Abstract
The authors sought to assess whether viral load (VL) monitoring frequency was associated with differential rates of virologic failure (VF) among HIV Outpatient Study (HOPS) participants seen during 1999 to 2013, who had maintained VL <50 copies/mL, CD4 counts ≥300 cells/mm3, and been prescribed a stable combination antiretroviral regimen for at least 2 years. The authors required VL and CD4 testing to have occurred regularly for the entire 2-year period. The authors assessed rates of VF comparing patients who maintained a frequent VL testing (≥3 VLs) to those who shifted to a less frequent schedule (2 VL) after the 2-year period. Virologic failure was observed among 116 of 573 participants. The authors did not detect statistically significant difference in frequency of VF among patients undergoing frequent (21.0%) versus less frequent VL testing (19.6%), even after multivariable adjustment. Biannual VL monitoring for stable patients with aviremia could generate substantial cost savings without the increased risk of VF.
Keywords: HIV, diagnostics, viral load
Background
HIV-infected persons who have access to medical care and are prescribed combination antiretroviral therapy (cART) are increasingly living longer and experience lower rates of treatment failure.1,2 Intuitively, it is reasonable that the requirement for laboratory monitoring of stable patients might be less frequent, yet there are sparse data to support this notion. Monitoring plasma HIV RNA levels (viral load [VL]) is recommended as part of routine care for HIV-infected persons, and achieving and maintaining VL suppression are considered critical indicators of successful response to cART.3 Current US Department of Health and Human Services (DHHS) treatment guidelines recommend obtaining routine VL testing every 3 to 4 months, except for patients who have had undetectable VLs while on cART for more than 2 to 3 years and who are stable clinically and immunologically; for such patients, VL testing frequency may be decreased to every 6 months.3 This DHHS recommendation received an AIII rating, meaning that it was of strong strength but was based only on expert opinion. Because the financial cost of laboratory diagnostics represents a substantial portion of total health care costs for HIV-infected persons, biannual VL frequency could result in significant reductions in financial costs of laboratory diagnostics, provided that such monitoring strategy is proven to be sufficient and safe (ie, not associated with adverse events). Recognizing the DHHS Panel’s recommendation, the lack of scientific literature on this subject, and the recently expanded World Health Organization recommendation for reduced frequency of VL monitoring to every 6 months in low- and middle-income countries,4 we investigated the clinical consequences of performing biannual VL testing among clinically and immunologically stable cART-treated patients followed in a large observational HIV cohort study in the United States.
Methods
The HIV Outpatient Study Cohort
The HIV Outpatient Study (HOPS) cohort is an ongoing, open, prospective cohort study since 1993 that has followed over 10 000 HIV-infected adults (aged 18 years and older) receiving HIV outpatient care.5 Participating clinics provide care to a total of approximately 3000 HOPS participants each year. The 11 clinics included in the present analyses were located in 8 US cities: Tampa, Florida; Washington, District of Columbia; Denver, Colorado (3 sites); Oakland, California; San Leandro, California; Chicago, Illinois (2 sites); Stonybrook, New York; and Philadelphia, Pennsylvania. The HOPS protocol has been approved and renewed annually by the Centers for Disease Control and Prevention (CDC) and each participating institution’s ethical review board. All study participants have provided written informed consent. All HOPS clinicians have extensive experience treating HIV-infected patients. Information was abstracted from outpatient charts at each visit, entered electronically by trained staff, compiled centrally, and reviewed and edited before being analyzed. Abstracted information includes demographic characteristics, risk factors for HIV infection, diagnoses, prescribed medications, laboratory values (including CD4 counts and VLs), mortality, and hospitalization records (primarily from discharge summaries).
