Abstract
During mouse gastrulation, cells in the primitive streak undergo epithelial-mesenchymal transformation and the resulting mesenchymal cells migrate out laterally to form mesoderm and definitive endoderm across the entire embryonic cylinder. The mechanisms underlying mesoderm and endoderm specification, migration, and allocation is poorly understood. In this study, we focused on the function of mouse Cripto, a member of the EGF-CFC gene family that is highly expressed in the primitive streak and migrating mesoderm cells on embryonic day 6.5. Conditional inactivation of Cripto during gastrulation leads to varied defects in mesoderm and endoderm development. Mutant embryos display accumulation of mesenchymal cells around the shortened primitive streak indicating a functional requirement of Cripto during the formation of mesoderm layer in gastrulation. In addition, some mutant embryos showed poor formation and abnormal allocation of definitive endoderm cells on embryonic day 7.5. Consistently, many mutant embryos that survived to embryonic day 8.5 displayed defects in ventral closure of the gut endoderm causing cardia bifida. Detailed analyses revealed that both the Fgf8-Fgfr1 pathway and p38 MAP kinase activation are partially affected by the loss of Cripto function. These results demonstrate a critical role for Cripto during mouse gastrulation, especially in mesoderm and endoderm formation and allocation.
Keywords: Cripto, mouse gastrulation, cell migration, mesoderm, endoderm, Fgf and p38
Introduction
Before gastrulation, mouse embryonic cells are organized into a cylindrical structure called the egg cylinder that consists of two layers, the inside epiblast (also known as embryonic ectoderm) and the outside visceral endoderm (Beddington and Robertson, 1999; Tam and Behringer, 1997). At the onset of gastrulation, cells on one side of the epiblast delaminate and undergo epithelial-mesenchymal transition (EMT) leading to the formation of a morphologically distinguishable area called the primitive streak (Beddington and Robertson, 1999; Tam and Loebel, 2007). The emerging primitive streak expands distally and forms a characteristic structure termed the node on the distal end (Beddington, 1994; Tam and Loebel, 2007). The anterior tip of the primitive streak (APS), and later the node, serves as the trunk organizer capable of inducing the posterior region when transplanted onto a host embryo (Beddington, 1994). The anterior visceral endoderm (AVE), located in the anterior region of the embryo, is essential for anterior neural formation and patterning (Beddington, 1998; Beddington and Robertson, 1998, 1999; Kimelman, 2006; Shawlot et al., 1999; Thomas et al., 1998). While expanding toward the distal end of the embryo, the primitive streak cells also migrate out laterally to form an intervening layer of mesoderm between the visceral endoderm and epiblast, that will be further specified to cardiac and lateral plate mesoderm, axial mesoderm and paraxial mesoderm (Harvey, 2002; Lawson et al., 1991; Shawlot et al., 1999; Tam and Behringer, 1997; Tam and Tan, 1992). Following the migration and allocation of mesoderm cells, definitive endoderm cells also migrate out of the primitive streak to form endoderm-derived tissues and organs such as the gut (Tam et al., 2007). In addition, cells from the primitive streak also move proximally to form extra-embryonic mesoderm (Downs et al., 2004).
Numerous signaling and regulatory factors have been found to be involved in early embryonic induction and patterning. Among them, the components of the Nodal and Wnt pathways are particularly important. Nodal, a member of TGF-β superfamily, and its intracellular transducers, Smad2 and Smad3, are required for various aspects of early embryonic events such as A-P establishment, mesoderm and endoderm induction and patterning, and axial midline formation (Brennan et al., 2001; Conlon et al., 1994; Dunn et al., 2004; Lowe et al., 2001; Nomura and Li, 1998; Schier and Shen, 2000; Varlet et al., 1997; Vincent et al., 2003; Waldrip et al., 1998; Weinstein et al., 1998; Zhou et al., 1993). Consistently, loss of Nodal inhibitors such as Drap1 and Lefty1 results in elevated Nodal activity and an expanded primitive streak as well as excess mesoderm in mice (Iratni et al., 2002; Meno et al., 1999). Loss of β-catenin function in mice affects the formation of AVE as well as the primitive streak and its derivatives (Huelsken et al., 2000), whereas mutation of the Wnt3 gene abolishes only the primitive streak without affecting AVE formation (Liu et al., 1999). Interestingly, the AVE induces anterior neural formation by producing Nodal and Wnt antagonists such as Cer1 and DKK1 indicating that suppression of Nodal and Wnt activities in the anterior epiblast is essential for head formation and patterning (Glinka et al., 1998; Mao et al., 2001; Piccolo et al., 1999; Semenov et al., 2001). Therefore, precise spatial and temporal regulation of Nodal and Wnt activity is critical for embryonic induction and patterning. Additional pathways and factors important for early embryogenesis in the mouse include Shh (Chiang et al., 1996), BMPs and their antagonists Chordin and Noggin (Bachiller et al., 2000; Fujiwara et al., 2002; Hogan, 1996), transcription factors Lhx1(Shawlot and Behringer, 1995; Shawlot et al., 1999), Foxa2 (Ang and Rossant, 1994), Mixl1(Hart et al., 2002) and many others (Tam and Loebel, 2007).
