Abstract
Myeloid-derived suppressor cells (MDSC) and Th17 cells were found to expand in collagen-induced arthritis (CIA) significantly. Two subsets of MDSC, polymorphonuclear (PMN) and mononuclear (MO), were detected and their ratios varied during the development of CIA. The depletion of MDSC in vivo resulted in suppression of T-cell proliferation and decreased IL-17A and IL-1β production. The adoptive transfer of MDSC restored the severity of arthritis and Th17 cell differentiation. The depletion of MDSCs on day 35 resulted in arthritis amelioration without reaching a significant difference. Furthermore, MDSCs from CIA mice had higher production of IL-1β and promoted Th17 cell differentiation. The expansion of MDSCs in the peripheral blood of rheumatoid arthritis (RA) patients was in correlation with increased Th17 cells and disease activity DAS28. These results support the hypothesis that MDSC may play a significant proinflammatory role in the pathogenesis of CIA and RA by inducing Th17 development in an IL-1β-dependent manner.
Keywords: Myeloid-derived, suppressor cells, Collagen-induced arthritis, Th17 cells, IL-1β
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease of unknown etiology that affects small diarthrodial joints with chronic inflammation, cartilage destruction, and bone erosions [1]. Recent advances in therapy have dramatically reduced morbidity for RA. However, there is still a significant number of RA patients who are resistant to the current therapy and to further reduction in disability [1,2].
Th17 cell is a CD4+ T-cell subset characterized by the production of inflammatory cytokine IL-17 [3,4], and they have been shown to be involved in many human autoimmune diseases, including RA, psoriasis, and multiple sclerosis [5]. The production of IL-17 has been observed to be elevated in RA murine models as well as in RA patients [6,7]. In collagen-induced arthritis (CIA), IL-17 correlates with joint damage [8]. IL-17-deficient mice are protected from CIA [9], and treatment with a neutralizing IL-17 antibody reduces joint inflammation, cartilage destruction, and bone erosion in CIA [10].
In recent years, myeloid-derived suppressor cells (MDSCs) have attracted considerable attention in the context of tumor because of its suppressive effect in tumor immunity [11,12]. MDSCs were originally described as a heterogeneous population of immature cells with immunosuppressive functions in tumor-bearing hosts. The immunosuppressive mechanisms of MDSC are complex [13]. In mice, MDSCs are characterized by the expression of cell surface makers CD11b and Gr-1. Two functionally subsets have been described, Ly6G+Ly6Clowpolymorphonuclear (PMN) and Ly6G−Ly6Chighmononuclear (MO) cells [14–16]. In humans, MDSCs lack the characteristic markers and have been identified as CD14+HLA-DR−/low [17,18], or Lin−CD11b+CD33+HLA-DR− [19]. In addition to their role in tumor immunology, MDSCs were shown to regulate immune response in animal models of autoimmune diseases [17,20], infectious diseases [21] and trauma [22].
Although the immunosuppressive function of MDSC in tumor has been well established [12], their regulation in autoimmune diseases has not yet been well defined. In experimental autoimmune encephalomyelitis (EAE), MDSCs have been found in some studies to increase dramatically in the spleen and to exert an immunosuppressive role [20,23]. These cells were found to ameliorate demyelination and delay disease onset after in vivo transfer through inhibiting Th1 and Th17 cells [20]. In other studies, MDSCs were found to be recruited into the central nervous system and play a pathogenic role [24,25]. In addition, MDSCs were found to contribute to the pathogenesis of EAE by driving Th17 cells differentiation [26]. In proteoglycan-induced arthritis, MDSCs from synovial fluid of the affected joints, in contrast to splenic MDSCs, limited the expansion of auto-reactive T cells in vitro [27]. However, their specific roles in other experimental models and in RA patients, particularly the in vivo function, remain to be elucidated.
From this brief discussion, it is clear that Th17 cells and MDSCs play significant pathogenic roles in autoimmune disorders, but the relationship between these two types of immune cell remains to be elucidated. In this study, we examined the pathogenic role of MDSC in CIA and their role in the differentiation of Th17 cells.
2. Materials and methods
2.1. Animals
Male DBA/1J mice, 8–10 weeks old, were purchased from SLAC Laboratory Animal Centre (Shanghai, China). Mice were housed under specific pathogen-free conditions. All animal experiments were approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University, and all experiments were carried out in accordance with the National Institute of Health Guide for Care and Use of Animals.
2.2. Induction and assessment of CIA
Lyophilized bovine type II collagen (CII, Chondrex, USA) was dissolved overnight at 4 °C in 0.05 M acetic acid at a concentration of 2 mg/ml. An equal volume of CII was emulsified with complete Freund’s adjuvant (CFA) containing 4 mg/ml of inactivated mycobacterium tuberculosis (Chondrex, USA). The emulsion was prepared and kept on ice before injection. The mice were injected intradermally at the base of the tail with 100 μl of the emulsion containing 100 μg of CII on day 0. On day 21, mice received a booster injection of 100 μg of CII emulsified with incomplete Freund’s adjuvant (IFA).
Mice were monitored by independent examiners who were blinded as to their treatment every other day for signs of arthritis onset and for clinical score. Arthritis was graded on a scale of 0–4 scales as follows: grade 0, no swelling and no erythema; grade 1, slight swelling and erythema; grade 2, moderate swelling and edema; grade 3, severe swelling and pronounced edema; and grade 4, severe swelling and edema with joint rigidity. Each limb was scored independently. The maximum score is 16 for each mouse [28].
