Abstract
OBJECTIVE
To determine whether clinically accessible parameters early in the course of youth-onset type 2 diabetes predict likelihood of durable control on oral therapy.
RESEARCH DESIGN AND METHODS
TODAY was a randomized clinical trial of adolescents with type 2 diabetes. Two groups, including participants from all three treatments, were defined for analysis: 1) those who remained in glycemic control for at least 48 months of follow-up and 2) those who lost glycemic control before 48 months. Outcome group was analyzed in univariate and multivariate models as a function of baseline characteristics (age, sex, race/ethnicity, socioeconomic status, BMI, waist circumference, Tanner stage, disease duration, depressive symptoms) and biochemical measures (HbA1c, C-peptide, lean and fat body mass, insulin inverse, insulinogenic index). Receiver operating characteristic curves were used to analyze HbA1c cut points.
RESULTS
In multivariate models including factors significant in univariate analysis, only HbA1c and insulinogenic index at randomization remained significant (P < 0.0001 and P = 0.0002, respectively). An HbA1c cutoff of 6.3% (45 mmol/mol) (positive likelihood ratio [PLR] 3.7) was identified that optimally distinguished the groups; sex-specific cutoffs were 6.3% (45 mmol/mol) for females (PLR 4.4) and 5.6% (38 mmol/mol) for males (PLR 2.1).
CONCLUSIONS
Identifying youth with type 2 diabetes at risk for rapid loss of glycemic control would allow more targeted therapy. HbA1c is a clinically accessible measure to identify high risk for loss of glycemic control on oral therapy. Adolescents with type 2 diabetes unable to attain a non–diabetes range HbA1c on metformin are at increased risk for rapid loss of glycemic control.
Introduction
TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) was the first and largest randomized clinical trial to examine approaches to management of youth-onset type 2 diabetes. Among the primary findings of TODAY was that metformin monotherapy was inadequate for maintenance of glycemic control in almost half of participants after median time to failure of ∼11 months (1). Furthermore, there were distinct sex and racial/ethnic differences in the pattern of failure overall and across treatment arms. This degree of failure on monotherapy was higher than expected from studies of adults with type 2 diabetes (2,3) and only partially prevented by the addition of rosiglitazone. Taken together, the findings suggest that type 2 diabetes among adolescents can be more rapidly progressive than in adults (4–7).
On the other hand, ∼50% of TODAY participants maintained durable glycemic control regardless of treatment group. This suggests that type 2 diabetes among adolescents is a heterogeneous disorder grossly divided between those who are and are not able to maintain glycemic control on oral therapy. If so, it would be of substantial clinical importance to properly classify patients early in the course of the disease so that appropriate treatment can be planned.
In this analysis, we address the hypothesis that readily available demographic and metabolic characteristics determined early in the course of type 2 diabetes can distinguish adolescents who will display durable glycemic control from those at risk for loss of glycemic control on oral therapy.
Research Design and Methods
TODAY Design and Primary Findings
TODAY clinical trial rationale, design, and methods have been reported in detail (8) and are described briefly. Between July 2004 and February 2009, 699 youth aged 10–17 years, diagnosed with type 2 diabetes according to American Diabetes Association (ADA) criteria (9) for <2 years, with BMI ≥85th percentile, and negative for diabetes autoantibodies, were enrolled (10). After screening to determine eligibility, subjects completed a 2- to 6-month prerandomization run-in period in which they demonstrated mastery of standard diabetes education, were weaned from nonstudy diabetes medications, demonstrated tolerance of metformin 500–1,000 mg twice daily, maintained HbA1c <8% (<64 mmol/mol) monthly for at least 2 months on metformin alone, and demonstrated adherence to study medication and visit attendance (11). Participants were randomized to one of three treatment arms: metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle program. Study baseline data were collected after run-in and before start of randomized treatment assignment. Participants attended clinic visits for medical management every 2 months in the first year and quarterly thereafter. The goal of diabetes medical management was to maintain HbA1c levels as close to the normal range as possible in order to reduce long-term diabetes complications. During the trial, the investigators and the participants were blinded to specific HbA1c values but were informed if HbA1c was 1) ≥8% (≥64 mmol/mol), 2) 6–8% (42–64 mmol/mol) and increased from previous value by ≥0.8% (≥8.7 mmol/mol), or 3) ≤6% (≤42 mmol/mol) (target). The primary objective was to compare the three treatment arms on time to treatment failure, defined as either HbA1c ≥8% (≥64 mmol/mol) over a 6-month period or inability to wean from temporary insulin therapy within 3 months after acute metabolic decompensation.
