Abstract
P2Y12 receptor (P2Y12-R) signaling is mediated through Gi, ultimately reducing cellular cAMP levels. Because cAMP is a central modulator of arginine vasopressin (AVP)-induced water transport in the renal collecting duct (CD), we hypothesized that if expressed in the CD, P2Y12-R may play a role in renal handling of water in health and in nephrogenic diabetes insipidus. We found P2Y12-R mRNA expression in rat kidney, and immunolocalized its protein and aquaporin-2 (AQP2) in CD principal cells. Administration of clopidogrel bisulfate, an irreversible inhibitor of P2Y12-R, significantly increased urine concentration and AQP2 protein in the kidneys of Sprague–Dawley rats. Notably, clopidogrel did not alter urine concentration in Brattleboro rats that lack AVP. Clopidogrel administration also significantly ameliorated lithium-induced polyuria, improved urine concentrating ability and AQP2 protein abundance, and reversed the lithium-induced increase in free-water excretion, without decreasing blood or kidney tissue lithium levels. Clopidogrel administration also augmented the lithium-induced increase in urinary AVP excretion and suppressed the lithium-induced increase in urinary nitrates/nitrites (nitric oxide production) and 8-isoprostane (oxidative stress). Furthermore, selective blockade of P2Y12-R by the reversible antagonist PSB-0739 in primary cultures of rat inner medullary CD cells potentiated the expression of AQP2 and AQP3 mRNA, and cAMP production induced by dDAVP (desmopressin). In conclusion, pharmacologic blockade of renal P2Y12-R increases urinary concentrating ability by augmenting the effect of AVP on the kidney and ameliorates lithium-induced NDI by potentiating the action of AVP on the CD. This strategy may offer a novel and effective therapy for lithium-induced NDI.
Keywords: collecting ducts, cyclic AMP, diabetes insipidus, vasopressin, extracellular, nucleotides, hypothalamus
Water absorption in renal collecting duct (CD) is regulated by arginine vasopressin (AVP), through its V2 receptor (R) and cyclic AMP signaling, resulting in increased expression of aquaporin-2 (AQP2) and its apical membrane targeting in the CD cells.1 AVP action on CD can be modulated by the autocrine/paracrine agents, such as extracellular nucleotides.2,3 The AVP-V2-R-AQP2 pathway is disrupted in acquired nephrogenic diabetes insipidus (NDI), resulting in the development of resistance of CD to AVP.4 The most common acquired NDI is caused by chronic usage of lithium (Li) for the treatment of bipolar disorder, which affects 2% of the general population of the United States. The exact mechanism(s) by which CD develops AVP resistance in Li-induced NDI is not established.5–8 Current therapies for Li-induced NDI are prone to adverse effects.7,9–11 Understanding the mechanism(s) of Li-induced NDI or developing modalities to reverse the AVP resistance of CD will result in new and safer therapies.
Previously we reported that purinergic signaling mediated by ATP/UTP-activated P2Y2 receptor (P2Y2-R) plays a significant role in the genesis of NDI. First, using a rat model, we identified a potential role for P2Y2-R in acquired NDI induced by Li treatment or postobstructive uropathy.12,13 P2Y2-R is known to exert tonic inhibitory effect on the AVP-V2-R-AQP2 pathway.14,15 Therefore, to further elucidate the role of P2Y2-R, we used mice with genetic deletion of P2Y2-R and found that these mice are significantly resistant to Li-induced NDI.16,17 Although promising, currently there are no Food and Drug Administration (FDA)-approved or experimental drugs that can selectively block P2Y2-R in vivo. Hence, we examined the ADP-activated P2Y12-R as another potential P2Y receptor subtype that may oppose AVP-induced water flow in the CD. Phylogenetically P2Y12-R is distinct from P2Y2-R and related receptors.18 P2Y12-R is expressed predominantly in blood platelets and microglia and astrocytes in the brain. P2Y12-R signaling in platelets is mediated through Gi, inhibiting adenylyl cyclase (AC) and activation of class I phosphoinositol 3-kinase. P2Y12-R is also coupled to Gq, which through phospholipase C activation increases intracellular Ca2+ needed for platelet activation.19 Both inhibition of AC activity and activation of phospholipase C are known to decrease the sensitivity of CD to AVP.2 Hence, we hypothesized that if expressed in the kidney, P2Y12-R may play a role in renal handling of water and in pathophysiology of NDI. The availability of an FDA-approved and time-tested drug, clopidogrel bisulfate, to selectively block P2Y12-R allowed us to test our hypothesis in rat models. Clopidogrel is an oral thienopyridine class of drug that irreversibly inhibits P2Y12-R. It is a prodrug activated in the liver by cytochrome P450 enzymes (e.g., CYP2C19) generating its active metabolite, which constitutes approximately 15% of the ingested drug molecule. In this study we document that P2Y12-R is expressed in the rat kidney, and its pharmacologic blockade increases urine concentrating ability and significantly ameliorates Li-induced NDI in rats.
