Abstract
OBJECTIVE
Premixed insulin is a commonly prescribed formulation for the outpatient management of patients with type 2 diabetes. The safety and efficacy of premixed insulin formulations in the hospital setting is not known.
RESEARCH DESIGN AND METHODS
In a prospective, open-label trial, we randomized general medicine and surgery patients to receive a basal-bolus regimen with glargine once daily and glulisine before meals (n = 33) or premixed human insulin (30% regular insulin and 70% NPH insulin) twice daily (n = 39). Major outcomes included differences in daily blood glucose (BG) levels and frequency of hypoglycemic events (<70 mg/dL) between treatment groups.
RESULTS
At the first prespecified interim analysis, the study was stopped early because of an increased frequency of hypoglycemia >50% in patients treated with premixed human insulin. A total of 64% of patients treated with premixed insulin experienced one or more episodes of hypoglycemia compared with 24% in the basal-bolus group (P < 0.001). There were no differences in mean daily BG level after the first day of insulin treatment (175 ± 32 vs. 179 ± 43 mg/dL, P = 0.64) between groups. A BG target between 80 and 180 mg/dL before meals was achieved in 55.9% of BG readings in the basal-bolus group and 54.3% of BG readings in the premixed insulin group (P = 0.23). There was no difference in the length of hospital stay or mortality between treatment groups.
CONCLUSIONS
Inpatient treatment with premixed human insulin resulted in similar glycemic control but in significantly higher frequency of hypoglycemia compared with treatment with basal-bolus insulin regimen in hospitalized patients with diabetes.
Introduction
Several observational studies (1–4) have shown that hyperglycemia, in patients with and without a history of diabetes, is associated with significant adverse effects, including increased mortality, infection rates, and hospital stay. Randomized clinical trials in critically ill and non–critically ill patients have reported that improved glycemic control can reduce the number of hospital complications and systemic infections, the length of hospital stay, and hospitalization cost (5–8). Clinical guidelines (9,10) recommend the use of basal-bolus insulin regimen for the management of hyperglycemia in non–intensive care unit (ICU) settings. A basal-bolus regimen with once-daily long-acting insulin and rapid-acting insulin analogs before meals is effective in improving glycemic control and in reducing the rate of hospital complications in non-ICU patients with type 2 diabetes (5,11). Despite the benefits of the basal-bolus regimen, many health care professionals consider this approach to be difficult to implement and inconvenient due to the high number of injections and the risk of hypoglycemia (12,13).
In many countries in Europe, Asia, and Latin America, premixed insulin is among the most frequently prescribed treatment formulations in patients with type 2 diabetes (14–16). Premixed insulin formulations include conventional (e.g., biphasic human insulin 30% regular insulin and 70% NPH insulin) and newer premixed human analogs (e.g., biphasic insulin aspart 30/70 or insulin lispro mix 25/75). Premixed insulin formulations are prescribed for many patients with type 2 diabetes because of their proven efficacy in improving glycemic control (17–20), fewer daily injections, and better postprandial glucose control compared with basal insulin regimens (20,21). In the “Schema survey” (16), a cross-sectional survey among 1,263 patients treated by 450 diabetes experts in France, it was reported that premixed insulin formulations were prescribed in 45.5% of type 2 patients. Some ambulatory studies, however, have reported a higher risk of hypoglycemia with the use of premixed insulin formulations compared with basal insulin analogs (21). In the hospital setting, the use of premixed insulin has been reported to be effective in improving hyperglycemia in patients receiving enteral nutrition support (22).
It is not known whether patients with type 2 diabetes treated with premixed insulin prior to hospital admission should be switched to a basal-bolus insulin regimen or whether it is safe to continue with the same formulation during the hospital stay. Because of the large number of patients receiving premixed insulin treatment at hospital admission, we conducted this study to compare the efficacy and safety of a premixed insulin regimen (30% regular insulin and 70% NPH insulin) twice daily with a basal-bolus insulin regimen (glargine once daily and glulisine before meals) in general medicine and surgery patients with type 2 diabetes.