The protocol was approved by an External Evaluation Committee convened by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health and by the institutional review boards of each participating institution. All participants provided written informed consent, and minor children confirmed assent according to local guidelines.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
Analysis Samples and Measures
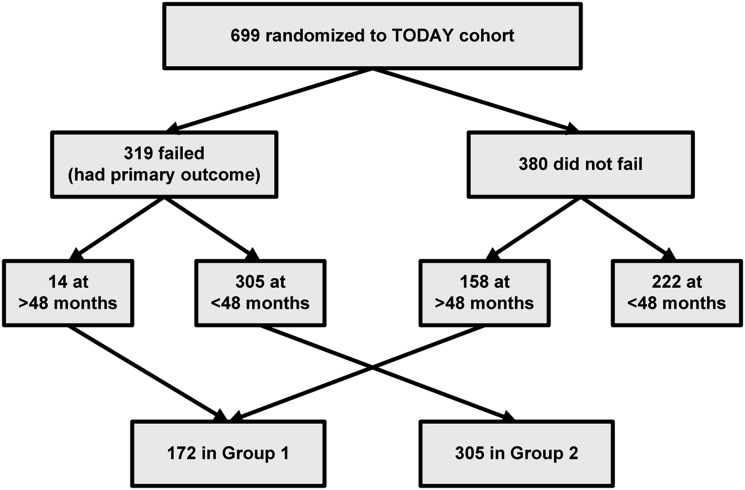
Two outcome groups were defined for analysis. Group designation was assigned irrespective of original treatment arm. Group 1 participants remained in glycemic control (i.e., did not reach the primary outcome, as defined above) for at least 48 months of follow-up, and group 2 reached the primary outcome before 48 months; beyond 48 months, sample size was inadequate for analysis and data are not included.
Demographic and baseline characteristics in the analysis were age, sex, race/ethnicity (classified as non-Hispanic black [NHB], Hispanic [H], non-Hispanic white [NHW], or other), household annual income, highest education level of parents or guardians, first-degree family history of diabetes, Tanner stage (by physician examination), months since diagnosis of type 2 diabetes, and presence of depressive symptoms based on the Children’s Depression Inventory (12) for participants age ≤15 years or the Beck Depression Inventory (13) for participants age ≥16 years.
Baseline measures were BMI, HbA1c at screening and randomization, C-peptide, and adiposity. Laboratories were performed by a central laboratory (8). Insulin sensitivity and β-cell function were derived from the 2-h oral glucose tolerance test as follows: insulin inverse = 1/insulinmin 0, and insulinogenic index = (insulinmin 30 − insulinmin 0)/(glucosemin 30 − glucosemin 0). Measures of adiposity were waist circumference and DXA measures of fat (kg) and lean (kg) body mass; in approximately one-quarter of the subjects, DXA scans were excluded owing to weight limitations (weight >140 kg).
Statistical Analysis
Logistic regression was used to model outcome group as a function of demographic variables, baseline characteristics, and baseline measures in univariate and multivariate analyses. Receiver operating characteristic (ROC) curve analyses were performed to identify optimal baseline HbA1c cut points predictive of failure (as indicated by outcome group). The standard logistic regression model and the trapezoidal rule method were used to compute the total area under the curve (AUC) and its associated 95% CI in the overall analysis sample by sex, by race/ethnicity (NHB, H, NHW), and for the six sex-by-race/ethnicity combinations. Sensitivity and specificity were computed and the Youden index method was used to select the optimal threshold point from the ROC curve (14). The Youden index [maximum (sensitivity + specificity − 1)] maximizes the vertical distance from the line of equality to identify the point on the curve farthest from chance (15,16) and maximizes the classification rate. The positive likelihood ratio (PLR) (sensitivity/1 − specificity) was used to determine whether a baseline HbA1c cutoff usefully changed the probability of failing oral therapy; PLR >1 indicates the test result is associated with increased probability (17). P values <0.05 were considered significant. P values were considered exploratory and were not adjusted for multiple testing; the study was powered only for the time-to-failure primary outcome.
Results
The assignment of participants into the two analysis groups is shown in Fig. 1.
Figure 1.