Compared with mesoderm induction and patterning, little is known about the migration of mesoderm and endoderm. The Fgf8-Fgfr1 pathway plays a central role in driving mesoderm cell migration and is mediated, at least in part, by transcription factors Snai1 and Tbx6 (Ciruna and Rossant, 2001; Deng et al., 1994; Sun et al., 1999; Yamaguchi et al., 1994). In this pathway, Snai1 is mainly responsible for EMT, because it is able to repress E-Cadherin gene expression (Barrallo-Gimeno and Nieto, 2005; Batlle et al., 2000; Cano et al., 2000) and more importantly, Snai1 mutant mouse embryos form a layer between the epiblast and the visceral endoderm, of which the cells are more epithelial than mesenchymal in character and retain E-Cadherin expression, demonstrating a functional requirement of Snai1 for EMT during gastrulation (Carver et al., 2001). Since an intervening epithelial cell layer still forms in Snai1 mutant mouse embryos, it appears that cell migration out of the primitive streak is not necessarily dependent on EMT. Studies with p38 interacting protein (p38IP) revealed that the activation of p38 MAP kinase is required for efficient EMT in the primitive streak and subsequent lateral migration of mesoderm cells (Zohn et al., 2006). This observation is consistent with previous reports that p38-α MAP kinase is required for embryonic branching angiogenesis, a process involving endothelial cell migration (Adams et al., 2000; Mudgett et al., 2000). Activation of the p38-mediated pathway in the primitive streak requires p38IP and NCK interacting kinase (NIK), but is independent of Fgf8 signaling. Moreover, Eomesodermin is required for EMT during mouse gastrulation that is independent of the Fgf8 pathway (Arnold et al., 2008). However, the relationship between Eomesodermin and the p38 pathway has not been reported.
Cripto is the founding member of the EGF-CFC family and functions as an essential co-factor for several TGF-β superfamily members such as Nodal during vertebrate embryogenesis (Dono et al., 1993; Schier and Shen, 2000; Shen, 2007; Shen and Schier, 2000; Shen et al., 1997). Cripto acts as both ligand and co-receptor in facilitating Nodal signaling (Yan et al., 2002). In addition, Cripto can block the function of Activin, also a member of TGF-β superfamily, by forming a complex with its type II receptor (Gray et al., 2003). During mouse development, Cripto is initially expressed in the epiblast before gastrulation (Ding et al., 1998). During gastrulation, Cripto is highly expressed in the primitive streak, migrating mesoderm, the node and axial mesendoderm (Chu et al., 2005; Ding et al., 1998). In Cripto-null mutant embryos, the prospective AVE is specified in the distal end, but is incapable of anterior migration (Ding et al., 1998). Similarly, the prospective trunk organizer is initiated in the proximal end of the epiblast, but fails to translocate to the posterior region, resulting in the lack of primitive streak, embryonic mesoderm and endoderm (Ding et al., 1998). These results indicate that Cripto is capable of regulating embryonic cell migration at pre-gastrulation stage. Experiments with a Cripto hypomorphic allele revealed that it is also required for the formation of axial midline structures, particularly the anterior definitive endoderm and prechordal mesoderm (Chu et al., 2005). However, the function of Cripto during early gastrulation, particularly in the formation of mesoderm layer, remains elusive.
Materials and methods
Mouse lines
The CriptoLacZ null allele used in this study has been described before (Ding et al., 1998). The Criptoflox allele was generated by removing the floxed PGK-Neo region in the previously reported Cripto3loxP allele (Chu et al., 2005) using the EIIa-Cre line.
The generation of the Criptoflox/flox allele is schematically illustrated in supplementary figure 1. The following PCR primer pairs were used to genotype the Criptoflox allele: 5′-GTG GTA AGT AAT TCC TCT TTC-3′ (forward) and AGG AAC ATT CCA ATG GCC TTG (reverse) detect the second loxP site yielding a 500 bp band for the floxed allele and a 400 bp band for the wild type allele; 5′-AGC CAT CTC ACC AGC CTT CA-3′ (forward) and 5′-ACC TCC CCA CCA TCC A-3′ (reverse) were used to determine the first loxP site producing a 450 bp band for the floxed allele and a 580 bp band for the wild type allele.
The Sox2-Cre line was purchased from the Jackson Laboratory (Hayashi et al., 2002) and the Fgf8LacZ, a Fgf8 null mutant strain generated by Dr. Gail Martin’s group, was purchased from MMRRC.
In situ hybridization
Whole mount in situ hybridization was carried out according to Shen (Shen, 2001) and double in situ hybridization was based on Cai (Cai et al., 2003).The post-hybridized embryos were sectioned and counter-stained with nuclear fast red from Vector Laboratories (catalog # H-3403). For each probe, more than 10 embryos were analyzed.
Immunostaining
Activation of p38 MAP kinase was detected by immunostaining using a rabbit monoclonal antibody from Cell Signaling that specifically recognizes phospho-p38 (P-p38) (catalog # 4631L). E7.5 embryos were fixed with 4% paraformaldehyde at 4°C for 2-3 hours followed by OCT embedding for cryo-sectioning and stored at −80°C until use. Sections were incubated with primary antibody overnight using a dilution of 1:10 or 1:20 followed by incubation with Cy3-conjugated goat anti-rabbit secondary antibody from Jack Immuno Laboratories.
Results
Loss of Cripto function causes mesoderm cell accumulation around the primitive streak area during gastrulation
Cripto is highly expressed in the primitive streak and migrating mesoderm during early gastrulation (Ding et al., 1998) suggesting that Cripto may function during this process. However, development of Cripto-null mutant embryos is arrested at the pre-gastrulation stage due to failure in anterior-posterior polarity establishment (Ding et al., 1998). We therefore generated a floxed allele of Cripto as shown in supplementary figure 1. The resulting Criptoflox/flox mice are viable and develop normally. Crosses of Criptoflox/flox male mice with [Criptolacz/wt :Sox2-Cre] female mice, which contain a high level of maternal Cre activity (Hayashi et al., 2003; Vincent and Robertson, 2003), gave rise to a severe phenotype that was identical to Cripto-null mutant embryos, indicating the deletion of the floxed region disrupts the entire function of Cripto (supplementary figure 2 ). In addition, this Criptoflox allele has been used in a recent study examining the function of Cripto in skeletal muscle regeneration (Guardiola et al., 2012; Michael Shen, personal communication).
