Abstract
Objectives
Raltegravir (RAL)-containing antiretroviral therapy (ART) produced better immunologic and virologic responses than optimized background ART in clinical trials of heavily ART-experienced patients, but few data exist on long-term outcomes in routine HIV care.
Methods
We studied ART-experienced HIV outpatient study (HOPS) participants seen at 10 US HIV-specialty clinics during 2007–2011. We identified patients who started (baseline date) either continuous ≥30 days of RAL-containing or RAL-sparing ART, and used propensity score (PS) matching methods to account for baseline clinical and demographic differences. We used Kaplan–Meier methods and log-rank tests for the matched subsets to evaluate probability of death, achieving HIV RNA <50 copies/ml, and CD4 cell count (CD4) increase of ≥50 cells mm−3 during follow-up.
Results
Among 784 RAL-exposed and 1062 RAL-unexposed patients, 472 from each group were matched by PS. At baseline, the 472 RAL-exposed patients (mean nadir CD4, 205 cells mm−3; mean baseline CD4, 460 cells mm−3; HIV RNA <50 copies ml−1 in 61%; mean years on prescribed ART, 7.5) were similar to RAL unexposed. During a mean follow-up of over 3 years, mortality rates and immunologic and virologic trajectories did not differ between the two groups. Among patients with detectable baseline HIV RNA levels, 76% of RAL-exposed and 63% of RAL-unexposed achieved HIV RNA <50 copies ml−1 (P=0.51); 69 and 58%, respectively, achieved a CD4 increase ≥50 cells mm−3 (P=0.70).
Discussion
In our large cohort of US ART-experienced patients with a wide spectrum of clinical history, similar outcomes were observed when prescribed RAL containing versus other contemporary ART.
Keywords: Raltegravir, Mortality, Viremia, Viral load, Clinical outcomes, HIV cohort
Introduction
Raltegravir (RAL), an HIV-1 integrase strand transfer inhibitor (ISTI) was the first drug in this class approved by the FDA for use as part of combination antiretroviral therapy (ART), in October 2007.1 Approval was based on results from two placebo-controlled randomized clinical trials, BENCHMRK-1 and BENCHMRK-2, which were conducted among HIV-infected ART-experienced patients with triple antiretroviral drug class resistance and limited treatment options. These studies found that RAL plus optimized background ART provided better HIV RNA suppression than optimized background therapy alone.2,3 The ANRS 139 Trio and the Merck EAP 0518 studies confirmed high rates of virologic suppression associated with use of RAL-based antiretroviral regimens among ART-experienced patients with multidrug-resistant HIV infections.4,5 Further studies among ART-naïve patients found that RAL-based combination treatment resulted in rapid and potent antiretroviral activity and was well tolerated,6 leading to the recommendation for its use as standard for initial treatment of HIV.7 RAL remains a recommended drug for ART-experienced patients who are ISTI-naïve and experience virologic non-suppression or rebound or who desire regimen simplification.7,8
The use of ISTIs and drugs from other new classes, such as entry and fusion inhibitors, has increased steadily in the US in recent years.9 However, relatively few studies have described RAL use and immunologic, virologic, and clinical outcomes among ART-experienced patients outside of clinical trials in ‘real world’ HIV clinical practice.10–15 We sought to evaluate long-term outcomes associated with use of RAL-containing ART compared with other contemporary ART regimens among ART-experienced patients in our large multi-site US-based cohort of HIV-infected patients.
Methods
The HIV outpatient study
The HIV outpatient study (HOPS) is an ongoing, prospective observational study of HIV-infected patients in care followed at HIV-specialty clinics in the US, initiated in 1993. The HOPS methodology has been described previously.16 In brief, trained staff abstract patient data, including sociodemographic characteristics, diagnoses, treatments, and laboratory values from medical records and enter them into an electronic database for central processing and analysis. The institutional research review boards of the Centers for Disease Control and Prevention and the local participating sites have approved and reviewed the ethical conduct of this study yearly.
Study population
We analyzed data from HOPS participants seen at 10 HOPS clinics using the HOPS dataset as of 31 December 2012. For this study, observation time was truncated on 31 December 2011 to allow for ascertainment and abstraction of death events. We limited analyses to participants who had at least two HOPS-related encounters documented (i.e. clinic visits, hospitalizations, laboratory measurements, but not telephone calls) any time in the HOPS, of which one had to be during 2007–2011. We selected patients who at baseline (defined below) were ART experienced and had no history of RAL use. We defined as baseline date the first occurrence after 1 January 2007 when an ART-experienced patient started either: a continuous RAL-containing ART regimen of ≥30 days duration, or a continuous RAL-sparing ART regimen of ≥30 days duration. We termed these ART regimens as ‘qualifying regimens’. Patient follow-up continued throughout receipt of qualifying and subsequent ART regimens; observation for patients receiving RAL-containing regimen(s) was stopped when they discontinued RAL, and observation for patients receiving RAL-sparing regimen(s) was stopped when they started RAL; for all patients, observation was stopped if they discontinued ART. For survival analyses, end of observation time was at death or last patient contact plus 6 months (183 days), or 30 March 2012, whichever occurred first; only deaths that occurred within 183 days after last patient contact were considered.