Flow of participants into the two groups analyzed based on time to study primary outcome (failure to maintain glycemic control on randomized treatment assignment). Group 1 was followed to at least 48 months without having the primary outcome. Group 2 experienced the primary outcome within 48 months of follow-up.
Demographic and baseline characteristics in group 1 (n = 172) versus group 2 (n = 305) are shown in Table 1. Group 1 had a significantly higher percentage of NHW and lower of NHB, a lower prevalence of depressive symptoms at the time of entry into the study, and a lower percentage of first-degree relatives with diabetes; total annual household income (≥$50,000) barely missed statistical significance (P = 0.0512). There were no differences by sex, treatment group, age, duration of diabetes, Tanner stage, or highest household education at study entry. There was also no difference in Tanner stage by sex in the analysis sample (90.5% of females and 87.3% of males were Tanner stage 4 or 5). There was no difference at any time between groups 1 and 2 in percentage of participants with study medication adherence ≥80% (data not shown).
Table 1.
Demographic and baseline characteristics by analysis group
| Group 1: no PO <48 mo | Group 2: PO <48 mo | P | |
|---|---|---|---|
| n | 172 | 305 | |
| Treatment, n (%) | |||
| M | 53 (30.8) | 116 (38.0) | 0.2453 |
| M+R | 58 (33.7) | 86 (28.2) | |
| M+L | 61 (35.5) | 103 (33.8) | |
| Sex, n (%) | |||
| Female | 111 (64.5) | 193 (63.3) | 0.7845 |
| Male | 61 (35.5) | 112 (36.7) | |
| Age (years) | 13.8 (1.9) | 14.1 (2.1) | 0.1825 |
| Race/ethnicity, n (%) | |||
| NHB | 47 (27.3) | 117 (38.3) | 0.0323 |
| H | 70 (40.7) | 119 (39.0) | |
| NHW | 43 (25.0) | 48 (15.7) | |
| Other | 12 (7.0) | 21 (6.9) | |
| Months since diagnosis | 8.1 (6.2) | 8.7 (6.2) | 0.3752 |
| Depressive symptoms, n (%) | |||
| No | 154 (91.1) | 251 (83.4) | 0.0217 |
| Yes | 15 (8.9) | 50 (16.6) | |
| Tanner stage, n (%) | |||
| ≥4 | 155 (90.1) | 271 (88.9) | 0.6682 |
| ≤3 | 17 (9.9) | 34 (11.1) | |
| Household income ($), n (%) | |||
| Low (<25,000) | 62 (39.7) | 120 (45.0) | 0.0512 |
| Mid (25,000–49,999) | 46 (29.5) | 93 (34.8) | |
| High (≥50,000) | 48 (30.8) | 54 (20.2) | |
| Household education, n (%) | |||
| Less than high school | 46 (27.1) | 80 (26.5) | 0.4529 |
| High school, GED, business or technical school | 37 (21.8) | 85 (28.1) | |
| College, no degree | 57 (33.5) | 93 (30.8) | |
| College degree | 30 (17.6) | 44 (14.6) | |
| First-degree family history of diabetes, n (%) | |||
| No | 86 (50.6) | 100 (33.3) | 0.0003 |
| Yes | 84 (49.4) | 200 (66.7) | |
| BMI (kg/m2) | 34.0 (7.6) | 35.1 (7.5) | 0.1189 |
| Waist circumference (cm) | 107.1 (16.2) | 109.2 (17.0) | 0.1940 |
| HbA1c at screening (%) | 6.79 (1.64) | 8.05 (2.07) | <0.0001 |
| HbA1c at screening (mmol/mol) | 51 (17.9) | 64 (22.6) | <0.0001 |
| HbA1c at randomization (%) | 5.68 (0.55) | 6.39 (0.80) | <0.0001 |
| HbA1c at randomization (mmol/mol) | 39 (6.0) | 46 (8.7) | <0.0001 |
| C-peptide (ng/mL) | 3.71 (1.55) | 3.91 (1.64) | 0.1921 |
| DXA fat mass (kg) | 32.9 (10.0) | 33.0 (9.8) | 0.9453 |
| DXA lean mass (kg) | 55.6 (12.4) | 54.0 (11.0) | 0.2299 |
| Insulin inverse (mL/µU) | 0.045 (0.027) | 0.047 (0.037) | 0.7956† |
| Insulinogenic index (µU/mL per mg/dL) | 2.04 (2.18) | 1.12 (2.08) | <0.0001† |
Data are mean (SD) unless otherwise indicated. GED, General Equivalency Diploma; M, metformin alone; M+L, metformin plus lifestyle program; M+R, metformin plus rosiglitazone; mo, months; PO, primary outcome, i.e., failure to maintain glycemic control on randomized treatment assignment.