2.3. In vivo depletion of MDSC
The in vivo depletion of MDSC was performed either at the onset of arthritis (day 26) or at the peak of arthritis (day 35). Mice were injected intraperitoneally with 200 μg of anti-Gr-1-specific monoclonal antibodies (mAb) or with rat IgG2b as an isotype control (Bioxcell, USA) in 500 μl PBS at a 3-day interval. The depletion of MDSC was confirmed by flow cytometry.
2.4. Preparation of single-cell suspensions
Peripheral blood was obtained by cardiac puncture of mice under anesthesia. Mice were sacrificed by combination of anesthesia and blood loss. Single-cell suspensions were prepared from spleen and draining lymph nodes (DLN). Red blood cells from the spleen preparations were lysed with red blood cell lysing buffer (Sigma, USA).
Blood samples were collected from newly diagnosed RA patients and healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque by density gradient centrifugation within 2 h after sample collection. Fresh synovial tissue samples were obtained from patients with RA or osteoarthritis (OA) who underwent knee replacement surgery. Samples were minced and digested with type I collagenase (Sigma, USA) in serum-free Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA). The cells were filtered through a nylon mesh, washed extensively, and suspended in phosphate buffered saline (PBS). All patients with RA fulfilled the 1987 American College of Rheumatology criteria for the classification of RA [29]. Informed consent was obtained from all patients, and the experimental protocol was approved by the Institutional Review Board of our hospital.
2.5. Adoptive transfer
For adoptive transfer experiments, CD11b+Gr-1+MDSCs (purity > 92%) were sorted from the spleens of CIA mice on day 35. Some of the CD11b+Gr-1+MDSC were sorted from bone marrow. Ten days after MDSC depletion (day 35), CD11b+Gr-1+ cells (5 × 106) were transferred into CIA mice intraperitoneally twice at a 1-week interval.
2.6. Flow cytometry
Splenocytes were stained with FITC-anti-Gr-1, PE-anti-CD11b PE-Cy7-anti-CD11c, PerCP-Cy5.5-anti-CD3 (BD Pharmingen, USA) and APC-anti-Ly6G, PE-Cy7-anti-Ly6C, APC-anti-F4/80, and APC-anti-I-Ab (Biolegend, USA) antibodies. For intracellular cytokines staining, DLN cells and PBMC were stimulated with 50 ng/ml phorbolmyristate acetate (PMA) plus 500 ng/ml ionomycin (both from Sigma, USA) for 5 h in the presence of 10 μg/ml brefeldin A (eBiosciences, USA). Cells were fixed, permeabilized, and stained with PE-anti-IL-17A (BD Pharmingen, USA) after staining with FITC-anti-CD4 antibodies. To analyze the frequency of Foxp3+regulatory T cells (Treg) in DLN, after stained with FITC-anti-CD4 and PE-anti-CD25 (eBioscience, USA) antibodies, cells were fixed, permeabilized, and stained with PE-Cy5-anti-Foxp3 antibody (eBioscience, USA). Data were acquired using Beckman flow cytometer (USA).
2.7. In vitro T-cell response
Mice were euthanized under anesthesia condition on day 35 after the initial immunization. Single DLN cells were prepared and seeded in 24-well plates at a density of 1 × 106/well in complete medium containing RPMI 1640 (Gibco, USA), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM β2-mercaptoethanol, and 10% heat-inactivated FCS (Hyclone, USA), in the presence or absence of CII (50 μg/ml). Anti-CD3/CD28 mAbs were used as non-specific stimulation. For proliferation assays, cells were incubated with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, USA) according to the manufacture’s instructions. Cells were then washed extensively and resuspended in complete medium; 1 × 106 cells were seeded in a 96-well round-bottom plate, cultured at 37 °C in 5% CO2 for 4 days. Proliferation was measured by flow cytometry [30]. For enzyme-linked immunosorbent assay (ELISA) assay, cells were cultured for 3 days. Supernatants were collected and analyzed for IL-1β and IL-17A by ELISA.
2.8. Cell cultures
CD4+CD25−CD62L+ naïve T cells from normal mice and CD11b+Gr-1+MDSC from CIA on day 35 or normal mice were sorted by BD influx (BD Bioscience, USA) (purity > 92%). The 4 × 105 naïve CD4+T cells were cultured with or without 4 × 105 CD11b+Gr-1+MDSCs in a 96-well round-bottom plate in the presence of 5 μg/ml of anti-CD3 and 5 μg/ml of anti-CD28 Abs (both from Biolegend, USA). In some experiments, 300 ng/ml of IL-1 receptor antagonist (IL-1Ra, Prospec, Israel) was added. Cells were incubated at 37 °C in 5% CO2 for 3 days. Supernatants were collected for measurement of IL-17A and IL-1β production by ELISA.
2.9. Histopathology
For histological analysis, mice were euthanized on day 42 or day 49. Limbs were collected and fixed in 10% neutral buffered formalin for 24 h and decalcified in 10% EDTA solution for 4 weeks. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Safranin O-fast green staining was performed to evaluate cartilage integrity. Histopathological changes were separately scored by two independent researchers in a blinded manner as described before [31].
2.10. Cytokine detection
The levels of IL-1β and IL-17A in sera and supernatant were measured by ELISA kits (eBioscience, USA) according to the manufacturer’s instructions.
2.11. Statistical analysis
The Student unpaired t test was used to analyze parametric data and the Mann–Whitney U test was used to analyze clinical and histological CIA scores. The p values <0.05 were considered statistically significant.
