Abstract
Background
Despite the excess risk of mortality in young women (≤55 years) following acute myocardial infarction (AMI), little is known about young women’s health status (symptoms, functioning, quality of life) during the first year of recovery after an AMI. We examined gender differences in health status over time from baseline to 12-months post AMI.
Methods and Results
A total of 3,501 AMI patients (67% women) aged 18-55 years were enrolled from 103 United States/24 Spanish hospitals. Data were obtained by medical record abstraction/patient interviews at baseline hospitalization, 1- and 12-months post AMI. Health status was measured by generic [Short Form-12 (SF-12), and disease specific [Seattle angina questionnaire (SAQ)] measures. We compared health status scores at all three time points, and utilized longitudinal linear mixed effects analyses to examine the independent effect of gender, adjusting for time and selected covariates. Women had significantly lower health status scores than men at each assessment (all P-values <0.0001). Following adjustment for time and all covariates, women had SF-12 physical/mental summary scores that were −0.96 (95% CI: −1.59, −0.32) and −2.36 points lower (95% CI: −2.99, −1.72) than men, as well as worse SAQ physical limitations (−2.44 points lower; 95% CI: −3.53, −1.34), more angina (−1.03 points lower; 95% CI: −1.98, −0.07), and poorer quality of life (−3.51 points lower; 95% CI: −4.80, −2.22) than men.
Conclusions
Although both genders recover similarly following AMI, women have poorer scores than men on all health status measures; a difference that persisted throughout the entire year after discharge.
Keywords: Acute myocardial infarction, gender differences, women, prognosis, health status
INTRODUCTION
Young women (≤55 years) presenting with acute myocardial infarction (AMI) represent a unique, high-risk phenotype1, 2 with a significantly higher risk of in-hospital mortality than similarly aged men.3, 4 However, the health status (symptoms, functioning, quality of life)5 differences between men and women from the time of their AMI through the following year is poorly understood. Illuminating differences in aspects of health status is essential to developing tailored strategies to reduce potential gender-based disparities in outcomes.
The few published studies show that older women experience greater functional decline and a poorer health-related quality of life at 6-12 months after AMI as compared with men,6-11 but this research has not included a large number of young patients. Moreover, these studies have not compared the health status patterns of young women with men during the post-AMI recovery period, which can enable clinicians to know whether the disparity with regards to mortality also extends to patients’ symptoms, function and quality of life.11, 12
To address this gap in knowledge, we used data from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients), a prospective study of young women and men hospitalized with AMI, to examine the patterns of recovery in the year after AMI.13 The specific objectives of this study were to (1) assess gender differences in health status scores at three different time points (i.e. baseline, 1- and 12-months), including angina symptoms, disease-specific quality of life, physical, and mental functioning; (2) examine the change in health status from baseline to 12-months to describe the magnitude of recovery by gender, and (3) to examine the independent effect of gender on each health status score from baseline to 12-months, adjusting for time and important covariates. We hypothesized that young women would have a different pattern of recovery than similarly aged men. Additionally, we hypothesized that socio-demographic and clinical factors would attenuate many of the underlying difference in health status between women and men.
METHODS
Participants and Study Design
Between August 21st 2008 and May 1st 2012, we enrolled 18-55 year old patients hospitalized with AMI from 103 United States (US)/24 Spanish hospitals into the VIRGO study- named Infarto de miocardio en la Mujer Joven (IMJOVEN) in Spain (VIRGO US Grant: #5 R01 HL081153-05; VIRGO Spanish Grant: #081614). The VIRGO study was designed to investigate factors associated with higher mortality in young women with AMI.13 Patients were prospectively recruited and enrolled in the VIRGO study, which used a 2:1 female to male enrollment design to enrich the study’s inclusion of young women. Every attempt was made to enroll consecutive young women with an AMI at each center. After two women were recruited, the next young man with an AMI was recruited at each site. A total of 6,538 patients with AMI were screened at contributing sites, of which 3,572 were eligible and enrolled. The final cohort used in this study consisted of 2,985 patients from the US (2,009 women, 976 men), and 516 patients from Spain (340 women, 176 men) for a total of 3,501 patients (2,349 women, 1,152 men).
The VIRGO study has been previously described.13 In brief, AMI was confirmed by increased cardiac biomarkers (with at least one cardiac biomarker above the 99th percentile of the upper reference limit) within 24 hours of admission. We also required additional evidence of acute myocardial ischemia, including at least one of the following: symptoms of ischemia, electrocardiogram changes indicative of new ischemia (new ST-T changes, new or presumably new left bundle branch block, or the development of pathological Q waves). Patients must have presented directly to the enrolling site or been transferred within the first 24 hours of presentation to ensure primary clinical decision making occurred at the enrolling site. We excluded patients who were incarcerated, did not speak English or Spanish, were unable to provide informed consent or be contacted for follow-up, developed elevated cardiac markers because of elective coronary revascularization, or had an AMI as the result of physical trauma. Institutional Review Board approval was obtained at each participating institution, and patients provided informed consent for their study participation including baseline hospitalization and follow-up interviews.
Data Collection and Study Definitions
We collected patients’ baseline characteristics from medical chart abstraction and standardized in person interviews administered by trained personnel during the index AMI admission.13 The patient domains collected include detailed information on socio-demographics, cardiac risk factors, non-cardiac co-morbidities, disease severity, and in-hospital procedures (Table 1).
Table 1.
