (as described above). Finally, none of these estimates of gluconeogenesis would include the contributions of glycerol and other amino acids and therefore further underestimates net rates of gluconeogenesis (28,37).
Mass Isotopomer Analysis: Labeled Glycerol and Glucose
It was not until the development and application of methods using [2-13C]glycerol (12,47,48) and [U-13C]glucose (12,26,49,50) that we were able to more consistently quantitate the fraction of glucose formed from all potential gluconeogenic precursors. Mass isotopomer distribution analysis (MIDA) was originally developed to examine the biosynthesis and turnover of complex polymers (51). MIDA combined the precision and accuracy of mass spectrometry with combinatorial probability logistics to determine the biosynthesis of many biological polymers, including proteins, fatty acids, and ribonucleic acids (52). A labeled precursor subunit is administered in sufficient mass to permit the measurement of the relative abundance of isotopomers (polymers that have the same chemical formula and nominal mass but differing isotopic positions) using mass spectrometry (48,53).
Since glucose derived from gluconeogenesis is made of two three-carbon subunits (triose phosphates), Hellerstein and colleagues (48) adapted this technique to determine the fractional synthesis of glucose from a three-carbon precursor by administering [3-13C]lactate or [2-13C]glycerol, which labeled the triose phosphate pool and ultimately glucose. The principal underlying assumption is that there is complete equilibration of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) (48). However, as mentioned previously, lactate labeling of the true gluconeogeneic precursor pool is incomplete because of loss and exchange of carbon within the TCA cycle as well as lactate oxidation. Therefore, methods using labeled lactate resulted in variable and underestimated rates of gluconeogenesis, and correction equations were developed by Hetenyi et al. (37) to account for the loss of carbon in metabolic exchange from acetyl-CoA to oxaloacetate (54).
[2-13C]glycerol
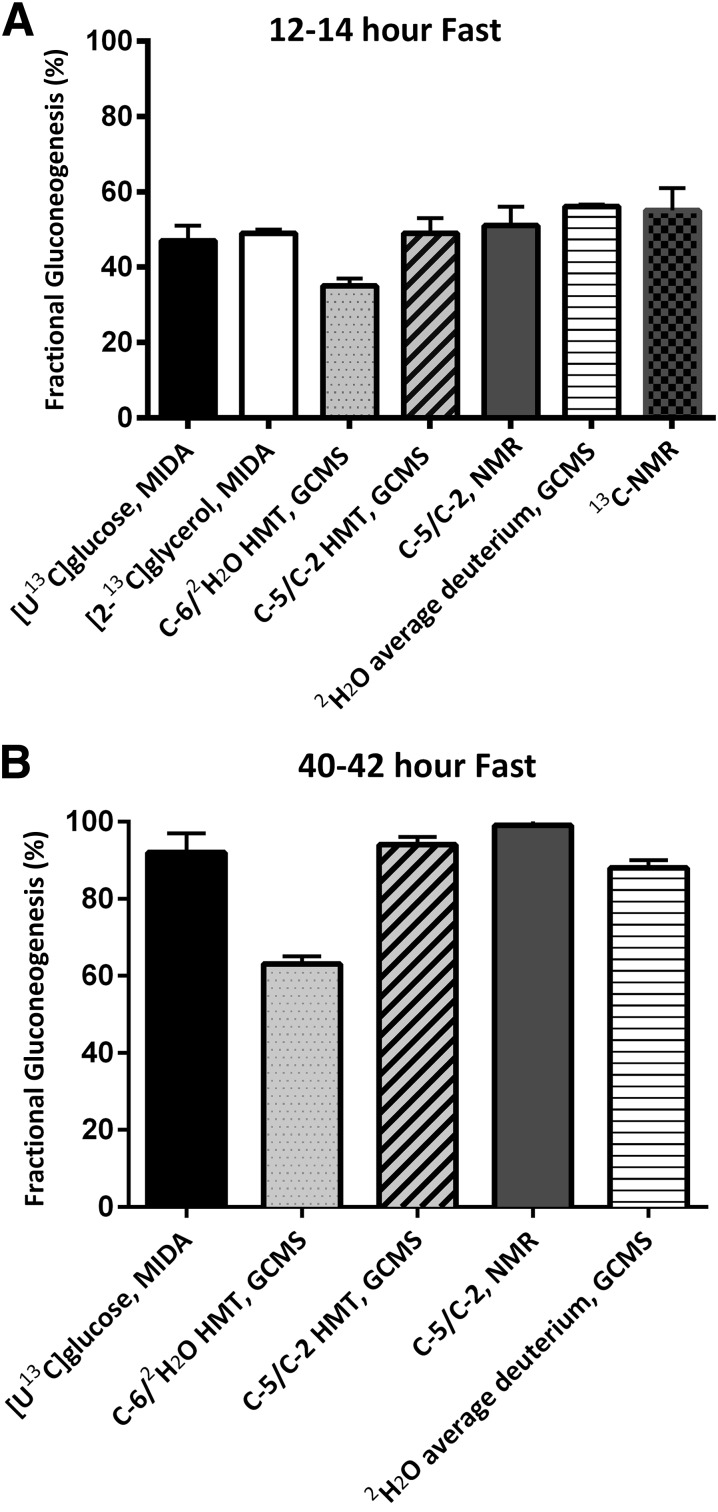
In contrast to lactate, [2-13C]glycerol was more efficient in estimating fractional gluconeogenesis but was very expensive to use because of the high rates of isotope infusion required to enrich sufficiently the triose phosphate pool. High pool enrichment was necessary to ensure accurate measurements of M+2 enrichments (the combination of two labeled substrates at the triose phosphate level) and modeling of the precursor-product relationship. Estimates of fractional gluconeogenesis, using [2-13C]glycerol, in healthy individuals ranged from 30 to 40% after an overnight fast and 60 to 90% after a 60–72-h fast (12,47) (Fig. 2).
Figure 2.
Estimates of fractional gluconeogenesis using various methods in healthy volunteers after 14-h fast (A) and after 40–42-h fast (B). Methods for panel A: [U-13C]glucose, MIDA (49); [2-13C]glycerol, MIDA (26); C-6/2H2O HMT, GCMS (71); C-5/C-2 HMT, GCMS (53); C-5/C-2, 2H-NMR (74); 2H2O average deuterium, GCMS (108); 13C-NMR (68). Methods for panel B: [U-13C]glucose, MIDA (58); C-6/2H2O HMT, GCMS (71); C-5/C-2 HMT, GCMS (53); C-5/C-2, 2H-NMR (74); 2H2O average deuterium, GCMS (2). Please note that C-6/2H2O HMT, GCMS reflects gluconeogenesis on pyruvate alone.
The primary assumption for MIDA probability analysis, i.e., the existence of a single triose phosphate pool, came under heavy debate. Isotopomer distributions of glucose and glucuronic acid using [U-13C]glycerol and urinary acetaminophen glucuronide indicated cellular heterogeneity in glycerol metabolism of two or more pools (55). Furthermore, ex vivo liver perfusion studies demonstrated differential isotopomer distributions of glucose within the different zones of perfused liver lobules, presumably leading to heterogeneous hepatic gluconeogenic activity (54). This model of metabolic zonation of the liver parenchyma suggests that hepatic glucose release occurs mainly in the periportal areas, whereas glucose utilization occurs predominantly in the perivenous zone (56). In addition to the aforementioned issues, high rates and long duration of tracer infusion were required, and discriminating the contribution of the infusion of the glycerol label from that of the unlabeled pool was often difficult. Therefore, MIDA analysis of [2-13C]glycerol became a less attractive option as an isotopic method for estimating gluconeogenesis in vivo.