Results
Expression of P2Y12-R in the Kidney
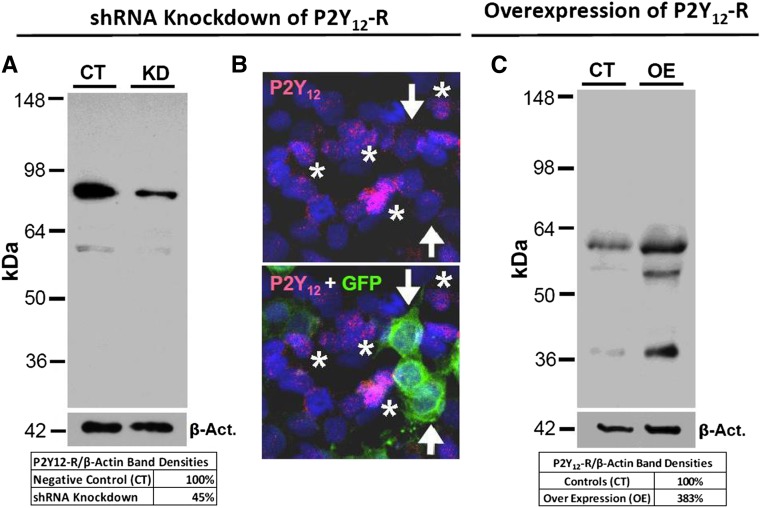
To immunolocalize P2Y12-R in the kidney, we designed, generated, and characterized a peptide-derived antibody specific for rat or mouse P2Y12-R (Supplemental Table 1). We evaluated the specificity of our antibody by immunoblotting and immunofluorescence (IF) microscopy in two different cell lines, namely inner medullary collecting duct-3 (IMCD3) (mouse renal CDs) and HEK293T (human embryonic kidney), and by using two different approaches: P2Y12-R gene knockdown and overexpression (Figure 1). For proper interpretation of the data presented in Figure 1, one needs to take into account that P2Y12-R exists in multiple forms (monomer, dimer, trimer, or oligomer), with each form in turn having different glycosylated subspecies, spanning from 35 to >220 kD in immunoblots.20 Furthermore, the expression of these multiple forms and glycosylated subspecies may differ depending on the nature of the cells under consideration. As shown in Figure 1A, the control IMCD3 cells showed a major band at approximately 80 kD and a faint band at approximately 60 kD, which match with the same size bands seen in rat kidney and brain (Figure 2B). In IMCD3 cells subjected to knockdown of P2Y12-R by shRNA (KD), there was an approximate 55% decrease in relative intensity of the 80 kD band and disappearance of 60 kD band. In parallel, IF imaging of IMCD3 cells showed labeling for P2Y12-R (red) only in green fluorescent protein (GFP)-negative untransfected cells (asterisks), but not in GFP-positive transfected cells (arrows) (Figure 1B). As shown in Figure 1C, in control HEK293T cells, our antibody recognized a clear band at approximately 60 kD, which matches with the same size band in IMCD3 cells (Figure 1A). In addition, we could see a faint band at approximately 38 kD, which matches with the same size band in the native rat kidney (Figure 2B). In cells over expressing P2Y12-R, both bands showed a marked increase in intensity, associated by the appearance of a new band at approximately 55 kD, which matches with the similar size band in human platelet lysates when probed with our P2Y12-R antibody (Supplemental Figure 1). Therefore, taken together, the data presented in Figure 1 validated the specificity of our P2Y12-R antibody. Because manipulation of P2Y12-R protein levels in the cells by knockdown or overexpression at the gene level matched with corresponding changes in the intensity of all of the detected bands, we can conclude that all detected bands are specific to P2Y12-R. Further validation of our P2Y12-R antibody was achieved by IF labeling of mouse microglial cells (Supplemental Figure 2).