Study Population
We included in the analyses patients enrolled in HOPS who had suppressed plasma HIV VLs (VL < 50 copies/mL) for at least 2 years and were prescribed a stable, nonsalvage cART regimen outside a research clinical trial for the same 2-year period. Regimens containing enfuvirtide or maraviroc were considered to be salvage regimens, and patients prescribed these regimens were excluded from the analyses. Eligible participants could be prescribed either their first or subsequent cART regimen, but the regimen must have been consistent throughout the 2-year period of viral suppression. Dose changes switches from component drugs to combination drugs and changes from emtricitabine to lamivudine (3TC) or vice versa were not considered changes to the regimen, but all other changes were not permitted and caused patients to be excluded from the analyses. Patients were further required to have regular VL and CD4 testing performed during this 2-year period: At least 3 VLs and 3 CD4 tests were required in each year with the first and the last being at least 6 months apart (see Figure 1). Finally, consistent with the latest DHHS guidelines on immunological stability and VL testing,3 all CD4 tests in the 2-year period must have had a result of at least 300 cells/mm3. The “index date,” the start of observation for this analysis, was defined as the date of the last VL obtained during the 2-year lead-in period as shown in Figure 1. Finally, in the year immediately following the index date, we required patients to have had at least 2 VL tests completed so that the predictor (exposure) variable of interest, namely, the frequency of VL testing, could be established.
Figure 1.
Schematic of analytic design, including definition of HIV viral load (VL) testing, frequency (exposure), and virologic failure (outcome). cART indicates combination antiretroviral therapy; VL, viral load.
Outcome and Independent Variable Definitions
The key predictor variable of interest was the frequency of VL testing performed in the year immediately following the index date. Patients who met the criteria (at least 3 VLs performed in the year at least 6 months between first and last) qualified as frequent testers, and those who had 2 VLs performed during the year were classified as less frequent testers (patients with only 1 VL were omitted from further analyses). The outcome of interest was time to virologic failure (VF), which we defined as at least 1 VL ≥ 200 copies/mL during the 2-year followup period after the index date (please see Figure 1). Observation terminated at the earliest among the following dates: date of VF, death, loss to follow-up, change in cART regimen, the end of 2 years of observation, or December 31, 2013.
Race/ethnicity was grouped into non-Hispanic/Latino white, non-Hispanic/Latino black or African American (hereinafter referred to as non-Hispanic/Latino black), Hispanic/Latino, and other/unknown. Participants’ HIV transmission risk was categorized based on self-reported behavior at HOPS enrollment. When multiple risk behaviors were reported, we classified risk hierarchically as follows: persons who inject drugs (IDUs); followed by gay, bisexual, and other men who have sex with men (collectively referred to as MSM), heterosexuals; and persons in other/unknown category. Health insurance status was defined based on self-reported type of health care payor closest to the index date. We defined nadir CD4 as the lowest value reported prior to the index date, and baseline VL and CD4 were the values closest to the index date during the preceding 6 months. Regimens were considered cART when meeting standard criteria as described previously.6 We categorized regimens as ≥3 nucleoside reverse transcriptase inhibitors (NRTIs); integrase inhibitor (INT) and NRTIs; nonnucleoside reverse transcriptase inhibitor (NNRTI) and NRTIs; and protease inhibitor (PI), NNRTI, and NRTIs; and PI and NRTIs. Tobacco smoking status was based on self-report at HOPS enrollment. One of the following was used to indicate coinfection with hepatitis C prior to the index date: a diagnosis of hepatitis C, a positive hepatitis C antibody test, a positive hepatitis C viral load, or a positive hepatitis C genotype test
Statistical Analyses
We analyzed observations through December 31, 2013, using data available as of June 30, 2014, to account for any delays in data abstraction and entry. Chi-square tests and 2-sided Wilcoxon tests were used to compare baseline characteristics of frequent VL testers and less frequent VL testers. We performed Kaplan-Meier time-to-event analyses as well as univariate Cox proportional hazard regressions to determine the association between VF during the 2-year follow-up period and the following baseline (at index date) characteristics: age, sex, race/ethnicity, HIV risk category, insurance, CD4, hepatitis C coinfection, and frequency of VL testing in the year immediately following the index date. All characteristics in univariate analyses were included in the multivariable model to adjust for potential confounding. All analyses were performed in SAS 9.3.