Research Design and Methods
In this randomized, prospective, open-label study, we recruited patients, ≥18 years of age, who had been admitted to general medicine and surgery services in two academic medical centers in Spain. We enrolled patients with a previous diagnosis of type 2 diabetes who had been treated with diet, any combination of oral antidiabetic agents, and/or insulin therapy. We excluded patients with type 1 diabetes, hyperglycemia without a previous diagnosis of diabetes, acute hyperglycemic emergencies, or severe hyperglycemia treated with intravenous insulin infusion on hospital admission, acute or chronic kidney disease (serum creatinine level >2 mg/dL), corticosteroid therapy, a history of severe or repeated hypoglycemic episodes, and pregnancy, and patients expected to require ICU or a hospital stay of <3 days. In addition, we excluded patients who expected to receive NPO for a significant period of time (i.e., after abdominal surgery). The ethics committee of each participating institution approved the study protocol. Informed consent was obtained from all enrolled patients who were informed about the nature, objectives, and potential risks of the study. A research coordinator at each institution following a computer-generated randomization table conducted randomization and treatment assignment.
Outcome Measures
The primary outcome of the study was to determine the differences in glycemic control between treatment groups as measured by the mean capillary daily blood glucose (BG) concentration during each day of hospital stay. Secondary objectives were to determine differences in the percentage of glucose measures between 80 and 180 mg/dL, the frequency and severity of hypoglycemic events, total daily insulin use, length of hospital stay, and glycemic variability between groups. Hypoglycemia was defined as a capillary BG level of <70 mg/dL, and was classified as severe if it involved loss of consciousness and/or seizures.
Insulin Treatment
Patients were randomized to receive a premixed human insulin formulation with 30% regular insulin and 70% NPH insulin (Mixtard 30; Novo Nordisk) or a basal-bolus regimen with glargine (Lantus; Sanofi) once daily and glulisine (Apidra; Sanofi) before meals. All oral antidiabetic agents were discontinued on admission to the hospital. Subjects treated with diet or oral agents prior to hospital admission were started at a total daily dose of 0.3 units/kg if their admission BG level was <150 mg/dL or at 0.4 units/kg if their hospital admission BG level was >150 mg/dL. Subjects treated with insulin prior to hospital admission were continued on their previous total daily dose.
Patients in the basal-bolus group received 50% of the total daily dose as glargine and 50% as glulisine. Glargine was administered as a single daily dose, and glulisine was divided into three equal doses that were administered before breakfast, lunch, and dinner. In the premixed insulin group, patients received two doses of premixed insulin, with 60% of the total daily dose administered before breakfast and 40% before dinner. Premixed human insulin was injected 30 min before meals. Patients in the premixed insulin group received a 2,000 calorie/day dietary regimen divided into five meals given at breakfast, midmorning snack, lunch, afternoon snack, and dinner. Patients in the basal-bolus group were provided with a dietary regimen with the same total number of calories but given in three meals per day. In addition, patients in both treatment groups with a BG level 2 h after dinner of <140 mg/dL were provided with a bedtime snack.
In both groups, the insulin dose was adjusted daily based on capillary BG levels to target glucose before meals between 80 and 140 mg/dL (Supplementary Fig. 1).
The capillary BG level was measured 2 h before and after breakfast, lunch, and dinner. In addition, capillary BG level was measured at any time if symptoms of hypoglycemia were noted. Laboratory tests, including basic biochemistry studies, lipid profiles, and HbA1c levels, were performed in all patients on the first day of hospital admission.
Glycemic variability was calculated by the following three methods: 1) determination of the mean daily SD of BG values, a measure of dispersion of glucose values about a measure of central tendency, which represents the overall glycemic variability during the entire stay (23); 2) determination of the coefficient of variation of glucose measures, which represents the average daily glycemic excursion (24); and 3) determination of the mean amplitude of glycemic excursions, which is the arithmetic mean of the absolute value of BG level excursions from glucose nadirs to peaks or vice versa (25,26).