3. Results
3.1. MDSCs and Th17 cells were expanded in mice with CIA
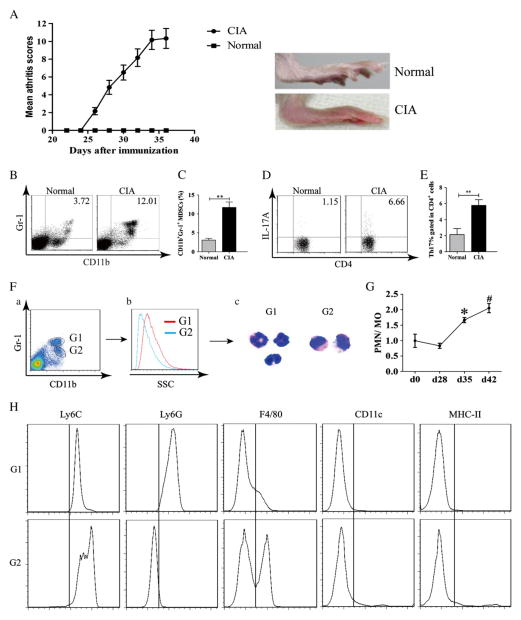
DBA/1J mice were immunized with type II collagen (CII) in CFA on day 0 and received a booster immunization with CII in IFA on day 21. Arthritis appeared on day 26, and the severity of arthritis peaked on day 35 after immunization (Fig. 1A). By day 35 after immunization, significantly more MDSCs were found by flow cytometric analysis to accumulate in spleen of CII-treated mice (Fig. 1B). The data from 6 mice are summarized in Fig. 1C. Similarly, the frequency of Th17 cells in the draining lymph nodes (DLN) was measured by flow cytometry (Fig. 1D). The percentage of Th17 cells was significantly elevated in the DLNs (Fig. 1E).
Figure 1.
CD11b+Gr-1+ MDSCs consist of two major subsets and were expanded with differentiation of Th17 cells in mice with CIA. (A) Mice were immunized with CII (100 μg) on day 0 and day 21, and clinical arthritis scores were recorded. Photograph on the right show a normal hind limb and one affected by CIA. (B–E) Mice were euthanized on day 35. Spleen and DLN were collected and single-cell suspensions were prepared and analyzed. (B) CD11b+Gr-1+ MDSCs in spleen were measured by flow cytometry and one representative experiment is shown. (C) Percentages of MDSC in the spleens of normal mice and those with CIA. (D) Th17 cells identified as IL-17A+ cells in DLN in normal, and CIA mice were measured by flow cytometry and one representative experiment is shown gating on CD4+ cells. (E) The percentages of Th17 cells in CD4+DNLs in normal mice and mice with CIA. (F) CD11b+Gr-1high and CD11b+Gr-1medium cells were sorted by flow cytometry and spun onto a slide and stained with Giemsa. (G) The ratios of CD11b+Gr-1high (G1) and CD11b+Gr-1medium(G2) cells in the spleen in CIA at different time points of CIA development are shown.(H) Two populations of cells as shown in panel F were stained with anti-Ly6C, anti-Ly6G, anti-F4/80, anti-CD11c, and anti-MHC-II mAbs. Data are summarized from 6 mice in each group and shown as mean ± SD. *p < 0.05, compared to day 28; #p < 0.05, compared to day 35; **p < 0.01.
3.2. Characterization of MDSC in CIA
The morphology and lineage surface markers of splenic MDSC were examined at day 35 after the initial immunization. As shown in Fig. 1F, two subsets of MDSCs were identified by flow cytometric analysis. They were characterized by CD11b+Gr-1high and CD11b+Gr-1medium, respectively. Giemsa stain of the sorted cells showed that CD11b+Gr-1high were polymorphonuclear (PMN) and CD11b+Gr-1medium were mononuclear (MO). The ratios of these two subsets varied during the development of arthritis (Fig. 1G). During arthritis progression, the ratios of CD11b+Gr-1high cells to CD11b+Gr-1medium cells increased. CD11b+Gr-1high subset expressed the common neutrophil marker Ly6G, whereas CD11b+Gr-1medium expressed the monocyte/macrophage marker Ly6C andF4/80. However, they are different from mature macrophage and dendritic cells by their low expression of MHC II (I-Ab) and CD11c (Fig. 1H).
3.3. Depletion of MDSC inhibited inflammatory response in mice with CIA
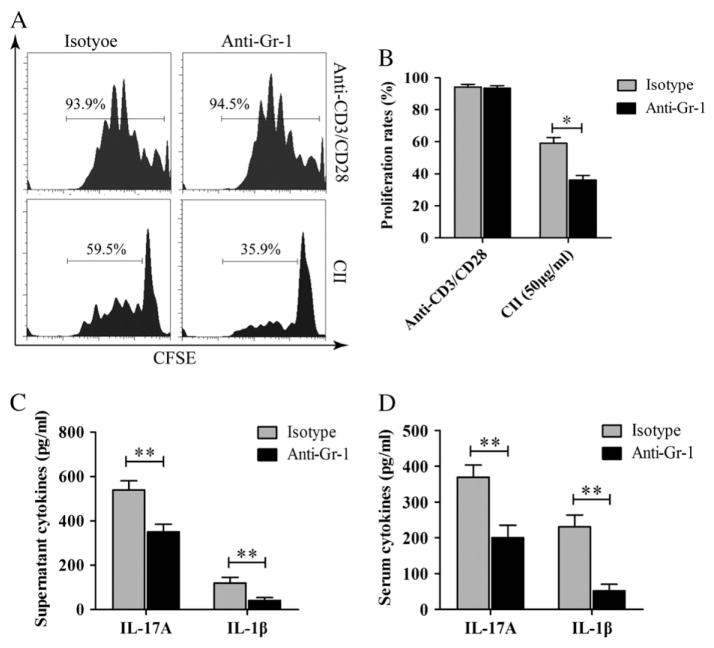
Anti-Gr-1 mAb was used to deplete MDSC in CII-immunized mice on day 26 after the initial immunization. At this time point, most treated mice had arthritis joint scores ≥2. The depletion of MDSC had a marked effect on T-cell responses to CII in the immunized mice as shown in Fig. 2A and B. Both cells isolated from the DLN of anti-Gr1-treated and isotype control Ab-treated mice responded equally well to anti-CD3/anti-CD28 mAbs (the upper panel of Fig. 2A). However, CII-specific T-cell response significantly decreased in anti-Gr-1 mAb-treated mice compared to those treated with isotype control Ab (lower panel of Fig. 2A and B). In addition, DLN cells were cultured in 96-well plate in the presence of CII (50 μg/ml) for 3 days and the secretion of IL-17A and IL-1β in the supernatant was determined. DLN cells from anti-Gr1-treated produced significantly less IL-17A and IL-1β compared to cells from isotype control Ab-treated mice (Fig. 2C). In consistence with the in vitro findings, serum levels of IL-17A and IL-1β were significantly lower in MDSC-depleted mice (Fig. 2D).