Patient characteristics for overall population and stratified by gender.
| Description | Total Sample N=3,501 |
Men N=1,152 (33%) |
Women N=2,349 (67%) |
P-value |
|---|---|---|---|---|
| Socio-Demographics (%) | ||||
| Age: Mean (SD) | 47±6.20 | 47±5.97 | 47±6.32 | 0.6906 |
| Age: Median (IQR) | 48 (8.00) | 48 (9.00) | 48 (8.00) | 0.2500 |
| Race | <0.0001 | |||
| Black | 15.71 | 9.81 | 18.60 | |
| White | 78.32 | 83.42 | 75.82 | |
| Other | 5.97 | 6.77 | 5.58 | |
| Hispanic* | 7.68 | 7.99 | 7.54 | 0.6378 |
| Marital Status | <0.0001 | |||
| With partner | 57.95 | 63.80 | 55.09 | |
| Without partner | 40.96 | 35.16 | 43.81 | |
| Other | 1.09 | 1.04 | 1.11 | |
| Education Status | 0.0871 | |||
| Unknown | 1.91 | 2.34 | 1.70 | |
| Less than high school | 5.28 | 4.08 | 5.87 | |
| High school graduate | 40.47 | 41.15 | 40.14 | |
| More than high school | 52.33 | 52.43 | 52.28 | |
| Employment/Working Status | <0.0001 | |||
| Working full-time | 51.04 | 66.67 | 43.38 | |
| Working part-time | 10.57 | 6.08 | 12.77 | |
| Not working | 38.39 | 27.26 | 43.85 | |
| Has health insurance | 79.95 | 77.86 | 80.97 | 0.0310 |
| Avoid healthcare due to cost | 30.16 | 28.47 | 30.99 | 0.1269 |
| Location: Spain | 14.74 | 15.28 | 14.47 | 0.5286 |
|
| ||||
| Cardiac Risk Factors (%) | ||||
| Smoked within last 30 days | 59.55 | 59.20 | 59.73 | 0.7657 |
| History of Diabetes | 34.82 | 26.74 | 38.78 | <0.0001 |
| History of hypertension | 63.32 | 62.33 | 63.81 | 0.3907 |
| History of dyslipidemia | 66.32 | 70.14 | 64.45 | 0.0008 |
| Family history of CAD | 71.55 | 70.23 | 72.20 | 0.2235 |
| BMI>30 | 48.07 | 43.58 | 50.28 | 0.0002 |
|
| ||||
| Medical History (%) | ||||
| Prior CAD | 19.19 | 20.49 | 18.56 | 0.1742 |
| Prior angina/stable CAD | 27.02 | 26.04 | 27.50 | 0.3609 |
| Prior stroke/TIA | 4.20 | 2.34 | 5.11 | 0.0002 |
| Prior PAD | 2.26 | 2.00 | 2.38 | 0.4682 |
| History of Sleep Apnea | 4.60 | 5.12 | 4.34 | 0.3010 |
| Congestive Heart Failure | 4.03 | 2.08 | 4.98 | 0.0000 |
| Renal dysfunction | 10.34 | 7.81 | 11.58 | 0.0006 |
|
| ||||
| Non-Cardiac Co-morbidities (%) | ||||
| History of alcohol abuse | 6.60 | 10.94 | 4.47 | <0.0001 |
| History of illicit drug use | 7.65 | 7.81 | 7.58 | 0.8060 |
| Autoimmune Disorders | 3.08 | 1.30 | 3.96 | <0.0001 |
| Chronic Obstructive Pulmonary | ||||
| Disease | 10.25 | 5.47 | 12.60 | <0.0001 |
| Depression | 39.93 | 23.87 | 47.81 | <0.0001 |
| Cancer | 3.37 | 1.91 | 4.09 | 0.0008 |
|
| ||||
| Disease Severity (%) | ||||
| Initial heart rate (BPM): Mean (SD) | 20.34 | 20.10 | 20.42 | 0.0037 |
| Initial heart rate (BPM): Median (IQR) | 24.00 | 24.00 | 25.00 | 0.0137 |
| Initial SBP (mmHg): Mean (SD) | 30.85 | 28.78 | 31.80 | 0.0482 |
| Initial SBP (mmHg): Median (IQR) | 38.00 | 35.00 | 39.00 | 0.0214 |
| ST-segment elevation AMI | 51.73 | 59.46 | 47.94 | <0.0001 |
| Ejection fraction <40% | 10.51 | 10.94 | 10.30 | 0.5647 |
| GRACE score: Median (QR) | 25.00 | 24.00 | 25.00 | 0.0165 |
| Peak troponin: Median (QR) | 27.34 | 35.78 | 22.55 | <0.0001 |
| Presentation 6 hrs. symptom onset | 57.75 | 63.54 | 54.92 | <0.0001 |
|
| ||||
| Hospital Procedures (%) | ||||
| Diagnostic angiography | 94.60 | 94.88 | 94.47 | 0.6116 |
| Primary PCI | 48.24 | 52.60 | 46.10 | 0.0003 |
| Use of coronary revascularization | ||||
| CABG | 8.43 | 10.16 | 7.58 | |
| PCI | 71.95 | 79.51 | 68.24 | <0.0001 |
| None | 19.62 | 10.33 | 24.18 | |
Abbreviations: BMI= body mass index, CAD= coronary artery disease, TIA= transient ischemic attack, PAD= peripheral artery disease, SBP= systolic blood pressure, AMI= acute myocardial infarction, PCI= percutaneous coronary intervention, CABG= coronary artery bypass grafting
In Spain, the default race/minority is classified as white, non-Hispanic.
In addition to the above variables collected from the medical record abstraction, in-hospital, 1- and 12-month mortality endpoints were also gathered for both the US/Spanish sites. Both generic (Short Form-12 [SF-12],14 Euro-Quality of life Scale [EQ-5D]),15 and disease specific (Seattle Angina Questionnaire [SAQ])16 health status instruments were administered to enrolled patients by trained study personnel at baseline, 1- and 12-month interviews. The SF-12 and SAQ have 4-week recall periods, while the EQ-5D inquires about patients’ current health at the time of the interview.