†Test performed on log transform to normalize distribution.
In univariate analysis, screening and baseline HbA1c and baseline insulinogenic index were identified as the only significant metabolic factors distinguishing those with durable glycemic control irrespective of treatment group; participants with durable control had significantly lower HbA1c and higher insulinogenic index. Baseline measures of insulin sensitivity (fasting C-peptide and insulin inverse), measures of body fatness (BMI, waist circumference, and DXA fat mass), and DXA lean mass were not different between groups. Calculation of oral disposition index did not contribute beyond insulinogenic index alone (data not shown).
In multivariate models including all baseline demographic and metabolic factors significant in univariate analysis, only HbA1c and insulinogenic index remained significant (P < 0.0001 and P = 0.0002, respectively). This was also true when treatment assignment was included in the model. With all other variable values held as fixed, there was a 16% increase in the odds of failing within 48 months of treatment for each 0.1% (1.1 mmol/mol) increase in HbA1c from study baseline (after stabilization of HbA1c <8% [<64 mmol/mol] during run-in but before start of study randomized treatment). Similarly, there was a 16% increase in the odds of failing within 48 months of treatment for each 0.1-unit decrease in insulinogenic index from study baseline.
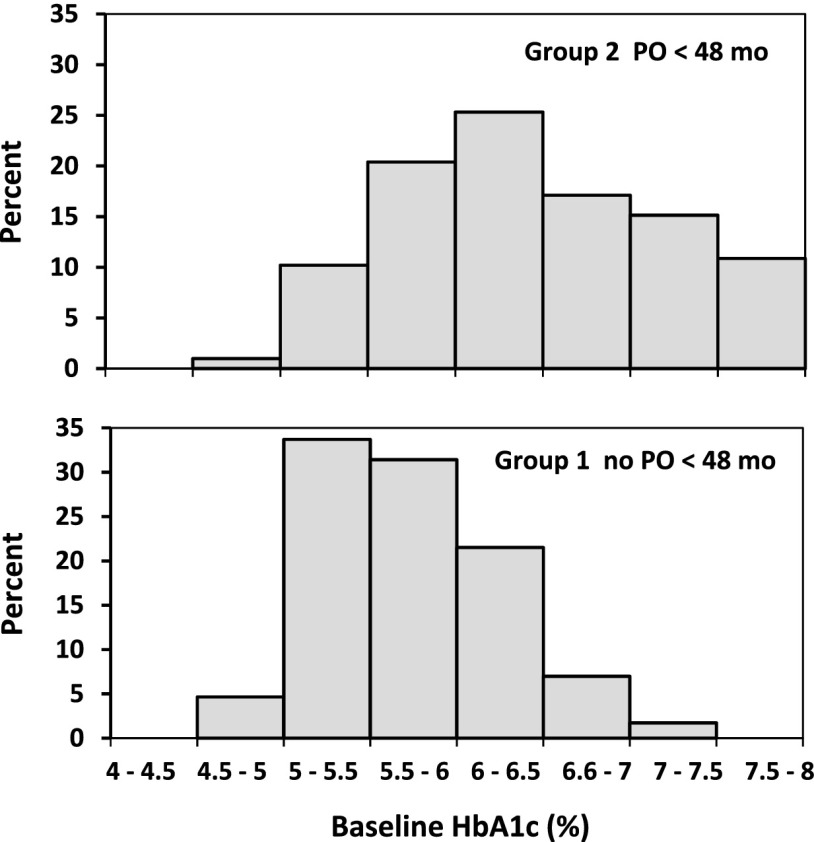
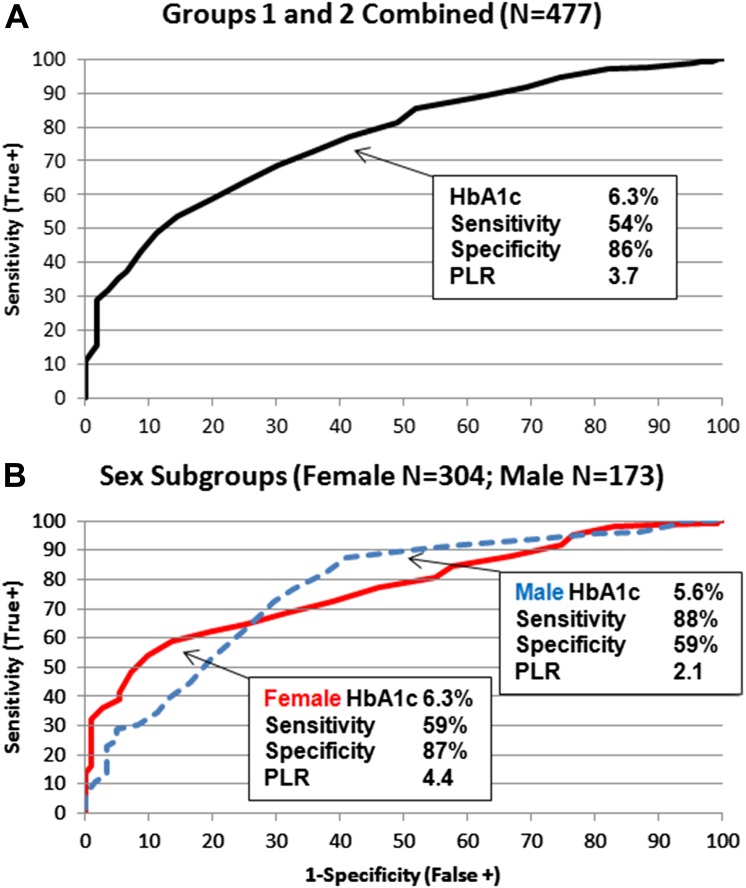
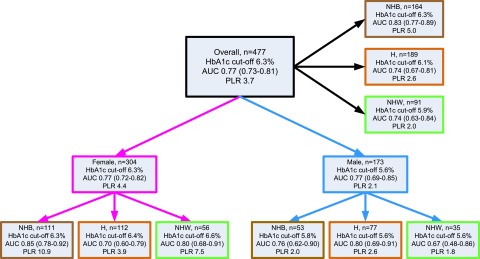