To investigate the function of Cripto during mouse gastrulation, we utilized the Criptoflox and CriptoLacZ lines in combination with the Sox2-Cre transgenic line, which expresses Cre activity throughout the epiblast and has been successfully used to study the function of Nodal during gastrulation (Hayashi et al., 2003). To avoid the high maternal Cre activity in the Sox2-Cre line as mentioned above (Hayashi et al., 2003; Vincent and Robertson, 2003), we crossed [Criptolacz/wt ; Sox2-Cre] males with Criptoflox/flox females. The majority of the resulting [Criptolacz/flox ; Sox2-Cre] embryos survived to gastrulation and post-gastrulation stages. As shown in Fig.1, using in situ hybridization, we examined the expression of Brachyury, a primitive streak marker, on E7.5. Compared with wild type embryos (Fig.1A), [Criptolacz/flox ; Sox2-Cre] embryos formed an abnormal primitive streak area that was shortened and widened (Fig.1D). Cross-sections of these embryos revealed that the mesoderm cells in wild type embryos migrated out of the posterior region and were spread over the entire embryo (Fig.1B). In marked contrast, mesoderm cells in the conditional mutant embryos accumulated in the posterior region within the abnormal primitive streak area (Fig.1E), indicating a defect in lateral migration of mesoderm. This defect was detectable as early as E6.5. On E6.5, the expression of Lim1 marks the anterior visceral endoderm (AVE) and newly-formed migrating mesoderm cells that have moved out of the posterior region (Fig.1C and G). However, in Cripto conditional mutant embryos on E6.5, Lim1 -positive mesoderm cells were restricted to the posterior region, even though the Lim1-expressing visceral endoderm cells had already migrated from the distal end to the anterior region (Fig.1F and I). Compared to the epithelial embryonic ectoderm cells, the cells adjacent to the abnormal primitive streak region in the conditional mutant embryos were typical mesenchymal cells with loose cell-cell adhesion, indicating that the cells had undergone EMT (Fig.1E). Consistently, the expression of E-Cadherin was down-regulated in mesoderm cells in these conditional mutant embryos (Fig.1H and J). In addition, Cripto-null mutant embryos can still form mesenchymal mesoderm cells in the extra-embryonic area (Ding et al., 1998; Kimura et al., 2001).We also found extensive formation of blood islands in Cripto-null mutant embryos (data not shown), indicating that even Cripto-null mutants undergo EMT. Therefore, the defects observed here were not due to deficient EMT.
Figure 1. Mesoderm cell migration defects in Cripto conditional mutant embryos.
In Situ hybridization showing the expression of Brachyury (A, B, D and E), Lim1 (C, F, G and I) and E-Cadherin (H and J) in wild type (Wt) and Cripto conditional knock (cKO) embryos. On E7.5, wild type embryos form the primitive streak, marked by Brachyury expression, in a narrow line across the embryo from the proximal to distal end (arrow in A). Mesenchymal mesoderm cells were found over the entire embryo (arrows in B). In contrast, Cripto conditional mutant embryos formed a shortened and widened patch of abnormal primitive streak (arrow in D) and mesenchymal mesoderm cells accumulated in the posterior region around the abnormal primitive streak (arrow in E). On E6.5, the migrating mesoderm cells, marked by Lim1 expression, in wild type embryo (C) have migrated out of the posterior region (arrows in G), whereas the Lim1-expressing cells in Cripto conditional mutant embryos (F) remained in the posterior region (arrow in I), although the expression of Lim1 in the visceral endoderm has moved to anterior region in both cases (arrow heads in G and I). Wt: wild type; cKO: conditional knock-out. The dashed lines in panels A, C, D and F represent the positions of cross-sections shown in panels B, E, G and I, respectively.
Fgf8-Fgfr1 pathway in mesoderm cells is partially affected by the loss of Cripto function
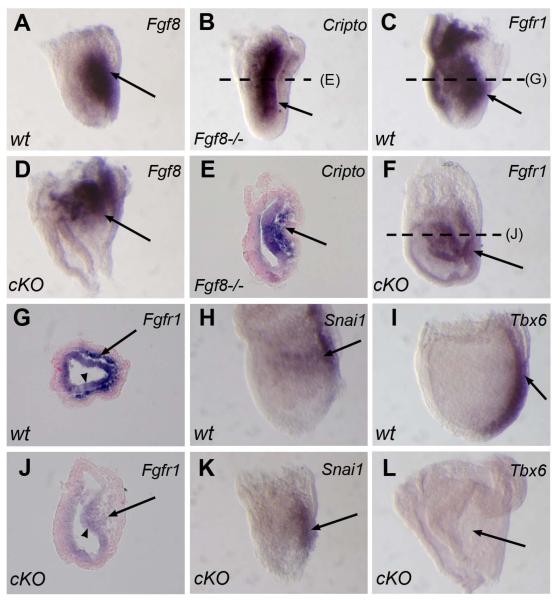
Previous studies with Fgf8 and Fgfr1 mutant embryos revealed their critical roles in EMT and mesoderm cell migration during gastrulation (Deng et al., 1994; Sun et al., 1999; Yamaguchi et al., 1994). Subsequent studies demonstrated that this function is mediated, at least in part, through regulation of Snai1 and Tbx6 expression (Ciruna and Rossant, 2001). Therefore, Fgf8 acts through Fgfr1 to regulate the expression of downstream target genes such as Snai1 and Tbx6 to control EMT and mesoderm cell migration during gastrulation (Ciruna and Rossant, 2001; Zohn et al., 2006). Because the Cripto conditional mutant embryos displayed defects in mesoderm cell migration that, to some degree, resembled the phenotype found in Fgf8 mutant embryos, we examined Fgf8 expression in Cripto conditional mutant embryos, but found no significant changes (Fig.2A and D), except that the expression in Cripto conditional mutant embryos tended to be more wide and proximally located due to the shortened and widened primitive streak. We then tested