Statistical Analysis
This was a noninferiority study design based on the hypothesis that the difference in mean daily BG levels between a basal bolus with insulin analogs and premixed human insulin would not be >18 mg/dL (1 mmol/L). A BG level difference of such a magnitude has been reported (5,11) as non–clinically significant and is typically smaller than significant treatment effects detected in other superiority trials. Given the data from the RABBIT medicine and surgery trials (5,11), it is reasonable to assume that the SD of the mean daily BG level is bounded by 40 mg/dL. Based on two-sample t tests or Wilcoxon tests, one-sided, α = 0.05, we estimated that 65 subjects per group (130 total) will be needed to achieve 80% power. The protocol included as stopping rule a rate of hypoglycemia (<70 mg/dL) in >50% of patients in either group. Following the first interim analysis, the study was stopped early because the frequency of hypoglycemia exceeded 50% in the premixed insulin group.
We report continuous variables as the mean ± SD in the case of normal distribution. Qualitative variables are described using absolute and relative frequencies (percentages). The hypoglycemia rate was calculated as the number of hypoglycemic events divided by the total number of subject-days of exposure. To study the association between qualitative variables, the χ2 test was used with Yates correction and Fisher exact test when required by the conditions. In the case of quantitative variables, the Kolmogorov-Smirnov test was used to determine the normality of the distributions. To study the differences between independent means, the parametric or nonparametric tests required by the application were used (Student t test or Mann-Whitney U test in case of two categories; ANOVA with Bonferroni post hoc test or Kruskal-Wallis H test for comparisons with more than two categories). The significance level was set conventionally at P ≤ 0.05. Using the Bonferroni post hoc test, the significance level was set at P ≤ 0.004. The data were processed using the SPSS version 17.0 statistical package.
Results
A total of 109 patients with type 2 diabetes were enrolled in this trial. Of them, 94 patients were randomized to receive a basal-bolus regimen with glargine and glulisine (n = 46) or to receive premixed insulin twice daily (n = 48). A total of 33 patients in the basal-bolus group and 39 patients in the premixed group completed the study (Supplementary Table 1). The clinical and demographic characteristics of both groups were similar, except for mean age, which was higher in the premixed group (Table 1). The mean ± SD duration of hospitalization was 17.4 ± 17 days in the basal-bolus group vs. 24.1 ± 18 in the premixed group (P = 0.13). There were no differences in the type of diabetes treatment prior to hospital admission between groups, with approximately half of the patients treated with insulin alone or in combination with oral agents. There were more patients in the medicine service randomized to receive a basal bolus than to receive premixed insulin (67% vs. 42.1%, P = 0.038).
Table 1.
Baseline clinical characteristics of the patients who completed the study
| Variable | Basal-bolus group | Premixed insulin group | P value |
|---|---|---|---|
| Patients, n | 33 | 39 | |
| Sex | 0.96 | ||
| Male | 10 (30.3) | 12 (30.8) | |
| Female | 23 (69.7) | 27 (69.2) | |
| Age, years | 67.5 ± 11.0 | 75.3 ± 9.5 | 0.002 |
| BMI, kg/m2 | 29.5 ± 6.9 | 27.3 ± 4.6 | 0.15 |
| Body weight, kg | 80.9 ± 22.3 | 74.0 ± 12.4 | 0.11 |
| Duration of diabetes, years | 13.4 ± 10.3 | 17.2 ± 11.3 | 0.19 |
| Hospital admission service | |||
| Medicine | 22 (66.7) | 16 (42.1) | 0.038 |
| Surgery | 11 (33.3) | 22 (57.9) | |
| Hospital LOS, days | 17.4 ± 17.3 | 24.1 ± 18.4 | 0.13 |
| Hospital admission diabetes therapy | 0.63 | ||
| Diet alone | 4 (12.1) | 7 (17.9) | |
| Oral agents | 13 (40.6) | 11 (28.2) | |
| Insulin alone | 8 (25.0) | 15 (38.5) | |
| Insulin and oral agents | 8 (25.0) | 6 (15.4) | |
| Primary diagnosis | 0.15 | ||
| Cardiovascular | 16 (48.5) | 13 (33.3) | |
| Pulmonary | 3 (9.1) | 1 (2.6) | |
| Soft-tissue and foot infection | 11 (33.3) | 23 (59) | |
| Other | 3 (9.1) | 2 (5.1) | |
| Glycemic control | |||
| HbA1c | 0.78 | ||
| % | 8.5 ± 1.8 | 8.6 ± 2.0 | |
| mmol/mol | 69.7 ± 19.9 | 71.1 ± 22.4 | |
| Hospital admission BG, mg/dL | 203.1 ± 105 | 221.3 ± 104.6 | 0.47 |
| Randomization BG, mg/dL | 221.2 ± 76 | 210.5 ± 57 | 0.52 |
Data are n (%) or mean ± SD, unless otherwise indicated. LOS, length of stay.