Propensity score analyses
ART-experienced patients who received RAL-containing regimens may have had more advanced HIV disease than patients who received regimens without RAL; this confounding by indication could distort the evaluation of RAL effectiveness regarding immunologic and virologic responses as well as clinical outcomes. To address this potential bias, we used propensity score (PS) matching17 to balance the two non-randomized treatment groups with respect to a variety of factors, following previously published methodologic guidance.18,19 For our purposes, a patient’s PS was defined as the probability he/she was prescribed a RAL-containing regimen given that person’s demographic characteristics, ART treatment history, lab measurements, and other data. Propensity score matching involves a two-step process: computing a PS and performing the matching.
First, to compute a PS we used multivariable logistic regression to model factors associated with receiving RAL. To account for a few covariates with a small fraction of missing data and to avoid bias if these data were not missing completely at random, we used the general location mixture model proposed by Mitra and Reiter.20 This approach uses multiply imputed data to handle the missing values with an additional covariate that assists in identifying patients who switched to RAL-sparing ART but would have been good candidates for RAL-containing ART. Furthermore, we incorporated a maximum likelihood-based estimation procedure into the logistic regression model21 to account for baseline HIV RNA values that were undetectable. We evaluated several PS models to identify one which fit the data best with a limited number of covariates.18 After fitting the PS model to each imputed dataset, the PS for a patient was assigned using the average probability of RAL initiation from the 10 imputed datasets.
Diverse demographic and clinical characteristics were considered in developing our PS models, both binary and continuous. The final binary variables, defined using data prior to or as of baseline, included the following: history of AIDS, completeness of ART history, history of mono/dual- ART exposure, men who have sex with men (MSM) HIV transmission risk category, Hispanic ethnicity, African-American or black race, private health insurance, having had an HIV phenotype performed, documentation of ≥1 major HIV genetic mutation per International AIDS Society (IAS)-USA 2008 guidelines, and documentation of any major mutation associated with resistance to nucleoside reverse transcriptase inhibitors (NRTIs). We also categorized patients according to whether the RAL-containing or RAL-sparing regimen they began at baseline contained any of the following novel agents: etravirine, maraviroc, enfuvirtide, and elvitegravir.
The final continuous variables selected for PS models, similarly determined using data prior to or as of baseline, included the following: age, plasma HIV RNA level, CD4 cell count, nadir CD4 cell count, number of prior ART drugs received, number of prior ART regimens, months since 1 January 2007 until baseline date, years of ART treatment, and years since HIV diagnosis. We also determined the number of ART drugs in the first qualifying ART regimen. Interactions between aforementioned variables were considered and included in the final model when they improved model fit (see Statistical Analyses). Variables measured at baseline that were not included in the PS model because they did not improve model fit were as follows: sex, alcohol and tobacco use, injection drug use (IDU) and heterosexual HIV transmission risk, other or unknown race, having public insurance, undetectable HIV RNA at baseline, history of stopping an ART due to toxicity, diagnosis of hepatitis B or C infection, history of having a genotype performed, having any major mutation associated with resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) or protease inhibitors (PIs), and CD8 cell count.
After deriving PS scores for all patients, we matched 1 : 1 patients who did and did not receive RAL with similar PS using a nearest-neighbor algorithm with a caliper of 0.1 (i.e. the largest difference in PSs between a match was 0.1 or 10%). Unmatched patients were excluded from subsequent analyses.
Statistical analyses
Assessments of the differences in group means and proportions before and after matching were performed with the t- and Pearson chi-square tests, respectively, to highlight the utility of the matching process in balancing the characteristics of patient subsets.22 The Wilcoxon rank sum was used to test for differences in median duration of therapy between the two groups. For each main outcome event (death, achieving HIV RNA <50 copies ml−1, and CD4 cell count increase ≥50 cells mm−3), we performed several analyses. For the subset of PS-matched patients, we considered a simple binary outcome (whether or not the event occurred) and additionally the event and occurrence time together (as a time-to-event outcome). We first constructed models for the entire observation time, and then censored the observation time at 12 months after the start of first qualifying regimen. We analyzed the percentage of patients with HIV virologic suppression in both RAL-exposure groups at various points in time. We then examined time to undetectable HIV RNA (<50 copies ml−1) and time to CD4 cell count increase ≥50 cells mm−3 for a subset of patients who had detectable HIV RNA levels at baseline. Binary outcomes were compared with the chi-square test, and time-to-event models with the log-rank test. Kaplan–Meier curves23 were used to depict survival distributions.
