Abstract
Zuojin Pill (ZJP), a traditional Chinese medicine formula, consists of Coptis chinensis Franch. and Evodia rutaecarpa (Juss.) Benth. in a ratio of 6:1 (w/w) and was first recorded in “Danxi’s experiential therapy” for treating gastrointestinal disorders in the 15th century. However, the poor solubility of alkaloids from ZJP restricted the protective effect in treating gastritis and gastric ulcer. The aim of the study was to investigate the protective mechanism of mucoadhesive microspheres loaded with alkaloids from C. chinensis Franch. and E. rutaecarpa (Juss.) Benth. on ethanol-induced acute gastric mucosal injury in rats. Surface morphology, particle size, drug loading, encapsulation efficiency, in vitro drug release, mucoadhesiveness, and fluorescent imaging of the microspheres in gastrointestinal tract were studied. The results showed that the mucoadhesive microspheres loaded with alkaloids could sustain the release of drugs beyond 12 hours and had gastric mucoadhesive property with 82.63% retention rate in vitro. The fluorescence tracer indicated high retention of mucoadhesive microspheres within 12 hours in vivo. The mucoadhesive microspheres loaded with alkaloids could reduce the gastric injury by decreasing the mucosal lesion index, increasing the percentage of inhibition and increasing the amount of mucus in the gastric mucosa in an ethanol-induced gastric mucosal injury rat model. Moreover, the mucoadhesive microspheres loaded with alkaloids reduce the inflammatory response by decreasing the levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), downregulating the mRNA expression of inducible nitric oxide synthase, TNF-α, and IL-1β in gastric mucosa. All the results indicate that mucoadhesive microspheres loaded with alkaloids could not only increase the residence time of alkaloids in rat stomach, but also exert gastroprotective effects through reducing the inflammatory response on ethanol-induced gastric mucosal damage. Thus, these microspheres could be developed as a potential controlled release drug for treatment of gastric ulcer.
Keywords: alkaloids, mucoadhesive microspheres, alginate, chitosan, anti-inflammatory, gastric mucosal injury
Introduction
Peptic ulcer disease, a common disease among the gastrointestinal disorders, occurs in any part of the digestive tract, especially in the stomach and proximal duodenum. Gastric ulcer is one of the most common peptic ulcers and could develop with the disbalance of some endogenous aggressive factors (gastric acid, pepsin, leukotrienes) and gastroprotective factors, such as mucus, bicarbonate, prostaglandins, mucosal blood flow, and some growth factors.1 Gastric ulcer is a multifactorial disease, and various factors such as bacterial infection with Helicobacter pylori and overingestion of alcohol and nonsteroidal anti-inflammatory drugs may lead to the gastric injury.2 Gastric ulcer could infringe on the submucosa, and therapeutic drugs are required to be able to penetrate the mucous layer and maintain high concentration in the stomach for better treatment. The conventional dosage forms, such as tablet, showed weak adhesion to the mucous surface because of its weight and rapid emptying from the stomach, thus restricting its use in the clinical treatment of gastric diseases. Mucoadhesive drug delivery systems, due to their ability to contact with the absorbing mucosa, could prolong residence time of drugs and improve the drug absorption and bioavailability in partial gastric mucosa, and microspheres which consist of one or more adhesive materials were chosen to treat gastric injury as they are lightweight and have smaller dose variation.3
Chitosan, a naturally derived polysaccharide, has been widely applied in biomedical fields as scaffolds for tissue engineering,4 wound dressing,5 and drug delivery systems.6 Alginate, another naturally occurring polysaccharide derived from the marine brown algae,7 shows a wide range of biomedical applications especially in drug delivery, cell immobilization, and tissue regeneration because of its outstanding properties such as biodegradability, biocompatibility, and chelating ability.8 Moreover, chitosan can form polyelectrolyte with alginate by electrostatic interactions, which could not only make up for the deficiency of alginate hydrogels but also improve the release performance and reinforce the bioadhesive property.9 Sodium alginate and chitosan are both marine-derived polysaccharides and offer many advantages over synthetic polymers since they interact under relatively mild temperature and pH. So, they have been chosen as the mucoadhesive materials as well as sustained delivery carriers.
Gastric ulcer is a complex pluricausal inflammatory disease and is known to develop due to the disruption of mucosal defense.10 In the inflammatory process, a number of inflammatory cytokines, chemokines, and other mediators, such as nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin 1β (IL-1β) are involved in the immune response.11,12 NO is a major product which is controlled by nitric oxide synthases (NOSs), such as inducible NOS (iNOS), endothelial NOS, and neuronal NOS.13 Most importantly, highly expressed iNOS could lead to organ destruction in some inflammatory and autoimmune diseases.14 NO could lead to oxidative burst,15 which would inflict endothelial damage.16 Moreover, the enhanced generation of NO by iNOS may contribute to the pathogenesis of various gastroduodenal disorders including peptic ulcer.17 As many enzymes and free radicals damage the gastric mucosa, these are believed to contribute to ulcer formation. Prostaglandin E2 (PGE2) is also another important mediator which is produced from arachidonic acid metabolites catalyzed by cyclooxygenase (COX)-2 in inflammatory responses.18 Current evidence suggests that nonsteroidal anti-inflammatory drugs exert their impairing effects on ulcer healing through the inhibition of COX isoforms, such as COX-2-selective inhibitors,19 thus supporting a significant involvement of COX-2 in ulcer repair. Moreover, PGE2 in ulcer tissue specimens has been reported to play a role in ulcer repair.20
Zuojin Pill (ZJP), a typical traditional Chinese medicine formula, consists of Rhizoma Coptidis (Coptis chinensis Franch. was used in the study) and Fructus Evodiae (Evodia rutaecarpa (Juss.) Benth. was used in the study) in the ratio of 6:1 (w/w). ZJP was first recorded in “Danxi’s Experiential Therapy” for treating gastrointestinal disorders in the 15th century. It is officially listed in the Chinese Pharmacopoeia as a prescription employed in patients suffering from gastric ulcer, gastroesophageal reflux disease, gastritis, and pyloric obstruction, among other disorders.21 Our previous study showed that alkaloids from C. chinensis Franch. and E. rutaecarpa (Juss.) Benth. could exert an anti-inflammatory effect by the inhibition of iNOS, COX-2, IL-6, IL-1β, and TNF-α expression through preventing the nuclear translocation of the NF-κB p50 and p65 subunits in RAW 264.7 cells,22 which provided evidence to understand the therapeutic effects of ZJP on gastritis, gastric ulcer, and other inflammatory diseases in clinic. Moreover, our results showed that alkaloids from C. chinensis Franch. and E. rutaecarpa (Juss.) Benth. produced an antidepressant-like effect via the central monoaminergic neurotransmitter system and 5-HT.23 Although ZJP was used for treating gastrointestinal disorders in the 15th century, the poor solubility of the alkaloids from the two herbs in ZJP restricted the protective effect of ZJP in treating gastritis and gastric ulcer. Furthermore, the protective mechanism of the alkaloids from ZJP on gastritis and gastric ulcer has not been fully clarified in vivo.
In this study, total alkaloids extracted from C. chinensis Franch. and E. rutaecarpa (Juss.) Benth. were loaded into the mucoadhesive microspheres to increase the residence time of alkaloids in stomach. The protective mechanism of microspheres loaded with alkaloids on ethanol-induced acute gastric mucosal injury was investigated in rats.