whether Cripto is a downstream target of Fgf8 in the primitive streak and migrating mesoderm. For this, we examined the expression of Cripto in Fgf8-null mutant (Fgf8lacz/lacz ) embryos, and found that Cripto expression was highly retained in the accumulated mesoderm cells in Fgf8lacz/lacz mutant embryos on E7.5, indicating that Cripto expression is not controlled by Fgf8 (Fig.2B and E). In contrast, we found that the expression of Fgfr1 was severely affected in the mesoderm cells in the Cripto conditional mutant embryos. In wild type embryos, Fgfr1 was highly expressed throughout the entire embryo on E7.5 including the epithelial embryonic ectoderm and mesenchymal mesoderm cells, with stronger expression in the mesoderm compared to ectoderm (Fig.2C and G). The expression in mutant embryos was reduced overall, and the sections showed that the remaining Fgfr1 expression was found largely in the embryonic ectoderm, with little expression in mesoderm cells (Fig.2F and J). In wild type embryos, the expression in the mesoderm is even higher than that in the ectoderm (Fig.2C and G). We also examined Fgfr1 expression in Cripto null mutant embryos on E7.5 and found that Fgfr1 expression was retained in the ectoderm of Cripto null embryos (data not shown). Therefore, the expression of Fgfr1 is differentially down-regulated in mesoderm cells in these Cripto conditional mutant embryos. We then examined the expression of Snai1 and Tbx6, two downstream target genes of Fgf8/Fgfr1 and found that the expression of Tbx6 was completely abolished in all the [Criptolacz/flox:Sox2-Cre] embryos examined with abnormal streak morphology (n>10) (Fig.2I and L), whereas Snai1 was still expressed in the mutant embryos (Fig.2H and K). Moreover, we found Snail1 was also expressed in Cripto null mutant embryos on E7.5 (data not shown), indicating that even the complete loss of Cripto function does not abolish Snail1 expression. These results indicated that the Fgf8-Fgfr1 pathway is partially affected by the conditional inactivation of Cripto.
Figure 2. The Fgf8-Fgfr1 pathway is partially affected in Cripto conditional mutant embryos.
(A and D) Fgf8 expression on E7.5 does not show significant differences between wild type (A) and Cripto conditional mutant (D) embryos, except that mutant embryos (D) show more expression in the proximal end due to the shortened primitive streak. (B and E) Cripto is highly expressed in Fgf8 null mutant embryos on E7.5 including the accumulated mesoderm cell mass in the posterior region (arrow in E). (C, F, G and J) On E7.5, Fgfr1 is highly expressed in wild type embryos (arrow in C), including both mesoderm cells (arrow in G) and embryonic ectoderm cells (arrow head in G). Notably, the expression in the mesoderm is higher than the expression in the embryonic ectoderm. However, in Cripto conditional mutant embryo, the expression is largely reduced (arrow in F). Compared to the expression in the embryonic ectoderm (arrow head in J), the expression in the mesoderm is much weaker (arrow in J). (H, I, K and L) In situ hybridization showing the expression of Snai1 (H and K) and Tbx6 (I and L), two known Fgf8-Fgfr1 downstream genes, in wild type and conditional mutant embryos on E7.5. The expression of Snai1 in the mutant embryo (K) is unchanged compared to wild type embryos, whereas the expression of Tbx6 is completely abolished (L). Wt: wild type; cKO: conditional knock-out; Fgf8−/−: Fgf8 null mutant. The dashed lines in panels B, C, and F represent the positions of cross-sections shown in panels E, G and J, respectively.
Cripto function regulates p38 MAP kinase activation in embryonic ectoderm
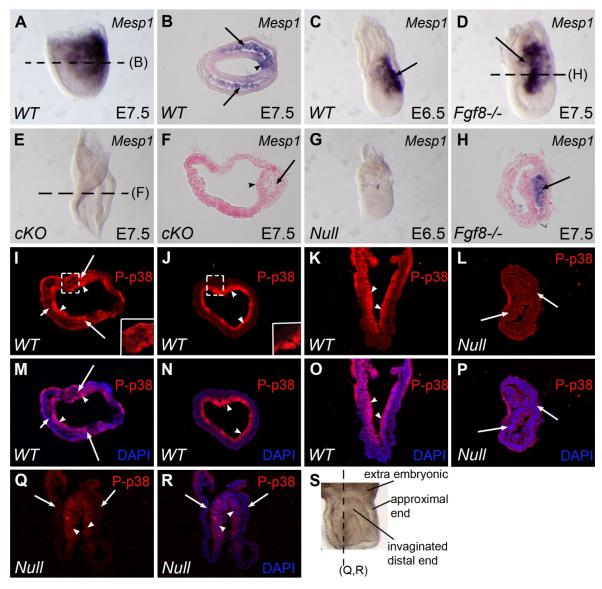
During our search for genes responsible for the migration defect in Cripto conditional mutant embryos, we found that the expression of Mesp1, a primitive streak- and mesoderm-expressed gene responsible for cardiac mesoderm cell migration (Saga et al., 1999) was completely abolished at the early streak stage in all the [Criptolacz/flox:Sox2-Cre] embryos examined with abnormal streak morphology (n>10) (Fig.3A, B, E and F). Consistently, we found that Mesp1 expression was undetectable in Cripto null mutant embryos on E6.5 (Fig.3C and G), whereas the expression of other primitive streak markers such as Fgf8, Lim1 and goosecoid was still retained, although in the proximal end (Ding et al., 1998), Therefore, Cripto is required for Mesp1 expression at both the pre-gastrulation and gastrulation stages. We then examined the expression of Mesp1 in Fgf8-null mutant embryos to determine if Mesp1 expression is Fgf8 dependent, and found that Mesp1 was highly expressed in these mutant embryos on E7.5 indicating that Mesp1 expression is not dependent on the Fgf8-Fgfr1 pathway (Fig.3D and H). Therefore, Cripto may function through other pathways in addition to Fgf8-Fgfr1. The p38 MAP kinase pathway is another major pathway reported to play critical roles during EMT and mesoderm cell migration (Zohn et al., 2006). Loss of p38 interacting protein (p38IP) negatively affects the activation of p38 MAP kinase in the embryonic ectoderm and mesoderm cells on E7.5 and leads to mesoderm migration defects and a delay in EMT (Zohn et al., 2006). We investigated the activation of p38 MAP kinase in wild type and Cripto conditional mutants by immunostaining using a phospho-p38 (P-p38) specific antibody. In E7.5 wild type embryos, we found that positive signals were uniformly present in all embryonic ectoderm cells examined by both cross and frontal sections (Fig.3I-K and M-O). However, the signals in mesoderm cells were less uniform and were position dependent. In the proximal region, the majority of mesoderm cells were P-p38 positive with only a few negative cells (Fig.3I and M). In contrast the signals were absent in mesoderm cells in the distal region (Fig.3J and N). This is slightly different from a previous report which showed uniform p38 activation in mesoderm cells on E7.5 (Zohn et al., 2006). We believe this discrepancy is probably due to stage differences as well as the positional differences mentioned above. To better evaluate the function of Cripto in activating p38 MAK kinase in embryonic ectoderm cells, we examined the activation of p38 in Cripto-null mutant embryos on E7.5. If loss of Cripto function indeed affects p38 activation in embryonic ectoderm cells, it should be more pronounced in Cripto null mutant embryos. We found that in most of the cross sections of Cripto null mutant embryo sections did not contain P-p38 positive cells on E7.5 (Fig.3L and P). Consistently, the frontal sections showed that P-p38 positive cells were present only in a small area on the distal end, whereas most areas along proximal-distal axis were devoid of P-p38 (Fig.3Q and R). Therefore, loss of Cripto function can significantly affect the activation of p38 MAP kinase, at least in embryonic ectoderm cells. The remaining activation of p38 in Cripto null mutants could be due to functional compensation from Cryptic (Chu and Shen, 2010). We also examined the expression of p38IP mRNA in Cripto mutant embryos and found no changes compared to wild type embryos (data not shown).
Figure 3. Loss of Cripto function abolishes Mesp1 expression and dramatically reduces p38 MAP kinase activation in the embryonic ectoderm.
(A-H) In Situ hybridization showing high expression of Mesp1 in wild type embryos on E7.5 (A and B) and E6.5 (C) including the primitive streak (arrow head in B) and mesoderm cells (arrows in B), whereas no expression is detected in E7.5 conditional mutant embryos (E and F) in the primitive streak (arrow head in F) and the accumulated mesoderm cells (arrow in F). In addition, the expression is completely abolished in E6.5 Cripto null mutant embryo (G). In contrast, Mesp1 is still highly expressed in Fgf8 null mutant embryos (arrows in D and H). (I – R) Immunostaining using P-p38 specific antibody showing p38 MAP kinase activation in E7.5 wild type (I-K and M-O) and Cripto null mutant (L, P, Q,R) embryos. Panel S is the whole mount view of a Cripto null embryo corresponding to the staining shown in panels Q and R. Note that the distal end of this embryo invaginated upwards as indicated by the lines. The dashed line indicates the position and direction of section Q and R. Since the Cripto null embryos do not form embryonic mesoderm and endoderm, the tissues shown here are embryonic ectoderm cells. In wild type embryos, as shown by both transverse (I, J, M, and N) and frontal sections (K and O), embryonic ectoderm cells are uniformly positive for P-p38 (arrow heads in I-K and M-O). In some areas, most of the mesoderm cells are positive for P-p38 (arrows in I and M), but a few mesoderm cells are negative (short arrows in I and M). In distal areas, the entire mesoderm layer is P-p38 negative (J and N). In Cripto null mutant embryos, some transverse sections show a complete lack of P-p38 cells in the embryonic ectoderm (arrows in L and P), and frontal sections show that most of the ectoderm cells are P-p38 negative (arrows in Q and R), except for a small region around the ectoderm on distal end (arrow heads in Q and R). Wt: wild type; cKO: conditional knock-out; Fgf8−/−: Fgf8 null mutant; Null: Cripto null mutant. The dashed lines in panels A, D and E represent the positions of cross-sections shown in panels B, H and F, respectively. Note: Since invaginations always occur in the distal ectoderm of E7.5 Cripto null mutant embryo as reported before and also shown by the arrowheads in (U), the proximal-distal axes in (R, T) were indeed oriented in top-bottom manner, although they look like bottom-top.
Consistent with our observation, a recent study revealed that Nodal affects AVE differentiation by activating p38 MAP kinase in visceral endoderm before gastrulation (Clements et al., 2011). These investigators also noticed that P-p38 was undetectable in the visceral endoderm of Cripto null mutant embryo by immunostaining on E5.5 (Clements et al., 2011), demonstrating that Cripto can affect p38 activation in visceral endoderm.
