Abstract
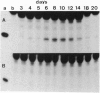
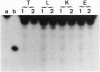
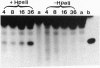
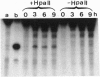
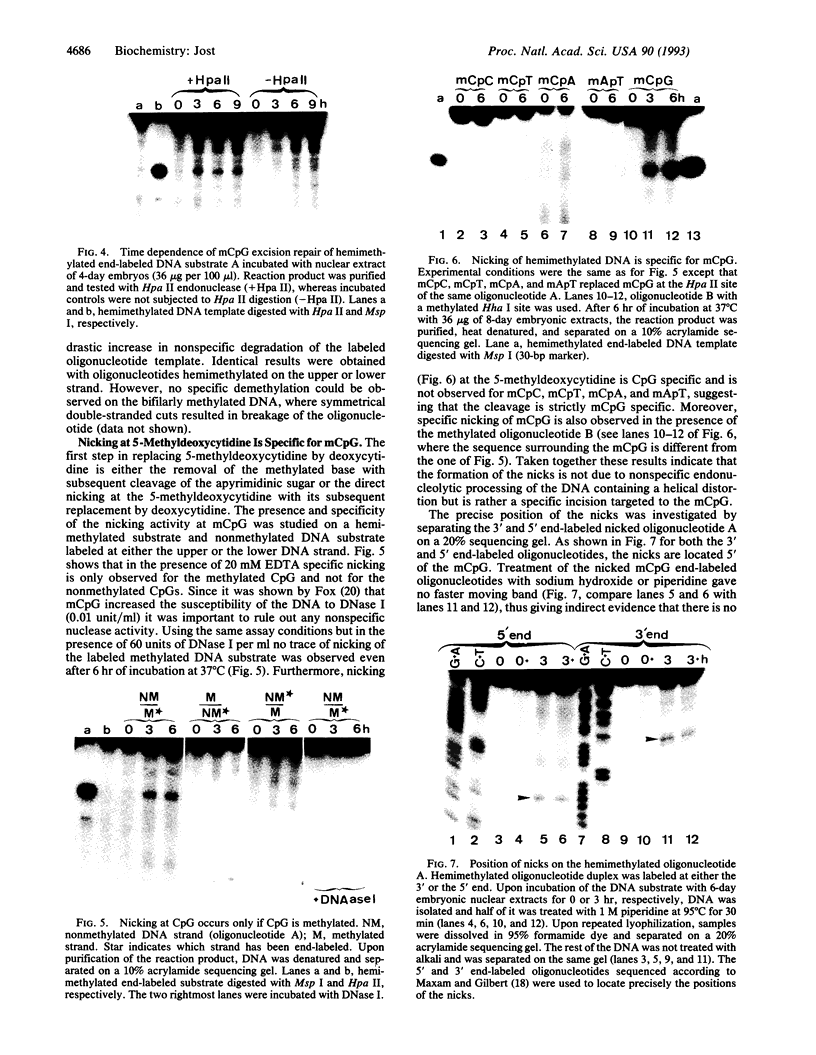
Here I show that nuclear extracts of chicken embryos can promote the active demethylation of DNA. The evidence shows that in hemimethylated DNA (i.e., methylated on one strand only) demethylation of 5mCpG occurs through nucleotide excision repair. The first step of demethylation is the formation of specific nicks 5' from 5-methyldeoxycytidine. Nicks are also observed in vitro on symmetrically methylated CpGs (i.e., methylated on both strands) but they result in breakage of the oligonucleotide with no repair. No specific nicks are observed on the nonmethylated CpG. Nicks are strictly 5mCpG specific and do not occur on 5mCpC, 5mCpT, 5mCpA, or 6mApT. The effect of nonspecific nuclease(s) has been ruled out. The nicking of mCpG takes place in the presence of 20 mM EDTA irrespective of the nature of the sequence surrounding the 5mCpG. No methylcytosine glycosylase activity could be detected. The repair is aphidicolin and N-ethylmaleimide resistant, suggesting a repair action by DNA polymerase beta. In extracts of chicken embryos, the excision repair of mCpG is highest between the 6th and the 12th day of development, whereas it is barely detectable in nuclear extracts from different organs of adults. The possible implications of 5mCpG endonuclease activity in active demethylation of DNA during differentiation is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bestor T. H., Hellewell S. B., Ingram V. M. Differentiation of two mouse cell lines is associated with hypomethylation of their genomes. Mol Cell Biol. 1984 Sep;4(9):1800–1806. doi: 10.1128/mcb.4.9.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Fox K. R. The effect of HhaI methylation on DNA local structure. Biochem J. 1986 Feb 15;234(1):213–216. doi: 10.1042/bj2340213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Mechanisms for the control of gene activity during development. Biol Rev Camb Philos Soc. 1990 Nov;65(4):431–471. doi: 10.1111/j.1469-185x.1990.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Holliday R. The inheritance of epigenetic defects. Science. 1987 Oct 9;238(4824):163–170. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Hofsteenge J. The repressor MDBP-2 is a member of the histone H1 family that binds preferentially in vitro and in vivo to methylated nonspecific DNA sequences. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9499–9503. doi: 10.1073/pnas.89.20.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Saluz H. P., Pawlak A. Estradiol down regulates the binding activity of an avian vitellogenin gene repressor (MDBP-2) and triggers a gradual demethylation of the mCpG pair of its DNA binding site. Nucleic Acids Res. 1991 Oct 25;19(20):5771–5775. doi: 10.1093/nar/19.20.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Paroush Z., Keshet I., Yisraeli J., Cedar H. Dynamics of demethylation and activation of the alpha-actin gene in myoblasts. Cell. 1990 Dec 21;63(6):1229–1237. doi: 10.1016/0092-8674(90)90418-e. [DOI] [PubMed] [Google Scholar]