Materials and methods
Materials and animals
Sodium alginate, chitosan (with 90% deacetylation degree), and fluorescein isothiocyanate (FITC) were obtained from Sangon Biotech, Co., Ltd. (Shanghai, People’s Republic of China). Liquid paraffin was supplied by Tianjin Beifang Tianyi Chemical Reagent Company (Tianjin, People’s Republic of China). Span-80 and Tween-80 were purchased from Sigma-Aldrich (St Louis, MO, USA). Micronized CaCO3 (40 nm) was provided by Beijing Wangyong Technology Co., Ltd. (Beijing, People’s Republic of China). The rhizomes of C. chinensis Franch. and the dry ripe fruit of E. rutaecarpa (Juss.) Benth. were obtained from Baokang Hospital of Tianjin University of Traditional Chinese Medicine (Tianjin, People’s Republic of China) and authenticated by Prof Yuan-Lu Cui at Tianjin University of Traditional Chinese Medicine according to the Chinese Pharmacopoeia (2010 edition). The standards of berberine chloride, evodiamine, and rutaecarpine with purity of over 98% were all purchased from the National Institute for Control of Pharmaceutical and Biological Products (Beijing, People’s Republic of China). Epiberberine (98%) and coptisine (98%) were supplied by Chengdu Must-Technology Co., Ltd. (Chengdu, People’s Republic of China). Jatrorrhizine (98%) and palmatine (98%) were provided by Phytomarker Company (Tianjin, People’s Republic of China). Omeprazole capsules were obtained from Ruikang pharmaceutical Co., Ltd. (Beijing, People’s Republic of China). Methanol, ethanol, and acetonitrile of HPLC (high-performance liquid chromatography) grade were supplied by Tianjin Concord Technology Co., Ltd. (Tianjin, People’s Republic of China). Phosphoric acid and triethylamine of analytical grade were obtained from Tianjin Chemical Reagent Company (Tianjin, People’s Republic of China). TNF-α and IL-1β rat ELISA Kit were obtained from eBioscience (San Diego, CA, USA). Prostaglandin E2 Parameter Assay Kit was obtained from R&D Systems (Minneapolis, MN, USA). BCA protein assay kit was obtained from Thermo (San Jose, CA, USA). Mammalian cell lysis kit and UNIQ-10 column Trizol total RNA extraction kit were bought from Sangon Biotech. Improm-II Reverse Transcription System was purchased from Promega Corporation (Madison, WI, USA). FastStart Universal SYBR Green Master (ROX) kit was purchased from Roche (Mannheim, Germany).
Male Sprague-Dawley (SD) rats (weighing 200–220 g) were obtained from Beijing Vital River Laboratories Animal Technology Co., Ltd. (Beijing, People’s Republic of China). Male ICR mice (weighing 18–22 g) were purchased from Beijing HuaFuKang Bio-Technology Co., Ltd. (Beijing, People’s Republic of China). The animals were kept under controlled light (12 hours light/12 hours dark, lights on at 7 am). Ambient temperature and relative humidity were maintained at 24°C±1°C and 55%±5%, respectively. Animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Laboratory Animals, and the protocol was approved by the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (TCM-2009-037-E15).
Total alkaloids extracted from C. chinensis and E. rutaecarpa and quantitative analysis of seven alkaloids
Preparation and quantitative analysis of seven alkaloids from C. chinensis and E. rutaecarpa have been published in our previous paper.22
Preparation of mucoadhesive microspheres loaded with alkaloids
Alginate–chitosan microspheres loaded with alkaloids were prepared by internal gelation of alginate with ion (Ca2+) and coating chitosan outside. Sodium alginate solution (1.5%, w/v) was dissolved in deionized water with magnetic stirring for 6 hours at 45°C. Nano-CaCO3 (1%, w/v) and alkaloids (0.15 g) were added into 20 mL sodium alginate solution in turn by a homogenizer, the mixture was dropped into 100 mL paraffin oil containing 1% of Span-80 to form uniform-sized droplets. After 15 minutes emulsification, 0.5 mL glacial acetic (dispersed in 5 mL paraffin oil) was added, which led to dissolution of insoluble CaCO3 to release free Ca2+, and the gelation process was continued for 15 minutes with stirring at 300 rpm. The calcium alginate gel microspheres were collected and washed three times with 1% (volume fraction) Tween 80 solution and two times with deionized water by centrifugation (1,000 rpm, 3 minutes, 25°C), followed by a membrane forming step where the particles were dispersed in a 1.0% (w/v) chitosan solution (pH 5.5) and allowed to react for 10 minutes under magnetic stirring. Finally, the alginate–chitosan microspheres loaded with alkaloids were washed three times with deionized water and lyophilized for 24 hours.
Characterization of the microspheres
Morphological examination
The surface morphology of alginate–chitosan microspheres loaded with alkaloids was observed using an optical microscope (Nikon Ti-U inverted biological microscope, Tokyo, Japan). Environmental scanning electron microscopy (ZEISS EVOLS-15, Jena, Germany) was used to compare the surface characteristics of dry microspheres.
Particle size measurement
The average size of microspheres and size distribution curves were determined by using a particle-size analyzer. Dry microspheres (4 mg) were suspended in distilled water and ultrasonicated for 10 seconds. A drop of suspension was placed on a clean glass slide, and the microspheres were counted under an optical microscope. A minimum of 200 microspheres was counted per batch.
Differential scanning calorimetry analysis
Differential scanning calorimetry (DSC) curves were recorded using a DSC7 differential scanning calorimeter (Perkin-Elmer, Norwalk, CT, USA). The samples of sodium alginate, chitosan, chitosan and alginate physical mixture, and chitosan-coated alginate microspheres were freeze-dried. Lyophilized samples (5–8 mg) were heated from 30°C to 350°C at a heating rate of 10°C/minute under constant purging of nitrogen at 20 mL/minute. An empty cup was used as reference and thermograms were then obtained.
Fourier transform infrared spectroscopy
The Fourier transform infrared spectroscopy (FTIR) transmission spectra were recorded using a FTS 3000 spectrophotometer (Bio-Rad, Richmond, CA, USA) in the wavenumber range of 4,000–400 cm−1 using KBr pellets. The samples were freeze-dried, and a total of 2% (w/w) of samples were mixed with potassium bromide. The mixtures were ground into powders, and disks were compressed for scanning. Each sample was assessed in triplicate at a minimum.
Drug release study of mucoadhesive microspheres loaded with alkaloids in vitro
The release behaviors of seven alkaloids loaded in microspheres were investigated in the simulated gastric fluid (hydrochloric acid–sodium chloride solution containing pepsin at pH 1.2±0.1) as described by Anal and Stevens.9 The detailed experiment was carried out according to Chinese Pharmacopoeia (2010). Alginate–chitosan microspheres loaded with alkaloids (0.7 g) were placed in 200 mL of dissolution medium with magnetic stirring at 50 rpm and 37°C±0.5°C. At scheduled time intervals, 1 mL of sample solution was withdrawn and filtered through a 0.22 μm pore size Millipore filter, and then an equal volume of fresh dissolution medium was added. The samples were diluted suitably and analyzed by HPLC to calculate the accumulated release percentage. Determination of alkaloid content in microspheres was performed by HPLC method as mentioned in the previous study.22
Evaluating the mucoadhesive properties of microspheres loaded with alkaloids in vitro and in vivo
The mucoadhesive properties of the microspheres in vitro were assessed according to the method designed by Akiyama et al24 with slight modifications. Briefly, the mucoadhesive properties were analyzed by determination of the amount of microspheres sticking to mucosa after being rinsed using the rat’s stomach in vitro. Three SD rats were fasted overnight with free access to water and dissected after sacrifice. The stomachs were removed and opened along the greater curvature, then rinsed thoroughly with normal saline and fixed on the microscopic slide. A certain amount of microspheres (10 mg) were scattered uniformly on the surface of stomach mucosa. Then, the stomach mucosa with microspheres was put in an airtight container and maintained at a certain relative humidity and room temperature. The mucosa was taken out and fixed on a plastic foam support at an angle of 45° after 20 minutes. The stomach tissue was rinsed with pH 1.2 simulated gastric fluids for 5 minutes at a rate of 6 mL/minute. The microspheres adhering on the gastric mucosa were weighed and the percentage of remaining microspheres calculated.