Figure 2.
MDSC depletion at the onset of CIA decreased inflammatory response in CIA mice. Mice were euthanized on day 35. DLNs were collected and single-cell suspensions were prepared. (A) DLN cells were labeled with CFSE and cultured in 96-well round-bottom plate in the presence of CII (50 μg/ml) for 4 days. Anti-CD3/CD28 Abs were used as polyclonal stimulation. Proliferation responses were measured by flow cytometry. (B) Cell proliferation rates in response to anti-CD3/anti-CD28 mAbs and to CII by DLN cells from CII-immunized mice treated with either anti-Gr-1 mAb or isotype control Ab were shown. Data from three experiments were presented. (C) DLN cells from CII immunized mice treated with either anti-Gr-1 mAb or isotype control were cultured in 96-well plate in the presence of CII for 3 days. Supernatants were collected, and the concentration of IL-17A and IL-1β was measured by ELISA. Data from three experiments were presented. (D) Sera were collected when mice were sacrificed, and the concentrations of IL-17A and IL-1β were measured by ELISA. Results are summarized from data of 6 mice in each group and shown as mean ± SD; *p < 0.05 and **p < 0.01.
3.4. MDSC depletion at the initiation of arthritis inhibited the development of CII-induced arthritis and the induction of Th17 response
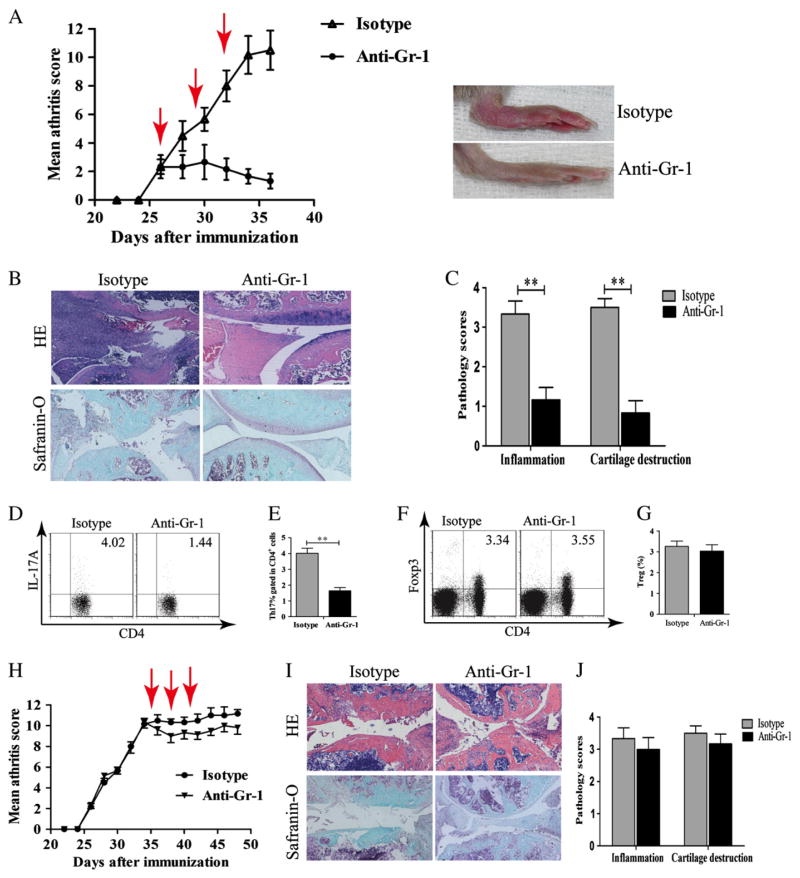
Following the depletion of MDSC, the disease severity score significantly decreased in anti-Gr-1 mAb-treated mice with marked reduction of swelling (Fig. 3A). Histological analysis of the hind paw showed a significant decrease in inflammatory cell infiltration, bone erosion, and cartilage destruction in MDSC-depleted mice (Fig. 3B and C). In addition, MDSC depletion resulted in the down-regulation of Th17 cells response. The frequency of Th17 cells decreased in MDSC-depleted mice (Fig. 3D and E). However, the depletion of MDSC had no effects on the Foxp3+Treg cell population (Fig. 3F and G). When CII-immunized mice were treated with anti-Gr-1 mAb from day 35, clinical scores and pathology severity were slightly ameliorated. However, the amelioration did not reach statistical difference (Fig. 3H–J).
Figure 3.