Results
We identified 3156 HOPS participants with at least 2 years of viral suppression, of whom 2168 remained on a single cART regimen during that 2-year period and 972 maintained consistent VL testing (defined as at least 3 VLs per year at least 6 months apart). We excluded from the analyses 61 patients whose CD4 fell below 300 cells/mm3 during that 2-year period, and a further 69 patients who did not maintain regular CD4 monitoring (defined as at least 3 CD4 tests per year at least 6 months apart), thus resulting in 842 participants. Of those, 573 had sufficient data to be classified as either frequent VL testers or less frequent VL testers in the year immediately following the 2-year period of viral suppression.
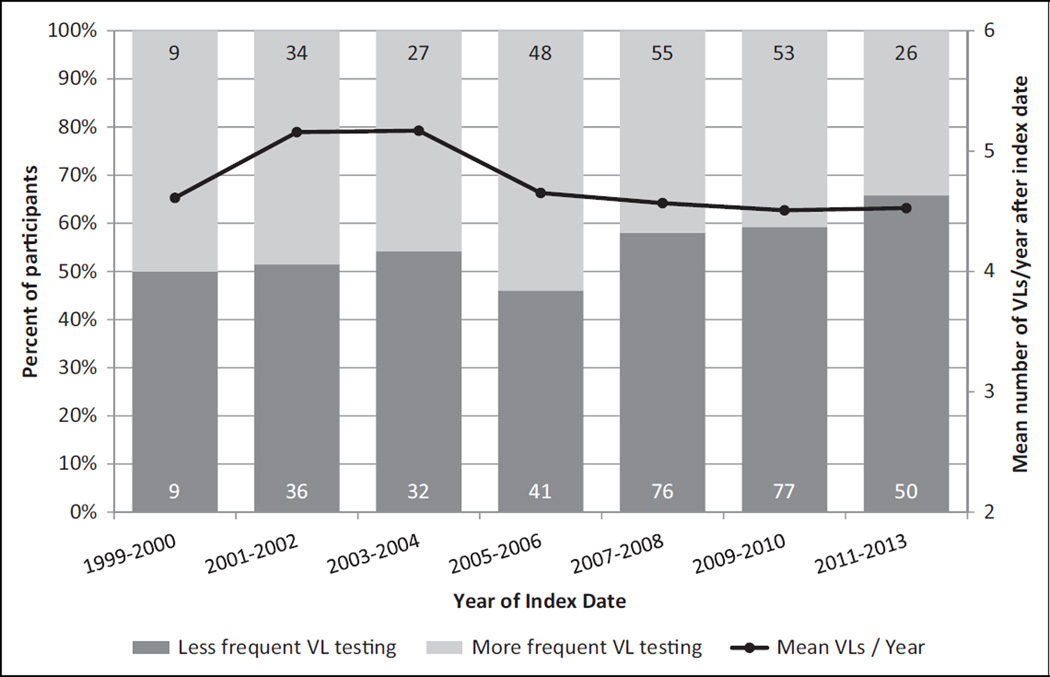
The 573 participants included in this analysis had the following characteristics at the index date: median (interquartile range [IQR]) age 46.1 years (IQR 39.9–53.5), 80.6% male, 62.5% non-Hispanic/Latino white, 66.1% MSM, and median CD4 599 cells/mm3 (IQR 442–809). We classified the main exposure variable for the 573 participants in the analysis as follows: 252 (44.0%) had frequent VL testing performed in the year after the index date, while 321 (56.0%) had less frequent testing performed (Table 1). Those with frequent testing and less frequent testing did not differ significantly by age, sex at birth, race/ethnicity, HIV risk category, AIDS status, nadir or baseline CD4, cART classification, history of smoking, or hepatitis C coinfection. There was a numerical difference in the frequency of private insurance coverage between the 2 groups, although this difference did not reach statistical significance (P = .06). Over the study period, the percentage of patients who underwent ≤2 VL tests during the year following the index date increased (Figure 2), reaching 66% among individuals whose index date was 2011 to 2013. The mean number of VL tests per patient, however, remained similar: 4.6 tests/year in 1999 to 2000 versus 4.5 in 2011 to 2013.