FITC-labeled chitosan-coated microspheres were prepared to evaluate the mucoadhesive properties of microspheres loaded with alkaloids in vivo. Fifteen ICR mice were divided into five groups randomly and fasted overnight with free access to water. Mice were orally administrated microspheres coated with FITC-labeled chitosan (6 mg/20 g) and observed at 0, 2, 4, 8, and 12 hours. Mice were anesthetized by intraperitoneal injection of chloral hydrate before the test and sacrificed at scheduled time points, then the stomach, duodenum, and jejunum were observed with a Kodak in vivo Imaging System.
Gastroprotective study in rats
Ethanol-induced gastric mucosal injury and administration in rats
The SD rats were deprived of food, but had free access to water for 24 hours before ulcer induction. Gastric mucosal lesions were induced with absolute ethanol at a dose of 5 mL/kg by oral administration. Rats were divided into six groups randomly: normal control (normal saline), model group (ethanol), omeprazole (20 mg/kg) + ethanol, microspheres (0.02 mg/kg) + ethanol, microspheres (0.05 mg/kg) + ethanol, and microspheres (0.18 mg/kg) + ethanol. Microspheres were suspended in normal saline. All animals were pretreated with omeprazole or microspheres orally once a day for 3 days and were fasted overnight with free access to water until experiment. Two hours after the last administration, rats were gavaged with ethanol. The rats were sacrificed and their stomachs were immediately removed after 4 hours.
Determination of mucosal lesion index and percentage inhibition
The rats’ stomachs were opened along the greater curvature and rinsed thoroughly with normal saline and then fixed to determine the mucosal lesion index (MLI) and percentage inhibition. An observer who was unfamiliar with the treatment regimen performed this operation. MLI and percentage inhibition were calculated according to Liu et al’s25 study and scored as follows: if hemorrhagic erosion <1 mm, three small dot hemorrhages (score 1 each); calculated by multiplying the length (mm) and width (mm) when hemorrhagic erosion >1 mm (score 1 every mm2), the sum of the MLI for each animal was calculated as mean mucosal lesions score. The percentage of inhibition (%) was calculated according to the following formula:26
Measurement of mucus production
The gastric mucosa of each rat was gently scraped using a glass slide and any extra moisture was removed using a filter paper. The collected mucus was weighed by precision electronic balance.27
Histopathology
For pathological examinations, all gastric tissues were fixed in 10% buffered formalin solution followed by dehydration in a graded series of alcohols and xylene, and then immersed in paraffin. The pathological sections were sliced along the longitudinal axis, and 5 μm thick sections were prepared and stained with hematoxylin–eosin by standard procedure for histological examination. The stained sections of each test sample were examined using light microscopy (OLYMPUS, Tokyo, Japan).
Effects of mucoadhesive microspheres loaded with alkaloids on inflammatory response in ethanol-induced acute gastric mucosal injury
Following the macroscopic analysis, production of PGE2, TNF-α, and IL-1β as well as the mRNA expression of iNOS, TNF-α, and IL-1β were determined in rat stomach tissues.
Production of PGE2, TNF-α, and IL-1β in gastric mucosa
Production of PGE2, TNF-α, and IL-1β in gastric mucosa were detected by enzyme-linked immunosorbent assay. Gastric mucosal homogenates were prepared by treating mixed mucosal tissues with lysis buffer at a ratio of 1:10 (mg/μL), and these were then homogenized on ice using a homogenizer and incubated for 30 minutes on ice. Then, the samples were centrifuged at 12,000 rpm at 4°C for 20 minutes and the supernatants were collected for biochemical analysis. The protein concentrations of the homogenized mucosa solutions were determined using a BCA protein assay kit according to the manufacturer’s instruction. Samples were analyzed and the concentrations of PGE2, TNF-α, and IL-1β were corrected as picograms per milligram protein.
Quantitative real-time RT-PCR for the mRNA expression of iNOS, TNF-α, and IL-1β
Total RNA was isolated using a Sangon UNIQ-10 column Trizol total RNA extraction kit according to the instructions of the manufacturer. RNA was reversely transcribed using an ImProm-II Reverse Transcription System cDNA synthesis kit (Promega Corporation, Madison, WI, USA). The real-time reverse transcription polymerase chain reaction (RT-PCR) oligonucleotide primers used for rat iNOS, TNF-α, and IL-1β are shown in Table 1. The reactions were set up in duplicates in 25 μL total volumes with 1 μL of each primer (0.3 μM final concentrations), 12.5 μL of FastStart Universal SYBR Green Master (ROX) (Roche), and 1 μL of template. The PCR cycle was as follows: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, 60°C for 1 minute, and a melt curve analysis was performed at the end of each experiment to verify that a single product per primer pair was amplified. The amplification and analysis were performed using an ABI Prism 7,500 real-time PCR system. Samples were analyzed using the relative CT method. The fold increase or decrease was determined relative to a blank control after normalizing to a housekeeping gene using 2−ΔΔCT.28,29
Table 1.
Real-time RT-PCR oligonucleotide primers
| Gene | Primer | Sequence (5′–3′) | PCR product (bp) |
|---|---|---|---|
| GAPDH (NM_017008.4) | Forward | AGACAGCCGCATCTTCTTGT | 142 |
| Reverse | TGATGGCAACAATGTCCACT | ||
| iNOS (NM_012611.3) | Forward | CACCTTGGAGTTCACCCAGT | 170 |
| Reverse | ACCACTCGTACTTGGGATGC | ||
| TNF-α (NM_012675.3) | Forward | TGACCCCCATTACTCTGACC | 142 |
| Reverse | CGTGTGTTTCTGAGCATCGT | ||
| IL-1β (NM_031512.2) | Forward | AAAAATGCCTCGTGCTGTCT | 127 |
| Reverse | GGGATTTTGTCGTTGCTTGT |
Abbreviations: TNF-α, tumor necrosis factor-α; IL-1β, interleukin 1β; iNOS, inducible nitric oxide synthase; RT-PCR, reverse transcription polymerase chain reaction.
Statistical analysis
All values are represented as mean ± standard deviation excluding results given in gastroprotective study in rats that were expressed with mean ± standard error. Statistically significant differences between groups were determined by one-way analysis of variance followed by Scheffe’s multiple range test. The criterion for statistical significance was P<0.01 or P<0.05.
Results
Characterization of alginate–chitosan microspheres loaded with alkaloids
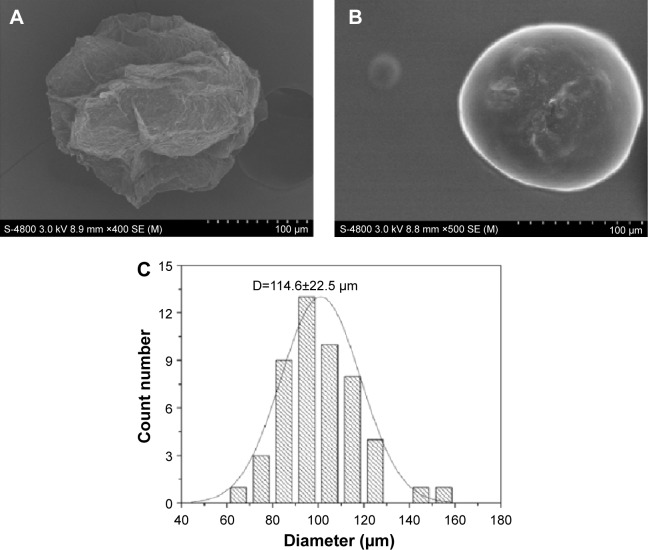
As shown in Figure 1, the scanning electron microphotographs showed that the surface of microsphere after freeze-drying became rough and had many wrinkles. The particle size of the microspheres was approximately 114 μm, indicating that microspheres showed a narrow size distribution (Figure 1C), which would improve the adhesion and mobility in vivo.