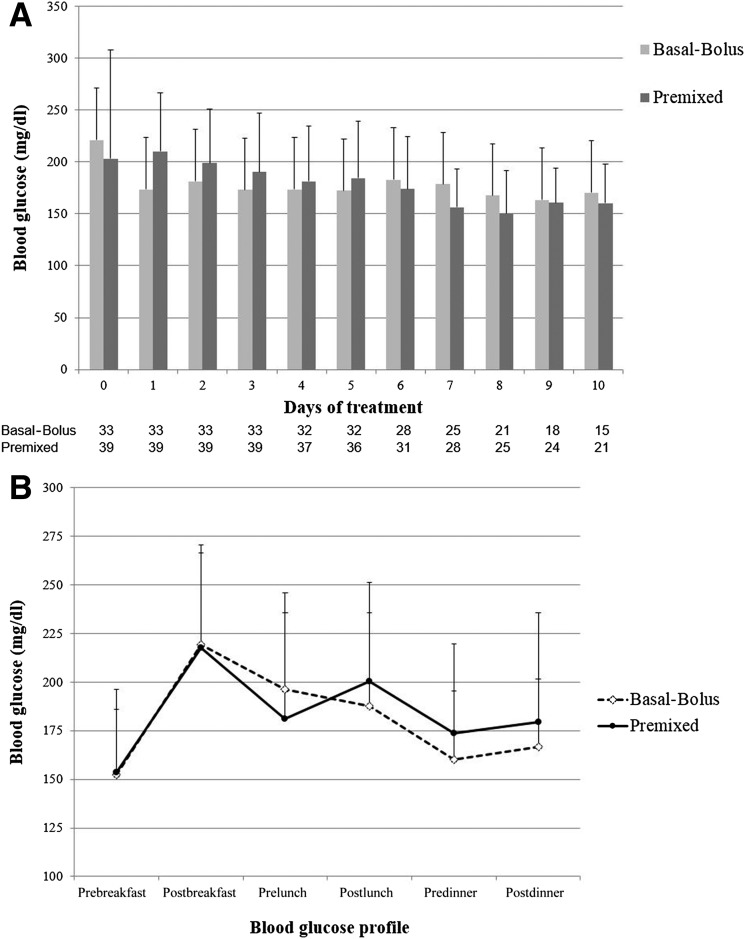
The mean capillary BG level on hospital admission was similar in both groups (203.1 ± 105 mg/dL in the basal-bolus group vs. 221.3 ± 105 mg/dL in the premixed group; P = 0.47). Both treatment regimens resulted in prompt and sustained improvement in the mean daily BG concentration during the hospital stay. There were no differences between groups in daily BG concentration, mean fasting, premeal, or after-meal BG levels (Fig. 1A and B). In addition, we observed no differences in the percentage of subjects with glucose values between 80 and 180 mg/dL during treatment.
Figure 1.
Difference in glycemic control between patients treated with basal-bolus and premixed insulin formulation. A: Changes in mean daily BG concentration. Day 0, randomization day. B: Differences in mean BG (in mg/dL) before and 2 h after meals.