Table 1.
Characteristics of Study Participants by Frequency of Viral Load Testing, the HIV Outpatient Study, 1999 to 2013.a
| Total in analytic cohort | Total 573 |
Frequent VL Testersb 252 |
Less Frequent VL Testers 321 |
P Value | |||
|---|---|---|---|---|---|---|---|
| Median years observed prior to index datec (IQR) | 5.6 (3.2–9.4) | 5.7 (3.1–9.0) | 5.5 (3.3–9.4) | .97 | |||
| Age at index datec, years, n (%) | .10 | ||||||
| <35 | 52 | 9.1 | 19 | 7.5 | 33 | 10.3 | |
| 35–49 | 323 | 56.4 | 138 | 54.8 | 185 | 57.6 | |
| ≥50 | 198 | 34.6 | 95 | 37.7 | 103 | 32.1 | |
| Median age at index date, years (IQR) | 46.1 (39.9–53.5) | 47.4 (40.7–53.9) | 45.4 (39.3–52.7) | .066 | |||
| Sex at birth, n (%) | .37 | ||||||
| Female | 111 | 19.4 | 53 | 21.0 | 58 | 18.1 | |
| Male | 462 | 80.6 | 199 | 79.0 | 263 | 81.9 | |
| Race/ethnicity, n (%) | .35 | ||||||
| Non-Hispanic/Latino white | 358 | 62.5 | 153 | 60.7 | 205 | 63.9 | |
| Non-Hispanic/Latino black | 130 | 22.7 | 54 | 21.4 | 76 | 23.7 | |
| Hispanic/Latino | 62 | 10.8 | 33 | 13.1 | 29 | 9.0 | |
| Other/unknown | 23 | 4.0 | 12 | 4.8 | 11 | 3.4 | |
| HIV risk transmission category, n (%) | .77 | ||||||
| Heterosexual | 113 | 19.7 | 51 | 20.2 | 62 | 19.3 | |
| IDU | 46 | 8.0 | 21 | 8.3 | 25 | 7.8 | |
| MSM | 379 | 66.1 | 162 | 64.3 | 217 | 67.6 | |
| Other/unknown | 35 | 6.1 | 18 | 7.1 | 17 | 5.3 | |
| Insurance, n (%) | .065 | ||||||
| Private | 340 | 59.3 | 135 | 53.6 | 205 | 63.9 | |
| Public | 184 | 32.1 | 90 | 35.7 | 94 | 29.3 | |
| Self-pay/none | 38 | 6.6 | 20 | 7.9 | 18 | 5.6 | |
| Other/unknown | 11 | 1.9 | 7 | 2.8 | 4 | 1.2 | |
| AIDS at index date, n (%) | .97 | ||||||
| No | 262 | 45.7 | 115 | 45.6 | 147 | 45.8 | |
| Yes | 311 | 54.3 | 137 | 54.4 | 174 | 54.2 | |
| Year of first HOPS visit, n (%) | .61 | ||||||
| Before 1997 | 138 | 24.1 | 61 | 24.2 | 77 | 24.0 | |
| 1997–2000 | 158 | 27.6 | 70 | 27.8 | 88 | 27.4 | |
| 2001–2004 | 149 | 26.0 | 73 | 29.0 | 76 | 23.7 | |
| 2005–2012 | 128 | 22.3 | 48 | 19.0 | 80 | 24.9 | |
| Median year of first HOPS visit, (IQR) | 2000 (1997–2004) | 2000 (1997–2003) | 2000 (1997–2004) | .22 | |||
| Nadir CD4 at index date, cells/mm3, n (%) | .40 | ||||||
| <50 | 93 | 16.2 | 42 | 16.7 | 51 | 15.9 | |
| 50–199 | 135 | 23.6 | 66 | 26.2 | 69 | 21.5 | |
| 200–349 | 175 | 30.5 | 71 | 28.2 | 104 | 32.4 | |
| 350+ | 170 | 29.7 | 73 | 29.0 | 97 | 30.2 | |
| Median nadir CD4 at index date, cells/mm3 (IQR) | 242 (109–374) | 237 (107–380) | 251 (111–369) | .61 | |||
| CD4d at index date, cells/mm3, n (%) | .43 | ||||||
| 300–349 | 57 | 9.9 | 30 | 11.9 | 27 | 8.4 | |
| 350–499 | 140 | 24.4 | 58 | 23.0 | 82 | 25.5 | |