[U-13C]glucose
Mass isotopomer analysis of [U-13C]glucose was proposed as a suitable alternative to the isotope methods discussed above (57). This method allowed simultaneous measurements of total glucose turnover (M+6), fractional gluconeogenesis (MIDA calculation of primarily the M+1, M+2, and M+3 masses of glucose), estimates of the recycling of glucose (Cori cycle), and perhaps hexomonophosphate shunt activity using the M+4 masses (27). Depending on the exact MIDA analysis used, gluconeogenic calculations included various correction factors to account for precursor pool estimation. For example, Hellerstein et al. (47) developed correction equations to estimate the enrichment at the level of the triose phosphates, whereas Tayek and Katz (49,58) formulated equations to estimate the loss and exchange of labeled carbon via the TCA cycle that were based on further lactate isotopomer analyses. Alternatively, our laboratory reported the reciprocal pool model, which was based on the ratio of 12C to 13C in the labeled glucose molecules derived from gluconeogenesis (M1–M5) (31,34). Regardless of the correction equation used, estimates of fractional gluconeogenesis were comparable (45–60% in healthy individuals after an overnight fast) (34) (Fig. 2). Despite these advantages, this approach was not widely used because of the high isotope cost (high rates of isotope infusion combined with an expensive isotope) required to obtain sufficient enrichments in the M1–M5 masses of glucose (6–8% enrichment of the [U-13C]glucose).
13C-NMR Spectroscopy
At around the same time, a new method was published to quantify the components of glucose production by measuring glucose turnover and glycogenolysis as the primary determinants. Although direct estimates of hepatic glycogen metabolism were one of the first methods used to quantify glycogen metabolism in the liver, this ex vivo method was limited in its applicability because of the significant risks associated with liver biopsy (59–61). Shulman and colleagues (62–68) revolutionized this approach by using noninvasive 13C-NMR spectroscopy, which identified the natural abundance of 13C in the liver glycogen.
Assuming the liver functions as a homogenous unit (which it may not), all liver glycogen should be evenly distributed and detectable by NMR (62,63). Liver glycogen concentration (mmol/L) was derived from spectra measurements of glycogen C-1 glycosyl units coupled with a liver volume measurement (64). When these measures were made across a defined period of time, the change in glycogen content could be estimated and rates of glycogenolysis calculated. With the simultaneous administration of a separate glucose tracer to measure glucose turnover, rates of gluconeogenesis were calculated as the difference between total glucose production and the estimated rate of glycogenolysis.
The 13C-NMR spectroscopy analysis has a good safety profile, no radiation exposure, and the ability to perform serial studies to measure glycogenoglysis without residual isotope labeling occurring in subsequent studies. Importantly, the calculations are straightforward and do not require estimations of precursor pool sizes or complex correction equations for substrate carbon flux. Additionally, in vivo measurements of glycogen flux could be quantified for the first time during the fed and fasted state, which gave insight into the normal physiologic range of glycogen concentrations (65,66). Fractional gluconeogenesis in healthy individuals averages 50–60% and 65–75% after 12- and 23-h fast, respectively (64,67,68) (Fig. 1).
These rates of fractional gluconeogenesis are comparable to results obtained from MIDA analysis. However, the NMR technique is associated with marked intersubject variability even in individuals under the same conditions and with similar phenotypes (68). Although intrasubject coefficient of variation was only ∼7%, reproducibility of glycogen concentration measurements may also be compounded by ±5% variation in spectral noise (64,68). The main disadvantages of 13C-NMR that may hinder its widespread applicability are the need for expensive equipment or “magnet” time and the technical expertise required for analysis.
Deuterium Oxide
Conceptually, estimates of fractional gluconeogenesis utilizing deuterium oxide (2H2O) are simple and straightforward and address many of the limitations imposed by the previous methods described. The principle of the deuterated water method is based on the hydrogen/deuterium labeling of glucose from body water during the process of gluconeogenesis. Deuterated water is administered orally to enrich the body water to 0.3–0.5%. At this enrichment, deuterium would be incorporated into the precursors of gluconeogenesis, first by labeling reduced triphosphopyridine nucleotide/triphosphopyridine nucleotide (TPNH/TPN) and reduced diphosphopyridine nucleotide/diphosphopyridine nucleotide (DPNH/DPN) pools. Subsequently, deuterium would label the intermediate molecules in the gluconeogenic process as a result of the hydration and dehydration of specific molecular bonds (69). Deuterium labeling could occur on any carbon in the glucose molecule with the assumption that deuterium body water enrichment reached a steady state and there was complete exchange between the deuterium in body water and the hydrogens bound to the carbons in glucose. Absolute estimates of gluconeogenesis were subsequently made using a glucose tracer (commonly [6,6-2H2]glucose, [1-13C]glucose, or [3H]glucose) to estimate the rate of total glucose appearance.
The deuterated water approach relies on identifying the pattern of 2H incorporation in plasma glucose, reflecting the hydrogen exchange during the formation of glucose. Obviously, all of the H on the OH groups of glucose is readily exchangeable. In addition, the H covalently bound to carbon 2 on glucose is readily exchangeable with the extensive isomerization of glucose-6-phosphate with fructose-6-phosphate (glucose cycling) (70). Enrichment of deuterium on carbon 2 of glucose may have contributions from gluconeogenesis (via fructose-6-phosphate→glucose-6-phosphate→glucose) as well as from glycogenolysis (glycogen→glucose-1-phosphate→glucose-6-phosphate→glucose) but has good agreement with the enrichment of the labeled body water pool measured independently using isotope-ratio mass spectrometry. Therefore, deuterium labeling on carbon 2 is a useful marker of total body water but not of gluconeogenesis. Carbon 2 enrichment is often used as a surrogate for the enrichment in total body water (the deuterium precursor pool) (53,71). In contrast, deuterium labeling of other carbons 1, 3, 4, 5, and 6 reflects gluconeogenesis.
Hexamethylenetetramine Method
On the basis of original work done in rat liver using tritiated water (3H2O) (72,73), Landau et al. (71) developed a method in 1995 using deuterium oxide to estimate fractional gluconeogenesis as the ratio of deuterium enrichment on carbon 6 of glucose (product) to deuterium enrichment on carbon 2 of glucose (precursor). Quantifying the labeled carbon 6 of glucose involved a complex multistep process to amplify the low deuterium enrichment and enable accurate measurement by a mass spectrometer. The labeled glucose was degraded to isolate specifically the deuterium covalently bonded to C-6 in the form of formaldehyde. The formaldehyde was combined with ammonia to form hexamethylenetetramine (HMT), which was purified by distillation for analysis by GCMS. This process facilitated the incorporation of six formaldehyde molecules derived from C-6 glucose carbons. Thus, the HMT molecule labeled with deuterium was magnified six times and could be easily measured by GCMS.
Identifying the glucose fragment labeled at carbon 6 was chosen for two reasons. First, this fragment should represent the equilibration of hydrogens bound if all the glucose was formed via pyruvate. Second, it was assumed (and subsequently proven) that the hydrogens on carbon 6 did not exchange with body water after the conversion of glycogen to glucose (71). Therefore, these measurements were based on the following assumptions: the majority of glucose is formed via pyruvate, and the two hydrogens on carbon 3 of PEP are completely interchangeable with the deuterium in body water. However, hydrogen exchange may be incomplete at carbon 3, and this method also did not include the contributions from glycerol, a well-known substrate for gluconeogenesis, at the level of the triose phosphate pool (74,75).