Short Form-12 (SF-12)
The SF-12 has been demonstrated to be a valid and reliable instrument and is the most widely used generic health status instrument to quantify patients’ mental/physical functional status.14 This instrument measures overall physical/mental health status through 12 items, which are answered along various varying length Likert scales. Both the SF-12 physical (PCS)/mental (MCS) component summary scores were calculated for this study and range from 0-100, with higher scores indicating greater functioning. A score of 50 represents the US population average, with a standard deviation of 10 points. A mean score of ≥5 to 10 is considered clinically significant.17, 18
Euro-QoL Scale (EQ-5D)
The EQ-5D is a standardized measure of health status, which provides a simple and generic measure of health for clinical assessment,15 and has been validated in AMI patients.19 This questionnaire has two parts, the first being a descriptive section that classifies patients into one of 243 health states consisting of the following dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with each dimension consisting of three possible levels (i.e. 1-3) representing ‘no problems- extreme problems.’ The second part is a 20cm visual analog scale (EQ-VAS) that ranges from ‘best imaginable state- worst state’ anchored at 100 and 0 respectively,20 with higher scores indicating better health states. The mean (standard deviation) minimally important difference for the EQ-5D utility index score is 0.040 (0.026).21, 22
Seattle Angina Questionnaire (SAQ)
The SAQ is a 19-item disease specific health related quality of life measure for patients with CAD that has demonstrated validity, reliability and clinical responsiveness,16, 23 and is predictive of mortality and re-hospitalization.24, 25 The five clinically relevant domains of the SAQ include physical limitation, angina stability, angina frequency, treatment satisfaction, and quality of life. For the purpose of this study the physical limitation, angina frequency, treatment satisfaction and quality of life domains were used. Each domain of the SAQ scores range from 0 to 100 points, with higher scores indicating higher levels of functioning, fewer symptoms and a greater quality of life or treatment satisfaction. A mean difference of greater than 5 points between groups is considered clinically significant16.
Statistical Analysis
Frequencies for categorical variables and means with standard deviations or medians with interquartile range for continuous variables were calculated. Statistical differences between women and men were determined using chi-squared, t-tests, and Wilcoxon Rank Sum tests, where appropriate. Mean scores at baseline, 1- and 12-months were calculated and plotted between women and men for the SF-12, EQ-5D, and the SAQ, and the change from baseline to 12-months were represented as density plots.
To examine the independent effect of gender on health status over time, while taking into account the effect of time, we utilized longitudinal linear mixed effects analyses (LME) fitted to each health status outcome.6, 11, 12 This model allows us to examine health status patterns from baseline to 12-months post AMI. We included random effects for the intercept and the slope of time (in which the random effect of the slope of time accounts for the difference in time trend within a patient; and the random effect of the intercept accounts for the effect of the repeated measurements within a patient), respectively6. For the LME analysis, patients were organized into a multi-level longitudinal structure (patient-time) according to their health score measurements at the three time points (i.e. baseline, 1- and 12-months).
We first examined female gender alone with the random effect for the intercept in the unadjusted model. We then sequentially adjusted for other important covariates.26 The first model (Adjusted model #1) included female gender and time, with the random effect for the slope of time and intercept. The second model (Adjusted model #2) added socio-demographics (age, race, Hispanic origin, and marital status) to model #1. The third model (Adjusted model #3) added socio-economic status (education level, employment status, insurance status and site location) to model #2. The fourth model (Adjusted model #4) added cardiovascular risk factors (current smoking, hypertension, diabetes, dyslipidemia, obesity (body mass index [BMI] ≥30kg/m2) to model #3. The fifth model (Adjusted model #5) added medical history (prior coronary artery disease [CAD], prior angina, prior stroke/transient ischemic attack [TIA], prior peripheral artery disease [PAD], history of sleep apnea, congestive heart failure, and renal dysfunction) to model #4. The sixth model (Adjusted model #6) added non-cardiac co-morbidities (chronic obstructive pulmonary disease [COPD]/lung disease, illicit drug use, autoimmune, history of alcohol abuse, depression, and cancer) to model #5. The final model (Adjusted model #7) further adjusted for disease severity (initial heart rate [HR], initial systolic blood pressure [SBP], ST-elevation myocardial infarction [STEMI], ejection fraction <40%, global registry of acute coronary events (GRACE)27 score, peak troponin, and presentation within 6 hours) to model #6. In the final model we assessed the interaction between gender and time to explicitly examine if the effect of gender on the health status scores changed over time. We reported the parameter estimates (PE) and its 95% confidence interval (CI) as well as the significance testing of the gender effect in each LME model. Forest plots were used to graphically demonstrate the association of gender with health status throughout these serial stages of adjustment.
All missing data was assumed to be missing at random. For covariates considered in each model, missing values were rare (<3% in the study cohort). For the covariates considered in each model, missing values were imputed as the most common values for categorical variables and as median values for continuous variables. A p-value < 0.05 was considered statistically significant. All analyses were done in SAS version 9.3.
RESULTS
Study Sample
At 1-month, 91.8% of women and 91.8% of men were interviewed; and at 12-months 83.0% of women and 80.6% of men were interviewed. Characteristics of those who were interviewed at 12-months versus those who were not interviewed are presented in Supplemental Table 1. In brief, those that were interviewed were older, more likely to be white, to be partnered, had more than a high school level of education, were more likely to be working, and to have health insurance. Those that were interviewed were also slightly healthier, having fewer risk factors and co-morbidities (i.e. smoking, hypertension, diabetes, prior CAD/angina, stroke).
Baseline Characteristics
Baseline characteristics of the total sample (N=3,501), and stratified by gender (N=2,349 67% women) are shown in Table 1. The median age was 48 years old. The majority of patients in this study had a self-identified race of white, were married, had more than a high school education, worked full time, and had private health insurance. The most common risk factor was family history of CAD, followed by dyslipidemia, hypertension, smoking, obesity, and diabetes.
Although both genders were of similar age, women were more likely to be black, single, to work part time or not at all, and to have health insurance as compared with men. In addition, women were more likely to have diabetes mellitus, obesity, prior stroke/TIA, congestive heart failure, renal dysfunction, chronic lung disease, autoimmune disorders, cancer, and depression, but were less likely to have dyslipidemia or alcohol abuse.