| 500+ | 376 | 65.6 | 164 | 65.1 | 212 | 66.0 | |
| Median CD4 at index date, cells/mm3 (IQR) | 599 (442–809) | 589 (416–795) | 612 (451–830) | .28 | |||
| Availability of complete ARV history, n (%) | .26 | ||||||
| No | 124 | 21.6 | 60 | 23.8 | 64 | 19.9 | |
| Yes | 449 | 78.4 | 192 | 76.2 | 257 | 80.1 | |
| ARV start date known, n (%) | .85 | ||||||
| No | 24 | 4.2 | 11 | 4.4 | 13 | 4.0 | |
| Yes | 549 | 95.8 | 241 | 95.6 | 308 | 96.0 | |
| Class of cART regimen at index, n (%) | .34 | ||||||
| ≥3 NRTIs | 22 | 3.8 | 9 | 3.6 | 13 | 4.0 | |
| INT + NRTIs | 18 | 3.1 | 4 | 1.6 | 14 | 4.4 | |
| NNRTI + NRTIs | 304 | 53.1 | 143 | 56.7 | 161 | 50.2 | |
| NNRTI + PI + NRTIs | 11 | 1.9 | 5 | 2.0 | 6 | 1.9 | |
| PI + Enh + NRTIs | 104 | 18.2 | 46 | 18.3 | 58 | 18.1 | |
| PI + NRTIs | 114 | 19.9 | 45 | 17.9 | 69 | 21.5 | |
| Median years since HIV diagnosis (IQR) | 9.6 (5.3–14.6) | 10.1 (5.6–14.3) | 8.9 (5.3–14.8) | .55 | |||
| Median years since AIDS diagnosis (IQR) | 7.1 (4.3–11.2) | 7.1 (3.7–11.2) | 7.0 (4.7–11.2) | .78 | |||
| Median years on cART up to index date (IQR) | 5.6 (3.4–9.2) | 5.6 (3.3–9.0) | 5.6 (3.5–9.4) | .48 | |||
| History of smoking tobacco, n (%) | .33 | ||||||
| No | 191 | 33.3 | 76 | 30.2 | 115 | 35.8 | |
| Yes | 265 | 46.2 | 124 | 49.2 | 141 | 43.9 | |
| Unknown | 117 | 20.4 | 52 | 20.6 | 65 | 20.2 | |
| Hepatitis C coinfection as of index date, n (%) | .20 | ||||||
| Yes | 107 | 18.7 | 53 | 21.0 | 54 | 16.8 | |
| No | 466 | 81.3 | 199 | 79.0 | 267 | 83.2 | |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; cART, combination antiretroviral therapy; Enh, enhancers; IDU, injection drug user; INT, integrase inhibitors; IQR, interquartile range; MSM, gay, bisexual, and other men who have sex with men; NNRTIs, nonnucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; VL, viral load.
N = 573.
Frequent VL testers: patients who had at least 3 viral load tests in the year after index date with at least 6 months between the first and last test. Less frequent VL testers: patients who had 2 viral load laboratory tests in the year after index date.
Index date is the end date of the 2-year lead-in period after which primary exposure (VL testing frequency) is defined and occurrence of viral failure assessed (see Figure 1).
Closest value to index from values documented 6 months prior to 3 months post index date.
Figure 2.
Mean number of viral load tests and the frequency of viral load testing performed in the year after index date, the HIV Outpatient Study (N = 573). VL indicates viral load. Embedded in the bars are numbers of participants represented.