Finally, we tested for mean differences between patient groups in mean log10 transformed plasma HIV RNA levels and mean CD4 cell counts in the 12 months after initiating RAL-containing or RAL-sparing ART. Matched participants without available values in the first 12 months were excluded. Each regression model included the following covariates defined as of baseline: age in years, HIV transmission risk group, race/ethnicity, type of insurance, history of AIDS diagnosis, years since HIV diagnosis, diagnosis of hepatitis B or C infection, known ART history, history of mono- or dual-NRTI exposure, number of ART regimens received, prior receipt of genotype or phenotype test, presence of any novel antiretroviral agents, and number of ART drugs in the first qualifying ART regimen. The mean differences and 95% confidence intervals (CIs) are reported. A method accounting for data below the limit of detection24 was used for HIV RNA, whereas CD4 counts were analyzed with a linear mixed model.25 Associations with a p value <0.05 were considered significant. Models were fit in SAS 9.3 (SAS Institute, Inc, Cary, NC, USA) and figures were created with the ggplot2 package26 in R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
In the HOPS dataset as of 31 December 2012, there were 10 179 patients, of whom 1846 ART-experienced patients met criteria for analysis. Patients were excluded, hierarchically, if they (a) did not have two visits recorded in the HOPS (n=677 excluded); (b) did not have at least one visit from 1 January 2007 to 31 December 2011 (n=5381 excluded); (c) remained ART-naïve during 2007–2011 (n=243 excluded); (d) did not start a new qualifying ART regimen of ≥30 days duration during 2007–2011 (n=1979 excluded); or (e) used RAL in any prior ART regimen (n=53 excluded).
Among the 1846 eligible ART-experienced patients, there were 784 ART-experienced patients who began a RAL-containing regimen and 1062 patients who began a RAL-sparing regimen between 1 January 2007 and 30 March 2011. Of these, 472 patients from each group were matched by PS. Whereas in the entire study population (N=1846), patients on RAL-containing and RAL-sparing regimens differed by many demographic (e.g. age, race/ethnicity, HIV risk group) and clinical characteristics (e.g. nadir CD4 cell count, years since HIV diagnosis, ART treatment history and history of genotypic and phenotypic resistance testing), patients in PS-matched subset did not differ on these characteristics (all P>0.10, Table 1). The 472 patients who received RAL-containing regimens had a mean nadir CD4 cell count of 205 cells mm−3, mean baseline CD4 cell count of 460 cells mm−3, mean of 13.0 years since their HIV diagnosis, had been prescribed a mean of 6.8 antiretroviral agents over a mean of 7.5 years before baseline, and 54% of them had been exposed to mono- or dual-NRTIs (Table 1). Thirty-nine patients prescribed RAL-containing ART had received a novel agent in their first qualifying regimen: 7 enfuvirtide, 8 maraviroc, 24 etravirine, 1 ancriviroc, and 1 prescribed both enfuvirtide and etravirine.
Table 1.
Characteristics of patients who started antiretroviral regimens with vs. without raltegravir, the HOPS, 2007–2011
| Variable at baseline* | Eligible population RAL
|
Eligible population non-RAL
|
P | Matched subset RAL
|
Matched subset non-RAL
|
P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Value | N | Value | N | Value | N | Value | |||
| Age (years) | 784 | 47.7 | 1062 | 45.5 | <0.001 | 472 | 47.6 | 472 | 48.0 | 0.53 |
| Male sex | 784 | 79.3 | 1062 | 74.3 | 0.01 | 472 | 77.1 | 472 | 79.2 | 0.43 |
| Race/ethnicity | ||||||||||
| Black, non-Hispanic/Latino | 784 | 30.5 | 1062 | 37.0 | 0.004 | 472 | 31.8 | 472 | 32.8 | 0.73 |
| Hispanic/Latino | 784 | 10.5 | 1062 | 11.3 | 0.57 | 472 | 10.2 | 472 | 10.4 | 0.91 |
| Other/unknown | 784 | 2.4 | 1062 | 4.5 | 0.02 | 472 | 3.0 | 472 | 3.8 | 0.47 |