Figure 1.
Characterization of specificity of P2Y12-R antibody. (A) Western analysis of knockdown (KD) of P2Y12-R showing receptor protein abundance in IMCD3 cells transfected with scrambled (CT) or P2Y12-R specific shRNA (KD), relative to the respective protein abundances of β-actin; the table below the blots shows the percent P2Y12-R protein remaining after KD. (B) IF detection of GFP (green) and P2Y12-R (red) in the IMCD3 cells transfected with shRNA plasmids specific for P2Y12-R. Cell nuclei were stained with propidium iodide (blue). Upper panel shows P2Y12-R labeling in a profile of cells, whereas the lower panel is overlay of GFP labeling on the same profile of cells. Note a near complete KD of P2Y12-R expression (arrows in the upper panel) in transfected cells (arrows in the lower panel). Asterisks indicate nontransfected cells showing P2Y12-R labeling (red). (C) P2Y12-R protein abundance in control (CT) or human P2Y12-R overexpressing (OE) HEK293T cells relative to the respective protein abundances of β-actin; the table below the blots shows the percent overexpression of P2Y12-R protein. Act., actin.
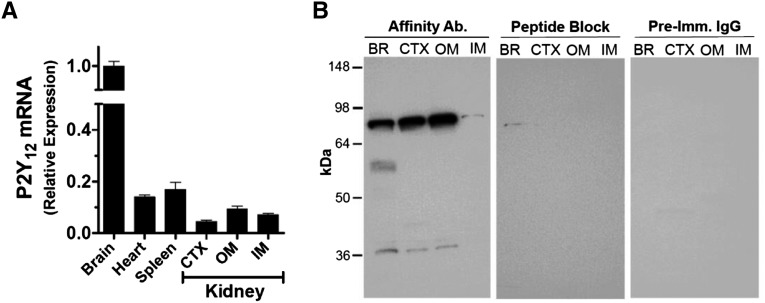
Figure 2.
P2Y12-R expression in rat kidney. (A) P2Y12-R mRNA expression relative to that of β-actin in the three major regions of the kidney and other organs of rat. CTX, cortex; OM, outer medulla; IM, inner medulla. Bars show mean±SEM of triplicate real-time PCR assays, plotted relative to the expression levels in the brain. (B) P2Y12-R protein in the rat kidney and brain. Identical immunoblots prepared by applying equal amounts of protein (30 µg) from solubilized homogenates of rat brain (BR), renal cortex (CTX), or outer medulla (OM) or inner medulla (IM) were incubated with the affinity purified P2Y12-R antibody (Ab.) or the antibody blocked by the immunizing peptide or with protein A-purified preimmune (Imm.) IgG as marked on the top of the panels.
Figure 2B shows identical P2Y12-R protein bands in rat kidney and brain, which are ablated by the blocking peptide and could not be visualized when the blot was probed with preimmune IgG fraction. In the kidney samples our antibody identified two bands (one weak band at 38–40 kD, another strong band at 80 kD) in the cortex and outer medulla and one weak band (approximately 80 kD) in the inner medulla. On the basis of the predicted molecular weight of the P2Y12-R protein (38 kD; 347 amino acid residues), it appears that the weak band at 38–40 kD is the monomeric form of the receptor protein, whereas the strong band at 80 kD represents a dimeric form. Real-time RT-PCR detected P2Y12-R mRNA in the renal cortex and outer and inner medullas of Sprague–Dawley rats (Figure 2A).