Alternative techniques to determine the pattern of C-1 and C-3 labeling were developed but were not adopted because of various nongluconeogenic exchange reactions and/or kinetic isotope effects influencing the sites differently. Deuterium enrichment on carbon 3 was unsuitable because of severe isotope effects at this position during the isomerase reaction, which resulted in underestimates of gluconeogenesis (76–78). Similarly, the hydrogen on carbon 1 may be exchanged during gluconeogenesis and glycogenolysis, and deuterium enrichments were typically highest at this carbon position (74,79).
In 1996, Landau et al. (53) published the C5-HMT method, which addressed the major criticisms of the C6-HMT approach while still allowing for amplification of the labeled glucose (on carbon 5 and 2) with HMT. The C5-HMT method assumed that the precursor for labeling of carbon 5 on glucose was the carbon 2 on GAP, which undergoes complete isomerization with DHAP (55) (Fig. 2). Additionally, in the formation of glucose via pyruvate, total body water is also the precursor pool for the hydrogen required in the conversion of PEP to 2-phosphoglycerate. Carbon 5 was believed to represent ideally gluconeogenesis because there was no exchange of the hydrogen bound to carbon 5 of the glucose with water during glycogenolysis. Therefore, the ratio of enrichment on C-5 to C-2 on glucose, or C-5 to body water deuterium enrichment at steady state, was a better measure of fractional gluconeogenesis.
Reliance on the ratio of C-5 to C-2 to determine fractional gluconeogenesis is based on the assumption that the deuterium at C-2 equilibrates rapidly during the glucose-6-phsophate to fructose-6-phosphate isomerization. The enrichment at C-2 would result from hydrogen exchange during the process of gluconeogenesis and glycogenolysis. As shown by Landau et al. (53), this C-2 enrichment is equivalent to 2H enrichment in body water at steady state.
However, steady-state enrichment of deuterium in body water may take up to 3 h (22). Under non–steady-state and hyperinsulinemic-euglycemic clamp conditions, some authors propose using the ratio of C-5 to C-2 instead of C-5 to 2H2O to determine fractional gluconeogenesis. Prior to steady-state deuterium enrichment in body water, Allick et al. (22) and Chandramouli et al. (80) demonstrated differences in the enrichment ratios of C-5 to C-2 and C-5 to 2H2O that resulted in differences in rates of gluconeogenesis. Specifically, Chandramouli et al. (80) examined three subjects and showed the enrichment at the ratio of C-5 to C-2 remained relatively constant ∼1 h after 2H2O administration. Similarly, Basu et al. (81) also found differences in the ratio of C-5 to C-2 and C-5 to 2H2O during an insulin clamp. Therefore, under non–steady-state conditions, these authors propose using the ratio of C-5 to C-2 rather than C-5 to 2H2O as an index of fractional gluconeogenesis.
We believe that complete mixing of 2H2O in the body water pool is important since this method relies on the deuterium incorporation on glucose molecules synthesized from the body water pool to be a measure of fractional gluconeogenesis. If the body water pool is not mixed uniformly, glucose molecules synthesized via gluconeogenesis may not be labeled equally as different zones/compartments exist with different 2H2O enrichments. Nevertheless, even if the ratio of C-5 to C-2 was proven valid during the period prior to achieving substrate and isotopic near steady state, fractional gluconeogenesis cannot be translated to absolute rates of gluconeogenesis unless the glucose tracer used to measure glucose turnover has reached the steady state (three to five biologic half-lives).
The C5-HMT method has been used extensively over the last two decades to estimate gluconeogenesis in a variety of patients under different clinical conditions. Major strides have been made in understanding the role of gluconeogenesis under physiological (healthy children, premature infants, and fasted states [36,53,75,82,83]) and pathological states (such as obesity, prediabetes, and type 2 diabetes [84–87]). As 2H2O is an inexpensive tracer and the amounts needed for a human study are small, the deuterium method is very attractive. But the analytical costs are very high in technician time and the requirement for frequent repeated analysis of the same sample(s). The procedure required to measure the C-5 enrichment involves deproteination, the formation of HMT, several HPLC and fractional distillation steps, and finally HMT quantification by GCMS. Initially, there were up to 42 analytical steps, which required a minimum of two full days of sample preparation, and only limited numbers of samples could be handled simultaneously. Unfortunately, mishaps in any one of these steps could lead to failure to recover a sufficient amount of product that would not be recognized until the end of the analytical process. In addition, this method requires moderately large amounts of plasma to obtain sufficient mass of glucose for this complicated analysis and even more to allow for repeated processing of sample, should that be necessary. Finally, the relatively large volumes of plasma required make studies in infants and children challenging and preclude its use in small animal models, particularly mice. Thus, although attractive from a theoretical perspective, this method is also fraught with many potential and real analytical problems.
Average Deuterium Method
A simple and reproducible method, which required 30 μL or less of plasma for analysis, was published by Chacko et al. (33) in 2008 utilizing the average labeling of deuterium covalently bound to the carbons on a newly formed glucose molecule. The average deuterium approach involves oral administration of deuterated water in a fashion identical to that of the C5-HMT method and requires an independent infusion of a glucose tracer to measure the rate of appearance of glucose. In contrast to the C5-HMT method, the average deuterium method accounted for the possibility of small physiological variations in C-H glucose labeling (69,71,88,89) by identifying a unique glucose fragment that includes all of the covalently bonded H (or deuterium) on carbons 1, 3, 4, 5, and 6. Of note, the C-2 carbon is not retained in the fragment analyzed (90).
The method assumes that with the low deuterium enrichment of body water (0.3–0.5%), it is statistically unlikely that more than one deuterium will be found on any single glucose molecule and that each carbon in the glucose molecule will have approximately the same statistical probability for being labeled. GCMS is used to measure the specific enrichment of the glucose fragment, m/z 169, that contained all of the pertinent C-H bound hydrogens (covalently bound C-H hydrogens on 1, 3, 4, 5, and 6 of glucose and excludes that on carbon 2) (90–92). Therefore, as the carbons traversed the gluconeogeneic pathway, they are labeled with deuterium to measure gluconeogenesis, independent of a specific carbon source (2,33). Thus, we have good evidence that this method provides a measure of the total flux through the gluconeogeneic pathway, i.e., from lactate, pyruvate, amino acids, and glycerol. We believe near equal labeling is highly likely because of repeated cycling of substrates and a series of isomerization/equilibration reactions regardless of whether deuterium labeling occurs at the same or different carbons when using single or multiple substrates (53,76–78,93). This has recently been confirmed by 2H-NMR (see comparative section below) [74].