| White, non-Hispanic/Latino | 784 | 56.6 | 1062 | 47.2 | <0.001 | 472 | 55.1 | 472 | 53.0 | 0.51 |
| HIV risk group | ||||||||||
| MSM | 784 | 61.9 | 1062 | 51.3 | <0.001 | 472 | 57.2 | 472 | 57.8 | 0.84 |
| IDU | 784 | 6.9 | 1062 | 10.2 | 0.014 | 472 | 8.5 | 472 | 9.3 | 0.65 |
| Other/unknown | 784 | 5.7 | 1062 | 8.1 | 0.05 | 472 | 7.2 | 472 | 7.2 | 1.00 |
| Heterosexual | 784 | 25.5 | 1062 | 30.4 | 0.02 | 472 | 27.1 | 472 | 25.6 | 0.61 |
| Private insurance | 784 | 57.1 | 1062 | 51.5 | 0.02 | 472 | 53.4 | 472 | 54.7 | 0.70 |
| CD4 cell count (cells/mm3)† | 668 | 446 | 916 | 457 | 0.48 | 400 | 460 | 394 | 459 | 0.97 |
| CD4 cell count <200 (cells/mm3) | 784 | 34.1 | 1062 | 30.4 | 0.10 | 472 | 33.5 | 472 | 32.8 | 0.84 |
| Log10 HIV viral load (copies/ml)† | 692 | 2.5 | 896 | 2.6 | 0.05 | 412 | 2.4 | 402 | 2.4 | 0.96 |
| HIV viral load <50 (copies/ml) | 665 | 54.1 | 814 | 58.2 | 0.11 | 398 | 57.5 | 389 | 61.4 | 0.27 |
| Nadir CD4 cell count (cells/mm3)† | 759 | 190 | 1048 | 229 | <0.001 | 449 | 205 | 463 | 201 | 0.74 |
| Hepatitis C | 784 | 13.3 | 1062 | 16.6 | 0.050 | 472 | 15.3 | 472 | 16.5 | 0.59 |
| Years since HIV diagnosis | 784 | 13.9 | 1062 | 11.2 | <0.001 | 472 | 13.0 | 472 | 13.0 | 0.97 |
| Number of ARTs in the regimen | 784 | 3.5 | 1062 | 3.2 | <0.001 | 472 | 3.3 | 472 | 3.3 | 0.47 |
| Prior ART history completely known | 784 | 36.2 | 1062 | 41.9 | 0.014 | 472 | 38.8 | 472 | 39.8 | 0.74 |
| Prior number of antiretrovirals taken | 784 | 7.9 | 1062 | 5.8 | <0.001 | 472 | 6.8 | 472 | 6.7 | 0.49 |
| Prior number of regimens taken | 784 | 7.4 | 1062 | 4.8 | <0.001 | 472 | 6.2 | 472 | 6.0 | 0.51 |
| Mono/dual ART exposure history | 784 | 57.9 | 1062 | 50.8 | 0.003 | 472 | 54.4 | 472 | 56.4 | 0.56 |
| Months since January 1, 2007 | 784 | 26.8 | 1062 | 18.2 | <0.001 | 472 | 25.1 | 472 | 25.7 | 0.45 |
| Years ART treated | 784 | 8.2 | 1062 | 6.4 | <0.001 | 472 | 7.5 | 472 | 7.5 | 0.97 |
| Genotype done | 784 | 63.9 | 1062 | 45.1 | <0.001 | 472 | 55.5 | 472 | 50.8 | 0.15 |
| Phenotype done | 784 | 33.5 | 1062 | 14.2 | <0.001 | 472 | 21.8 | 472 | 21.0 | 0.75 |
| Any use of novel antiretrovirals‡ | 784 | 20.9 | 1062 | 2.7 | < 0.001 | 472 | 8.3 | 472 | 6.2 | 0.26 |
Note:
Value denotes a mean for continuous variables, and a proportion for categorical variables. Matching was done using propensity-score methods (see Methods). N’s indicate number of persons (denominator) providing data for a given variable.
Values were imputed in the final multiple logistic model to derive propensity scores.
As part of first qualifying regimen with novel drug agents defined in the text.
IDU: injection drug users; MSM: men who have sex with men; RAL: raltegravir.
Among 472 PS-matched patients receiving RAL-containing regimens, the number and percentage starting them were 64 (14%) in 2007, 174 (37%) in 2008, 138 (29%) in 2009, 90 (19%) in 2010, and 6 (1%) in 2011. Among 472 matched participants on RAL-sparing regimens, the number and percentage starting them were 97 (21%) in 2007, 109 (23%) in 2008, 133 (28%) in 2009, 117 (25%) in 2010, and 16 (3%) in 2011. For the PS-matched participants, the median durations of first qualifying ART regimen were 24.2 versus 21.1 months for RAL-exposed and -unexposed patients, respectively, and the median durations of therapy in each treatment group were 31.8 versus 22.4 months, respectively (Wilcoxon rank sum test, P<0.001). There were no statistically significant differences in the durations of initial RAL-containing and RAL-sparing regimens within each calendar year (Fig. 1). Among 472 patients receiving RAL-containing therapy, 266 (56%) discontinued RAL altogether before last contact in the HOPS. The median available follow-up for mortality analyses was 42.4 months for patients prescribed RAL-containing therapy versus 37.8 months for patients prescribed RAL-sparing therapy (P=0.04).