On arrival, women had a higher median heart rate, higher systolic blood pressure, and significantly lower peak troponin levels than men. Women also had a higher GRACE score, were more likely than men to present with a non-STEMI, and were more likely to present to the hospital more than 6 hours after symptom onset (Table 1). During their hospitalization, women were significantly less likely to receive primary percutaneous coronary intervention (PCI), or revascularization procedures (i.e. PCI, coronary artery bypass grafting [CABG]) compared to similarly aged men.
Mortality
The 1-month mortality rate was low in women and men (0.5% vs. 0.7%, P=0.8). At 1-year the rates were still low and women were more likely to die than men, but the difference did not achieve statistical significance (2.3% in women vs. 1.4% in men, P=0.10).
Health Status Scores Over Time
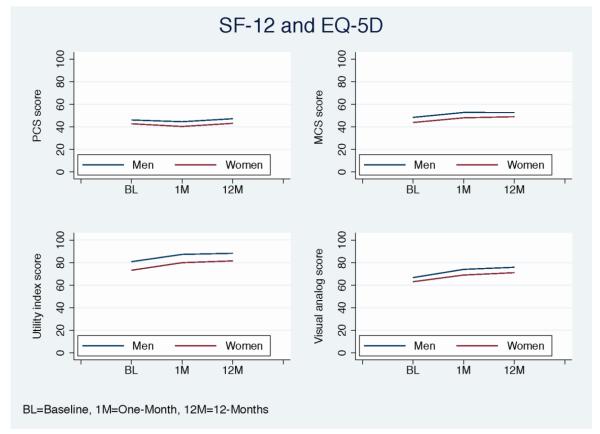
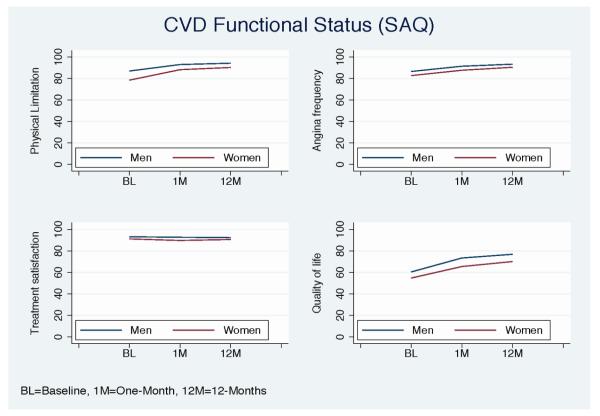
As shown in Table 2, women had significantly lower observed generic and disease-specific health status scores than men at baseline, 1- and 12-months post AMI. At 12-months, women had significantly poorer physical (mean [SD]: 43.2±12.5 vs. 47.2±11.2), and mental (49.0±11.4 vs. 52.7±9.5) generic health status than men as per the SF-12 (P<0.0001 for all). In addition, women scored significantly poorer on the EQ-5D index score (81.5±20.2 vs. 88.2±18.1) and VAS score (71.1±21.6 vs. 75.8±18.7) (P<0.0001 for all). Women also reported more disease-specific physical limitations (90.2±20.0 vs. 94.2±15.5), worse angina (90.4±17.6 vs. 93.3±14.6), less treatment satisfaction (90.5±15.5 vs. 92.3±14.0), and poorer quality of life (70.1±23.6 vs. 76.8±21.6) than men as quantified by the SAQ (P<0.0001 for all). For all domains, the lower health status scores in women persisted throughout the entire period of observation, in comparison to men (Figures 1-2).
Table 2.
Baseline, 1- and 12-month health status scores (Mean ± SD) stratified by gender for overall population*.
| Men | Women | P-value | ||
|---|---|---|---|---|
| General Health (SF-12) PCS | Baseline | 46.2±11.3 | 42.8±12.2 | <0.0001 |
| 1-month | 44.6±11.1 | 40.4±11.7 | <0.0001 | |
| 12-month | 47.2±11.1 | 43.2±12.5 | <0.0001 | |
|
| ||||
| General Health (SF-12) MCS | Baseline | 48.5±11.5 | 43.9±12.8 | <0.0001 |
| 1-month | 52.8±9.3 | 48.1±11.1 | <0.0001 | |
| 12-month | 52.7±9.5 | 49.0±11.4 | <0.0001 | |
|
| ||||
| SAQ Physical Limitations | Baseline | 86.9±20.7 | 78.5±26.9 | <0.0001 |
| 1-month | 93.0±15.7 | 88.2±21.0 | <0.0001 | |
| 12-month | 94.2±15.5 | 90.2±20.0 | <0.0001 | |
|
| ||||
| SAQ Angina Frequency | Baseline | 86.6±18 | 82.7±21.5 | <0.0001 |
| 1-month | 91.4±15.8 | 87.6±18.5 | <0.0001 | |
| 12-month | 93.3±14.6 | 90.4±17.6 | <0.0001 | |
|
| ||||
| SAQ Quality of Life | Baseline | 60.4±22.0 | 54.7±24.7 | <0.0001 |
| 1-month | 73.4±22.7 | 65.4±25.8 | <0.0001 | |
| 12-month | 76.8±21.6 | 70.1±23.6 | <0.0001 | |
|
| ||||
| SAQ Treatment Satisfaction | ||||
| Baseline | 93±1±10.7 | 91.1±13.7 | <0.0001 | |
| 1-month | 92.6±12.1 | 89.6±15.1 | <0.0001 | |
| 12-month | 92.3±14.0 | 90.5±15.5 | 0.0037 | |
|
| ||||
| HRQOL (EQ-5D) UI Score | Baseline | 80.8±20.0 | 73.2±23.3 | <0.0001 |
| 1-month | 87.3±15.7 | 79.9±18.9 | <0.0001 | |
| 12-month | 88.2±18.1 | 81.5±20.2 | <0.0001 | |
|
| ||||
| HRQOL (EQ-5D) VAS score | Baseline | 66.7±20.0 | 63.0±22.0 | <0.0001 |
| 1-month | 73.9±19.7 | 68.9±21.6 | <0.0001 | |
| 12 month | 75.8±18.7 | 71.1±21.6 | <0.0001 | |
Abbreviations: PCS= physical component summary score, MCS= mental component summary score, SAQ= Seattle angina questionnaire, UI= utility index score, VAS= visual analog score
P-values derived from unadjusted student t-test.