Figure 1.
Optical micrographs and SEM micrographs of alginate–chitosan microspheres: (A) freeze-dried microsphere; (B) microsphere loaded with alkaloids; (C) particle size distribution of alkaloid-loaded microspheres.
Abbreviation: SEM, scanning electron microscope.
The DSC characteristics of (a) alginate, (b) chitosan, (c) physical mixture of chitosan and sodium alginate, and (d) chitosan-coated alginate microspheres were recorded and are shown in Figure 2. The DSC curve of alginate showed a sharp exothermic peak at 246.4°C which was attributed to rupture of polymer framework, and stood for the alginate degradation temperature (Figure 2a). The thermogram of chitosan exhibited an exothermic peak at approximately 306.12°C, which indicated that the chitosan was degraded at this temperature (Figure 2b). From the DSC scanning curve of the physical mixture of chitosan and sodium alginate, we found that it was the simple sum of the two polymers’ absorption peaks (Figure 2c). However, the characteristic peaks of the two polymers disappeared and new peaks emerged in the thermogram of chitosan-coated alginate microspheres, which indicated the interaction between chitosan and alginate (Figure 2d).
Figure 2.
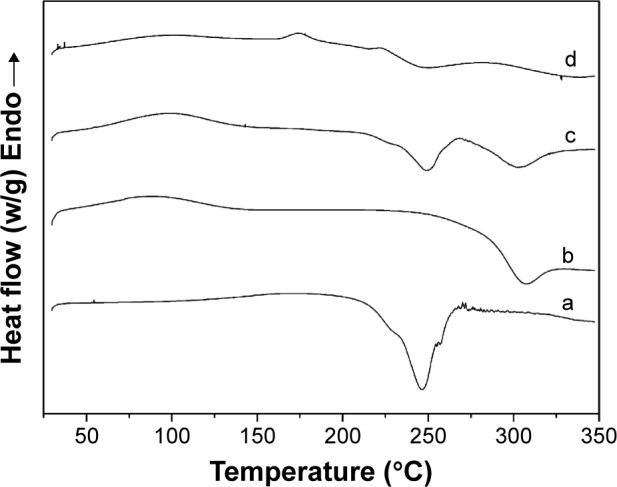
DSC thermograms of (a) sodium alginate, (b) chitosan, (c) physical mixture of chitosan and sodium alginate, and (d) alkaloid-loaded microspheres.
Abbreviation: DSC, differential scanning calorimetry.
FTIR analysis allows for the observation of vibration modes for the specific groups of compounds analyzed. The spectra of (a) alginate, (b) chitosan, (c) physical mixture of chitosan and sodium alginate, and (d) chitosan-coated alginate microsphere are shown in Figure 3. The IR spectrum of alginate showed a wide band at 3,430 cm−1, which related to the characteristic absorption of hydroxyl groups; the peaks near 1,615 and 1,418 cm−1 were attributed to asymmetric and symmetric stretching vibrations of carboxyl groups (COO−), respectively. A band around 1,029 cm−1 (C–O–C stretching) was observed corresponding to its saccharide structure. The peaks at 1,615 cm−1 and 1,418 cm−1 shifted a little similarly when alginate interacted with chitosan. In the spectrum of chitosan, a broad band at 3,411 cm−1 as well as peaks at 1,651, 1,593, and 1,419 cm−1 belonged to the characteristic absorption of amino groups (−NH3+); the wide absorption peak observed at 1,086 cm−1 was the characteristic peak of –CH–OH. When chitosan interacted with alginate, there was a new absorption peak at 1,609 cm−1. The peaks at 1,593 cm−1 and 1,086 cm−1 disappeared, suggesting that the amine groups and carboxyl groups reacted, which would prove that the polyelectrolyte complex was formed between alginate and chitosan.
Figure 3.

FTIR spectra of (a) sodium alginate, (b) chitosan, (c) physical mixture of chitosan and sodium alginate, and (d) alkaloid-loaded microspheres.
Abbreviation: FTIR, fourier transform infrared spectroscopy.
There are mainly seven alkaloids in C. chinensis and E. rutaecarpa. To characterize the drug-loading properties of microspheres, the contents of seven alkaloids in microspheres were detected by HPLC. The results are shown in Table 2, and the content of total alkaloids loaded in microspheres was 1.21 mg/g.
Table 2.
The contents of seven alkaloids in microspheres (n=3)
| Number | Seven alkaloids | Content, mean ± SD (mg/kg) | Total alkaloid (mg/kg) |
|---|---|---|---|
| 1 | Epiberberine | 64.7±5.3 | |
| 2 | Jatrorrhizine | 37.6±5.1 | |
| 3 | Coptisine | 67.2±9.1 | |
| 4 | Palmatine | 45.9±5.8 | 1,210.0 |
| 5 | Berberine | 256.1±31.8 | |
| 6 | Evodiamine | 33.3±10.4 | |
| 7 | Rutaecarpine | 705.3±156.4 |
Abbreviation: SD, standard deviation.
Drug release studies in vitro
As shown in Figure 4, the release behavior of seven alkaloids from mucoadhesive microspheres was analyzed by HPLC. Seven alkaloids, except evodiamine, had similar release behavior and maintained sustained release at 12 hours in the simulated gastric fluid (pH 1.2±0.1). The delivery of drugs from the microspheres tended to stabilize after 8 hours and there was a small increase after 12 hours. Approximately 83.55% of rutaecarpine, 77.48% of epiberberine, 70.83% of palmatine, 60.97% of jatrorrhizine, 57.16% of coptisine, 53.13% of berberine, and 16.08% of evodiamine in the microspheres were released respectively after 12 hours.
Figure 4.
Release curves of seven alkaloids from alginate–chitosan microspheres loaded with total alkaloids extracted from Coptis chinensis and Evodia rutaecarpa.