MDSCs were proinflammatory and regulated Th17 differentiation. (A–G) Anti-Gr-1 Ab treatment was started from day 26 after the initial immunization. (A) Clinical scores and representative images of hind paws in CIA mice treated with anti-Gr-1 mAb or with isotype control Ab. Arrows in red indicate the time when anti-Gr-1 mAb or control Ab was given. (B) For histology assessment, mice were euthanized on day 42. Paraffin-embedded hind paws sections were stained with H&E or Safranin-O/fast green. (C) Pathology scores on hind paws sections. (D–G) Mice were euthanized on day 35. Draining lymph nodes (DLNs) were collected, and single-cell suspension was prepared. (D–E) The frequency of Th17 cells in DLNs was measured by flow cytometry. CD4+ cells were gated. (F–G) Percentages of Treg cells in DLN were measured by flow cytometry. (H–J) Treatments with anti-Gr-1 mAb or isotype control Ab were started on day 35. (H) Clinical scores in CIA mice treated with anti-Gr-1 mAb or isotype control Abs. Arrows in red indicate when anti-Gr-1 mAb or control Ab treatments were given. (I) Histology assessment was carried out on mice euthanized on day 49 after the initial CII immunization. (J) Pathology scores on hind paws sections are shown. Results are summarized from data of 6 mice in each group and shown as mean ± SD. **p < 0.01.
3.5. Adoptive transfer of MDSC in MDSC-depleted mice restored arthritis and Th17 cells response
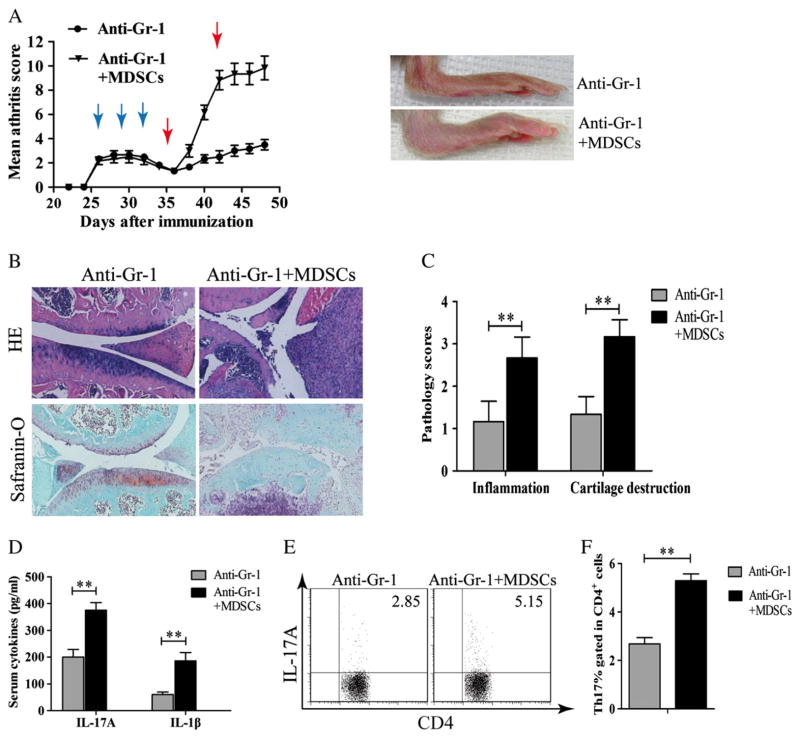
To further investigate the roles of MDSC in CIA, CIA mice were treated with anti-Gr-1 mAb to deplete MDSC. The treated mice were then given MDSCs derived from CIA mice on day 35. The adoptive transfer of MDSC from mice with CIA increased disease severity with joint swelling, cell infiltration, bone erosion, and cartilage destruction (Fig. 4A, B and C). Proinflammatory cytokines IL-17A and IL-1β in the sera significantly increased in MDSC-transferred mice (Fig. 4D). Furthermore, the frequency of Th17 cells increased in DLN in MDSC-transferred mice (Fig. 4E and F).
Figure 4.
The adoptive transfer of MDSCs into CIA mice treated with anti-Gr1-mAb restored arthritis and Th17 cell response. (A–F) Mice were euthanized on day 49. (A) Clinical scores and representative images of hind paws from CIA mice received anti-Gr-1 Abs or anti-Gr-1 Ab followed by MDSC transfer. Blue arrows indicate the time when anti-Gr-1 treatments were given. Red arrows indicate the time when MDSC transfer was carried out. (B) Paraffin-embedded hind paws sections were stained with H&E or Safranin-O/fast green. (C) Pathology scores on hind paws sections of mice treated with anti-Gr1-mAb with or without MDSC transfer. (D) Cytokines of IL-17A and IL-1β were measured by ELISA on sera obtained from mice sacrificed on day 49. (E) The frequencies of Th17 cells in CD4+DLN from CIA mice received either anti-Gr-1 mAb or anti-Gr-1 mAb followed by MDSC transfer were measured by flow cytometry. (F) Percentages of Th17 cells in DLNs gated in CD4+ cells. Results are summarized from data of 6 mice in each group and shown as mean ± SD. **p < 0.01.