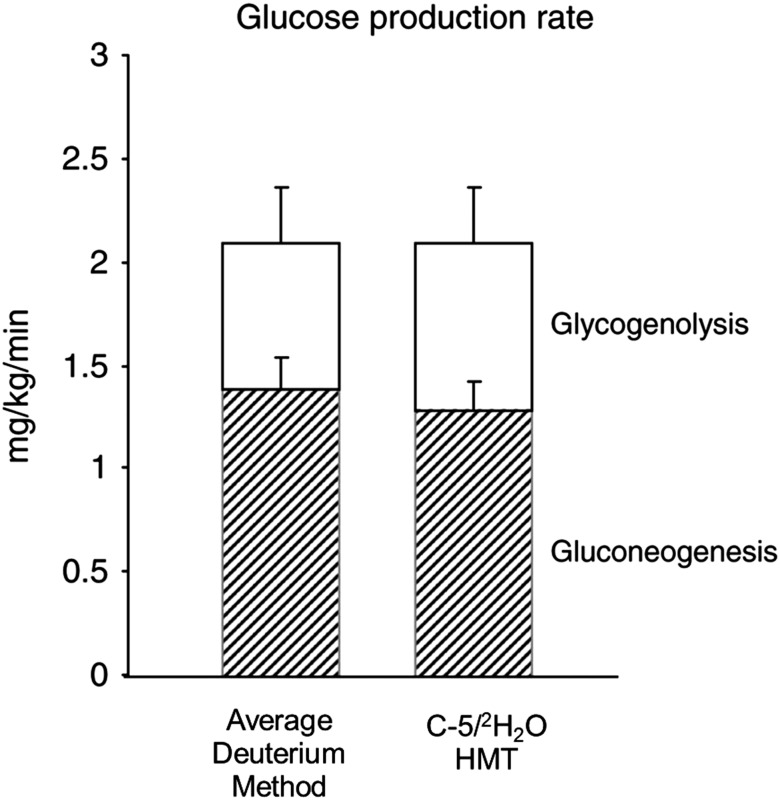
The average deuterium method has been validated under a variety of conditions using a number of samples at high, intermediate, and low fractional gluconeogenesis, such as during fasting (short and long term) and during administration of total parental nutrition (33). Even under all these conditions, the average deuterium method had a coefficient of variation of <3% and the results were comparable to those of the C5-HMT method when analyzed in the same laboratory using the same samples (32,33) (Fig. 3). Finally, the average deuterium enrichment measurement and that of a stable isotopic tracer of glucose can be carried out on as little as 30 μL of blood, therefore facilitating studies of gluconeogenesis in murine animal models (94), other small or large laboratory animals, and very premature human infants (32). Other GCMS analytical methods that could potentially quantify deuterium labeling on glucose carbons are under study but have not yet been validated or replicated by others (95).
Figure 3.
Comparative analyses of average deuterium and C-5/2H2O methods. Glucose production rate in premature infants receiving total parenteral nutrition for 20 h, gluconeogenesis (hatched bars) and glycogenolysis (white bars). Gluconeogenesis was measured by the average deuterium method (33) and C-5/2H2O method (53), respectively. There was no significant difference between the estimates of gluconeogenesis obtained by the two methods. Data are mean ± SE. Reprinted with permission from Chacko and Sunehag (32).
In Vitro 2H-NMR
It is important to note that identifying the deuterium-labeled glucose fragment is commonly performed by GCMS, but this is not the sole analytical method available. Analysis of deuterium NMR provides measures of relative 2H enrichment in vitro after oral deuterium administration (0.5% 2H total body water enrichment). Using this method, steady-state plasma samples are deproteinized and converted to monoacetone glucose in a multistep process that could take up to 32 h prior to spectral analysis (76,77). Thereafter, in vitro spectra analysis identified the seven carbon-bound deuterons in a single 2H-NMR spectrum of monoacetone glucose. One distinct advantage of this method is the ability to determine deuterium enrichment at each carbon of glucose. At present, large volumes of plasma (20–30 mL) and long duration of spectra accumulation (5–20 h) hinder the widespread acceptance and utilization of this in vitro 2H-NMR approach (76). Nevertheless, costs are comparable to 13C-NMR, and with the development of custom-built NMR probes, total cost may be comparable to GCMS methods.
Critical Comparison of Analytical Methodologies
We have undertaken comparative analyses of most of the methods described, all of which demonstrate relatively good agreement (32,33,35). Most recently, we demonstrated that the average deuterium method was comparable with C5-HMT (Fig. 3) in conditions of low and high substrate concentrations (32,33). Similarly, others have shown good agreement in estimating fractional rates of gluconeogenesis when using 2H-NMR and 13C-NMR methodology (74,96) (Fig. 2). Only a few studies have compared GCMS with NMR and yield slightly different results. In one study, estimates of C-5/C-2 deuterium enrichments using 2H-NMR and GCMS were comparable (96). However, these results were not replicated in a separate laboratory and NMR appeared to underestimate C-5/C-2 enrichment compared with mass spectroscopy (74).
Analyzing carbon-specific enrichments with 2H-NMR also provides additional insight into the possible variation of deuterium labeling that provides the basis of the C-5/C-2 methodological assumptions. In vitro 2H-NMR spectra analysis of glucose labeled from deuterated water identified comparable labeling in glucose (C-1, C-3, C-4, C-5, and C-6) to glucose (C-3, C-4, and C-5) (97). In separate analyses, 2H-NMR spectra analysis revealed only slight differences in deuterium labeling at carbon 1, 3, 4, 5, and 6 (74,98). The authors then compared calculations of fractional gluconeogenesis by using either the average enrichment on carbons 1, 3, 4, 5, and 6 (average method) or the ratio of C-5 to C-2. Of note, these calculations were based on NMR data from Burgess et al. (98) and not direct measurements of deuterium enrichment by GCMS. Despite the subtle differences in deuterium enrichment on carbons 1–6, estimates of fractional gluconeogenesis, after a 24-h fast, were comparable when either the ratios of C-5 to C-2 or the average method calculations were used. However, when the fast was extended to 48 h, a 7% difference in rates of fractional gluconeogenesis emerged between the two methods. Burgess et al. (98) speculated that the difference between the average method and ratios of C-5 to C-2 was secondary to systematic errors in the average method that were only exposed under extreme conditions when gluconeogenesis approached 100%, i.e., after 48 h of fasting. Yet, it is important to note that the 7% difference between methods is within the margin of error for their NMR measurements (CV 6.7 ± 3%) (98,99).
Bederman et al. (100) also directly compared rates of gluconeogenesis using the GCMS average method with the C-5/2H2O HMT method in fasted male Sprague-Dawley rats. Rates of gluconeogenesis calculated by the average method were 30% lower after short-term (8 h) and long-term (13 h) fasting conditions. Bederman et al. (100) suggested that the average method underestimates rates of gluconeogenesis and the difference between the two methodologies is related to differences in enrichment of the fragments unique to each method. As described above, these results are in direct contradiction to a number of other head-to-head comparisons of the two methods when performed in our laboratory and by other investigators (33,98,99).
It is also not clear if the negligible differences in deuterium on various glucose carbons are because of real differences in labeling during gluconeogenesis or the variation in the analytical methods themselves. Measurement techniques with higher levels of precision are required to verify if such subtle differences of deuterium labeling between various glucose carbon atoms are real. Furthermore, if the small differences in deuterium enrichment on different glucose carbons are real, it is not known if that would make any meaningful differences in the estimates of gluconeogenesis and thus the conclusions.
Limitations of Using Deuterium Oxide
Oral deuterium oxide is a useful and reliable tool for understanding gluconeogenesis in vivo, but there are several limitations that must be considered when using this isotopic technique. First, deuterium oxide has a relatively long biological half-life (7–8 days) (101), which presents a practical dilemma when comparing rates of gluconeogenesis before and after short-term intervention. Deuterium enrichment of total body water can be detected up to 4 weeks after a single administration, and this abundance should be considered when designing subsequent experiments. It is also important to consider glycogen cycling, i.e., deuterium-labeled glucose, which is stored as glycogen and then released at a later time (19).
Additionally, deuterium exchange is assumed to be equal and complete with total body water. Equilibration should occur with the hydrogens bound to the glucose carbons at the level of pyruvate as well as at the level of GAP and DHAP. Inadequate exchange would result in underestimations of gluconeogenesis. This effect is further magnified if plasma sampling is performed too soon (<4 h after administration) and there is incomplete equilibration of deuterium in body water (22).