Figure 2 shows a shift in the distributions of baseline HbA1c for groups 1 and 2. Because HbA1c is easily obtained in the clinical setting, this shift suggested a clinically practical basis for distinguishing between the groups. Overall, the Youden index identified an HbA1c cutoff of 6.3% (45 mmol/mol) that maximized correct classification of participants (Fig. 3A), with sensitivity 54%, specificity 86%, and PLR 3.7. Figure 3B shows a sex difference in HbA1c operator characteristics. Although both sexes had the same AUC (0.77), the ROC curves crossed, indicating that HbA1c had higher specificity in females and higher sensitivity in males. The Youden index identified HbA1c of 6.3% (45 mmol/mol) for females (sensitivity 59%, specificity 87%, and PLR 4.4) and 5.6% (38 mmol/mol) for males (sensitivity 88%, specificity 59%, and PLR 2.1) as the optimal cutoffs for classifying the risk of loss of durable control. This sex difference in optimal cutoff was true across race/ethnicity; the Youden index identified optimal HbA1c cutoffs of 6.3, 6.4, and 6.6% (45, 46, and 49 mmol/mol) for NHB, H, and NHW females, respectively, but 5.8% (40 mmol/mol) for NHB and 5.6% (38 mmol/mol) for H and NHW males. Figure 4 summarizes HbA1c cutoffs, AUC, and PLR overall and by sex and race/ethnicity in the sample of groups 1 and 2 combined. (Supplementary Table 1 gives sensitivity, specificity, and PLR overall by sex, by race/ethnicity, and by the interaction of sex with race/ethnicity.)
Figure 2.
Distribution of baseline HbA1c in each of the analysis groups. Baseline HbA1c was measured after a run-in period in which participants had to maintain HbA1c <8% (<64 mmol/mol) monthly for at least 2 months on metformin alone in order to remain eligible for randomization in the clinical trial. The graphic suggests that HbA1c on metformin monotherapy is a clinically practical indicator of ability to maintain glycemic control on oral agents. mo, months; PO, primary outcome, i.e., failure to maintain glycemic control on randomized treatment assignment.
Figure 3.