Cellular Localization of P2Y12-R in the Rat Kidney
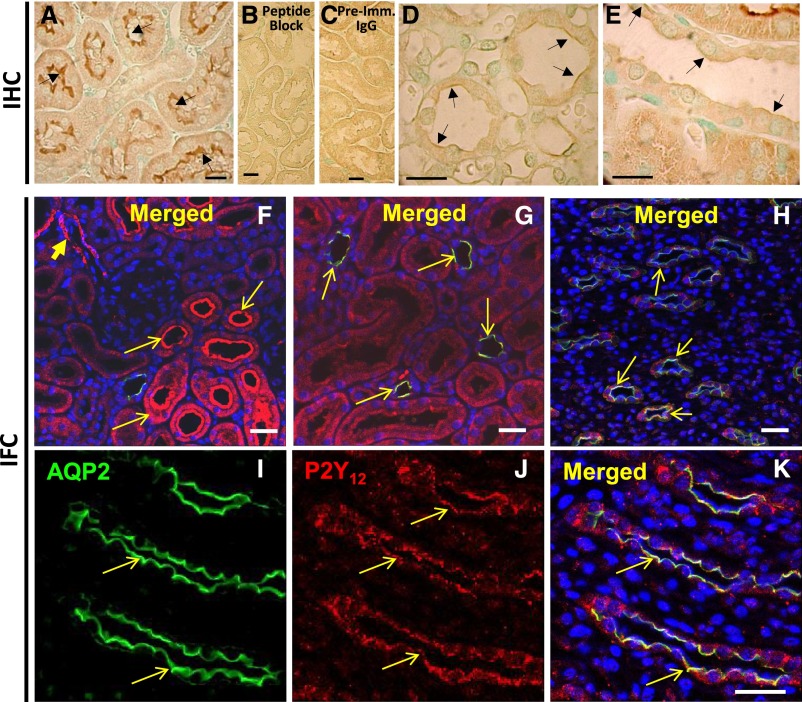
Immunoperoxidase reaction revealed strong labeling for P2Y12-R on the brush border of the proximal tubule in the cortex (Figure 3A), which was ablated by peptide block and was not seen when probed with preimmune IgG (Figure 3, B and C). Weak labeling was found at the apical domain of the CD in the medulla and cortex (Figure 3, D and E). Confocal IF imaging confirmed expression of P2Y12-R over the brush border of proximal tubules, revealed labeling in arterioles (Figure 3F), and showed P2Y12-R protein in AQP2 positive cells in the cortex (Figure 3, G) and medulla (Figure 3, H–K), with an apparent localization of both proteins on the apical membrane of the CD cells (Figure 3, G, H, and K). Similar observations were made in IF when P2Y12-R antibody from Alomone Labs was used (Supplemental Figure 3).
Figure 3.
Immunolocalization of P2Y12-R protein in rat kidney. Upper row: Immunoperoxidase labeling for P2Y12-R: (A) labeling of brush border membrane of proximal tubules (PT); (B) peptide-block ablated the labeling in PT; (C) substitution of antibody with preimmune (Imm.) IgG showed no labeling in the PT (arrows); weak labeling on the apical aspect of the CD in the medulla (D), and cortex (E) (arrows). Middle and lower rows: IF labeling of AQP2 (green) and P2Y12-R (red): (F) and (G) are low magnification profiles of cortical region, showing labeling of arterioles (arrowhead in F), proximal tubules (arrows in F), and cortical CDs showing merging of labeling of AQP2 and P2Y12-R (arrows in G). (H) Low magnification profile of inner medullary region showing labeling of CDs for AQP2 and P2Y12-R. (I)–(K) High magnification images of inner medullary region showing labeling of medullary CDs for AQP2 or P2Y12-R or merging of both. Cell nuclei were labeled with DAPI. Bars are 20 µm in length.