Figure 1.
Mean health status scores from baseline to 12-months stratified by gender for the SF-12 and EQ-5D health status measures (women=red, men=blue).
Figure 2.
Mean health status scores from baseline to 12-months stratified by gender for the SAQ health status measure (women=red, men=blue).
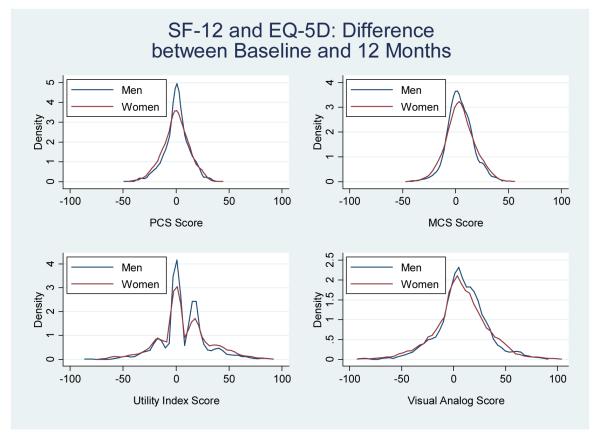
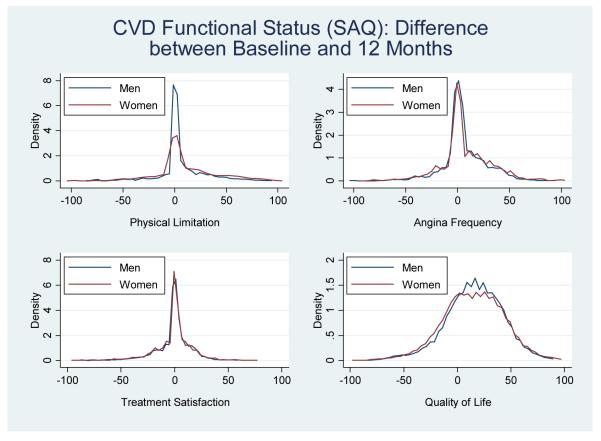
Change in Health Status Scores from Baseline to 12-months
Although young women had worse health status scores at baseline, 1- and 12-months, the change and/or magnitude in health status from baseline to 12-months was not statistically different between genders for all measures, aside from the SAQ physical limitations domain (Figures 3-4). Specifically, young women reported significantly more change from baseline to 12-months on the physical limitations domain (i.e. women gained 10 points compared with men who gained only 6 points) (10.5±28.7 vs. 6.12±21.7, P<0.001).
Figure 3.
Density plots showing the distribution and/or the change in health status scores from baseline to 12-months stratified by gender for the SF-12 and EQ-5D health status measures (women= red, men=blue).
Figure 4.
Density plots showing the distribution and/or the change in health status scores from baseline to 12-months stratified by gender for the SAQ health status measure (women= red, men=blue).
Independent effect of Gender on Health Status Scores
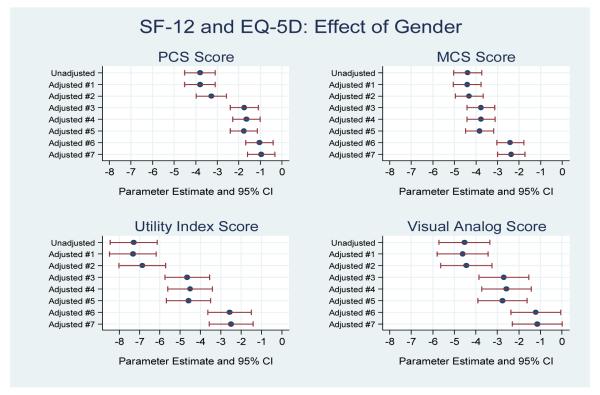
Women reported a SF-12 PCS score that was −3.80 points lower than men in the unadjusted analysis (95% CI PE: −4.51, −3.09, P<0.001). Following adjustment for time and all other covariates (socio-demographics, SES, cardiovascular risk factors, medical history, non-cardiac co-morbidities and disease severity), women had a PCS score that was −0.96 points lower than men (95% CI: −1.60, −0.32; P=0.003) (Figure 5). Women also reported a poorer MCS score that was −4.39 points lower than men in the unadjusted analysis (95% CI: −5.04, −3.74; P<0.0001). After adjustment, women still had a lower MCS score than men (−2.36 points, 95% CI: −3.00, −1.73; P<0.001) (Figure 5, Supplemental Table 2).
Figure 5.
The independent effect of gender on the SF-12 and EQ-5D health status scores, from baseline to 12-months, adjusting for time and other important covariates. Parameter estimates and 95% confidence intervals shown.
Compared with men, women reported a poorer EQ-5D utility index score that was −7.29 points lower than men (95% CI: −8.44, −6.13; P<0.0001), and a poorer VAS score which was −4.53 points lower than men (95% CI: −5.72, −3.34; P<0.001) in unadjusted analyses. Following adjustment, women had a utility index score that was −2.50 points lower than men (95% CI: −3.57, −1.43, P<0.001), and although women reported a VAS score that was −1.14 points lower than men, this was no longer significant in the final model (95% CI: −2.31, 0.02, P=0.05) (Figure 5).
In the model only including gender, women reported an SAQ physical limitations score that was −6.00 points lower than men in unadjusted analysis (95% CI: −7.14, −4.86, P<0.001). After adjustment, women had a physical limitations score that was −2.44 points lower than men (95% CI: −3.53, −1.34, P<0.001) (Figure 6, Supplemental Table 2).
Figure 6.
The independent effect of gender on the SAQ health status measure, from baseline to 12-months, adjusting for time and other important covariates. Parameter estimates and 95% confidence intervals shown.