- Paroush Z., Keshet I., Yisraeli J., Cedar H. Dynamics of demethylation and activation of the alpha-actin gene in myoblasts. Cell. 1990 Dec 21;63(6):1229–1237. doi: 10.1016/0092-8674(90)90418-e. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How gene activators work. Sci Am. 1989 Jan;260(1):40–47. doi: 10.1038/scientificamerican0189-40. [DOI] [PubMed] [Google Scholar]
- Razin A., Levine A., Kafri T., Agostini S., Cantoni G. L. DNA hypomethylation and differentiation of Friend erythroleukemia cells. Gene. 1988 Dec 25;74(1):139–141. doi: 10.1016/0378-1119(88)90270-3. [DOI] [PubMed] [Google Scholar]
- Razin A., Levine A., Kafri T., Agostini S., Gomi T., Cantoni G. L. Relationship between transient DNA hypomethylation and erythroid differentiation of murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9003–9006. doi: 10.1073/pnas.85.23.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Szyf M., Kafri T., Roll M., Giloh H., Scarpa S., Carotti D., Cantoni G. L. Replacement of 5-methylcytosine by cytosine: a possible mechanism for transient DNA demethylation during differentiation. Proc Natl Acad Sci U S A. 1986 May;83(9):2827–2831. doi: 10.1073/pnas.83.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Webb C., Szyf M., Yisraeli J., Rosenthal A., Naveh-Many T., Sciaky-Gallili N., Cedar H. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2275–2279. doi: 10.1073/pnas.81.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluz H. P., Jiricny J., Jost J. P. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer R., Kafri T., O'Connell A., Eisenberg S., Breslow J. L., Razin A. Methylation changes in the apolipoprotein AI gene during embryonic development of the mouse. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11300–11304. doi: 10.1073/pnas.88.24.11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Nowak J. A., Maniloff J. Mycoplasma restriction: identification of a new type of restriction specificity for DNA containing 5-methylcytosine. J Bacteriol. 1986 Jan;165(1):219–225. doi: 10.1128/jb.165.1.219-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasler J. M., Hake L. E., Johnson P. A., Alcivar A. A., Millette C. F., Hecht N. B. DNA methylation and demethylation events during meiotic prophase in the mouse testis. Mol Cell Biol. 1990 Apr;10(4):1828–1834. doi: 10.1128/mcb.10.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S. Eukaryotic DNA polymerases. Annu Rev Biochem. 1991;60:513–552. doi: 10.1146/annurev.bi.60.070191.002501. [DOI] [PubMed] [Google Scholar]
- Weisinger G., Korn A. P., Sachs L. Protein that induces cell differentiation causes nicks in double-stranded DNA. FEBS Lett. 1986 May 5;200(1):107–110. doi: 10.1016/0014-5793(86)80520-8. [DOI] [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. Mismatch-specific thymine DNA glycosylase and DNA polymerase beta mediate the correction of G.T mispairs in nuclear extracts from human cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A., Seldran M., Jost J. P. An estrogen-dependent demethylation at the 5' end of the chicken vitellogenin gene is independent of DNA synthesis. Nucleic Acids Res. 1984 Jan 25;12(2):1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. R., Tilghman S. M. Induction of alpha-fetoprotein synthesis in differentiating F9 teratocarcinoma cells is accompanied by a genome-wide loss of DNA methylation. Mol Cell Biol. 1984 May;4(5):898–907. doi: 10.1128/mcb.4.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]