Administration of Clopidogrel Ameliorated Li-Induced NDI
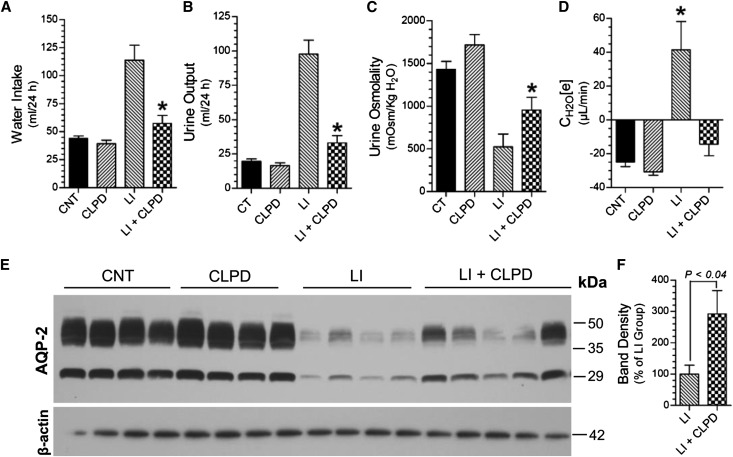
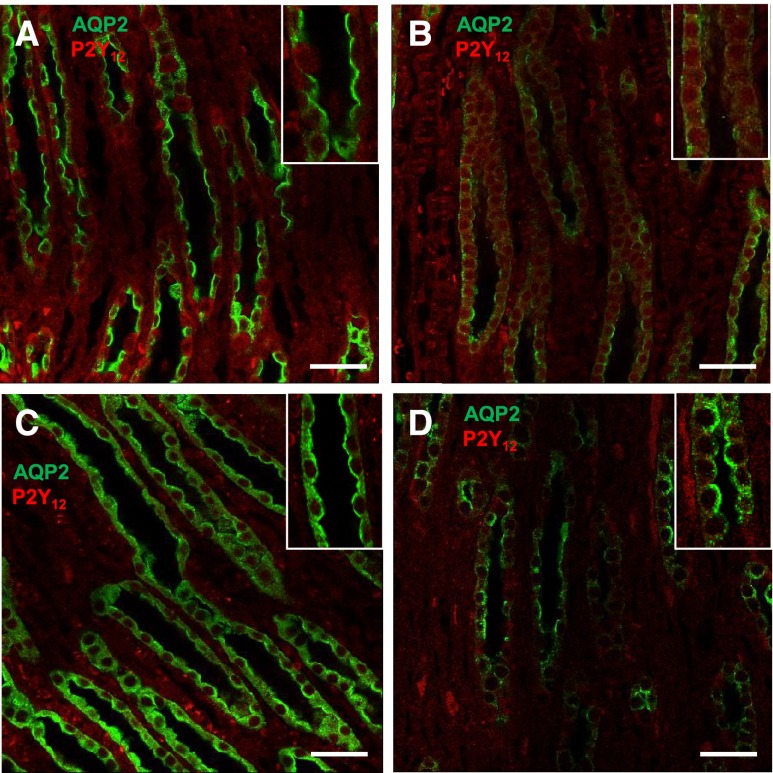
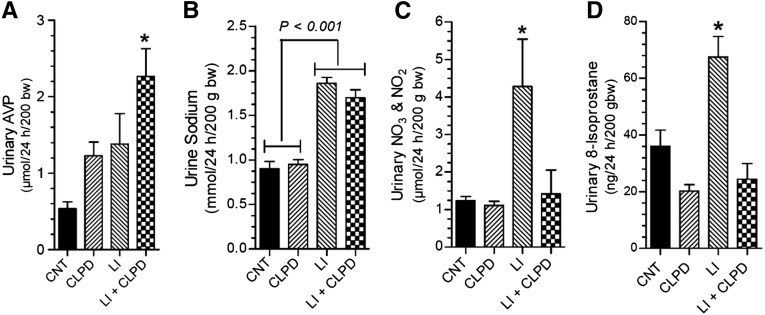
Administration of clopidogrel significantly ameliorated polydipsia, polyuria, and decrease in urine osmolality (Figure 4, A–C) and reversed the increase in electrolyte-free water excretion (Figure 4D) induced by Li feeding to rats for 2 weeks. These were associated with significant improvement in Li-induced decrease in AQP2 protein in the inner medulla (Figure 4, E and F). IF labeling for AQP2 protein in the inner medullas of control rats was predominantly on the apical aspect of the medullary CD cells (Figure 5A). In Li-treated rat kidneys the AQP2 labeling in the medullary CD was scanty (Figure 5B). After clopidogrel administration, AQP2 labeling was seen all over the cells, with relatively higher intensity on the apical domain (Figure 5C). In rats coadministered with Li and clopidogrel, the intensity of AQP2 labeling improved with predominant apical labeling (Figure 5D).