Women were also more likely to report having angina with a SAQ angina frequency score that was −3.47 points lower than men in unadjusted analyses (95% CI: −4.43, −2.52, P<0.001). After adjustment, women had an angina frequency score that was −1.03 points lower than men (95% CI: −1.99, −0.07, P=0.03) (Figure 6).
In unadjusted analyses, women reported an SAQ treatment satisfaction score that was −2.34 points lower than men (95% CI: −3.07, −1.61, P<0.001). Following full adjustment for time and all covariates (socio-demographics, SES, cardiovascular risk factors, medical history, non-cardiac co-morbidities and disease severity) women continued to have a score that was −1.22 points lower than men (95% CI: −1.99, −0.46, P=0.001).
Lastly, in unadjusted analyses, women reported a SAQ quality of life score that was −6.57 points lower than men (95% CI: −7.90, −5.23, P<0.001). After adjustment for time and all covariates, women still had a score that was −3.51 points lower than men (95% CI: −4.80, −2.22, P<0.001).
When assessing the gender versus time interaction in the fully adjusted model, there were no significant differences in generic health status scores over time as measured by the SF-12 PCS/MCS, the EQ-5D utility index/VAS score domains, or disease specific health status as measured by the SAQ angina frequency, treatment satisfaction, and quality of life domains except the SAQ physical limitations domain. This suggests that the worse health status experienced by women persists from the time of their AMI throughout the year after recovery. As an alternative primary analysis to the LME models described above, we used inverse probability weighting and found no meaningful differences between the two approaches.
DISCUSSION
We demonstrate that young women with AMI have worse health status outcomes than similarly aged men. Although both genders recover similarly over time with regards to their health status, women have significantly poorer scores than men on all health status domains, from baseline to 12-months post AMI. The worse outcomes are similar to the differences present at the time of the AMI, with a similar magnitude of change in health status scores in both genders, but no reduction in the worse health status experienced by women from the time of their AMI to 12-months. After adjustment for time and important socio-demographic and clinical variables, women had health status scores that were significantly lower than similarly aged men on nearly all measures (i.e. women have more angina symptoms, poorer disease-specific quality of life, as well as poorer physical/mental functioning). These findings show that women have a similar pattern of health status recovery following AMI compared with men. The poorer health status scores observed in women are not entirely explained by the large number of factors prospectively captured in VIRGO.13
This study extends the prior literature in several important ways. First, this study is the first and largest study of gender differences in health status following admission for AMI in young patients. Second, no prior study has provided such a comprehensive evaluation of health status. The sparse literature on young women with AMI has predominately focused on in-hospital3, 4, 28 and short-term mortality,29 which alone are insufficient to capture patient experiences following AMI. Understanding the recovery after AMI necessitates examining a wide range of health outcomes and over a period of 12 months. The very few longer-term studies have been limited by methodology, the use of mainly generic health status instruments (i.e. SF-12), small sample size, and did not focus on young women (≤55 years),11, 12, 26, 30-35 thus they do not provide an adequate picture of how younger women, a previously established vulnerable population, recover over time. Third, we were able to demonstrate worse outcomes for women and had, with the male controls, the ability to determine whether there were differences in the magnitude of change over time and at baseline hospitalization for AMI. Finally, we are able to show that the effect of female gender remains significant, even after adjusting for a range of important confounders, which persists over the year following AMI.
For the first time, we show that young women report significantly poorer physical and mental functioning, more symptoms of angina, and a poorer quality of life over a 12-month period following AMI, when compared with similarly aged men. Further, unadjusted health status scores for both genders reached the threshold for what is defined as a clinically relevant difference for the SAQ quality of life, physical limitations, and angina frequency domains, but not for the treatment satisfaction domain (i.e. mean difference >5 points on the SAQ).16 Similarly, health status scores reached the threshold for a clinically significant difference on the SF-12 MCS and PCS score.17, 18, 36 The study provides novel information that may explain the persistent finding of worse AMI outcomes of young women compared with men. In prior studies, focused on mortality and largely conducted more than 10 years ago, younger women have consistently found to have higher mortality than men.3, 4, 37 This difference has not been explained by socio-demographic or clinical factors and thus far the mechanism underlying this disparity has been unexplained.1, 2 In our study of AMI survivors, we demonstrate that women have a different health status profile at the time of their AMI. In addition to having a heavier burden of comorbidity, they have worse health status, which largely accounts for the worse outcomes at 12-months. The challenge moving forward is to determine what interventions might prevent deterioration in health status or assist those with a low health status at baseline in order to improve outcomes.
The mechanism for the poorer health status observed in young women post AMI is not clear. Despite adjusting for a broad range of confounders, women continued to have a poorer health status compared with men, which implies that there may be other potential factors at play. Firstly, this cohort has a substantial burden of disease before the AMI, and thus these events are not occurring in a healthy group, with a greater encumbrance in women. It is probable that hormonal and genetic explanations are at play.1, 2, 38 Indeed, the association of hormonal status on health status outcomes is an understudied area of research, but remains a long-term goal of the VIRGO study.13 It has been previously demonstrated that young women present with atypical symptoms, in comparison to men,4 and have more complications post AMI such as bleeding events, longer lengths of stay and more 30-day hospitalizations after the index event,1, 3, 4 leading to a greater susceptibility to poor outcomes following discharge.
Psychosocial explanations may account, at least in part, for a portion of the gender-based differences in health status. Younger women may have differential lifestyle changes following AMI compared with men, including secondary effects from drugs/prescribed medications, and fewer opportunities to attend cardiac rehabilitation programs.38 Adjusting to a new life situation may hamper their ability to successfully return to work following AMI,39, 40 likely coupled with greater caregiving demands.41 This is a concern, as caregiving responsibilities have been shown to predict hospitalization and/or adverse outcomes following a cardiac event.42-44 In addition, fewer social supports together with increased anxiety and a greater inclination to stress,45, 46 are all likely to influence women’s health status and/or recovery post AMI.38, 47 Collectively, lifestyle changes, return to work (i.e. thus resuming a normal lifestyle), caregiving responsibilities- and the role of social support all warrant further evaluation in future studies of this population.