Cripto is required for embryonic endoderm development
In addition to mesoderm, cells that migrate out of the primitive streak also form the definitive endoderm layer that is essential for many organs such as the gut (Lawson et al., 1991; Nagy, 2003; Tam et al., 2007; Tam and Loebel, 2007). Some of the Cripto conditional mutant embryos survived to late stage and displayed cardia bifida, in which cardiac progenitors failed to migrate towards the midline to form a linear heart tube, a defect also seen in the zebrafish Oep mutant embryos (Griffin and Kimelman, 2002) (Fig.4A, B, D and E). To ensure that these mutant embryos underwent gastrulation, it is necessary to examine the cardia bifida in relation to A-P polarity establishment, mesoderm and endoderm formation. We therefore carried out in situ hybridization analyses using four probes simultaneously: Mlc2a for myocytes; Shh for axial mesoderm, ventral midline and endoderm; Meox1 for paraxial mesoderm; and Otx2 for anterior neural tissue. Mlc2a expression is shown in red and the other three are dark purple. As shown in Fig. 4, mutant embryos at E8.5 stained positively for all four marker genes, indicating that they underwent gastrulation and mesoderm formation, although Otx2 expression showed midline defects in anterior region (Fig. 4A, B, D and E). Similar to what occurs in Zebrafish Oep mutants, the cardia bifida in these mutants was probably caused by foregut endoderm defects, in particular defects in the ventral closure of the foregut (Fig.4E). At early E7.5, the conditional mutant embryos expressed Sox17, a key regulatory gene in endoderm cell formation (Kanai-Azuma et al., 2002; Seguin et al., 2008), indicating the formation of endoderm cells. However, Sox17-expressing cells were missing in a region corresponding to the ventral midline region including the future gut (Tam et al., 2007) (Fig.4C and F). Therefore, the endoderm cells were formed in the mutant embryos, but the regional allocation was perturbed, especially with regard to the future gut region. This endoderm cell allocation defect could be directly due to the cell migration defects in the primitive streak. Consistently, we found that the expression of Mixl1 was altered in these mutant embryos. Mixl1 is highly expressed in the primitive streak and the adjacent mesendoderm cells (Fig.4G and H). However, in severe mutants that resemble Cripto-null mutants with no sign of the primitive streak and embryonic mesendoderm, Mixl1 expression was exclusively found in the extra-embryonic region (Fig.4I). In embryos that formed an abnormal primitive streak and mesendoderm, no expression of Mixl1 was found in the primitive streak region and the adjacent mesoendoderm except for a narrow band near the extra embryonic region (Fig.4K and L). Considering the importance of Mixl1 in the formation and allocation of endoderm cells (Hart et al., 2002; Tam et al., 2007), this mis-expression of Mixl1 may be responsible for the endoderm defects found in Cripto conditional mutant embryos. The importance of Oep in Zebrafish endoderm formation has been well documented (Griffin and Kimelman, 2002; Schier et al., 1997), and previous studies have reported the function of Cripto in mouse anterior definitive endoderm formation (Chu et al., 2005). The findings reported here extend previous studies and reveal a conserved role of EGF-CFC genes in general endoderm development, especially the regional allocation of endoderm cells.
Figure 4. Loss of Cripto function affects definitive endoderm cell allocation during gastrulation.
(A and D) whole-mount view of wild type (A) and Cripto conditional mutants (D) embryos on E8.5 showing expression of Otx2 (arrowheads), Meox-1 (arrows), Shh (short arrows) and Mlc2a (red). (B and E) Cross sections of (A) and (D) showing Meox-1 positive paraxial mesoderm tissue, somites (short arrows), Shh positive axial midline structure, notochord (arrowheads) and foregut endoderm cells expressing Shh (arrows). (C and F) whole-mount view showing Sox17-expressing endoderm cells form a patch in wild type embryo on early E7.5 including the area corresponding to the future ventral foregut (arrow in C), whereas Sox17-expressing cells are present in E7.5 Cripto conditional mutant embryos (arrow heads in F), but were missing from the area corresponding to the future ventral gut (arrow in F). (G-L) Mixl1 is highly expressed in the E7.5 wild type embryos (G) including the primitive streak (arrow head in H) and the adjacent mesendoderm cells (arrow in H). For severely affected E7.5 Cripto conditional mutant embryos that have no primitive streak and mesendoderm cells, the expression of Mixl1 was found only in the proximal extra-embryonic region (arrow in I), and for less severely affected embryos that do form mesendoderm layer (arrow in J), only a trace level of Mixl1 expression was found in the primitive streak (arrow heads in K) and no signals were found in the adjacent mesendoderm (arrow in K). Only the extra-embryonic region (short arrow in J) and the area near the edge of the embryonic region (arrow head in J) showed positive signals in mesendoderm cells (arrow in L). Wt: wild type; cKO: conditional knock-out. The dashed lines in panels A, D,G and J represent the positions of cross-sections shown in panels B, E, H and (L,K), respectively.
Discussion
During mesoderm formation, epithelial epiblast cells undergo EMT and the resulting mesenchymal cells migrate out of the primitive streak area. It has been demonstrated that Snai1 is critical for EMT during mouse gastrulation (Carver et al., 2001). Snai1 mutant mouse embryos display defects in EMT and the cells originating from the primitive streak are more epithelial than mesenchymal in character and retain E-Cadherin expression (Carver et al., 2001). Surprisingly, these epithelial cells do not accumulate around the primitive streak area, but migrate out and form an intervening cell layer between the epiblast and the visceral endoderm (Carver et al., 2001). Therefore, it appears that an EMT defect is not sufficient to cause cell migration failure. In Fgfr1 and Fgf8 mutants, cells accumulated around the primitive streak area and retained E-Cadherin expression, indicating defects in both EMT and mesoderm cell migration (Ciruna and Rossant, 2001; Deng et al., 1994; Sun et al., 1999). Since an EMT defect is not sufficient to cause cell accumulation around the primitive streak as shown by Snai1 mutants (Carver et al., 2001), the cell migration defect in Fgf8 and Fgfr1 mutants may not be caused by the EMT defect. Snai1 and Tbx6 are two downstream targets of the Fgf8-Fgfr1 pathway during gastrulation, and down-regulation of Snai1 expression is responsible for the EMT defect in Fgfr1 and Fgf8 mutants (Ciruna and Rossant, 2001; Sun et al., 1999).