More recently, it was also proposed that deuterium estimates of gluconeogenesis may be overestimated if there is sizeable exchange of the lower carbons of fructose-6-phosphate via transaldolase exchange and enrichment on C-5 in the absence of net gluconeogenesis (102). Notably, the transaldolase exchange could also influence the labeling pattern when using other labeled precursor approaches and may affect estimates using the MIDA approach (34,47). Yet estimates of fractional gluconeogenesis via deuterated water methods are comparable to those using 13C-NMR, which are not influenced by transaldolase reactions (83). However, if the transaldolase exchange is affecting quantitative measurements, it appears to occur at the same rate in individuals with and without diabetes and would overestimate gluconeogenesis to the same extent provided the studies are carried out with fidelity to a common protocol (103).
Glucose cycling refers to glucose phosphorylated to glucose-6-phosphate and then dephosphorylated by glucose-6-phosphatase to glucose. During the process of conversion back to glucose, deuterium could label carbon 2 during equilibration of glucose-6-phosphate and fructose-6-phosphate. Glycogenolysis would be overestimated as the fractional contribution of carbon 2 is used in the gluconeogenesis equation. In addition, glucose cycling was reported to be greater in individuals with type 2 diabetes (104). However, glucose cycling is minimal as evidenced by the fact that the deuterium enrichment on carbon 2 is equivalent to total deuterium enrichment in body water (53) and there were no observed differences in carbon 2 enrichment between individuals with or without diabetes (84).
Finally, no one has extensively explored the effects of the hexomonophosphate shunt on the labeling of glucose released from the liver. Using [U-13C]glucose in lactating women, we found evidence of hexomonophosphate shunt activity in determining the carbon source for milk galactose. Additional studies will be required to evaluate the relative importance of the hexomonophosphate shunt under a number of metabolic conditions (27).
Clinical Significance of Measuring Gluconeogenesis
Measurements of gluconeogenesis have been pivotal in elucidating normal physiology and understanding the pathophysiological role of the liver (and kidney) in many diseases such as obesity, metabolic acidosis, and diabetes. In this review, we are not able to expound fully on the myriad experiments involving gluconeogenesis and the significant advances that have been made in this field. However, there are three key clinical areas that are worth mentioning.
First, improvement in techniques and the use of stable isotopes enabled quantification of the relative contribution of gluconeogenesis to fasting in a wide range of individuals under a variety of different conditions, including healthy individuals (53,58,74), premature infants (31,35,105), children (36,75,82), lactating mothers (2,12,26), and individuals with type 1 and type 2 diabetes (106–108). Overall, in healthy individuals, gluconeogenesis contributes 40–60% to total glucose production after an overnight fast (Fig. 2A), with comparable estimates in lactating mothers and children. With prolonged fasting, fractional gluconeogenesis progressively increases and is >90% after 48 h of fasting (Fig. 2B).
Second, glucose production via the gluconeogenic pathway remains operative not only during fasting and exercise but also during relative and absolute periods of hyperinsulinemia (for example during a meal or a hyperinsulinemic clamp [2,68,109]). Earlier experiments to quantify glucose production during an insulin clamp suggested that gluconeogenesis is completely suppressed to zero (or below) (110). This was most likely due to a combination of factors such as isotopic impurity, underestimation of glucose turnover corrected with high-performance liquid chromatography purification of [6-3H]glucose, high rates of glucose infusion, and inaccuracies in the measurement of specific radioactivity (111). However, more recent studies utilizing a number of isotopic methodologies have elucidated the complex relationship of the metabolic effects of insulin on the components of hepatic glucose production. Using 2H-NMR and arterio-venous difference techniques, Cherrington and colleagues (6) demonstrated that acute physiological increases in insulin concentration rapidly inhibit glucose production by suppressing glycogen breakdown. Within 30 min of the insulin infusion, marked decreases in PEPCK mRNA expression were observed with a modest and nonsustained decrease in gluconeogenic flux. Similarly, with the use of deuterated water techniques in healthy individuals and individuals with diabetes, it is now known that although glycogenolysis is totally suppressed, as demonstrated by Cori and Cori (7) in the 1940s, there is little or no suppression of gluconeogenesis under physiological or supraphysiological hyperinsulinemic conditions (2,26,68,109,112).
Finally, these techniques have provided new insights into research in human obesity and type 2 diabetes and contributed greatly to our understanding of the role of liver and hepatic insulin resistance in these diseases. Increased rates of gluconeogenesis are a key and very early pathological feature of type 2 diabetes (108). Depending on the stable isotope technique used and the study design, absolute rates of gluconeogenesis will differ by methodology and there is no available “normal” range. Yet regardless of the methodology used, there is great consensus that increased rates of gluconeogenesis are a primary feature of fasting hyperglycemia and gluconeogenesis is increased up to 40% in individuals with diabetes (49,87,106,108,113–115). These newer techniques are also sensitive enough to detect increased fractional rates of gluconeogenesis in obesity (82,87,113) and prediabetes (86,116) prior to the onset of marked hyperglycemia. Last, in vivo measurements of gluconeogenesis have been useful in examining the physiological targets of important antihyperglycemic agents, such as metformin (117,118) and liraglutide (119).
Conclusions
In summary, the newer methodologies to measure gluconeogenesis, including deuterium oxide, MIDA, and NMR analysis, are simpler, more affordable, and less invasive than those originally used in humans. These newer methods have allowed investigators to advance the field of glucose physiology and pathophysiology (obesity and type 2 diabetes) by examining the role of gluconeogenesis in these diseases. As we lack a true “gold standard” for measuring gluconeogenesis in vivo, all of the currently available methods must be considered reasonable estimates but not precise quantitative measurements of fractional gluconeogenesis. Absolute comparisons of rates of gluconeogenesis may be problematic between centers using the same tracer and can be even more difficult within the same center when different tracer methods are used. Perhaps by the 100th anniversary of the initial description of the Cori cycle, we may have a better understanding as to how to accurately measure the rates of gluconeogenesis in vivo under a variety of physiological and clinical conditions in humans.
Article Information
Acknowledgments. The authors sincerely thank the patient volunteers and their families who participated in the studies and those of other investigators and the research nurse and staff at the Metabolic Research Unit, Children’s Nutrition Research Center, Baylor College of Medicine and other institutions. The authors thank S. Sharma (Department of Pediatrics, Baylor College of Medicine) for her dedication and technical support of the studies undertaken by the group.
Funding. This work is a publication of the U.S. Department of Agriculture/Agricultural Research Service Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. S.T.C. is supported by the intramural department at the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health. The authors’ studies reported in this review were supported by the Marilyn Fishman Endocrine Fellows Foundation (grant to S.T.C.), 2RO1-HD-037957 (A.L.S.), 1RO1-HD-044609 (A.L.S.), 5T32-DK-063873 (M.W.H.), 5RO1-DK-055478 (M.W.H.), and the U.S. Department of Agriculture (CRIS 6250-51000 to M.W.H.).