Hypoglycemia and Trial Suspension
Following a planned interim safety analysis after the enrollment of half of the patients, the trial was terminated early owing to an increased frequency of hypoglycemic events, which was >50% (prespecified stopping rule) in the premixed insulin therapy group compared with basal-bolus insulin therapy group. Hypoglycemia occurred in 8 patients (24.2%) treated with glargine/glulisine insulin and in 25 patients (64.1%) treated with premixed insulin during the hospital stay (P = 0.001) (Table 2). In the glargine/glulisine group, five patients (15.2%) had a BG level between 60 and 69 mg/dL, five patients (15.2%) had a BG level between 40 and 59 mg/dL, and only two patients (6.1%) had a BG level <40 mg/dL. In the premixed insulin group, 17 patients (43.6%) had BG levels between 60 and 69 mg/dL (P = 0.009 vs. basal-bolus group), 18 patients (46.2%) had BG levels between 40 and 59 mg/dL (P = 0.005 vs. basal-bolus group), and only 1 patient had BG levels <40 mg/dL (P = 0.45 vs. basal-bolus group). Patients treated with premixed insulin had a greater number of hypoglycemic events throughout the day. Differences in hypoglycemia were significantly greater before lunch and during the night between midnight and 6:00 a.m. between patients treated with premixed insulin compared with those treated with a basal-bolus regimen (Table 2).
Table 2.
Efficacy and safety variables in patients treated with basal-bolus and premixed insulin regimens
| Basal-bolus regimen | Premixed reimen | P value | |
|---|---|---|---|
| Glycemic control | |||
| Mean BG concentration, the first day of therapy, mg/dL | 173.4 ± 54 | 210.2 ± 56 | 0.007 |
| Mean BG concentration, after first day of therapy, mg/dL | 175.1 ± 32 | 179 ± 43 | 0.64 |
| BG readings 80–180 mg/dL, % | 55.9 | 54.3 | 0.23 |
| Insulin therapy | |||
| Total, units/kg/day | 0.5 ± 0.3 | 0.7 ± 0.3 | 0.014 |
| Basal insulin, units/day | |||
| Glargine in basal-bolus group and NPH insulin in premixed group | 25.5 ± 15.4 | 38.8 ± 16.6 | 0.001 |
| Rapid insulin, units/day | |||
| Glulisine in basal-bolus and regular insulin in premixed group | 19.8 ± 8.4 | 16.6 ± 7.1 | 0.63 |
| Hypoglycemic events (BG concentration <70 mg/dL) | |||
| BG tests per patient/day | 5.7 ± 0.8 | 5.6 ± 0.8 | 0.716 |
| Overall, patients with hypoglycemia | 8 (24.2) | 25 (64.1) | 0.001 |
| Events, n | 20 | 65 | |
| BG readings, % | 1.4 | 3.2 | |
| Fasting or prebreakfast hypoglycemia | 1 (3.0) | 5 (12.8) | 0.134 |
| Events, n | 1 | 5 | |
| Morning or prelunch | 1 (3.0) | 12 (30.8) | 0.002 |
| Events, n | 5 | 19 | |
| Afternoon or predinner | 5 (15.2) | 8 (20.5) | 0.556 |
| Events, n | 7 | 12 | |
| Evening, after dinner to midnight | 4 (12.1) | 8 (20.5) | 0.341 |
| Events, n | 6 | 13 | |
| Night, midnight to 6:00 a.m. | 1 (3.0) | 9 (23.1) | 0.014 |
| Events, n | 1 | 16 |
Data are mean ± SD or n (%), unless otherwise indicated.
The frequency of hypoglycemia was higher in patients who were treated with insulin prior to hospital admission. Among patients receiving insulin therapy prior to hospital admission, 23 patients (62.9%) had at least one episode of hypoglycemia, whereas only 10 patients (28.6%) who were not receiving home insulin therapy had at least one episode of hypoglycemia (P = 0.04). None of the episodes of hypoglycemia was associated with loss of consciousness or seizures. In multivariable analyses, we did not find an association between the presence of hypoglycemic events and age, gender, duration of diabetes, HbA1c level, hospital admission service (medicine or surgery), and length of hospital stay.