In this study, we investigated the function of Cripto during mouse gastrulation by conditional deletion of Cripto allele using Sox2-Cre. Sox2-Cre drives Cre gene expression throughout the epiblast, and the resulting the [Criptolacz/flox:Sox2-Cre] embryos should, in principle, display pre-gastrulation defects like Cripto null mutant embryos. However, we obtained only a few [Criptolacz/flox:Sox2-Cre] embryos that look like null mutant embryos, the majority of [Criptolacz/flox ; Sox2-Cre] embryos displayed late phenotype or no phenotype. This was probably due to the variation of Cre activity among [Criptolacz/flox:Sox2-Cre] embryos and the non-cell autonomous nature of mouse Cripto function (Chu et al., 2005). Analysis of the resulting [Criptolacz/flox:Sox2-Cre] revealed that conditional inactivation of Cripto function during gastrulation caused cells to accumulate around the primitive streak area. Unlike the Fgf8 and Fgfr1 mutants, these accumulated cells were typical mesenchymal cells in morphology, with loose cell-cell adhesion and no expression of E-Cadherin, indicating EMT occurred normally in Cripto conditional mutants. Furthermore, previous studies revealed that Cripto null mutant embryos can still form mesenchymal mesoderm cells in the extra-embryonic region (Ding et al., 1998; Kimura et al., 2001), and we also found extensive blood cell formation in Cripto null mutant embryos (data not shown), indicating the EMT process is not affected even by the complete loss of Cripto function. We also found that the expression of Fgfr1 was down-regulated in mesoderm/mesenchymal cells in Cripto conditional mutant embryos. Moreover, the expression of Tbx6, a target of the Fgf8-Fgfr1 pathway, was abolished in Cripto conditional mutants, whereas the expression of Snai1, another target of the Fgf8-Fgfr1 pathway was unaffected. In addition, we showed that Snai1 expression was retained in Cripto null mutant embryos, further confirming that loss of Cripto does not down-regulate Snai1 expression. Therefore, the Fgf8-Fgfr1 pathway was partially affected by the loss of Cripto function. Considering the importance of the Fgf8-Fgfr1 pathway for mesoderm cell migration out of the primitive streak area as discussed above, disruption of this pathway may contribute to the cell accumulation in Cripto conditional mutants.
In addition to the Fgf8-Fgfr1 pathway, our results showed that loss of Cripto function affects activation of p38 MAP kinase, and activation of p38 is essential for mesoderm cell migration during mouse gastrulation (Zohn et al., 2006). Interestingly, a recent report demonstrated that p38 activation in visceral endoderm by Nodal is required for anterior visceral endoderm differentiation and migration (Clements et al., 2011). AVE is first specified on the distal end before migration to the anterior end (Thomas et al., 1998). Clements and colleagues demonstrated that this process requires Nodal stimulated p38 activation in visceral endoderm on E5.5 (Clements et al., 2011). They also found that p38 activation in visceral endoderm was undetectable in Cripto null mutant embryos as determined by P-p38 immunostaining, indicating that Cripto function is required for p38 activation in visceral endoderm at the pre-gastrulation stage (Clements et al., 2011). However, in Cripto null mutant embryos, the AVE is still specified in the distal end, but fails to migrate to anterior end (Ding et al., 1998). Because the differentiation of AVE still occurs in Cripto null mutant embryos, there should be a basal level of p38 activation maintained in the visceral endoderm of Cripto null mutant embryos. It is possible that in Cripto null mutant embryos, activation of p38 in the visceral endoderm is significantly reduced to a level below the sensitivity of P-p38 immunostaining, but sufficient to promote AVE differentiation in the distal end. In other words, the differentiation of AVE requires a low level of p38 activation, whereas the migration of AVE from the distal end to the anterior region requires a higher level of p38 activation, and Cripto is required only for high level activation of p38 in visceral endoderm. This situation may also applies to the epiblast or embryonic ectoderm, where loss of Cripto function leads to a low level of p38 activation sufficient only for EMT, a process of cell differentiation, but inadequate to promote the subsequent mesoderm cell migration. In p38IP mutant embryos, p38 activation may be reduced to a greater extent than in Cripto mutants and leads to defects in both EMT and cell migration as reported (Zohn et al., 2006). Therefore, Cripto interacts with both the Fgf8-Fgfr1 and p38 MAP kinase pathways to regulate mesoderm migration during gastrulation.
The phenotype described here resembles Nodalnr/nr embryos, a severe Nodal hypomorphic mutant embryo, which displays massive accumulated mesoderm cells expressing Snai1 within a wider and shorter region (Ben-Haim et al., 2006). This is consistent with the notion that Cripto acts as a co-factor for Nodal during early embryogenesis (Chu and Shen, 2010; Shen and Schier, 2000).
During mouse gastrulation, cells from the primitive streak also contribute the definitive endoderm (Lawson et al., 1991; Nagy, 2003; Tam et al., 2007; Tam and Loebel, 2007). The defects in primitive streak cell migration should, in principle, affect the formation of embryonic endoderm. Consistently, we observed that a large portion of Cripto conditional mutant embryos that survived to late stage displayed defects in the venture closure of foregut endoderm. Interestingly, these mutant embryos also suffered cardia bifida, a defect caused by ventral migration failure of cardiac progenitor cells. It is known that Mesp1 is required for this process (Saga et al., 1999), and Cripto function is required for its expression as shown by the current study. Therefore, the heart malformation observed here could be caused by direct defects in cardiac progenitor cell migration. In addition, the formation of a linear heart tube along the ventral midline is also dependent on the ventral foregut development, which was disrupted in these mutants. Therefore, the cardia bifida could be due to multiple mechanisms.
In conclusion, Cripto plays a critical role during mouse gastrulation, especially in mesoderm and endodermformation. This function of Cripto is partially mediated through pathways involving Fgf receptor 1 and p38 MAP kinase.
Supplementary Material
Highlights.
Mesoderm migration is a fundamental, but poorly understood question.
Only a limited genes and pathways identified such as Fgf and p38 MAP.
We found mouse Cripto gene is required for this process.