There were 116 (20.2%) participants who experienced VF during the 2-year follow-up period: 53 (21.0%) persons with frequent VL testing compared to 63 (19.6%) persons less frequent VL testing (chi-square P value .71). Frequency of virologic testing during the year after index date was not associated with subsequent VF in univariate (hazard ratio [HR] 1.1, 95% confidence interval [CI] 0.8–1.7) or multivariable Cox proportional hazards regression models (adjusted HR: 1.2, 95% CI: 0.8–1.7; Table 2). Other factors were similarly not associated with time to VF, including age, sex, race/ethnicity, HIV risk category, insurance, baseline CD4, or hepatitis C coinfection in either univariate or multivariable models.
Table 2.
Association of Frequency of Viral Testing with Time to Virologic Failure,a the HIV Outpatient Study, 1999 to 2013.b
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Characteristic | Hazard Ratio | P Value | Hazard Ratio | P Value |
| Age at index date,c years | ||||
| <35 | 1.3 (0.7–2.6) | .43 | 1.3 (0.6–2.6) | .47 |
| 35–49 | 1.4 (0.9–2.2) | .09 | 1.5 (0.9–2.2) | .09 |
| ≥50 | Referent | Referent | ||
| Sex at birth | ||||
| Female | 1.0 (0.6–1.6) | .93 | 1.0 (0.5–1.9) | .90 |
| Male | Referent | Referent | ||
| Race/ethnicity | ||||
| Non-Hispanic/Latino white | Referent | Referent | ||
| Non-Hispanic/Latino black | 1.2 (0.8–1.9) | .35 | 1.2 (0.8, 2.0) | .40 |
| Hispanic/Latino | 0.8 (0.4–1.5) | .52 | 0.8 (0.4–1.5) | .45 |
| Other/unknown | 1.2 (0.5–2.9) | .72 | 0.9 (0.4–2.3) | .85 |
| HIV risk transmission category | ||||
| Heterosexual | 1.0 (0.6–1.6) | .94 | 1.1 (0.5–2.2) | .79 |
| IDU | 0.9 (0.5–1.8) | .80 | 1.0 (0.4–2.4) | .94 |
| MSM | Referent | Referent | ||
| Other/unknown | 1.3 (0.6–2.5) | .51 | 1.3 (0.6–2.8) | .54 |
| Insurance | ||||
| Private | Referent | Referent | ||
| Public | 0.7 (0.5–1.2) | .19 | 0.7 (0.4–1.1) | .14 |
| Self-pay/none | 1.5 (0.8–2.8) | .20 | 1.4 (0.7–2.8) | .29 |
| Other/unknown | 1.2 (0.4–3.8) | .77 | 1.2 (0.4–4.0) | .74 |
| CD4d at index date, cells/mm3 | ||||
| 300–349 | 0.8 (0.4–1.6) | .50 | 0.9 (0.4–1.8) | .70 |
| 350–499 | 1.2 (0.8–1.8) | .46 | 1.3 (0.9–2.0) | .21 |
| 500+ | Referent | Referent | ||
| Hepatitis C coinfection at index date | ||||
| Yes | 1.0 (0.6–1.5) | .89 | 1.1 (0.6–1.8) | .78 |
| No | Referent | Referent | ||
| VL testinge | ||||
| Frequent | 1.1 (0.8–1.7) | .46 | 1.2 (0.8–1.7) | .36 |
| Less frequent | Referent | Referent | ||
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; cART, combination antiretroviral therapy; Enh, enhancers; IDU, injection drug user; INT, integrase inhibitors; IQR, interquartile range; MSM, gay, bisexual, and other men who have sex with men; NNRTIs, nonnucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; VL, viral load.
Virologic failure is the first viral load test ≥200 copies/mL.
N = 573.
Index date is the end date of the 2-year lead-in period after which primary exposure (VL testing frequency) is defined and occurrence of viral failure assessed (see Figure 1).
Closest value to index from values documented 6 months prior to 3 months post index date.