Figure 1.

Boxplots of first qualifying ART regimen duration stratified by patients who started raltegravir-containing and -sparing regimens, the HOPS, 2007–2011. Note: Panels denote the beginning year and numbers to the right indicate sample size of the boxplot.
During available follow-up for evaluation of mortality (i.e. on and after baseline) in the PS-matched subset, among patients prescribed RAL-containing regimens, 90 (19.1%) received ART that contained one or more of other novel antiretroviral agents: enfuvirtide (n=11), maraviroc (n=27), etravirine (n=67), and SCH351 (n=1); among patients prescribed RAL-sparing regimens, 40 (8.5%) received ART that contained one or more of other novel antiretroviral agents: enfuvirtide (n=7), maraviroc (n=6), and etravirine (n=28). In terms of PIs included in cART in the PS-matched subset, the use of ritonavir-boosted PIs was less common among patients prescribed RAL-containing regimens than RAL-sparing regimens (28.6 vs. 53.4%, P<0.001) but the frequency of use of darunavir, a relatively newer and potent PI available at the time of this analysis, was similar (20.1 vs. 18.4%, P=0.56).
In PS-matched analyses, 22 (4.7%) of 472 of patients prescribed RAL-containing regimens died during follow-up as compared with 20 (4.2%) of 472 of patients prescribed RAL-sparing regimens (log-rank P=0.85, Fig. 2). The corresponding mortality rates (per 100 person years) were 1.37 (95% CI, 0.90–2.09) and 1.30 (95% CI, 0.83–2.02), respectively. In analyses restricted to first 12 months of observation, 8 (1.7%) versus 9 (1.9%) of patients died, respectively (log-rank P=0.81).
Figure 2.
Kaplan–Meier survival curves (with 95% confidence intervals) for time to death from start of qualifying raltegravir-containing and -sparing regimens for propensity score-matched patient subset, the HOPS, 2007–2011.
In PS-matched time-to-event analyses limited to patients with detectable baseline HIV RNA levels, 128 (75.7%) of 169 patients who received RAL-containing ART regimens achieved an undetectable HIV RNA level compared with 94 (62.7%) of 150 patients who were followed while prescribed RAL-sparing regimens (log-rank P=0.51). In analyses restricted to the first 12 months of observation, 115 (68.0%) versus 85 (56.7%) of patients achieved undetectable HIV RNA, respectively (log-rank P=0.24, Fig. 3).
Figure 3.

Kaplan–Meier survival curves (with 95% confidence intervals) for time to HIV RNA suppression within the first 12 months after start of qualifying raltegravir-containing and -sparing regimens for propensity score-matched patients without HIV RNA suppression at baseline, the HOPS, 2007–2011.
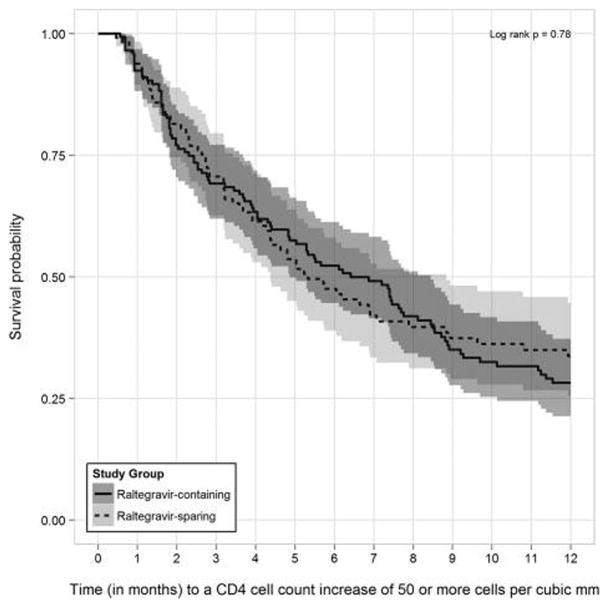
In PS-matched time-to-event analyses among patients with detectable baseline HIV RNA levels, 116 (68.6%) of 169 patients prescribed RAL-containing regimens achieved a CD4 cell count increase of ≥50 cells mm−3 during observation compared with 87 (58.0%) of 150 patients followed while prescribed RAL-sparing regimens (log-rank P=0.70). In analyses restricted to the first 12 months of observation, 96 (56.8%) versus 69 (46.0%) of patients achieved similar CD4 increases, respectively (log-rank P=0.78, Fig. 4).