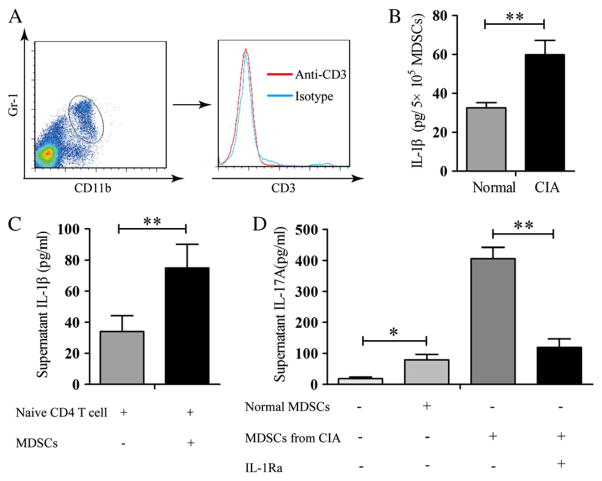
3.6. MDSCs from mice with CIA enhanced IL-17A production by naïve CD4+ T cells stimulated with anti-CD3/anti-CD28 mAbs in an IL-1β-dependent manner
As shown in Fig. 2C, DLN cells from mice immunized with CII produced more IL17A and IL-1β. The relationship between IL-17A and IL-1β was investigated. Sorted MDSCs that were not contaminated with CD3+ T cells (Fig. 5A) from both normal mice and mice with CIA, and the supernatants were assayed for IL-1β. MDSCs from mice with CIA contained significantly more IL-1β (Fig. 5B). Supernatant from naïve T cells stimulated with anti-CD3/anti-CD28 mAbs in the presence of MDSCs from mice with CIA had more IL-1β in comparison with those in the presence of MDSCs from normal mice (Fig. 5C). Naïve CD4+ T cells stimulated with anti-CD3/anti-CD28 mAbs produced little IL-17A (Fig. 5D). In the presence of MDSCs from control mice, there was significant more IL-17A (Fig. 5D). In comparison with the supernatant from stimulated T cells in the presence of MDSC from control mice, more than a 4-fold increase in IL-17A was detected in stimulated T cells in the presence of MDSC from mice with CIA. This increase was inhibited in the presence of IL-1Ra, suggesting that the induction of the Th17 cell response by MDSCs was dependent on IL-1β.
Figure 5.
MDSC promoted Th17 cell differentiation from naïve CD4+ T cells in an IL-1β-dependent manner. (A) Mice were euthanized on day 35. Splenic cells stained with anti-CD11b and anti-Gr-1 mAbs were sorted by flow cytometry. Sorted CD11b+Gr-1+ were stained with anti-CD3 mAb. (B) Cell lysates were prepared from CD11b+Gr-1+ cells isolated from mice with CIA or normal mice. IL-1β concentrations in the lysates were determined by ELISA. (C) Naïve CD4+CD25−CD62L+ T cells from normal mice and CD11b+Gr-1+MDSCs from CIA or normal mice were sorted by flow cytometry. Naïve CD4+T cells were stimulated with anti-CD3/anti-CD28 mAbs either in the presence or in the absence of CD11b+Gr-1+MDSC. IL-1β concentrations in the culture supernatant were determined by ELISA. (D) MDSCs derived from normal or CIA mice were co-cultured with naïve CD4+ T cells in the presence of anti-CD3/CD28 Ab for 3 days with or without IL-1Ra (300 ng/ml). The concentration of IL-17A in supernatant was measured by ELISA. The results represent three independent experiments. Data are shown as mean ± SD. *p < 0.05 and **p < 0.01.
3.7. MDSCs and Th17 cells were both increased in the peripheral blood and synovium of RA patients
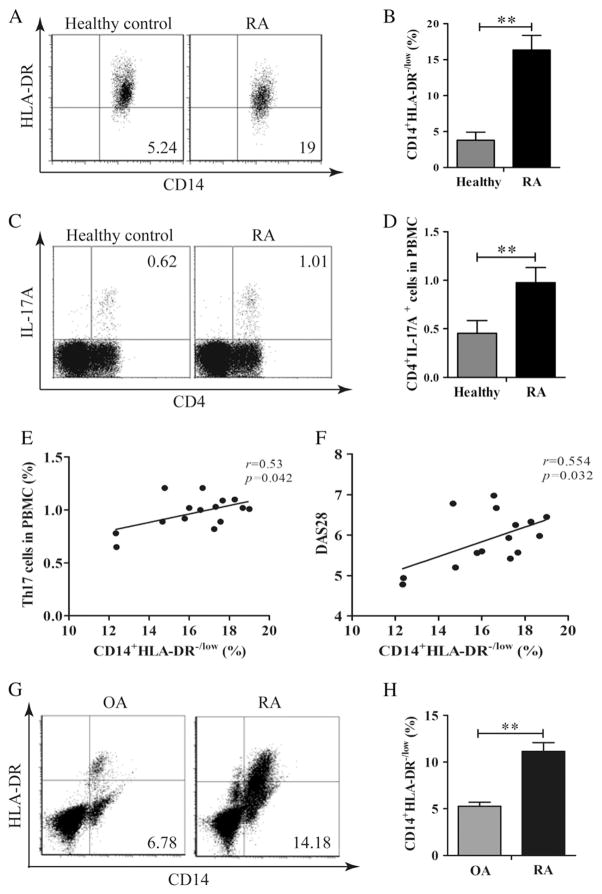
Human MDSC lacks characteristic markers and expresses different markers compared to murine MDSC. CD14+HLA-DR−/low cells in cancer and autoimmune diseases in humans were reported to exert a similar function as murine MDSC [17,18]. The peripheral blood from newly diagnosed RA patients and healthy controls were analyzed. The frequency of CD14+HLA-DR−/low cells in RA patients’ peripheral blood was significantly higher compared to that in healthy controls (Fig. 6A and B). Furthermore, the expansion of Th17 cells in RA patients was noted as shown in Fig. 6C and D. There was a significant correlation between circulating Th17 cells and CD14+HLA-DR−/low cells (Fig. 6E). In addition, there was a significant correlation between circulating CD14+HLA-DR−/low cells and RA disease activity as measured by DAS28 (Fig. 6F). Synovial tissues were obtained from patients with RA and OA, who underwent knee replacement. Single-cell suspension was made by collagenase I digestion. As shown in Fig. 6G and H, the frequency of CD14+HLA-DR−/low cells in the RA synovium was significantly higher compared to that of OA patients. Unfortunately, not enough cells were obtained to permit us to determine the frequency of Th17 cells in RA and OA synovial tissues.