The contents of this publication do not necessarily reflect the views of policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. Government.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.T.C. and M.W.H. researched the data and wrote and reviewed the manuscript. S.K.C. and A.L.S. contributed to the discussion and reviewed and edited the manuscript. All authors gave final approval of the version to be published. M.W.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ekberg K, Landau BR, Wajngot A, et al. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 1999;48:292–298 [DOI] [PubMed] [Google Scholar]
- 2.Mohammad MA, Sunehag AL, Chacko SK, Pontius AS, Maningat PD, Haymond MW. Mechanisms to conserve glucose in lactating women during a 42-h fast. Am J Physiol Endocrinol Metab 2009;297:E879–E888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab 2011;14:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess SC, He T, Yan Z, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 2007;5:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel VT, Beddow SA, Iwasaki T, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci U S A 2009;106:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramnanan CJ, Edgerton DS, Rivera N, et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 2010;59:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cori CF, Cori GT. Glycogen formation in the liver from d- and l-lactic acid. J Biol Chem 1929;81:389–403 [Google Scholar]
- 8.Cori GT, Cori CF. Glucose-6-phosphatase of the liver in glycogen storage disease. J Biol Chem 1952;199:661–667 [PubMed] [Google Scholar]
- 9.Reichard GA Jr, Jacobs AG, Kimbel P, Hochella NJ, Weinhouse S. Blood glucose replacement rates in normal and diabetic humans. J Appl Physiol 1961;16:789–795 [DOI] [PubMed] [Google Scholar]
- 10.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF Jr. Liver and kidney metabolism during prolonged starvation. J Clin Invest 1969;48:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atwell ME, Waterhouse C. Glucose production from fructose. Diabetes 1971;20:193–199 [DOI] [PubMed] [Google Scholar]
- 12.Tigas S, Sunehag A, Haymond MW. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Metab 2002;87:302–307 [DOI] [PubMed] [Google Scholar]
- 13.Haymond MW, Karl IE, Feigin RD, DeVivo D, Pagliara AS. Hypoglycemia and maple syrup urine disease: defective gluconeogenesis. Pediatr Res 1973;7:500–508 [DOI] [PubMed] [Google Scholar]
- 14.Haymond MW, Karl IE, Clarke WL, Pagliara AS, Santiago JV. Differences in circulating gluconeogenic substrates during short-term fasting in men, women, and children. Metabolism 1982;31:33–42 [PubMed] [Google Scholar]
- 15.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009;297:E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherrington AD, Williams PE, Shulman GI, Lacy WW. Differential time course of glucagon’s effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes 1981;30:180–187 [DOI] [PubMed] [Google Scholar]
- 17.Edgerton DS, Cardin S, Emshwiller M, et al. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 2001;50:1872–1882 [DOI] [PubMed] [Google Scholar]
- 18.Bier DM. The use of stable isotopes in metabolic investigation. Baillieres Clin Endocrinol Metab 1987;1:817–836 [DOI] [PubMed] [Google Scholar]
- 19.Stingl H, Chandramouli V, Schumann WC, et al. Changes in hepatic glycogen cycling during a glucose load in healthy humans. Diabetologia 2006;49:360–368 [DOI] [PubMed] [Google Scholar]
- 20.Kacerovsky M, Jones J, Schmid AI, et al. Postprandial and fasting hepatic glucose fluxes in long-standing type 1 diabetes. Diabetes 2011;60:1752–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bier DM, Leake RD, Haymond MW, et al. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 1977;26:1016–1023 [DOI] [PubMed] [Google Scholar]
- 22.Allick G, van der Crabben SN, Ackermans MT, Endert E, Sauerwein HP. Measurement of gluconeogenesis by deuterated water: the effect of equilibration time and fasting period. Am J Physiol Endocrinol Metab 2006;290:E1212–E1217 [DOI] [PubMed] [Google Scholar]
- 23.Toffolo G, Cobelli C. The hot IVGTT two-compartment minimal model: an improved version. Am J Physiol Endocrinol Metab 2003;284:E317–E321 [DOI] [PubMed] [Google Scholar]
- 24.Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C. Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab 2006;291:E800–E806 [DOI] [PubMed] [Google Scholar]
- 25.Chiasson JL, Liljenquist JE, Lacy WW, Jennings AS, Cherrington AD. Gluconeogenesis: methodological approaches in vivo. Fed Proc 1977;36:229–235 [PubMed] [Google Scholar]
- 26.Kaplan W, Sunehag AL, Dao H, Haymond MW. Short-term effects of recombinant human growth hormone and feeding on gluconeogenesis in humans. Metabolism 2008;57:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad MA, Maningat P, Sunehag AL, Haymond MW. Precursors of hexoneogenesis within the human mammary gland. Am J Physiol Endocrinol Metab 2015;308:E680–E687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab 2002;282:E428–E434 [DOI] [PubMed] [Google Scholar]
- 29.Goodman AD, Fuisz RE, Cahill GF Jr. Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest 1966;45:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamm DE, Fuisz RE, Goodman AD, Cahill GF Jr. Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest 1967;46:1172–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chacko SK, Ordonez J, Sauer PJJ, Sunehag AL. Gluconeogenesis is not regulated by either glucose or insulin in extremely low birth weight infants receiving total parenteral nutrition. J Pediatr 2011;158:891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacko SK, Sunehag AL. Gluconeogenesis continues in premature infants receiving total parenteral nutrition. Arch Dis Child Fetal Neonatal Ed 2010;95:F413–F418 [DOI] [PubMed] [Google Scholar]
- 33.Chacko SK, Sunehag AL, Sharma S, Sauer PJJ, Haymond MW. Measurement of gluconeogenesis using glucose fragments and mass spectrometry after ingestion of deuterium oxide. J Appl Physiol (1985) 2008;104:944–951 [DOI] [PubMed] [Google Scholar]
- 34.Haymond MW, Sunehag AL. The reciprocal pool model for the measurement of gluconeogenesis by use of [U-(13)C]glucose. Am J Physiol Endocrinol Metab 2000;278:E140–E145 [DOI] [PubMed] [Google Scholar]
- 35.Sunehag AL, Haymond MW, Schanler RJ, Reeds PJ, Bier DM. Gluconeogenesis in very low birth weight infants receiving total parenteral nutrition. Diabetes 1999;48:791–800 [DOI] [PubMed] [Google Scholar]
- 36.Sunehag AL, Treuth MS, Toffolo G, et al. Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: an evaluation of their reproducibility. Pediatr Res 2001;50:115–123 [DOI] [PubMed] [Google Scholar]
- 37.Hetenyi G Jr, Lussier B, Ferrarotto C, Radziuk J. Calculation of the rate of gluconeogenesis from the incorporation of 14C atoms from labelled bicarbonate or acetate. Can J Physiol Pharmacol 1982;60:1603–1609 [DOI] [PubMed] [Google Scholar]
- 38.Katz J. Determination of gluconeogenesis in vivo with 14C-labeled substrates. Am J Physiol 1985;248:R391–R399 [DOI] [PubMed] [Google Scholar]
- 39.Consoli A, Nurjhan N, Capani F, Pangburn T, Lapenna D, Gerich J. Limitations in the use of [2-14C]acetate for measuring gluconeogenesis in vivo. Diabetes 1993;42:732–737 [DOI] [PubMed] [Google Scholar]
- 40.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 1989;38:550–557 [DOI] [PubMed] [Google Scholar]