ROC curves and HbA1c cutoffs, sensitivity, specificity, and PLR based on the Youden index over all participants (A) and by sex (B). PLR >1 indicates that the test result is associated with increased probability of failing oral therapy.
Figure 4.
The Youden index HbA1c cutoffs, AUC and its 95% CI, and PLR overall and by sex and racial/ethnic subgroups (“other” race/ethnicity not presented). AUC is a measure of diagnostic accuracy ranging from 0.0 to 1.0, where 0.5 is equivalent to a coin toss (the “curve” looks like a diagonal line); AUC values 0.6–0.7 represent poor ability to predict failure, values 0.7–0.8 represent fair ability, and 0.8–0.9 represent good ability. The TODAY cohort is predominantly female and minority, which affects the overall estimates. The male cutoffs are 0.5–1.0% below the female. The AUC 95% CI includes 0.50 only for NHW males (n = 35) indicating that the cutoff is equivalent to simply guessing. A PLR (sensitivity/1 − specificity) >1 indicates that the test result (HbA1c) is associated with presence of the disease, and the larger the PLR, the greater the likelihood of disease.
Since the ADA recommends a treatment target of 6.5% (48 mmol/mol), this value is often used by diabetes health care providers as a cutoff for determining whether to alter therapy. We compared the cutoff of 6.3 vs .6.5% (45 vs. 48 mmol/mol) in our analysis sample. Sensitivity for identification of participants who will lose glycemic control was 54 vs. 43%, while specificity rose from 86 to 91%. The PLR for an HbA1c of 6.3% (45 mmol/mol) was 3.7 vs. 5.0 for 6.5% (48 mmol/mol). At an HbA1c of 5.9% (41 mmol/mol), the PLR for loss of glycemic control was still >2. As expected from the sex-specific differences in Youden index, a cutoff of 6.5% (48 mmol/mol) had even lower sensitivity for identifying those likely to fail among males (35%, PLR 3.0) than females (48%, PLR 6.7). At an HbA1c of 6.0% (42 mmol/mol), the PLR for both males and females was >2.
Conclusions
The TODAY primary outcome analysis demonstrated that type 2 diabetes presenting during adolescence was characterized by high rates of loss of glycemic control over a relatively brief period of time compared with adults (1). Although addition of rosiglitazone to metformin reduced the rate of loss of glycemic control by 23% over the course of the study, the median time to glycemic failure was the same regardless of treatment assignment. Furthermore, the incidence of loss of glycemic control plateaued over time regardless of treatment assignment. Taken together, these observations suggest that there may be subsets of adolescents with type 2 diabetes characterized as those at risk of losing glycemic control rapidly, those who will maintain glycemic control for a prolonged period of time, and those who may respond to addition of a second oral agent with improved maintenance of glycemic control. In particular, the early ability to distinguish patients who maintain glycemic control from those who are going to rapidly lose glycemic control would allow clinicians to identify high-risk patients who would benefit from closer monitoring and earlier intensification of therapy while supporting a less aggressive approach for those who are likely to maintain good glycemic control on metformin alone.
To examine this question, we analyzed the effect of demographic, anthropometric, and biochemical characteristics of the participants at the time of their randomization into TODAY on the likelihood of loss of glycemic control during follow-up. In univariate analysis, NHB race/ethnicity, depressive symptoms, first-degree family history of diabetes, and lower family income were all associated with rapid loss of glycemic control, as might be expected from previous literature in adults (18–21). Surprisingly, age, duration of diabetes, BMI, waist circumference, fat mass, and lean body mass at randomization had no effect on outcome, though this may be due, in part, to the relatively narrow range of each of these measures in the TODAY cohort (22). Among metabolic measures, only screening and baseline HbA1c and insulinogenic index were associated with maintenance of glycemic control; insulin sensitivity did not differ between the groups. We did not explore screening HbA1c further because the heterogeneous nature of the screened cohort in terms of treatment modalities made generalization of findings to a clinical population difficult.