Figure 4.
Kaplan–Meier survival curves (with 95% confidence intervals) for time to CD4 increases ≥50 cells mm−3 within the first 12 months after start of qualifying raltegravir-containing and -sparing regimens for propensity score-matched patients without HIV RNA suppression at baseline, the HOPS, 2007–2011.
There were no significant differences in mortality risk by RAL use group among the subset of patients with detectable baseline HIV RNA levels, whether observed over the entirety of available observation time (10 [5.9%] vs. 7 [4.7%] of patients receiving RAL-containing and RAL-sparing regimens died during subsequent observation, respectively [log-rank P=0.81, data not shown]) or in the first 12 months (data not shown).
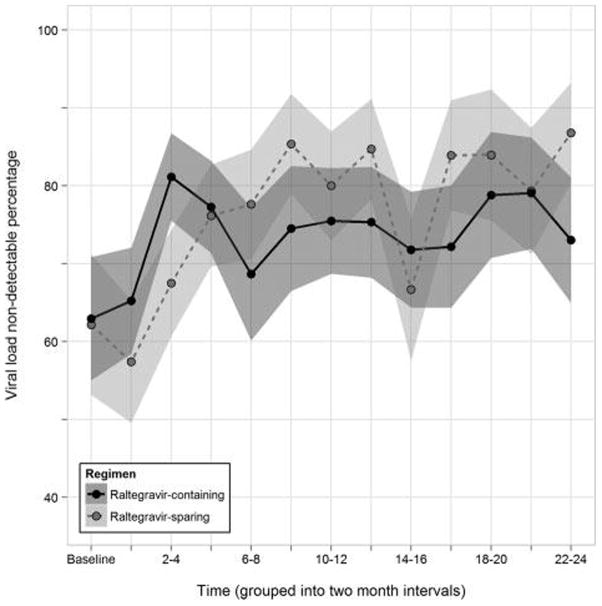
Finally, in analyses employing linear mixed models separately for HIV RNA levels (n=2286 measurements, 853 total participants) and CD4 cell count (n=2141 measurements, 816 total participants) over the first 12 months of follow-up, exposure to RAL was not associated with a statistically significant reduction in log10 HIV viral load (mean difference=−0.19, 95% CI: −0.50 to 0.11, P=0.21) or statistically significant improvement in CD4 cell count (mean difference=16.01, 95% CI: −49.66 to 107.80, P=0.39). The percentage of patients with undetectable HIV RNA also did not differ statistically (overlapping 95% CIs around estimates) by RAL exposure during the first 24 months of observation time (Fig. 5).
Figure 5.
Percentages and their 95% confidence intervals of patients with HIV RNA <50 copies ml−1 in moving 2-month intervals in propensity score matched patient subset, the HOPS, 2007–2011.
Discussion
Among ART-experienced contemporary participants in the multi-site US-based observational HOPS cohort, we did not detect statistically significant differences in survival, time to virologic suppression, or likelihood of CD4 cell count increases ≥50 cells mm−3 among patients who received RAL-containing ART regimens compared with similar patients who received RAL-sparing ART regimens. Among our diverse patients who had variable degrees of ART experience and were seen in real-world (non-clinical trial based) HIV clinic settings, outcomes among persons prescribed RAL-containing ART regimens did not appear to differ from those for counterparts prescribed other contemporary ART regimens. Our results, thus, differ from those of randomized clinical BENCHMRK trials evaluating benefits of RAL-inclusive ART regimens among extensively ART-experienced patients.2,3
The reasons for ART regimen changes were not systematically coded in medical records available to the HOPS, but our data suggest that many ART-experienced patients may have switched to RAL-containing or other ART regimens because of ART-related side effects or complications (e.g. dyslipidemia) or for regimen simplification, rather than due to virologic failure. Almost 60% of patients starting RAL-containing and RAL-sparing regimens in the matched patient subset had an HIV RNA <50 copies ml−1 at baseline, and subsequent mortality in both groups was about 1% annually, lending further support to the hypothesis that the populations of ART-experienced patients we studied were relatively healthy.
We found no marked differences in HIV RNA or CD4 cell count responses among treated patients who did versus did not receive RAL, in contrast to earlier studies that reported RAL-containing regimens were superior to optimized existing regimens among ART-experienced viremic patients.2,3 Our findings may have differed in part because our RAL recipients (both in the entire population and in the matched subset) were generally healthier and less heavily ART pretreated than patients studied in earlier clinical trials, and most were baseline virologically suppressed. Participants in the BENCHMRK trials2,27 had documented triple ART class resistance (by genotypic or phenotypic testing), baseline mean CD4 cell count of 151 cells mm−3 and were viremic with a mean plasma HIV RNA level of 4.6 log10 copies/ml. Nearly 40% of these patients received enfuvirtide as part of an optimized background ART regimen. By contrast, in our cohort, before and after propensity matching, our RAL-exposed patients’ mean baseline CD4 count was close to 450 cells mm−3 and mean HIV RNA level was 2.5 log10 copies ml−1. We did not require studied HOPS participants to have a documented ART triple drug class resistance, and very few of our patients (<2%) were prescribed enfuvirtide.