Evaluation of mucoadhesive properties in vitro
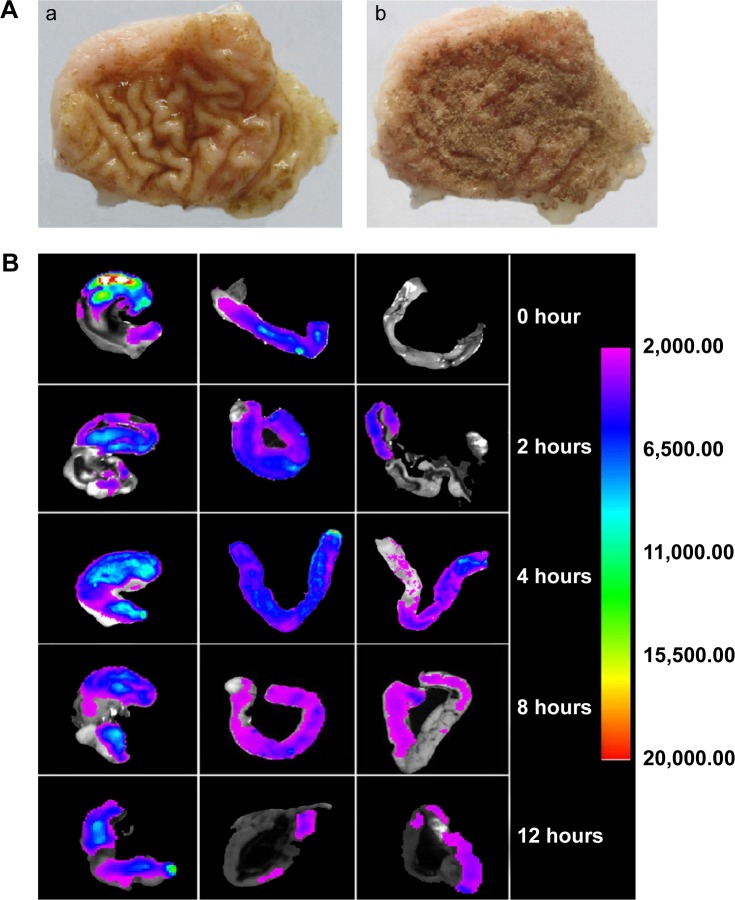
Mucoadhesive properties of microspheres were evaluated by wash-off test in vitro. As shown in Figure 5A, there was still a mass of microspheres sticking to gastric mucosa after being rinsed with simulated gastric fluid. The percentage of microspheres remaining on the gastric mucosa was 82.63%±1.42%, as determined by the adhesion test in vitro.
Figure 5.
Mucoadhesive properties (A) in vitro and (B) in vivo.
Notes: (A) Microspheres sticking to stomach mucosa (a) after rinsing and (b) before rinsing. (B) Characterizations of mucoadhesive properties of FITC-labeled chitosan-coated alkaloid-loaded microspheres in vivo (Left→Right: stomach, duodenum, and jejunum) varied from 0 to 12 hours.
Abbreviation: FITC, fluorescein isothiocyanate.
Evaluation of mucoadhesive properties in vivo
The FITC-labeled chitosan was used to study the mucoadhesive properties of microspheres in vivo. The labeling efficiency of FITC/chitosan was 38.5 μg (FITC)/mg (chitosan). As shown in Figure 5B, the microspheres could stick on the gastrointestinal tract when mice were orally administrated microspheres coated with FITC-labeled chitosan. At 8 hours, the fluorescence intensities of FITC-labeled microspheres on the surface of gastric mucosa were still relatively high, which indicated that the microspheres possess enhanced mucoadhesive properties. A small amount of microspheres could still be found in the stomach even after 12 hours, which indicated that the microspheres had good adhesive properties to stay on the gastric mucosal surface to protect it from damage.
Gastroprotective studies
MI, percentage inhibition, and mucus production
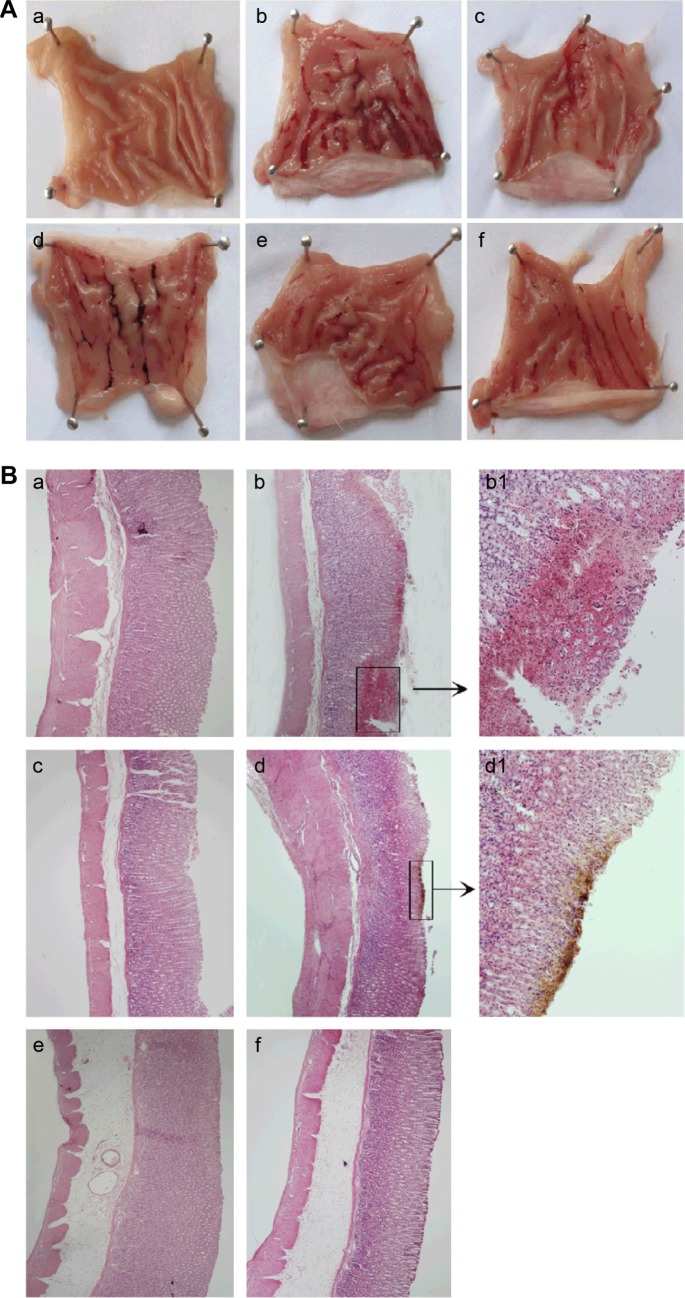
Since ethanol could easily penetrate into the gastric mucosa,30,31 and then influence blood circulation within the mucosa by altering protective factors,32,33 ethanol is regarded as the greatest contributor to gastric ulcer formation. The severity of gastric mucosal injury induced by ethanol is shown in Figure 6A. The model group showed macroscopic morphological changes, such as glandular area hyperemia, mucosal edema accompanied by dot and linear hemorrhage necrosis. A pathology score was used to evaluate whether pretreatment with microspheres loaded with alkaloids could protect against ethanol-induced gastric mucosal injury. As shown in Table 3, the model group displayed a significant increase of MI compared to control group (P<0.05); however, the group pretreated orally with omeprazole or mucoadhesive microspheres loaded with alkaloids could decrease the MI (P<0.05) and increase the percentage of inhibition significantly. As shown in Table 3, oral administration of ethanol could decrease the mucus production significantly compared to the normal group (P<0.05), but pretreatment with omeprazole or microspheres loaded with alkaloids could increase the amount of mucus in the gastric mucosa compared to model group.
Figure 6.
Effects of alkaloid-loaded microspheres on (A) gross appearance and (B) H&E-stained micrographs of stomach.
Notes: (A) (a) Normal control group, (b) model group, (c) omeprazole group (20 mg/kg), microsphere groups (d) at 0.02 mg/kg, (e) at 0.05 mg/kg, and (f) at 0.18 mg/kg. (B) H&E-stained micrographs of stomach from (a) normal control (40×), (b) model group (b-40×, b1-100×), (c) omeprazole at 20 mg/kg (40×), microsphere groups (d) at 0.02 mg/kg (d-40×, d1-100×), (e) at 0.05 mg/kg (40×), (f) at 0.18 mg/kg (40×).
Abbreviation: H&E, hematoxylin and eosin.
Table 3.