- 41.Landau BR, Wahren J, Previs SF, Ekberg K, Chandramouli V, Brunengraber H. Glycerol production and utilization in humans: sites and quantitation. Am J Physiol 1996;271:E1110–E1117 [DOI] [PubMed] [Google Scholar]
- 42.Consoli A, Nurjhan N, Reilly JJ Jr, Bier DM, Gerich JE. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest 1990;86:2038–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenssen T, Nurjhan N, Consoli A, Gerich JE. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest 1990;86:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landau BR, Chandramouli V, Schumann WC, et al. Estimates of Krebs cycle activity and contributions of gluconeogenesis to hepatic glucose production in fasting healthy subjects and IDDM patients. Diabetologia 1995;38:831–838 [DOI] [PubMed] [Google Scholar]
- 45.Perriello G, Pampanelli S, Del Sindaco P, et al. Evidence of increased systemic glucose production and gluconeogenesis in an early stage of NIDDM. Diabetes 1997;46:1010–1016 [DOI] [PubMed] [Google Scholar]
- 46.Garber AJ, Cryer PE, Santiago JV, Haymond MW, Pagliara AS, Kipnis DM. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest 1976;58:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellerstein MK, Neese RA, Linfoot P, Christiansen M, Turner S, Letscher A. Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J Clin Invest 1997;100:1305–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neese RA, Schwarz JM, Faix D, et al. Gluconeogenesis and intrahepatic triose phosphate flux in response to fasting or substrate loads. Application of the mass isotopomer distribution analysis technique with testing of assumptions and potential problems. J Biol Chem 1995;270:14452–14466 [DOI] [PubMed] [Google Scholar]
- 49.Tayek JA, Katz J. Glucose production, recycling, and gluconeogenesis in normals and diabetics: a mass isotopomer [U-13C]glucose study. Am J Physiol 1996;270:E709–E717 [DOI] [PubMed] [Google Scholar]
- 50.Katz J, Tayek JA. Recycling of glucose and determination of the Cori cycle and gluconeogenesis. Am J Physiol 1999;277:E401–E407 [DOI] [PubMed] [Google Scholar]
- 51.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. Am J Physiol 1992;263:E988–E1001 [DOI] [PubMed] [Google Scholar]
- 52.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol 1999;276:E1146–E1170 [DOI] [PubMed] [Google Scholar]
- 53.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 1996;98:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Previs SF, Fernandez CA, Yang D, Soloviev MV, David F, Brunengraber H. Limitations of the mass isotopomer distribution analysis of glucose to study gluconeogenesis. Substrate cycling between glycerol and triose phosphates in liver. J Biol Chem 1995;270:19806–19815 [DOI] [PubMed] [Google Scholar]
- 55.Landau BR, Fernandez CA, Previs SF, et al. A limitation in the use of mass isotopomer distributions to measure gluconeogenesis in fasting humans. Am J Physiol 1995;269:E18–E26 [DOI] [PubMed] [Google Scholar]
- 56.Jungermann K, Kietzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu Rev Nutr 1996;16:179–203 [DOI] [PubMed] [Google Scholar]
- 57.Kalderon B, Gopher A, Lapidot A. Metabolic pathways leading to liver glycogen repletion in vivo, studied by GC-MS and NMR. FEBS Lett 1986;204:29–32 [DOI] [PubMed] [Google Scholar]
- 58.Katz J, Tayek JA. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am J Physiol 1998;275:E537–E542 [DOI] [PubMed] [Google Scholar]
- 59.Nilsson LH. Liver glycogen content in man in the postabsorptive state. Scand J Clin Lab Invest 1973;32:317–323 [DOI] [PubMed] [Google Scholar]
- 60.Nilsson LH, Fürst P, Hultman E. Carbohydrate metabolism of the liver in normal man under varying dietary conditions. Scand J Clin Lab Invest 1973;32:331–337 [DOI] [PubMed] [Google Scholar]
- 61.Nilsson LH, Hultman E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest 1973;32:325–330 [DOI] [PubMed] [Google Scholar]
- 62.Sillerud LO, Shulman RG. Structure and metabolism of mammalian liver glycogen monitored by carbon-13 nuclear magnetic resonance. Biochemistry 1983;22:1087–1094 [DOI] [PubMed] [Google Scholar]
- 63.Gruetter R, Magnusson I, Rothman DL, Avison MJ, Shulman RG, Shulman GI. Validation of 13C NMR measurements of liver glycogen in vivo. Magn Reson Med 1994;31:583–588 [DOI] [PubMed] [Google Scholar]
- 64.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 1991;254:573–576 [DOI] [PubMed] [Google Scholar]
- 65.Magnusson I, Rothman DL, Jucker B, Cline GW, Shulman RG, Shulman GI. Liver glycogen turnover in fed and fasted humans. Am J Physiol 1994;266:E796–E803 [DOI] [PubMed] [Google Scholar]
- 66.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest 1998;101:1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 1992;90:1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen KF, Price T, Cline GW, Rothman DL, Shulman GI. Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. Am J Physiol 1996;270:E186–E191 [DOI] [PubMed] [Google Scholar]
- 69.Rose IA. Mechanism of the aldose-ketose isomerase reactions. Adv Enzymol Relat Areas Mol Biol 1975;43:491–517 [DOI] [PubMed] [Google Scholar]
- 70.Wajngot A, Chandramouli V, Schumann WC, Kumaran K, Efendić S, Landau BR. Testing of the assumptions made in estimating the extent of futile cycling. Am J Physiol 1989;256:E668–E675 [DOI] [PubMed] [Google Scholar]
- 71.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J Clin Invest 1995;95:172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rognstad R. Estimation of gluconeogenesis and glycogenolysis in vivo using tritiated water. Biochem J 1991;279:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuwajima M, Golden S, Katz J, Unger RH, Foster DW, McGarry JD. Active hepatic glycogen synthesis from gluconeogenic precursors despite high tissue levels of fructose 2,6-bisphosphate. J Biol Chem 1986;261:2632–2637 [PubMed] [Google Scholar]
- 74.Kunert O, Stingl H, Rosian E, et al. Measurement of fractional whole-body gluconeogenesis in humans from blood samples using 2H nuclear magnetic resonance spectroscopy. Diabetes 2003;52:2475–2482 [DOI] [PubMed] [Google Scholar]
- 75.Sunehag AL, Toffolo G, Treuth MS, et al. Effects of dietary macronutrient content on glucose metabolism in children. J Clin Endocrinol Metab 2002;87:5168–5178 [DOI] [PubMed] [Google Scholar]
- 76.Jones JG, Solomon MA, Cole SM, Sherry AD, Malloy CR. An integrated (2)H and (13)C NMR study of gluconeogenesis and TCA cycle flux in humans. Am J Physiol Endocrinol Metab 2001;281:E848–E856 [DOI] [PubMed] [Google Scholar]
- 77.Jones JG, Perdigoto R, Rodrigues TB, Geraldes CFGC. Quantitation of absolute 2H enrichment of plasma glucose by 2H NMR analysis of its monoacetone derivative. Magn Reson Med 2002;48:535–539 [DOI] [PubMed] [Google Scholar]
- 78.Leadlay PF, Albery WJ, Knowles JR. Energetics of triosephosphate isomerase: deuterium isotope effects in the enzyme-catalyzed reaction. Biochemistry 1976;15:5617–5620 [DOI] [PubMed] [Google Scholar]
- 79.Chandramouli V, Ekberg K, Schumann WC, Wahren J, Landau BR. Origins of the hydrogen bound to carbon 1 of glucose in fasting: significance in gluconeogenesis quantitation. Am J Physiol 1999;277:E717–E723 [DOI] [PubMed] [Google Scholar]