Study Implications
Our findings indicate that the health status of women is impaired at the time of an AMI and throughout the year of recovery compared with men. Improving outcomes may require a combination of attention toward their cardiovascular health, as well as any other factors that have impaired their health status in the pre-event setting (i.e. smoking, physical inactivity). Collectively, these findings indicate a better need to understand the outpatient care of young women at risk for AMI.
Limitations
This study should be interpreted in the context of several potential important limitations. First, to perform a longitudinal study with patient interviews requires patient consent and participation. As this occurs in these studies, some patients were lost to follow-up and some patients did not respond to requests for a follow-up interview. The percentages, however, were similar for women and men and it does not appear to have imposed a bias. Second, as this was an observational study the differences between women and men in health status may be due to residual confounding. However, our detailed data collection allowed us to adjust for an extensive range of patient-level factors that are typically not included in gender-based research. Third, as patients were required to be healthy at baseline to participate, the VIRGO cohort was unable to capture those patients who were too ill to be enrolled. Lastly, the mortality rates were quite low in this population and we lacked statistical power to evaluate differences in outcomes.
CONCLUSION
In conclusion, young women with AMI had lower health status at the time of their AMI and at 1-and 12-months later, in comparison to similarly aged men. Although both genders recover similarly over time, women have poorer scores than men on all health status measures at each time point. The effect of female gender persists following adjustment for time and an extensive set of potential confounders.
Supplementary Material
Acknowledgments
Funding Sources: The VIRGO study was supported by a 4-year National Heart, Lung, and Blood Institute grant [number 5R01HL081153). IMJOVEN was supported in Spain by grant PI 081614 from the Fondo de Investigaciones Sanitarias del Instituto Carlos III, Ministry of Science and Technology, and additional funds from the Centro Nacional de Investigaciones Cardiovasculares (CNIC). Dr. Krumholz is supported by grant U01 HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr Bueno was supported in part by grant BA08/90010 from the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III, Spain.
Footnotes
Disclosures: Mr. Wang and Dr. Krumholz work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Krumholz is chair of a cardiac scientific advisory board for UnitedHealth and is the recipient of research grants from Medtronic, Inc. and Johnson and Johnson through Yale University. In addition, Dr. Spertus is supported by grants from Gilead, Genentech, Lilly, Amorcyte, and EvaHeart, and has a patent Seattle Angina Questionnaire with royalties paid. Dr Bueno has received advisory/consulting fees from AstraZeneca, Bayer, Daichii-Sankyo, Eli-Lilly, Menarini, Novartis, Sanofi and Servier, and research grants from AstraZeneca.
References
- 1.Beckie T. Biopsychosocial determinants of health and quality of life among young women with coronary heart disease. Curr Cardiovasc Risk Rep. 2014;8:1–10. [Google Scholar]
- 2.Levit RD, Reynolds HR, Hochman JS. Cardiovascular disease in young women: A population at risk. Cardiol Rev. 2011;19:60–65. doi: 10.1097/CRD.0b013e31820987b5. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National registry of myocardial infarction 2 participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: Evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 5.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 6.Emery CF, Frid DJ, Engebretson TO, Alonzo AA, Fish A, Ferketich AK, Reynolds NR, Dujardin JP, Homan JE, Stern SL. Gender differences in quality of life among cardiac patients. Psychosom Med. 2004;66:190–197. doi: 10.1097/01.psy.0000116775.98593.f4. [DOI] [PubMed] [Google Scholar]
- 7.Dodson JA, Arnold SV, Reid KJ, Gill TM, Rich MW, Masoudi FA, Spertus JA, Krumholz HM, Alexander KP. Physical function and independence 1 year after myocardial infarction: Observations from the translational research investigating underlying disparities in recovery from acute myocardial infarction: Patients’ health status registry. Am Heart J. 2012;163:790–796. doi: 10.1016/j.ahj.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck CA, Joseph L, Belisle P, Pilote L. Predictors of quality of life 6 months and 1 year after acute myocardial infarction. Am Heart J. 2001;142:271–279. doi: 10.1067/mhj.2001.116758. [DOI] [PubMed] [Google Scholar]
- 9.Brink E, Grankvist G, Karlson BW, Hallberg LR. Health-related quality of life in women and men one year after acute myocardial infarction. Qual Life Res. 2005;14:749–757. doi: 10.1007/s11136-004-0785-z. [DOI] [PubMed] [Google Scholar]
- 10.Lacey EA, Walters SJ. Continuing inequality: Gender and social class influences on self perceived health after a heart attack. J Epidemiol Community Health. 2003;57:622–627. doi: 10.1136/jech.57.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worcester MU, Murphy BM, Elliott PC, Le Grande MR, Higgins RO, Goble AJ, Roberts SB. Trajectories of recovery of quality of life in women after an acute cardiac event. Br J Health Psychol. 2007;12:1–15. doi: 10.1348/135910705X90127. [DOI] [PubMed] [Google Scholar]
- 12.Wickholm M, Fridlund B. Women’s health after a first myocardial infarction: A comprehensive perspective on recovery over a 4-year period. Eur J Cardiovasc Nurs. 2003;2:19–25. doi: 10.1016/S1474-5151(02)00088-9. [DOI] [PubMed] [Google Scholar]
- 13.Lichtman JH, Lorenze NP, D’Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: Role of gender on outcomes of young ami patients (virgo) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware J, Jr., Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.The EuroQol Group Euroqol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the seattle angina questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 17.Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14:285–295. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Kosinski M, Keller SD, Lincoln RI. Sf-12: How to score the sf-12 physical and mental health summary scales. 3rd ed Quality Metric incorporated; Boston MA: 1998. [Google Scholar]