Frequent VL testers: patients who had at least 3 viral load tests in the year after index date with at least 6 months between the first and last test. Less frequent VL testers: patients who had 2 viral load laboratory tests in the year after index date.
Conclusion
In this large convenience sample of immunologically stable and virologically suppressed US HIV-infected persons prescribed cART, less frequent VL testing became more common over time. Having 2 VL tests per year was not associated with an increased rate of VF as compared with having at least 3 viral load tests per year at least 6 months apart. These data are similar to data reported by Romih and colleagues in Croatia involving a smaller cohort of 128 patients initiating cART, in which there was no difference in VL monitoring frequency between patients who did or did not experience VF.7 Another small randomized prospective study found no difference in VF rates among 165 individuals with CD4 ≥250 cells/mm3 and undetectable VLs for more than 12 months who were randomized to receive VL monitoring either every 4 or every 6 months.8
According to the US Center for Medicare and Medicaid Services, in 2014, the average VL test cost was US$116.9. We estimate that reducing testing frequency from 3 to 4 VLs per year to 2 times per year may save between US$116 and US$232 per patient-year. This value does not include the human resource costs in provider counseling time for each test or the costs associated with any morbidity associated with unnecessary phlebotomy.
Studies that examined the effects of reducing CD4 count measurements in persons with CD4 counts stably ≥350 cells/mm3 while virologically suppressed have demonstrated that reduced testing is safe (ie, few patients experience clinically meaningful immunologic declines that could be missed) and could result in large cost savings that could be applied to improve and expand clinical care for HIV-infected persons.10 Savings resulting from decreasing the frequency of CD4 monitoring could be used in other ways to improve clinical outcomes for HIV-infected persons.11 Similarly, decreasing the frequency of VL monitoring among clinically stable and effectively treated patients could result in cost savings that could be reinvested in improving other aspects of patient care.
Strengths of this analysis include its large sample size and duration of follow-up. Our findings are also subject to important limitations, including our inability to examine mortality as that outcome was too rare in our cohort. Although the patients included in our analysis were, in general, demographically well balanced between the 2 groups we compared, there may have been unaccounted for channeling bias (eg, patients perceived to have been more adherent had VL tested less frequently). We also could not assess the extent to which less frequent VL monitoring was an intentional choice on the part of clinicians or may have resulted from patients missing their clinical appointments or patient preference. To this point, we note that patients with private insurance tended to have less frequent viral load testing. It is possible that the lack of difference in VF rates could reflect 2 contrasting clinical scenarios. In one, there are more privately insured patients among the less frequent testers, and private insurance might be a surrogate marker for greater affluence and/or fewer comorbidities. Hence, less frequent VL testing might result from patients and clinicians recognizing that less frequent VL testing is sufficient in the presence of better clinical status or optimal adherence to care and ART. In the second scenario, less frequent VL testing could be the result of missed clinic visits, missed laboratory draws, and overall poorer adherence to ART and care. Further, our study participants were somewhat older than persons in the general US population of HIV-infected adults, and only a small percentage (9.1%) were under the age of 35 years, thus limiting our ability to generalize our conclusions for younger adult patients and precluding inferences to children and adolescents aged <18 years whom HOPS does not include. Finally, HOPS is conducted in well-established urban HIV specialty clinics, and, therefore, aspects of care, engagement in care, and laboratory monitoring may differ from less experienced HIV care environments. Because of these caveats, and because our study population was restricted, by design, to patients who continuously maintained CD4 ≥300 cells/mm3 during observation, we caution clinicians to carefully interpret our findings and allow for individualized VL testing schedules among their patients, which can be adjusted over time, as appropriate.
In summary, our findings from this large observational cohort study support current US recommendations for biannual VL monitoring among clinically stable and virologically suppressed HIV-infected patients.3 In our cohort, VL monitoring at this frequency was not associated with increased rates of VF during a 2-year follow-up period. While our experience may not be generalizable to all clinical care settings, we believe the biannual VL monitoring for the qualifying patients could generate substantial financial cost savings without jeopardizing patient safety, freeing the resources to expand, and improve other aspects of HIV care, such as supportive and ancillary services, including adherence and retention support.