Figure 6.
MDSC and Th17 cells were expanded in rheumatoid arthritis (RA). The frequency of CD14+HLA-DR−/low MDSCs in PBMCs from RA patients (n = 15) and healthy individuals (n = 15) was analyzed by flow cytometry. A representative experiment is shown in panel A. The percentages of HLA-DR−/low cells gated in CD14+ cells in PBMCs in these two populations are shown in panel B. The frequency of Th17 cells in PBMCs from RA patients or healthy individuals was analyzed by flow cytometry. A representative experiment is shown in panel C. The percentages of Th17 cells in PBMCs from RA patients and healthy individuals are shown in panel D. The correlation between MDSCs andTh17 cells in PBMCs from RA patients is shown in panel E. The correlation between MDSCs andDAS28 in RA patients is shown in panel F. Single cells were prepared from the synovial tissues from RA (n = 6) and OA (n = 6) patients. The frequency of CD14+HLA-DR−/low MDSC in these synovial cells was analyzed by flow cytometry. A representative experiment is presented in panel G. Percentages of CD14+HLA-DR−/low cells in the synovial tissues of RA and OA patients are presented in (H). **p < 0.01.
4. Discussion
MDSCs were originally described in tumor bearing mice many years ago [32], but their functional importance in the regulation of immune system has attracted researchers’ interests only in recent years. Accumulated evidence has shown that MDSCs are involved in a wide range of autoimmune diseases [17,19,33,34]. In mouse EAE model, both anti-inflammatory and pro-inflammatory functions of these cells have been reported [20,26]. In the case of CIA, one recent publication provided data suggesting that MDSCs play a suppressive regulatory role [35]. In the present investigation, we have demonstrated that MDSC depletion at the onset of CIA prevented the full development of CIA and the adoptive transfer of MDSCs isolated from mice with CIA restored the arthritis severity of CIA in mice treated with anti-Gr-1 mAb. Evidence has also been obtained to show that the depletion of MDSCs reduced DLN cells’ capacity to mount a CII-specific response and this depletion had no effect on T-cell proliferation induced by ant-CD3/anti-CD28 mAbs. These observations suggested that MDSC play a proinflammatory role in the development of CIA.
In this investigation, the anti-Gr-1 treatment in CII-immunized mice resulted in the decrease of Th17 cells in the DLN and the decrease in serum IL-17A and IL-1β. These decreases were reversed with transfer of MDSCs from mice with CIA. In addition, anti-Gr-1 treatment did not resulted in any changes in the Treg cell population. These results suggest that Th17 cells play a major inflammatory role in CIA and are in agreement with the data published by other investigators [36,37]. These results further implicate a relationship between IL-17A and IL-1β. Our experiments provide additional data to indicate that MDSCs from mice with CIA are a potent source of IL-1β. Incubation of the MDSCs from mice with CIA with naïve T cells further increase the production of IL-1β. In addition, IL-1Ra significantly inhibited the production of IL-17A by naïve T cells that were stimulated with anti-CD3/anti-CD28 mAbs in the presence of MDSCs. These data support the conclusion that the development of Th17 cells in CIA is regulated by IL-1β produced in part by MDSCs.
The relationship between MDSC and Th17 cells was also investigated in RA patients. In humans, MSDC is identified as a CD14+HLA-DR−/low cell population. The percentage of CD14+HLA-DR−/low MDSC was found to be significantly increased in the peripheral blood of newly diagnosed active RA patients compared to normal controls. In the synovium, the percentage of this cell population was much higher in RA in comparison with that from OA patients. In addition, the percentage of CD14+HLA-DR−/low MDSC was correlated with the expansion of Th17 cells and with disease activity, suggesting the involvement of MDSC during early RA development. These results are in agreement with those obtained in the murine CIA model. Overall, the results from this investigation would support the potential of targeting IL-1β and/or IL-17 for RA therapy. Indeed, IL1Ra has been shown to be effective in the treatment of RA patients in a randomized clinical trial [38].
Strikingly, MDSCs from CIA mice have higher production of IL-1β compared to normal mice. This is not unexpected in view of the heterogeneity of the MDSC population. The heterogeneity of the MDSCs is further highlighted by the changes in the ratio between granulocytic and mononuclear subpopulations within MDSC during the course of the development of CIA.
The finding that MDSCs are proinflammatory in CIA in the present investigation differs from those by Fujii et al. [35]. The possibility is that MDSCs isolated on day 35 from the initial immunization was investigated. Our results showed that the depletion of MDSCs late in the disease course did not result in higher arthritis scores or in more severe inflammation or cartilage destruction. However, it is of note that there was mild decrease in disease scores although this decrease did not reach statistical significance. Thus, in our experimental system, MDSCs were proinflammatory. The difference between our results and those of Fujii et al. can best explained by the observation that in their model, there was a recovery phase while in our model such a phase was not observed although both studies utilized DBA/1J mice. Their MDSCs were isolated from the beginning of the recovery phase when inflammation is down-regulated. It is also worthy of noting that MDSCs isolated by Fujii et al. contained more than 97% of CD11b+Ly6G+Ly6Clow polymorphic nuclear MDSCs and that our MDSCs have a large population of CD11b+Ly6G−Ly6Chighmononuclear cells. Noting the differences in the kinetics of CIA development and the differences in the MDSC populations studied, the divergent results are expected. The differences between our results and those of Fujii et al. are likely due to the differences in the animal facilities from which DBA/1J mice were obtained. These differences further underscore the heterogeneity of MDSCs and the importance of studying the functional differences between the polymorphic nuclear and mononuclear subsets within MDSC. It is of note that while this manuscript is being prepared, a paper with similar findings as those reported here was published by Guo et al. [39] on line ahead of publication in Ann Rheum Dis on November 4th, 2014 with CIA induced in C57Bl/6.