In multivariate analysis, only baseline HbA1c and insulinogenic index remained significant irrespective of treatment assignment. Studies in adults (2,3) and previous studies in the TODAY cohort (23,24) have shown that β-cell function is a strong determinant of HbA1c. Therefore, HbA1c already reflects information about β-cell function. Although both measures were significant predictors of durable control, HbA1c is clinically more accessible than determination of insulinogenic index. Similarly, although demographic factors were shown to contribute to diabetes outcome in univariate analysis, it may be that the HbA1c value already reflects these contributions, and these factors, therefore, disappeared as significant contributors to prediction of glycemic control in multivariate analysis in this cohort. Given the limitations of our data, we can only speculate on how these factors affect HbA1c, perhaps through genetic, hormonal, nutritional, or behavioral influences on β-cell function. This analysis suggests that HbA1c obtained after a short course of metformin monotherapy in adolescents with type 2 diabetes can function as a simple clinical measure to predict outcome and allow more effective targeting of therapy to those at highest risk for loss of glycemic control.
The next question is whether there is a specific HbA1c cutoff value that is useful as a predictor of likely outcome. ROC analysis identified an HbA1c of 6.3% (45 mmol/mol) as the optimal Youden cutoff for correctly categorizing an individual’s risk for loss of glycemic control on oral therapy, with an AUC of 0.77. The optimal cutoff was similar for females, reflecting the >60% representation of females in the cohort, but was substantially lower for males. This cutoff is lower than the ADA-recommended treatment target of 6.5% (48 mmol/mol) and probably lower than the level at which most clinicians would consider addition of a second agent. At a minimum, the analysis suggests that an HbA1c cutoff of 6.5% (48 mmol/mol) has low sensitivity for identifying those adolescents; a youth with HbA1c >6.5% (>48 mmol/mol) after a few months on metformin had a >70% chance of experiencing loss of glycemic control in a relatively short time on oral therapy alone. Indeed, both boys and girls with an HbA1c >6.0% (>42 mmol/mol) had approximately double the risk for failure as individuals with an HbA1c <6.0% (<42 mmol/mol). Together these data indicate that adolescents with type 2 diabetes who do not attain a non–diabetes range HbA1c after a few months on metformin are at increased risk for losing glycemic control, and the clinicians caring for these youth should not be reassured that HbA1c is “in target.” These individuals may benefit from closer monitoring or earlier initiation of supplemental therapy, despite an HbA1c that meets current targets. For example, females with baseline HbA1c higher than their optimal cutoff of 6.3% (45 mmol/mol) treated with rosiglitazone and metformin had a failure rate of 52% compared with 83% for metformin alone (Supplementary Data). Although the future role of rosiglitazone in the treatment of youth with type 2 diabetes remains unclear, these results point out the potential benefits of initiating combination therapy in selected patients who do not reach the optimal cutoff after a short course of metformin. Conversely, a youth with HbA1c <6.0% (<42 mmol/mol) after a few months on metformin had a better than 70% chance of remaining in control on monotherapy for at least 4 years.
These findings do not suggest that there is benefit to treating patients to a lower HbA1c than the ADA target; we did not perform an intervention trial with different targets to address this question. Rather, the analysis suggests that HbA1c at the end of a relatively short course of metformin in these adolescents may reflect the severity of the underlying disorder and may be a marker of risk. Given the association of durable control with insulinogenic index and our previous demonstration that HbA1c is strongly determined by insulin secretion in these youth (23,24), those who are not able to get their HbA1c into the non–diabetes range within a few months are likely to have substantial deterioration in β-cell function already.
This analysis has a number of important strengths. First, although the analysis sample is composed of participants in a randomized clinical trial with rigorous eligibility criteria who remained in follow-up for at least 4 years, the demographics of the analyzed sample are comparable with demographics of the general population of youth-onset type 2 diabetes in the U.S. (25). Second, the participants in TODAY have been extensively characterized, allowing analysis of a broad set of demographic, anthropometric, and biochemical measures. Third, the cohort was followed longitudinally and all diabetes care was provided to the participants, allowing for analysis of causal relationships not possible in small and/or cross-sectional studies. On the other hand, despite the relatively large size of the TODAY cohort, the sample size is too small for more than exploratory analysis of sex-by-racial/ethnic subgroups. In addition, the ROC analysis yields AUC values in the range indicating fair predictive ability for HbA1c, suggesting that there are other factors involved in determining likelihood of failure that are not fully reflected in HbA1c. However, despite this limitation, HbA1c remains the strongest predictor of likelihood of success or failure on metformin monotherapy in youth with type 2 diabetes and is an easily obtained clinical measure that may provide important clinical insight.