Over half of our patients entered observation while virologically suppressed and therefore probably switched to RAL-containing regimens to minimize ART-related toxicities or to simplify ART regimens, although we did not have systematic data on reasons for regimen switches. Nonetheless, among 169 HOPS RAL recipients with detectable HIV RNA levels at baseline, 135 (80%) achieved RNA levels <50 copies ml−1 by 48 weeks, compared with 62% of patients in the BENCHMRK study population by 48 weeks,2 indicating excellent rates of response in the HOPS cohort. The virologic responses of baseline-viremic HOPS patients on RAL were comparable to those observed in an earlier and smaller study of triple-class-experienced patients in the Swiss HIV cohort.12 Finally, while optimized background regimens in the BENCHMRK studies could include darunavir, tipranavir, and enfuvirtide, they did not include the then new CCR5 receptor antagonist, maraviroc.
Although this study’s extended follow-up time gave us an opportunity to assess mortality, a long-term outcome rarely observed in clinical trials, our analyses were limited by a relatively small number of death events. In this or similar patient populations, a substantially larger sample would be required to detect other than a very strong association between use of RAL-containing regimens and mortality, or to detect a modest associations with immunologic or virologic trajectories. A strength of our observational cohort study was in evaluating the effectiveness of RAL-containing therapies for virologic and immunologic outcomes in a non-clinical trial setting for a diverse patient population (>20% women, >30% black, >40% not MSM) and at different clinical stages of HIV disease and variable prior ART experience. The multi-site HIV-infected patient populations like ours include persons prescribed a variety of NNRTIs and PIs, who frequently have comorbidities (including chronic diseases), lifestyle risk factors (e.g. IDU), and variable adherence to prescribed regimens, all of which influence the results regarding the effectiveness of cART. The PS methods enabled us to match on a large number of covariates simultaneously and to closely approximate the design of a randomized controlled trial while using observational data from this ‘real world’ clinic population. A limitation of the PS matching method lies in the trade-off between matching all patients and basing analyses and inferences on only a subset of ‘good’ matches.
In conclusion, among ART-experienced patients followed in HIV-specialty clinics with access to many ART drug options, we found that immunologic, virologic, and clinical outcomes did not differ between patients who switched to RAL-containing regimens during 2007–2011 compared with similar patients who switched to other contemporary ART regimens.
Acknowledgments
Funding Contracts 200-2001-00133, 200-2006-18797, and 200-2011-41872 from the Centers for Disease Control and Prevention.
Footnotes
Disclaimer Statements
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Contributors We wish to acknowledge the contributions of the HIV Outpatient Study (HOPS) Investigators, which include the following persons and sites: Kate Buchacz, John T. Brooks, Marcus D. Durham, Division of HIV/AIDS Prevention, National Center for HIV, STD, Viral Hepatitis and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA; Harlen Hays, Kathleen C. Wood, Darlene Hankerson, Rachel Hart, Thilakavathy Subramanian, Carl Armon, Bonnie Dean, Dana Franklin, Cerner Corporation, Vienna, VA, USA; Frank J. Palella, Joan S. Chmiel, Saira Jahangir, Conor Daniel Flaherty, Jerian Denise Dixon-Evans, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA; Kenneth A. Lichtenstein, Cheryl Stewart, National Jewish Health, Denver, CO; John Hammer, Kenneth S. Greenberg, Barbara Widick, Rosa Franklin, Rose Medical Center, Denver, CO, USA; Bienvenido G. Yangco, Kalliope Chagaris, Infectious Disease Research Institute, Tampa, FL, USA; Doug Ward, Troy Thomas, Matt Starr, Dupont Circle Physicians Group, Washington, DC, USA; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, State University of New York (SUNY), Stony Brook, NY, USA; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, Princess Graham, Temple University School of Medicine, Philadelphia, PA, USA; Richard M. Novak, Andrea Wendrow, Renata Smith, University of Illinois at Chicago, Chicago, IL, USA; Benjamin Young, Barbara Widick, Mia Scott, APEX Family Medicine, Denver, CO, USA.
Ethics approval The institutional research review boards of the Centers for Disease Control and Prevention and the local participating sites have approved and reviewed the ethical conduct of the HIV Outpatient Study (HOPS) study yearly.