Loss of Cripto function affects both the Fgf8-Fgfr1 and p38 MAP kinase pathway.
Therefore, Cripto could be a part of the link connecting the two pathways.
Acknowledgements
We thank Dr. Michael Shen for his supports to this study. We also thank Dr. Dennis Warner and Dr. Michael Shen for critical reading of the manuscript. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103453, and research grants from National Institutes of Health (DE015565 and DE016845) and Kentucky Science & Engineering Foundation (KSEF-148-502-10-258) to J.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008;135:501–511. doi: 10.1242/dev.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Beddington R. Cripto-analysis of embryonic codes. Nature. 1998;395:641, 643. doi: 10.1038/27086. [DOI] [PubMed] [Google Scholar]
- Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chu J, Ding J, Jeays-Ward K, Price SM, Placzek M, Shen MM. Non-cell-autonomous role for Cripto in axial midline formation during vertebrate embryogenesis. Development. 2005;132:5539–5551. doi: 10.1242/dev.02157. [DOI] [PubMed] [Google Scholar]
- Chu J, Shen MM. Functional redundancy of EGF-CFC genes in epiblast and extraembryonic patterning during early mouse embryogenesis. Dev Biol. 2010;342:63–73. doi: 10.1016/j.ydbio.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Clements M, Pernaute B, Vella F, Rodriguez TA. Crosstalk between Nodal/Activin and MAPK p38 Signaling Is Essential for Anterior-Posterior Axis Specification. Curr Biol. 2011;21:1289–1295. doi: 10.1016/j.cub.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Dono R, Scalera L, Pacifico F, Acampora D, Persico MG, Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development. 1993;118:1157–1168. doi: 10.1242/dev.118.4.1157. [DOI] [PubMed] [Google Scholar]
- Downs KM, Hellman ER, McHugh J, Barrickman K, Inman KE. Investigation into a role for the primitive streak in development of the murine allantois. Development. 2004;131:37–55. doi: 10.1242/dev.00906. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Vincent SD, Oxburgh L, Robertson EJ, Bikoff EK. Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development. 2004;131:1717–1728. doi: 10.1242/dev.01072. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Dehart DB, Sulik KK, Hogan BL. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development. 2002;129:4685–4696. doi: 10.1242/dev.129.20.4685. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci U S A. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nat Cell Biol. 2002;4:821–825. doi: 10.1038/ncb862. [DOI] [PubMed] [Google Scholar]
- Guardiola O, Lafuste P, Brunelli S, Iaconis S, Touvier T, Mourikis P, De Bock K, Lonardo E, Andolfi G, Bouche A, Liguori GL, Shen MM, Tajbakhsh S, Cossu G, Carmeliet P, Minchiotti G. Cripto regulates skeletal muscle regeneration and modulates satellite cell determination by antagonizing myostatin. Proc Natl Acad Sci U S A. 2012;109:E3231–3240. doi: 10.1073/pnas.1204017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PP, Elefanty AG, Robb L. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- Harvey RP. Patterning the vertebrate heart. Nat Rev Genet. 2002;3:544–556. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Tenzen T, McMahon AP. Maternal inheritance of Cre activity in a Sox2Cre deleter strain. Genesis. 2003;37:51–53. doi: 10.1002/gene.10225. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iratni R, Yan YT, Chen C, Ding J, Zhang Y, Price SM, Reinberg D, Shen MM. Inhibition of excess nodal signaling during mouse gastrulation by the transcriptional corepressor DRAP1. Science. 2002;298:1996–1999. doi: 10.1126/science.1073405. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kimura C, Shen MM, Takeda N, Aizawa S, Matsuo I. Complementary functions of Otx2 and Cripto in initial patterning of mouse epiblast. Dev Biol. 2001;235:12–32. doi: 10.1006/dbio.2001.0289. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot WS, Robertson EJ, Schier AF, Hamada H. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–298. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci U S A. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo: a laboratory manual. (3rd edition) 2003 3rd ed. [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- Seguin CA, Draper JS, Nagy A, Rossant J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. doi: 10.1016/j.stem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Wakamiya M, Kwan KM, Kania A, Jessell TM, Behringer RR. Lim1 is required in both primitive streak-derived tissues and visceral endoderm for head formation in the mouse. Development. 1999;126:4925–4932. doi: 10.1242/dev.126.22.4925. [DOI] [PubMed] [Google Scholar]
- Shen MM. Identification of differentially expressed genes in mouse development using differential display and in situ hybridization. Methods. 2001;24:15–27. doi: 10.1006/meth.2001.1152. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Shen MM, Schier AF. The EGF-CFC gene family in vertebrate development. Trends Genet. 2000;16:303–309. doi: 10.1016/s0168-9525(00)02006-0. [DOI] [PubMed] [Google Scholar]
- Shen MM, Wang H, Leder P. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development. 1997;124:429–442. doi: 10.1242/dev.124.2.429. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Tam PP, Khoo PL, Lewis SL, Bildsoe H, Wong N, Tsang TE, Gad JM, Robb L. Sequential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development. 2007;134:251–260. doi: 10.1242/dev.02724. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Tam PP, Tan SS. The somitogenetic potential of cells in the primitive streak and the tail bud of the organogenesis-stage mouse embryo. Development. 1992;115:703–715. doi: 10.1242/dev.115.3.703. [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Brown A, Beddington RS. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- Varlet I, Collignon J, Robertson EJ. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development. 1997;124:1033–1044. doi: 10.1242/dev.124.5.1033. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Robertson EJ. Highly efficient transgene-independent recombination directed by a maternally derived SOX2CRE transgene. Genesis. 2003;37:54–56. doi: 10.1002/gene.10226. [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng CX. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci U S A. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Yan YT, Liu JJ, Luo Y, E, C., Haltiwanger RS, Abate-Shen C, Shen MM. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol. 2002;22:4439–4449. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.