In our study, we have not attempted to identify a subset of MDSCs that is capable of blocking the expansion of Th17 expansion in CIA. Since CD11b+Gr-1+ MDSC can be differentiated into two distinct populations, i.e., granulocytic MDSCs that are Ly6G+Ly6Clow and monocytic MDSCs that are Ly6G−Ly6Chigh [14–16], it is tempted to conclude that monocytic MDSCs are pro-inflammatory. However, the heterogeneity within each of these two populations should also be considered. Similarly, the data on RA patients are correlative. It is unwise to conclude that all CD14+HLADR−/low monocytes are pro-inflammatory. From this discussion, it is important to study the functional differences between polymorphonuclear and mononuclear subsets of MDSC to resolve the differences of various studies in EAE and in autoimmune arthritis models regarding the pro-inflammatory and anti-inflammatory nature of MDSCs [20,23–27,35,39]. It may also be necessary to state that the heterogeneity within each population may complicate the interpretation of differing results in various experimental models.
5. Conclusions
In conclusion, we show that MDSCs increase significantly and are proinflammatory in CIA. MDSCs in CIA consist of two major subsets including PMN and MO cells, and the ratios of PMN/MO vary at different disease stages. The depletion of MDSC at early stage of CIA ameliorates joint destruction. Adoptive transferred MDSC into MDSC-depleted CIA mice reverses arthritis. In addition, MDSC in CIA mice promote Th17 cell differentiation, which is dependent on the production of IL-1β. Furthermore, MDSCs expand in PBMCs of RA patients and are correlated with DAS28 and Th17 cells. Understanding the role of MDSC in CIA and the mechanism involved may provide a potential treatment target for RA.
Acknowledgments
This work was supported by grants from the Guangzhou Science and Technology Planning Program (2012J4100085), the National Natural Science Foundation of China (81273278 and 81471598), the PhD Program Foundation of Ministry of Education of China (20120171110064), and the Guangdong Natural Science Foundation (S2012010008780, and S2011010004578). FG and SMF were supported by grants from the National Institutes of Health (R01-AR047988 and R01-AR049449) and a grant from the Alliance for Lupus Research.
Abbreviations
- MDSC
myeloid-derived suppressor cell
- CIA
collagen-induced arthritis
- RA
rheumatoid arthritis
- CII
type II collagen
- DAS28
disease activity score in 28 joints
- EAE
experimental autoimmune encephalomyelitis
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- DLN
draining lymph nodes
- PBMCs
peripheral blood mononuclear cells
- OA
osteoarthritis
- PMN
polymorphonuclear
- MO
mononuclear
Footnotes
Conflicts
All the authors have no interests to declare.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Sokka T, Kautiainen H, Pincus T, Verstappen SM, Aggarwal A, Alten R, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. 2010;12(2):R42. doi: 10.1186/ar2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28(4):445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar S, Cooney LA, White P, Dunlop DB, Endres J, Jorns JM, et al. Regulation of pathogenic IL-17 responses in collagen-induced arthritis: roles of endogenous interferon-gamma and IL-4. Arthritis Res Ther. 2009;11(5):R158. doi: 10.1186/ar2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164(5):2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54(4):1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 9.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 10.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. 81 e1–5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 19.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188(3):1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 21.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176(4):2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, et al. CD11b + Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179(8):5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 24.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113(14):3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, et al. CCR2 + Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain J Neurol. 2009;132(Pt 9):2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 26.Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b + Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189(9):4295–4304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egelston C, Kurko J, Besenyei T, Tryniszewska B, Rauch TA, Glant TT, et al. Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 2012;64(10):3179–3188. doi: 10.1002/art.34494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Rey E, Chorny A, Varela N, O’Valle F, Delgado M. Therapeutic effect of urocortin on collagen-induced arthritis by down-regulation of inflammatory and Th1 responses and induction of regulatory T cells. Arthritis Rheum. 2007;56(2):531–543. doi: 10.1002/art.22394. [DOI] [PubMed] [Google Scholar]
- 29.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 30.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 31.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11(9):936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 32.Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987;47(1):100–105. [PubMed] [Google Scholar]
- 33.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1 + CD11b + myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286(26):23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31(4):354–361. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Fujii W, Ashihara E, Hirai H, Nagahara H, Kajitani N, Fujioka K, et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol. 2013;191(3):1073–1081. doi: 10.4049/jimmunol.1203535. [DOI] [PubMed] [Google Scholar]
- 36.Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62(10):2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 37.Pernis AB. Th17 cells in rheumatoid arthritis and systemic lupus erythematosus. J Intern Med. 2009;265(6):644–652. doi: 10.1111/j.1365-2796.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 38.Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41(12):2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Guo C, Hu F, Yi H, Feng Z, Li C, Shi L, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205508. http://dx.doi.org/10.1136/annrheumdis-2014-205508 (Epub ahead of print) [DOI] [PMC free article] [PubMed]