The mean starting insulin dose was similar in both groups (0.46 ± 0.2 units/kg in the basal-bolus group vs. 0.46 ± 0.1 units/kg in the premixed group; P = NS), whereas at the end of treatment the mean daily insulin dose was significantly higher in the premixed group (0.72 ± 0.27 units/kg) compared with the basal-bolus group (0.55 ± 0.24 units/kg, P = 0.014). The difference in daily insulin requirements was based on the amount of basal component with a mean glargine dose of 25.5 ± 15 units/day and an NPH insulin dose of 38.8 ± 16 units/day (P = 0.001). There were no differences in the amount of short-acting insulin per day (glulisine 19.8 ± 8 units/day, regular insulin 16.6 ± 7 units/day, P = 0.63).
Treatment with a basal-bolus regimen was associated with lower glycemic variability compared with treatment with premixed insulin regimen (mean ± SD 51.19 ± 15.3 vs. 58.76 ± 14.8 mg/dL, P = 0.037; coefficient of variation 28.91 ± 7.77 vs. 32.73 ± 6.52 mg/dL, P = 0.026). There were no differences in the mean amplitude of glycemic excursions between the groups (90.58 ± 28.53 vs. 95.72 ± 21.36 mg/dL, P = 0.386) in patients receiving the basal-bolus and premixed insulin regimens, respectively.
Conclusions
This is the first randomized prospective trial comparing the efficacy and safety of a basal-bolus regimen with insulin glargine once daily and premeal insulin glulisine with a premixed human insulin regimen (30% regular insulin/70% NPH insulin) in hospitalized patients with type 2 diabetes. Both treatment regimens resulted in a rapid and significant improvement in the mean daily BG concentration and in the percentage of glucose readings within the target range of 80 and 180 mg/dL before meals. The study was stopped early because the frequency of hypoglycemia was >50% in patients treated with premixed human insulin.
The association between inpatient hyperglycemia, in patients with and without diabetes, and increased risk of hospital complications is well established (1–4). Recent clinical trials (9,10) have also shown that treatment with a basal-bolus regimen is effective in improving glycemic control in medicine and surgical patients with acceptable rates of hypoglycemia. The basal-bolus approach, however, requires subcutaneous administration of basal insulin given once or twice daily in combination with prandial and corrective doses of rapid-acting insulin given before meals. The complexity of this approach has limited its acceptance among physicians (12,13). Premixed insulin formulations are commonly prescribed because of their proven efficacy in improving glucose control (17–20) and the need for fewer daily injections (20,21). In agreement with previous studies in ambulatory settings, we found that treatment with premixed insulin resulted in equivalent glucose control but a higher frequency of hypoglycemic events compared with the basal-bolus regimen.
A major finding in our study is that treatment with premixed human insulin resulted in a threefold higher rate of hypoglycemic events compared with treatment with a basal bolus of insulin analogs. Randomized trials (12,13,27) have reported a prevalence of hypoglycemia in 10–32% of non-ICU patients treated with the basal-bolus regimen. The higher rate of hypoglycemia in this study is likely the result of the fixed ratio of the premixed formulation in patients with altered oral intake or with changing insulin requirements. Minimizing the rate of hypoglycemia events is of major importance in hospitalized patients because it has been shown to represent an independent risk factor of poor outcome and mortality (28–30).