- 80.Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol 1997;273:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 81.Basu R, Chandramouli V, Dicke B, Landau BR, Rizza RA. Plasma C5 glucose-to-2H2O ratio does not provide an accurate assessment of gluconeogenesis during hyperinsulinemic-euglycemic clamps in either nondiabetic or diabetic humans. Diabetes 2008;57:1800–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sunehag AL, Toffolo G, Campioni M, Bier DM, Haymond MW. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy, obese adolescents. J Clin Endocrinol Metab 2005;90:4496–4502 [DOI] [PubMed] [Google Scholar]
- 83.Krebs M, Brehm A, Krssak M, et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 2003;46:917–925 [DOI] [PubMed] [Google Scholar]
- 84.Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 2005;54:1942–1948 [DOI] [PubMed] [Google Scholar]
- 85.Bock G, Chittilapilly E, Basu R, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 2007;56:1703–1711 [DOI] [PubMed] [Google Scholar]
- 86.Basu R, Barosa C, Jones J, et al. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J Clin Endocrinol Metab 2013;98:E409–E417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gastaldelli A, Miyazaki Y, Pettiti M, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab 2004;89:3914–3921 [DOI] [PubMed] [Google Scholar]
- 88.Bock G, Schumann WC, Basu R, et al. Evidence that processes other than gluconeogenesis may influence the ratio of deuterium on the fifth and third carbons of glucose: implications for the use of 2H2O to measure gluconeogenesis in humans. Diabetes 2008;57:50–55 [DOI] [PubMed] [Google Scholar]
- 89.Rognstad R, Clark G, Katz J. Glucose synthesis in tritiated water. Eur J Biochem 1974;47:383–388 [DOI] [PubMed] [Google Scholar]
- 90.Guo ZK, Lee WN, Katz J, Bergner AE. Quantitation of positional isomers of deuterium-labeled glucose by gas chromatography/mass spectrometry. Anal Biochem 1992;204:273–282 [DOI] [PubMed] [Google Scholar]
- 91.Szafranek J, Pfaffenberger CD, Horning EC. The mass spectra of some per-O-acetylaldononitriles. Carbohydr Res 1974;38:97–105 [DOI] [PubMed] [Google Scholar]
- 92.Shalwitz RA, Beth TJ, MacLeod AM, Tucker SJ, Rolison GG. Use of 2H2O to study labeling in plasma glucose and hepatic glycogen during a hyperglycemic clamp. Am J Physiol 1994;266:E433–E437 [DOI] [PubMed] [Google Scholar]
- 93.Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem 2004;327:149–155 [DOI] [PubMed] [Google Scholar]
- 94.Chacko SK, Haymond MW, Sun Y, et al. Effect of ghrelin on glucose regulation in mice. Am J Physiol Endocrinol Metab 2012;302:E1055–E1062 [DOI] [PubMed] [Google Scholar]
- 95.Antoniewicz MR, Kelleher JK, Stephanopoulos G. Measuring deuterium enrichment of glucose hydrogen atoms by gas chromatography/mass spectrometry. Anal Chem 2011;83:3211–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burgess SC, Nuss M, Chandramouli V, et al. Analysis of gluconeogenic pathways in vivo by distribution of 2H in plasma glucose: comparison of nuclear magnetic resonance and mass spectrometry. Anal Biochem 2003;318:321–324 [DOI] [PubMed] [Google Scholar]
- 97.Schleucher J, Vanderveer PJ, Sharkey TD. Export of carbon from chloroplasts at night. Plant Physiol 1998;118:1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burgess SC, Chandramouli V, Browning JD, Schumann WC, Previs SF. Complicating factors in the application of the “average method” for determining the contribution of gluconeogenesis. J Appl Physiol (1985) 2008;104:1852–1853; author reply 1854–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chacko SK, Sunehag AL, Sharma S, Sauer PJJ, Haymond MW. Reply to Burgess, Chandramouli, Browning, Schumann, and Previs. J Appl Physiol 2008;104:1854–1855 [Google Scholar]
- 100.Bederman IR, Chandramouli V, Sandlers Y, Henderson L, Cabrera ME. Time course of hepatic gluconeogenesis during hindlimb suspension unloading. Exp Physiol 2013;98:278–289 [DOI] [PubMed] [Google Scholar]
- 101.Péronnet F, Mignault D, du Souich P, et al. Pharmacokinetic analysis of absorption, distribution and disappearance of ingested water labeled with D₂O in humans. Eur J Appl Physiol 2012;112:2213–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basu R, Barosa C, Basu A, et al. Transaldolase exchange and its effects on measurements of gluconeogenesis in humans. Am J Physiol Endocrinol Metab 2011;300:E296–E303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rajpal A, Dube S, Carvalho F, et al. Effects of transaldolase exchange on estimates of gluconeogenesis in type 2 diabetes. Am J Physiol Endocrinol Metab 2013;305:E465–E474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Efendić S, Wajngot A, Vranić M. Increased activity of the glucose cycle in the liver: early characteristic of type 2 diabetes. Proc Natl Acad Sci U S A 1985;82:2965–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sunehag AL. Parenteral glycerol enhances gluconeogenesis in very premature infants. Pediatr Res 2003;53:635–641 [DOI] [PubMed] [Google Scholar]
- 106.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506 [DOI] [PubMed] [Google Scholar]
- 107.Vella A, Shah P, Basu R, et al. Type I diabetes mellitus does not alter initial splanchnic glucose extraction or hepatic UDP-glucose flux during enteral glucose administration. Diabetologia 2001;44:729–737 [DOI] [PubMed] [Google Scholar]
- 108.Chung ST, Hsia DS, Chacko SK, Rodriguez LM, Haymond MW. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia 2015;58:596–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gastaldelli A, Toschi E, Pettiti M, et al. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes 2001;50:1807–1812 [DOI] [PubMed] [Google Scholar]
- 110.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630–E639 [DOI] [PubMed] [Google Scholar]
- 111.Schwenk WF, Butler PC, Haymond MW, Rizza RA. Underestimation of glucose turnover corrected with high-performance liquid chromatography purification of [6-3H]glucose. Am J Physiol 1990;258:E228–E233 [DOI] [PubMed] [Google Scholar]
- 112.Adkins A, Basu R, Persson M, et al. Higher insulin concentrations are required to suppress gluconeogenesis than glycogenolysis in nondiabetic humans. Diabetes 2003;52:2213–2220 [DOI] [PubMed] [Google Scholar]
- 113.Gastaldelli A, Baldi S, Pettiti M, et al. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes 2000;49:1367–1373 [DOI] [PubMed] [Google Scholar]
- 114.Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 2001;50:810–816 [DOI] [PubMed] [Google Scholar]
- 115.Wajngot A, Chandramouli V, Schumann WC, et al. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism 2001;50:47–52 [DOI] [PubMed] [Google Scholar]
- 116.Bock G, Dalla Man C, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006;55:3536–3549 [DOI] [PubMed] [Google Scholar]
- 117.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basu R, Shah P, Basu A, et al. Comparison of the effects of pioglitazone and metformin on hepatic and extra-hepatic insulin action in people with type 2 diabetes. Diabetes 2008;57:24–31 [DOI] [PubMed] [Google Scholar]
- 119.Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004;53:1187–1194 [DOI] [PubMed] [Google Scholar]
- 120.Puhakainen I, Koivisto VA, Yki-Järvinen H. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1992;75:789–794 [DOI] [PubMed] [Google Scholar]
- 121.Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes 1986;35:1326–1331 [DOI] [PubMed] [Google Scholar]
- 122.Burgess SC, Weis B, Jones JG, et al. Noninvasive evaluation of liver metabolism by 2H and 13C NMR isotopomer analysis of human urine. Anal Biochem 2003;312:228–234 [DOI] [PubMed] [Google Scholar]