Conflicts of interest Dr. Frank Joseph Palella has received consulting or speaking fees from Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, and Merck & Co. Dr. Frank Joseph Palella has also received research funding from Gilead Sciences. Other authors – no declared conflicts.
References
- 1.Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009;48(7):931–939. doi: 10.1086/597290. [DOI] [PubMed] [Google Scholar]
- 2.Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis. 2010;50(4):605–612. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eron JJ, Cooper DA, Steigbigel RT, Clotet B, Gatell JM, Kumar PN, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13(7):587–596. doi: 10.1016/S1473-3099(13)70093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yazdanpanah Y, Fagard C, Descamps D, Taburet AM, Colin C, Roquebert B, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49(9):1441–1449. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 5.Skiest DJ, Cohen C, Mounzer K, Haigney Z, Barker D, Gottlieb M, et al. Similar efficacy of raltegravir when used with or without a protease inhibitor in treatment-experienced patients. HIV Clin Trials. 2011;12(3):131–140. doi: 10.1310/hct1203-131. [DOI] [PubMed] [Google Scholar]
- 6.Lennox JL, DeJesus E, Lazzarin A STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial (vol 374, pg 796, 2009) Lancet. 2009;374(9707):2054. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 7.DHHS. Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed March 27, 2012];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012 http://www.aidsinfo.nih.gov/
- 8.Eron JJ, Cooper DA, Steigbigel RT, Clotet B, Yeni P, Strohmaier KM, et al. Association between first-year virological response to raltegravir and long-term outcomes in treatment-experienced patients with HIV-1 infection. Antivir Ther. 2015;20(3):307–15. doi: 10.3851/IMP2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. US trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157(5):325–363. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teague A, Scott C, Bower M, Gazzard B, Nelson M, Stebbing J. A single-center cohort experience of raltegravir in salvage patients failing therapy. J Acquir Immune Defic Syndr. 2010;53(5):666–667. doi: 10.1097/QAI.0b013e3181ba4845. [DOI] [PubMed] [Google Scholar]
- 11.Engsig FN, Gerstoft J, Kronborg G, Larsen CS, Pedersen G, Audelin AM, et al. Clinical, virological and immunological responses in Danish HIV patients receiving raltegravir as part of a salvage regimen. Clin Epidemiol. 2010;2:145–151. doi: 10.2147/clep.s10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherrer AU, von Wyl V, Fux CA, Opravil M, Bucher HC, Fayet A, et al. Implementation of raltegravir in routine clinical practice: selection criteria for choosing this drug, virologic response rates, and characteristics of failures. J Acquir Immune Defic Syndr. 2010;53(4):464–471. doi: 10.1097/QAI.0b013e3181bca4ec. [DOI] [PubMed] [Google Scholar]
- 13.Squires KE, Bekker LG, Eron JJ, Cheng B, Rockstroh JK, Marquez F, et al. Safety, tolerability, and efficacy of raltegravir in a diverse cohort of HIV-infected patients: 48-week results from the REALMRK study. AIDS Res Human Retroviruses. 2013;29(6):859–870. doi: 10.1089/aid.2012.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capetti A, Landonio S, Meraviglia P, Di Biagio A, Lo Caputo S, Sterrantino G, et al. 96 week follow-up of HIV-infected patients in rescue with raltegravir plus optimized backbone regimens: a multicentre Italian experience. Plos One. 2012;7:7. doi: 10.1371/journal.pone.0039222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manfredi R, Calza L, Marinacci G, Cascavilla A, Colangeli V, Salvadori C, et al. Raltegravir use prospectively assessed in a major HIV outpatient clinic in Italy: sample population, virological-immunological activity, and tolerability profile. Infez Med. 2014;22(4):288–295. [PubMed] [Google Scholar]
- 16.Moorman AC, Holmberg SD, Marlowe SI, Von Bargen JC, Yangco BG, Palella FJ, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: The HIV outpatient study (HOPS) Ann Epidemiol. 1999;9(6):349–357. doi: 10.1016/s1047-2797(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 18.Williamson E, Morley R, Lucas A, Carpenter J. Propensity scores: from naive enthusiasm to intuitive understanding. Stat Methods Med Res. 2012;21(3):273–293. doi: 10.1177/0962280210394483. [DOI] [PubMed] [Google Scholar]
- 19.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra R, Reiter JP. Estimating propensity scores with missing covariate data using general location mixture models. Stat Med. 2011;30(6):627–641. doi: 10.1002/sim.4124. [DOI] [PubMed] [Google Scholar]
- 21.Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. Int J Epidemiol. 2009;38(6):1674–1680. doi: 10.1093/ije/dyp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier M. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 24.Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed. 2004;74(3):255–260. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Laird NW, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 26.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- 27.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]