We acknowledge several limitations in our study, including a relatively small number of patients and the fact that we used only two doses of premixed insulin daily without a third dose or fixed insulin coverage at lunch time. There was a difference in age between the groups despite randomization, which could have influenced the frequency of hypoglycemia; however, in the multivariable analysis there was no relationship between the presence of hypoglycemia and age. In addition, we included patients with a hospital admission glucose concentration of >180 mg/dL, which may have reduced the number of hypoglycemic events with the use of premixed insulin. We also excluded patients with acute or chronic kidney failure, patients receiving corticosteroid therapy, and patients with a history of severe or repeated hypoglycemia. Patients meeting these exclusion criteria make up a substantial percentage of hospitalized patients. Another limitation is the lack of information on nutritional intake and missed meals, which represents a significant risk factor for hypoglycemia in the hospital setting. In this study, however, we excluded patients who were expected to receive NPO for a significant period of time. Finally, in our institutions, patients treated with NPH insulin and regular insulin regimens receive three main meals and snacks at midmorning and afternoon; this meal pattern was different from that provided to patients in the basal-bolus regimen group, who received only three main meals without snacks during the day. The higher number of meals (snacks) may have resulted in a lower frequency of hypoglycemia in the premixed group. In addition, we used premixed human insulin and did not use premixed insulin analog formulations, which may be associated with lower rates of hypoglycemia compared with human insulin formulations. Future studies should determine the safety and efficacy of premixed insulin analogs in the hospital setting.
In summary, basal-bolus insulin with glargine once daily and rapid-acting insulin analogs before meals represents a safer regimen than premixed human insulin for glucose control in non–critically ill patients with type 2 diabetes, while both treatments result in similar glycemic control. Despite the simplicity, premixed human insulin regimen is associated with greatly elevated rates of hypoglycemia and should be used with caution in the management of general medicine and surgery patients with type 2 diabetes.
Supplementary Material
Article Information
Funding. G.E.U. is supported in part by a research grant from the American Diabetes Association (1-14-LLY-36) and by Public Health Service Grant UL1-RR-025008 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources.
Duality of Interest. This investigator-initiated study was supported by an unrestricted grant from Sanofi Aventis (Asturias, Spain). E.D. has received unrestricted research support from AstraZeneca, Novo Nordisk, Sanofi, Pfizer, and Roche and has received consulting fees and/or honoraria for membership on advisory boards from AstraZeneca, Novo Nordisk, Lilly, Sanofi, GlaxoSmithKline, Pfizer, Almirall, Novartis, Abbott Laboratories, Esteve, and Merck Sharp & Dohme. E.M. has received unrestricted research support from Sanofi and has received consulting fees or/and honoraria for membership on advisory boards from Sanofi, Novo Nordisk, and Lilly. G.E.U. has received unrestricted research support for inpatient studies (to Emory University) from Sanofi, Merck, Novo Nordisk, and Boehringer Ingelheim and has received consulting fees or/and honoraria for membership on advisory boards from Novo Nordisk, Sanofi, Merck, and Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
The sponsors of the study were not involved in the study design, data collection, analysis or interpretation of the results, or preparation of the manuscript.
Author Contributions. V.B. and E.M. wrote the initial research proposal, reviewed the proposal and the study results, and wrote the article. L.S., M.G.R., C.S., M.D., M.R., F.C., and E.D. collected research data, reviewed and edited the research proposal and article, and contributed to the discussion. G.E.U. reviewed the proposal and the study results and wrote the article. E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02333851, clinicaltrials.gov.
Spanish Clinical Studies Agency clinical trial reg. no. EMT-INS-2012-01, reec.aemps.es.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0160/-/DC1.