In summary, the population of youth with type 2 diabetes is heterogeneous and consists of subsets of individuals who are more or less likely to have durable glycemic control on oral therapy. Furthermore, the analysis demonstrates that HbA1c after a few months of metformin monotherapy was the strongest predictor of response, likely reflecting underlying insulin secretion. Finally, these analyses indicate that adolescents with type 2 diabetes who do not attain a non–diabetes range HbA1c after a few months on metformin are at increased risk of losing glycemic control, and the clinician caring for these youth should not be reassured with an HbA1c “in target.” These findings suggest that HbA1c obtained after a short course of metformin monotherapy in adolescents with type 2 diabetes may be a simple clinical measure to predict short- and medium-term outcome and allow better targeting of therapy to those adolescents at highest risk for loss of glycemic control. In the future, long-term follow-up of the TODAY cohort will be used to confirm and readjust our analysis and understanding of early markers for both diabetes control and diabetes-related complications and comorbidities.
Supplementary Material
Article Information
Acknowledgments. The authors gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service institutional review boards or their members.
Funding. This work was completed with funding from NIDDK and the National Institutes of Health Office of the Director through grants U01-DK-61212, U01-DK-61230, U01-DK-61239, U01-DK-61242, and U01-DK-61254; from the National Center for Research Resources (NCRR) General Clinical Research Centers Program through grants M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards through grants UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University in St. Louis), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver). The NIDDK project office was involved in all aspects of the study, including design and conduct; collection, management, analysis, and interpretation of data; review and approval of the manuscript; and decision to submit the manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb (BMS); Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; and Sanofi. P.Z. is a consultant for Daiichi-Sankyo, Merck, Takeda, Lilly, and BMS. L.L.K. is a consultant for Takeda Pharmaceuticals. N.H.W. serves on data-monitoring committees for Novo Nordisk and Daiichi-Sankyo. D.W. is a consultant for Shire Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.Z. and K.H. researched data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. K.C.C., L.L.K., and N.H.W. researched data, contributed to the discussion, and reviewed and edited the manuscript. L.E.g. researched data and reviewed and edited the manuscript. L.L.L., B.L., and D.W. contributed to the discussion and reviewed and edited the manuscript. P.M. contributed to the discussion and reviewed the manuscript. K.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00081328, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0848/-/DC1.
A complete listing of the TODAY Study Group is included in the Supplementary Data.
References
- 1.Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 3.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010;33:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36:3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levitt Katz LE, Magge SN, Hernandez ML, Murphy KM, McKnight HM, Lipman T. Glycemic control in youth with type 2 diabetes declines as early as two years after diagnosis. J Pediatr 2011;158:106–111 [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 7.Ostgren CJ, Lindblad U, Ranstam J, Melander A, Råstam L; Skaraborg hypertension and Diabetees Project . Glycaemic control, disease duration and β-cell function in patients with type 2 diabetes in a Swedish community. Skaraborg Hypertension and Diabetes Project. Diabet Med 2002;19:125–129 [DOI] [PubMed] [Google Scholar]
- 8.Zeitler P, Epstein L, Grey M, et al.; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2005;28(Suppl. 1):S37–S42 [DOI] [PubMed] [Google Scholar]
- 10.Klingensmith GJ, Pyle L, Arslanian S, et al.; TODAY Study Group . The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care 2010;33:1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laffel L, Chang N, Grey M, et al.; TODAY Study Group . Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012;13:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs M. Children’s Depression Inventory. New York, Multi-Health Systems, 1992 [Google Scholar]
- 13.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX, Psychological Corporation, 1996 [Google Scholar]
- 14.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35 [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr 2011;48:277–287 [DOI] [PubMed] [Google Scholar]
- 16.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner M, Altman DG. Statistics With Confidence: Confidence Intervals and Statistical Guidelines. London, BMJ Books, 2000 [Google Scholar]
- 18.Naranjo D, Hessler DM, Deol R, Chesla CA. Health and psychosocial outcomes in U.S. adult patients with diabetes from diverse ethnicities. Curr Diab Rep 2012;12:729–738 [DOI] [PubMed] [Google Scholar]
- 19.Campbell JA, Walker RJ, Smalls BL, Egede LE. Glucose control in diabetes: the impact of racial differences on monitoring and outcomes. Endocrine 2012;42:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care 1999;22:403–408 [DOI] [PubMed] [Google Scholar]
- 22.Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacha F, Pyle L, Nadeau K, et al.; TODAY Study Group . Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes 2012;13:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamman RF, Bell RA, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37:3336–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.