Macroscopic analysis results of mucosa lesions induced by ethanol
| Groups | Pretreatment | Mucosal lesion index (score) | Percentage of inhibition (%) | Mean mucus weight (mg) |
|---|---|---|---|---|
| 1 | Normal control | 0 | – | 186.8±1.62 |
| 2 | Model group | 115.99±8.74* | – | 140.1±4.11* |
| 3 | Omeprazole (20 mg/kg) | 24.52±1.90** | 78.9 | 147.7±10.0 |
| 4 | Microspheres (0.02 mg/kg) | 46.47±11.14** | 59.9 | 135.3±8.87 |
| 5 | Microspheres (0.05 mg/kg) | 30.71±2.35** | 73.5 | 152.0±2.77 |
| 6 | Microspheres (0.18 mg/kg) | 38.73±6.07** | 66.6 | 171.6±6.10** |
Notes: Data are expressed as mean ± standard error mean.
P<0.05 compared to the control group;
P<0.05 compared to the model group.
Histological results
Histological observations results are shown in Figure 6B. There was no damage on the stomach of normal control group, but the model group treated with absolute ethanol showed serious injury of gastric mucosa, and the superficial gastric epithelium was disrupted and exfoliated. Moreover, extensive hemorrhage, edema, necrosis, and leukocyte infiltration were also observed. In some areas, damage cells could be observed in the submucosa. The gastric mucosa damages were markedly attenuated with only slight injury observed in superficial gastric epithelium on treatment with omeprazole or microspheres loaded with alkaloids. There was little damage in gastric mucosa of animals pretreated with omeprazole (20 mg/kg) or microspheres loaded with alkaloids (0.05, 0.18 mg/kg).
Effect of mucoadhesive microspheres loaded with alkaloids on production of PGE2, TNF-α, and IL-1β in ethanol-induced acute gastric mucosal injury
PGE2 was an effective vasodilator, which could not only inhibit the gastric acid secretion, but also stimulate mucus and bicarbonate secretion in the stomach to enhance the mucosal defense. As shown in Figure 7, the production of PGE2 in the gastric mucosa treated with ethanol was downregulated significantly (P<0.05) compared to normal control. Pretreatment with omeprazole or microspheres loaded with alkaloids could increase the PGE2 levels significantly (P<0.05) in the gastric mucosa compared to model group. The administration of ethanol produced plentiful gastric mucosal injuries, especially an increase in inflammatory mediators. In the process of acute-phase inflammation, a variety of mediators such as TNF-α and IL-1β were involved.34–36 As shown in Figure 7, the production of TNF-α and IL-1β in the model group was increased significantly (P<0.05) compared with normal group. In the groups pretreated with omeprazole (20 mg/kg) and microspheres loaded with alkaloids (0.05, 0.18 mg/kg), the production of TNF-α and IL-1β decreased significantly (P<0.05). The anti-inflammatory property on gastric ulcer was in accordance with the previous study of the ethanol extract from alkaloids on inhibiting the production of inflammatory factors.22
Figure 7.
Effects of alkaloid-loaded microspheres on production of PGE2, TNF-α, and IL-1β in ethanol-induced gastric mucosa lesions.
Notes: (A) PGE2, (B) TNF-α, and (C) IL-1β in gastric mucosal homogenates were measured by ELISA. Data were expressed with mean ± SE from three independent experiments. *P<0.05 (n=4) compared to the normal control group, **P<0.05 (n=4) compared to the model group.
Abbreviations: PEG2, Prostaglandin E2; OME, omeprazole; TNF-α, tumor necrosis factor-α; IL-1β, interleukin 1β; ELISA, enzyme-linked immunosorbent assay; SE, standard error.
Effect of mucoadhesive microspheres loaded with alkaloids on mRNA expression of iNOS, TNF-α, and IL-1β in ethanol-induced acute gastric mucosal injury
In the digestive systems, NO generated from iNOS is cytotoxic,37 and the effects of microspheres on mRNA expression of iNOS were investigated in this study. As shown in Figure 8, ethanol ingestion could induce severe gastric mucosal damage compared with the normal group by increasing the mRNA expression of iNOS. The mRNA expression of iNOS was significantly (P<0.05) downregulated by pretreating with omeprazole (20 mg/kg) or microspheres loaded with alkaloids (0.05, 0.18 mg/kg) compared to model group. There was no significance on concentration of 0.02 mg/kg compared to model group.
Figure 8.
Effects of alkaloid-loaded microspheres on mRNA expression of iNOS, TNF-α, and IL-1β in ethanol-induced gastric mucosa lesions.
Notes: The mRNA expressions of (A) iNOS, (B) TNF-α, and (C) IL-1β were measured by real-time RT-PCR. Data were expressed with mean ± SE from three independent experiments. *P<0.05 (n=4) compared to the normal control group; **P<0.05 (n=4) compared to the model group.
Abbreviations: OME, omeprazole; TNF-α, tumor necrosis factor-α; IL-1β, interleukin 1β; ELISA, enzyme-linked immunosorbent assay; SE, standard error; iNOS, inducible nitric oxide synthase; RT-PCR, reverse transcription polymerase chain reaction.
Since microspheres loaded with alkaloids were found to inhibit the production of TNF-α and IL-1β, the effects on mRNA expression of TNF-α and IL-1β were evaluated by real-time RT-PCR. As shown in Figure 8, the mRNA expression of TNF-α could be inhibited significantly (P<0.05) by pretreating with alkaloid-loaded microspheres (0.02, 0.05 mg/kg) or omeprazole (20 mg/kg) compared to model group. The mRNA expression of IL-1β was downregulated by alkaloid-loaded microspheres significantly (P<0.05) only at the concentration of 0.02 mg/kg.
Discussion
Various formulations for oral drug delivery in the treatment of gastric ulcers, such as tablets and capsules, have been challenged because of their incomplete eradication of H. pylori. One reason for the incomplete eradication of H. pylori may be due to the short residence time of drugs in the stomach and because effective treatment concentrations cannot be achieved in the gastric mucous layer or epithelial cell surfaces where H. pylori exists.38 Another reason might be the poor stability of drugs in gastric acid or poor permeability of the drugs across the mucus layer.39 In order to increase the residence time and adhesion of alkaloids from C. chinensis and E. rutaecarpa in stomach, alkaloids were loading into the alginate–chitosan microspheres, and the protective mechanism of microspheres on ethanol-induced acute gastric mucosal injury in rats was investigated.
The surface of microspheres after freeze-drying became rough and had many wrinkles, which would not only improve the adhesion and mobility in vivo, but also accelerate the release of drugs in targeted areas, such as gastric mucosa, which has many wrinkled areas. The DSC characteristics of microspheres showed that the characteristic peaks of the two polymers disappeared and new peaks emerged in the thermogram of chitosan-coated alginate microspheres, which indicated that the interaction between chitosan and alginate. FTIR analysis suggested that the amine groups and carboxyl groups reacted, which would prove that the polyelectrolyte complex was formed between alginate and chitosan. The release study showed that alginate–chitosan microspheres loaded with alkaloids had good controlled-release properties and could be an ideal candidate for drug delivery carrier.