References
- 1.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 2.Kosiborod M, Inzucchi SE, Spertus JA, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation 2009;119:1899–1907 [DOI] [PubMed] [Google Scholar]
- 3.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009;37:3001–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotagal M, Symons RG, Hirsch IB, et al.; SCOAP-CERTAIN Collaborative . Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015;261:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011;34:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:49–58 [DOI] [PubMed] [Google Scholar]
- 7.Schroeder JE, Liebergall M, Raz I, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev 2012;28:71–75 [DOI] [PubMed] [Google Scholar]
- 8.Munoz C, Villanueva G, Fogg L, et al. Impact of a subcutaneous insulin protocol in the emergency department: Rush Emergency Department Hyperglycemia Intervention (REDHI). J Emerg Med 2011;40:493–498 [DOI] [PubMed] [Google Scholar]
- 9.Umpierrez GE, Hellman R, Korytkowski MT, et al.; Endocrine Society . Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 10.Moghissi ES, Korytkowski MT, DiNardo M, et al.; American Association of Clinical Endocrinologists; American Diabetes Association . American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 12.Pérez A. Glycemic control at hospital: why does it not improve? Endocrinol Nutr 2012;59:153–154 [DOI] [PubMed] [Google Scholar]
- 13.Cook CB, Castro JC, Schmidt RE, et al. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med 2007;2:203–211 [DOI] [PubMed] [Google Scholar]
- 14.Kalra S, Balhara YP, Sahay BK, Ganapathy B, Das AK. Why is premixed insulin the preferred insulin? Novel answers to a decade-old question. J Assoc Physicians India 2013;61(Suppl.):9–11 [PubMed] [Google Scholar]
- 15.Rathmann W, Haastert B, Riebel P, et al. Prescription of insulin glargine in primary care practices in Germany. Exp Clin Endocrinol Diabetes 2007;115:252–256 [DOI] [PubMed] [Google Scholar]
- 16.Charbonnel B, Balarac N, Cazeneuve B, Augendre-Ferrante B, Le Thaï F, Drouin P; Schema survey . Which are the insulin treatment regimens used in France? The “Schema survey”. Diabetes Metab 2001;27:591–597 [PubMed] [Google Scholar]
- 17.Miser WF, Arakaki R, Jiang H, Scism-Bacon J, Anderson PW, Fahrbach JL. Randomized, open-label, parallel-group evaluations of basal-bolus therapy versus insulin lispro premixed therapy in patients with type 2 diabetes mellitus failing to achieve control with starter insulin treatment and continuing oral antihyperglycemic drugs: a noninferiority intensification substudy of the DURABLE trial. Clin Ther 2010;32:896–908 [DOI] [PubMed] [Google Scholar]
- 18.Schiel R, Müller UA, Rauchfub J, Sprott H, Müller R. Blood-glucose self-monitoring in insulin treated type 2 diabetes mellitus a cross-sectional study with an intervention group. Diabetes Metab 1999;25:334–340 [PubMed] [Google Scholar]
- 19.Eliasson B, Ekström N, Bruce Wirta S, Odén A, Fard MP, Svensson AM. Metabolic effects of Basal or premixed insulin treatment in 5077 insulin-naïve type 2 diabetes patients: registry-based observational study in clinical practice. Diabetes Ther 2014;5:243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilag LL, Kerr L, Malone JK, Tan MH. Prandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of type 2 diabetes: an evidence-based comparison. Clin Ther 2007;29:1254–1270 [PubMed] [Google Scholar]
- 21.Garber AJ, Ligthelm R, Christiansen JS, Liebl A. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab 2007;9:630–639 [DOI] [PubMed] [Google Scholar]
- 22.Hsia E, Seggelke SA, Gibbs J, Rasouli N, Draznin B. Comparison of 70/30 biphasic insulin with glargine/lispro regimen in non-critically ill diabetic patients on continuous enteral nutrition therapy. Nutr Clin Pract 2011;26:714–717 [DOI] [PubMed] [Google Scholar]
- 23.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006;105:244–252 [DOI] [PubMed] [Google Scholar]
- 24.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 2008;36:3008–3013 [DOI] [PubMed] [Google Scholar]
- 25.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–655 [DOI] [PubMed] [Google Scholar]
- 26.Meynaar IA, Eslami S, Abu-Hanna A, van der Voort P, de Lange DW, de Keizer N. Blood glucose amplitude variability as predictor for mortality in surgical and medical intensive care unit patients: a multicenter cohort study. J Crit Care 2012;27:119–124 [DOI] [PubMed] [Google Scholar]
- 27.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 2013;36:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagansky N, Levy S, Rimon E, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med 2003;163:1825–1829 [DOI] [PubMed] [Google Scholar]
- 29.Stagnaro-Green A, Barton MK, Linekin PL, Corkery E, deBeer K, Roman SH. Mortality in hospitalized patients with hypoglycemia and severe hyperglycemia. Mt Sinai J Med 1995;62:422–426 [PubMed] [Google Scholar]
- 30.Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009;32:1153–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.