Acknowledgments
BY has received consulting or speaking fees from Bristol-Myers Squibb, Gilead Sciences, Merck & Co., and ViiV Healthcare. BY has received research funding from Gilead Sciences and ViiV Healthcare. FP has received consulting or speaking fees from Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals and Merck & Co.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Centers for Disease Control and Prevention (contract nos. 200-2001-00133, 200-2006-18797 and 200-2011-41872). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Appendix
The HIV Outpatient Study (HOPS) Investigators include the following persons and sites: John T. Brooks, Kate Buchacz, Marcus D. Durham, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, GA; Harlen Hays, Kathleen C. Wood, Darlene Hankerson, Rachel L. D. Hart, Thilakavathy Subramanian, Dana Franklin, and Carl Armon, Cerner Corporation, Vienna, VA; Frank J. Palella, Joan S. Chmiel, Saira Jahangir, and Conor Flaherty, Feinberg School of Medicine, Northwestern University, Chicago, IL; Kenneth A. Lichtenstein and Cheryl Stewart, National Jewish Medical and Research Center, Denver, CO; John Hammer, Kenneth S. Greenberg, Barbara Widick, and Rosa Franklin, Rose Medical Center, Denver, CO; Bienvenido G. Yangco and Kalliope Chagaris, Infectious Disease Research Institute, Tampa, FL; Doug Ward and Troy Thomas, Dupont Circle Physicians Group, Washington, DC; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, and Jane Esteves, State University of New York (SUNY), Stony Brook, NY; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, and Princess Graham, Temple University School of Medicine, Philadelphia, PA; Richard M. Novak, Andrea Wendrow, and Renata Smith, University of Illinois at Chicago, Chicago, IL; Benjamin Young, Mia Scott, and Barbara Widick, APEX Family Medicine, Denver, CO.
Footnotes
These data were presented in part at the 20th International AIDS Conference, July 20–25, 2014, Melbourne, Australia.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FP has received research funding from Gilead Sciences. MS, RLDH, KB, and JTB do not have any associations that may pose a conflict of interest.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG, Gange SJ, Kitahata MM, et al. Trends in multidrug treatment failure and subsequent mortality among antiretroviral therapy-experienced patients with HIV infection in North America. Clin Infect Dis. 2009;49(10):1582–1590. doi: 10.1086/644768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed August 4, 2015]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Web site. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Section. [Google Scholar]
- 4.World Health Organization. Consolidated Guidelines on General HIV Care and the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2013. [Google Scholar]
- 5.Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354. doi: 10.1097/QAD.0b013e328304b38b. [DOI] [PubMed] [Google Scholar]
- 6.Buchacz K, Baker RK, Palella FJ, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24(10):1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 7.Romih V, Židovec Lepej S, Gedike K, Lukas D, Begovac J. Frequency of HIV-1 viral load monitoring of patients initially successfully treated with combination antiretroviral therapy. PLoS ONE. 2010;5(11):e15051. doi: 10.1371/journal.pone.0015051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanasi K, VSeshadri V, Parker D, Dykema S, Hussy J, Weissman S. Randomized-controlled trial of every 4 month versus every 6 months monitoring in HIV-infected patients controlled on highly active antiretroviral therapy. 2nd ID Week Conference (IDWeek 2013); October 2–6, 2013; San Francisco. Abstract 673. [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. Clinical diagnostic laboratory fee schedule. [Accessed August 4, 2015]; Web site. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab. html.
- 10.Hyle EP, Sax PE, Walensky RP. Potential Savings by Reduced CD4 Monitoring in Stable Patients With HIV Receiving Antiretroviral Therapy. JAMA Int Med. 2013;173(18):1746–1748. doi: 10.1001/jamainternmed.2013.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz MH. Directing Resources to where they are the most needed. JAMA Int Med. 2013;173(18):1748. doi: 10.1001/jamainternmed.2013.8590. [DOI] [PubMed] [Google Scholar]