- 19.Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the eq-5d quality of life instrument in patients after myocardial infarction. Qual Life Res. 2005;14:95–105. doi: 10.1007/s11136-004-0614-4. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JW, Johnson JA, Coons SJ. Us valuation of the eq-5d health states: Development and testing of the d1 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48:365–371. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]
- 22.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: Eq-5d and sf-6d. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 23.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 25.Krumholz HM, Peterson ED, Ayanian JZ, Chin MH, DeBusk RF, Goldman L, Kiefe CI, Powe NR, Rumsfeld JS, Spertus JA, Weintraub WS. Report of the national heart, lung, and blood institute working group on outcomes research in cardiovascular disease. Circulation. 2005;111:3158–3166. doi: 10.1161/CIRCULATIONAHA.105.536102. [DOI] [PubMed] [Google Scholar]
- 26.Garavalia LS, Decker C, Reid KJ, Lichtman JH, Parashar S, Vaccarino V, Krumholz HM, Spertus JA. Does health status differ between men and women in early recovery after myocardial infarction? J Womens Health (Larchmt) 2007;16:93–101. doi: 10.1089/jwh.2006.M073. [DOI] [PubMed] [Google Scholar]
- 27.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA, Global Registry of Acute Coronary Events I Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 28.Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CNB, Canto JG, Lichtman JH, Vaccarino V. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart. 2009;95:895–899. doi: 10.1136/hrt.2008.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacIntyre K, Stewart S, Capewell S, Chalmers JW, Pell JP, Boyd J, Finlayson A, Redpath A, Gilmour H, McMurray JJ. Gender and survival: A population-based study of 201,114 men and women following a first acute myocardial infarction. J Am Coll Cardiol. 2001;38:729–735. doi: 10.1016/s0735-1097(01)01465-6. [DOI] [PubMed] [Google Scholar]
- 30.Wiklund I, Herlitz J, Johansson S, Bengtson A, Karlson BW, Persson NG. Subjective symptoms and well-being differ in women and men after myocardial infarction. Eur Heart J. 1993;14:1315–1319. doi: 10.1093/eurheartj/14.10.1315. [DOI] [PubMed] [Google Scholar]
- 31.Mendes de Leon CF, Dilillo V, Czajkowski S, Norten J, Schaefer J, Catellier D, Blumenthal JA. Psychosocial characteristics after acute myocardial infarction: The enrichd pilot study. Enhancing recovery in coronary heart disease. J Cardiopulm Rehabil. 2001;21:353–362. doi: 10.1097/00008483-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Agewall S, Berglund M, Henareh L. Reduced quality of life after myocardial infarction in women compared with men. Clin Cardiol. 2004;27:271–274. doi: 10.1002/clc.4960270506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shumaker SA, Brooks MM, Schron EB, Hale C, Kellen JC, Inkster M, Wimbush FB, Wiklund I, Morris M. Gender differences in health-related quality of life among postmyocardial infarction patients: Brief report. Cast investigators. Cardiac arrhythmia suppression trials. Womens Health. 1997;3:53–60. [PubMed] [Google Scholar]
- 34.Pettersen KI, Reikvam A, Rollag A, Stavem K. Understanding sex differences in health-related quality of life following myocardial infarction. Int J Cardiol. 2008;130:449–456. doi: 10.1016/j.ijcard.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Bosworth HB, Siegler IC, Olsen MK, Brummett BH, Barefoot JC, Williams RB, Clapp-Channing NE, Mark DB. Social support and quality of life in patients with coronary artery disease. Qual Life Res. 2000;9:829–839. doi: 10.1023/a:1008960308011. [DOI] [PubMed] [Google Scholar]
- 36.Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Validation of the eq-5d in patients with a history of acute coronary syndrome. Curr Med Res Opin. 2005;21:1209–1216. doi: 10.1185/030079905X56349. [DOI] [PubMed] [Google Scholar]
- 37.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134:173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 38.Sjostrom-Strand A, Ivarsson B, Sjoberg T. Women’s experience of a myocardial infarction: 5 years later. Scand J Caring Sci. 2011;25:459–466. doi: 10.1111/j.1471-6712.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- 39.Jackson L, Leclerc J, Erskine Y, Linden W. Getting the most out of cardiac rehabilitation: A review of referral and adherence predictors. Heart. 2005;91:10–14. doi: 10.1136/hrt.2004.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biering K, Nielsen TT, Rasmussen K, Niemann T, Hjollund NH. Return to work after percutaneous coronary intervention: The predictive value of self-reported health compared to clinical measures. PLoS One. 2012;7:e49268. doi: 10.1371/journal.pone.0049268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navaie-Waliser M, Feldman PH, Gould DA, Levine C, Kuerbis AN, Donelan K. When the caregiver needs care: The plight of vulnerable caregivers. Am J Public Health. 2002;92:409–413. doi: 10.2105/ajph.92.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosca L, Mochari-Greenberger H, Aggarwal B, Liao M, Suero-Tejeda N, Comellas M, Rehm L, Umann TM, Mehran R. Patterns of caregiving among patients hospitalized with cardiovascular disease. J Cardiovasc Nurs. 2011;26:305–311. doi: 10.1097/JCN.0b013e3181f34bb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal B, Liao M, Christian A, Mosca L. Influence of caregiving on lifestyle and psychosocial risk factors among family members of patients hospitalized with cardiovascular disease. J Gen Intern Med. 2009;24:93–98. doi: 10.1007/s11606-008-0852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in u.S. Women: A prospective study. Am J Prev Med. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 45.Kristofferzon ML, Lofmark R, Carlsson M. Myocardial infarction: Gender differences in coping and social support. J Adv Nurs. 2003;44:360–374. doi: 10.1046/j.0309-2402.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- 46.Brezinka V, Kittel F. Psychosocial factors of coronary heart disease in women: A review. Soc Sci Med. 1996;42:1351–1365. doi: 10.1016/0277-9536(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 47.Bucholz EM, Strait KM, Dreyer RP, Geda M, Spatz ES, Bueno H, Lichtman JH, D’Onofrio G, Spertus JA, Krumholz HM. Effect of low perceived social support on health outcomes in young patients with acute myocardial infarction: Results from the virgo (variation in recovery: Role of gender on outcomes of young ami patients) study. J Am Heart Assoc. 2014;3:e001252. doi: 10.1161/JAHA.114.001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.