Mucoadhesive properties of microspheres played an important role in the sustained release of drug. Evaluation of mucoadhesive properties showed that there was still a mass of microspheres sticking to stomach mucosa after rinsing with simulated gastric fluid in vitro. The results indicated that microspheres had strong adhesion performance as most microspheres still remained on the gastric mucosa. Microspheres could stick to the stomach easily because of the natural biological folds of stomach and the adhesion properties of gastric mucosa. The FITC-labeled chitosan was used to investigate the mucoadhesive properties of microspheres in vivo. The results showed that a small amount of microspheres still could be found in the digestive tract even at 12 hours. Studies showed that chitosan could cross-link with mucin and the strong electrostatic interaction between them contributed to the good mucoadhesive property of chitosan-coated alginate microspheres. Hydrophilic functional groups such as hydroxyl groups in chitosan may form hydrogen bonds with sialic acid in mucin molecules. This interaction may be responsible for enhancing permeability of locally delivery drugs and facilitating more effective absorption.40,41
Ethanol could also produce acute hemorrhagic gastric erosion and lead to mucosal edema, cellular exfoliation, and inflammatory cell infiltration, which was the symbol of acute inflammatory reaction.42 The group pretreated with omeprazole or alginate–chitosan microspheres loaded with alkaloids could decrease the MI and increase the percentage of inhibition significantly. It was reported that stimulating the mucus production was also one of the mucosal defense mechanisms for protecting the surface epithelial cells and the mucosa against damage.43 In order to further investigate the possible mechanism of the protective effect in gastric mucosa, the effects of microspheres loaded with alkaloids on mucus production were investigated. Oral administration of ethanol could decrease the mucus production significantly compared to the normal group, but pretreatment with omeprazole or microspheres loaded with alkaloids could increase the amount of mucus in the gastric mucosa compared to the model group. The increase of gastric mucus may be one of the protective mechanisms involved with the antiulcer effects.
Neutrophils inflicted endothelial damage by generating various free radicals including NO that influenced neutrophil oxidative burst.44 The enhanced generation of NO by the iNOS might contribute to the pathogenesis of various gastroduodenal disorders including peptic ulcer.17 An increase in iNOS activity in the gastric mucosa was closely related to the development of gastric mucosal lesions. Ethanol ingestion could induce severe gastric mucosal damage by increasing the expression of the proinflammatory mediators such as iNOS mRNA. The mRNA expression of iNOS was downregulated by microspheres loaded with alkaloids compared to model group. The gastric mucosa has specific defense mechanisms for holding its structural integrity, and its epithelial surface can secrete a barrier consisting of mucin bicarbonate and prostaglandins.45 PGE2 was considered as a key defense factor in the gastrointestinal mucosa for gastric ulcer healing.46 Pretreatment with microspheres could increase the PGE2 level significantly in the gastric mucosa compared to model group. Moreover, PGE2 also could promote gastric ulcer healing by influencing other inflammatory mediators such as TNF-α, histamine, and IL-1β in macrophages and platelet-activating factor in mast cells.47 The administration of ethanol produced plentiful gastric mucosal injuries, especially an increase in inflammatory mediators. In the process of acute phase inflammation, a variety of mediators such as TNF-α and IL-1β were involved.36,48 The production of TNF-α and IL-1β were significantly decreased by pretreating with microspheres. The anti-inflammatory property on gastric ulcer was in accordance with the previous study of alkaloids on inhibiting the production of inflammatory factors. The mRNA expression of TNF-α and IL-1β was inhibited significantly when pretreated with microspheres. Alkaloids could decrease the production of TNF-α and IL-1β by inhibiting the mRNA expression of TNF-α and IL-1β.
It is noteworthy that the effects of microspheres loaded with alkaloids on the inflammatory response induced by ethanol were not dose-dependent. One reason may be attributed to the multiple pharmacological effects based on the interactions of its complex active constituents in alkaloids, as we can find that there are seven main alkaloids by HPLC method. Another reason was that the intestinal absorption mechanisms of alkaloids were differently related with P-glycoprotein, and their intestinal absorption was limited as there was involvement of P-glycoprotein in vivo. For example, the study of berberine indicated that it possessed a biphasic effect on regulation of P-glycoprotein ATPase activity (stimulation at low concentration while inhibition at high concentration).49 Furthermore, coptisine, jateorhizine, palmatine, and berberine are all P-glycoprotein substrates, and no obvious inhibitory effect was found on the activity of P-glycoprotein at concentrations of 1–100 μM,50 which may be one of the reasons for their low bioavailability. Finally, the pharmacokinetics of alkaloids could be another influencing factor. Studies have shown that after intravenous administration, plasma concentrations of jatrorrhizine showed a biphasic decline, dose-independent clearance and half-life of terminal elimination phase reduction, and a relatively large distribution volume. The metabolic property of jatrorrhizine was suggested to multiple metabolic pathways.51 Further research on metabolic processes of alkaloids will be developed in vivo in the future.
In conclusion, the mucoadhesive microspheres loaded with alkaloids were prepared with chitosan and alginate and possessed good release performances and gastric mucosa adhesion. The mucoadhesive alkaloid-loaded microspheres could decrease the production of TNF-α and IL-1β, downregulate the mRNA expression of iNOS, TNF-α, IL-1β and increase the production of gastroprotective factor PGE2 in gastric mucosa in an ethanol-induced gastric mucosal injury model in rats. Moreover, the mucoadhesive alkaloid-loaded microspheres could maintain sustained release at 12 hours and play an important role in reducing the gastric injury by decreasing the MI, increasing the percentage of inhibition, and increasing the amount of mucus in the gastric mucosa. Therefore, the results of our studies will provide strong scientific evidence for the mucoadhesive microspheres loaded with total alkaloids of C. chinensis and E. rutaecarpa to be developed as a new method for the prevention and treatment of gastric ulcer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81473542), the Specialized Research Fund for the Doctoral Program of Higher Education (20131210110008), and the Postdoctoral Science Foundation of China (no 2014M552639).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mota KS, Dias GE, Pinto ME, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14(3):979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao ChV, Ojha SK, Radhakrishnan K, et al. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004;91(2–3):243–249. doi: 10.1016/j.jep.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Hejazi R, Amiji M. Stomach-specific anti-H. pylori therapy. I: preparation and characterization of tetracyline-loaded chitosan microspheres. Int J Pharm. 2002;235(1–2):87–94. doi: 10.1016/s0378-5173(01)00985-1. [DOI] [PubMed] [Google Scholar]
- 4.Ji C, Khademhosseini A, Dehghani F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials. 2011;32(36):9719–9729. doi: 10.1016/j.biomaterials.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Ong SY, Wu J, Moochhala SM, et al. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29(32):4323–4332. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Tronci G, Ajiro H, Russell SJ, et al. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014;10(2):821–830. doi: 10.1016/j.actbio.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayanan RP, Melman G, Letourneau NJ, et al. Photodegradable iron(III) cross-linked alginate gels. Biomacromolecules. 2012;13(8):2465–2471. doi: 10.1021/bm300707a. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Kong T, Yeung KW, et al. Fabrication and characterization of monodisperse PLGA-alginate core-shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013;9(7):7410–7419. doi: 10.1016/j.actbio.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Anal AK, Stevens WF. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int J Pharm. 2005;290(1–2):45–54. doi: 10.1016/j.ijpharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Tarnawski A, Szabo IL, Husain SS, Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J Physiol Paris. 2001;95(1–6):337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 11.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 12.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 13.Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268(17):12231–12234. [PubMed] [Google Scholar]
- 14.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500(1–3):255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee M, Saluja R, Kanneganti S, et al. Biochemical and molecular evaluation of neutrophil NOS in spontaneously hypertensive rats. Cell Mol Biol (Noisy-le-grand) 2007;53(1):84–93. [PubMed] [Google Scholar]
- 16.Salvemini D, de Nucci G, Gryglewski RJ, Vane JR. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza MH, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53(6):791–796. doi: 10.1136/gut.2002.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris SG, Padilla J, Koumas L, et al. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23(3):144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, del Soldato P, Wallace JL. Divergent effects of new cyclooxygenase inhibitors on gastric ulcer healing: shifting the angiogenic balance. Proc Natl Acad Sci U S A. 2002;99(20):13243–13247. doi: 10.1073/pnas.202392199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konturek SJ, Konturek PC, Brzozowski T. Prostaglandins and ulcer healing. J Physiol Pharmacol. 2005;56(Suppl 5):5–31. [PubMed] [Google Scholar]
- 21.Pharmacopoeia CoN . Pharmacopoeia of the People’s Republic of China. Beijing, China: Press of Chemical Industry; 2010. [Google Scholar]
- 22.Wang QS, Cui YL, Dong TJ, et al. Ethanol extract from a Chinese herbal formula, “Zuojin Pill”, inhibit the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 mouse macrophages. J Ethnopharmacol. 2012;141(1):377–385. doi: 10.1016/j.jep.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Wang QS, Ding SL, Mao HP, et al. Antidepressant-like effect of ethanol extract from Zuojin Pill, containing two herbal drugs of Rhizoma Coptidis and Fructus Evodiae, is explained by modulating the monoaminergic neurotransmitter system in mice. J Ethnopharmacol. 2013;148(2):603–609. doi: 10.1016/j.jep.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama Y, Nagahara N, Kashihara T, et al. In vitro and in vivo evaluation of mucoadhesive microspheres prepared for the gastrointestinal tract using polyglycerol esters of fatty acids and a poly(acrylic acid) derivative. Pharm Res. 1995;12(3):397–405. doi: 10.1023/a:1016208703380. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Li XR, Fan JY, et al. Experimental research on effects of Xue Dan Wei Chang Pill on gastric ulcer. LiShiZhen Med Mater Med Res. 2010;21:301–302. [Google Scholar]
- 26.Salga MS, Ali HM, Abdulla MA, Abdelwahab SI. Gastroprotective activity and mechanism of novel dichlorido-zinc(II)-4-(2-(5-methoxybenzylideneamino)ethyl)piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chem Biol Interact. 2012;195(2):144–153. doi: 10.1016/j.cbi.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Sidahmed HM, Hashim NM, Amir J, et al. Pyranocycloartobiloxanthone A, a novel gastroprotective compound from Artocarpus obtusus Jarret, against ethanol-induced acute gastric ulcer in vivo. Phytomedicine. 2013;20(10):834–843. doi: 10.1016/j.phymed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang QS, Xiang Y, Cui YL, et al. Dietary blue pigments derived from genipin, attenuate inflammation by inhibiting LPS-induced iNOS and COX-2 expression via the NF-kappaB inactivation. PLoS One. 2012;7(3):e34122. doi: 10.1371/journal.pone.0034122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang QS, Cui YL, Wang YF, Chi W. Effects of compounds from bi-qi capsule on the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Ethnopharmacol. 2011;136(3):480–487. doi: 10.1016/j.jep.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Sowndhararajan K, Kang SC. Protective effect of ethyl acetate fraction of Acacia ferruginea DC. against ethanol-induced gastric ulcer in rats. J Ethnopharmacol. 2013;148(1):175–181. doi: 10.1016/j.jep.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Franke A, Teyssen S, Singer MV. Alcohol-related diseases of the esophagus and stomach. Dig Dis. 2005;23(3–4):204–213. doi: 10.1159/000090167. [DOI] [PubMed] [Google Scholar]
- 32.Choi EY, Hwang HJ, Kim IH, Nam TJ. Protective effects of a polysaccharide from Hizikia fusiformis against ethanol toxicity in rats. Food Chem Toxicol. 2009;47(1):134–139. doi: 10.1016/j.fct.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Ineu RP, Pereira ME, Aschner M, et al. Diphenyl diselenide reverses gastric lesions in rats: involvement of oxidative stress. Food Chem Toxicol. 2008;46(9):3023–3029. doi: 10.1016/j.fct.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Hasgul R, Uysal S, Haltas H, et al. Protective effects of Ankaferd blood stopper on aspirin-induced oxidative mucosal damage in a rat model of gastric injury. Toxicol Ind Health. 2014;30(10):888–895. doi: 10.1177/0748233712466134. [DOI] [PubMed] [Google Scholar]
- 35.Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. 2006;20(5):879–896. doi: 10.1016/j.berh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Kwiecien S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53(1):39–50. [PubMed] [Google Scholar]
- 37.Liu Y, Tian X, Gou L, et al. Protective effect of L-citrulline against ethanol-induced gastric ulcer in rats. Environ Toxicol Pharmacol. 2012;34(2):280–287. doi: 10.1016/j.etap.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Axon AT. The role of acid inhibition in the treatment of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:16–23. [PubMed] [Google Scholar]
- 39.Fontana G, Licciardi M, Mansueto S, et al. Amoxicillin-loaded polyethylcyanoacrylate nanoparticles: influence of PEG coating on the particle size, drug release rate and phagocytic uptake. Biomaterials. 2001;22(21):2857–2865. doi: 10.1016/s0142-9612(01)00030-8. [DOI] [PubMed] [Google Scholar]
- 40.Dhaliwal S, Jain S, Singh HP, Tiwary AK. Mucoadhesive microspheres for gastroretentive delivery of acyclovir: in vitro and in vivo evaluation. AAPS J. 2008;10(2):322–330. doi: 10.1208/s12248-008-9039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amiji MM. Tetracycline-containing chitosan microspheres for local treatment of Helicobacter pylori infection. Cellulose. 2007;14:3–14. [Google Scholar]
- 42.Ko JK, Cho CH, Lam SK. Adaptive cytoprotection through modulation of nitric oxide in ethanol-evoked gastritis. World J Gastroenterol. 2004;10(17):2503–2538. doi: 10.3748/wjg.v10.i17.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 44.Kristal B, Shurtz-Swirski R, Chezar J, et al. Participation of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation in patients with essential hypertension. Am J Hypertens. 1998;11(8):921–928. doi: 10.1016/s0895-7061(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 45.Luiz-Ferreira A, Cola M, Barbastefano V, et al. Healing, antioxidant and cytoprotective properties of Indigofera truxillensis in different models of gastric ulcer in rats. Int J Mol Sci. 2012;13(11):14973–14991. doi: 10.3390/ijms131114973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esplugues JV, Whittle BJ, Moncada S. Modulation by opioids and by afferent sensory neurones of prostanoid protection of the rat gastric mucosa. Br J Pharmacol. 1992;106(4):846–852. doi: 10.1111/j.1476-5381.1992.tb14423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelwahab SI. Protective mechanism of gallic acid and its novel derivative against ethanol-induced gastric ulcerogenesis: involvement of immunomodulation markers, Hsp70 and Bcl-2-associated X protein. Int Immunopharmacol. 2013;16(2):296–305. doi: 10.1016/j.intimp.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Shimoyama AT, Santin JR, Machado ID, et al. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(1):5–14. doi: 10.1007/s00210-012-0807-2. [DOI] [PubMed] [Google Scholar]
- 49.Najar IA, Sachin BS, Sharma SC, et al. Modulation of P-glycoprotein ATPase activity by some phytoconstituents. Phytother Res. 2010;24(3):454–458. doi: 10.1002/ptr.2951. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Qiu F, Jiang J, Gao C, Tan Y. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: involvement of P-glycoprotein. Xenobiotica. 2011;41(4):290–296. doi: 10.3109/00498254.2010.529180. [DOI] [PubMed] [Google Scholar]
- 51.Shi R, Zhou H, Ma B, et al. Pharmacokinetics and metabolism of jatrorrhizine, a gastric prokinetic drug candidate. Biopharm Drug Dispos. 2012;33(3):135–145. doi: 10.1002/bdd.1779. [DOI] [PubMed] [Google Scholar]