Figure 4.
Effect of clopidogrel administration on Li-induced polyuria and decrease in AQP2 protein abundance in rats. (A) Water intake; (B) urine output; (C) urine osmolality; and (D) electrolyte-free water clearance on the last day (day 13). CNT, control group; CLPD, clopidogrel-treated group; LI, Li-treated group; LI+CLPD, combined treatment with Li and clopidogrel. n=7 rats/group for (A), (B), and (C); n=4–5 rats for (D). *P<0.05 versus Li groups by ANOVA followed by Tukey–Kramer multiple comparison test [(A)–(D)]. (E) Representative immunoblot of AQP2 and β-actin proteins run with inner medullary tissue samples. (F) Densitometry of AQP2 protein bands in Li and Li+CLPD groups relative to β-actin protein bands; P value by Mann–Whitney nonparametric method.
Figure 5.
Effect of clopidogrel treatment on Li-induced cellular expression and disposition of AQP2 (green) and P2Y12-R (red) proteins in rat IMCD. Representative low magnification profiles of IF labeling for AQP2 and P2Y12-R in the renal medullas of rats treated with no drug (A), Li (B), clopidogrel (C), or a combination of Li and clopidogrel (D). Insets show corresponding higher magnification profiles. Bar 20 µm.
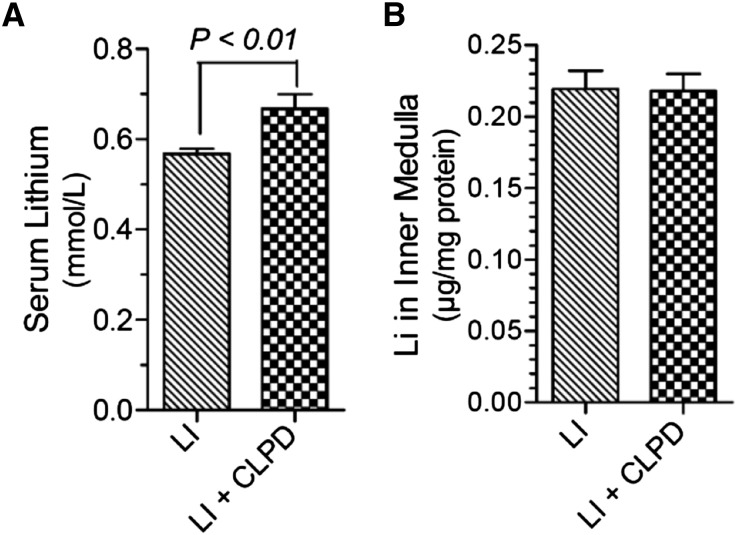
Clopidogrel administration augmented Li-induced increase in urinary AVP excretion (Figure 6A). Administration of clopidogrel had no significant effect on Li-induced natriuresis (Figure 6B), but effectively reversed the significant increases in urinary total nitrates/nitrites, an index of production of nitric oxide (Figure 6C), and 8-isoprostane, a measure of oxidative stress (Figure 6D). Finally, clopidogrel did not decreased lithium levels in the blood (Figure 7A) or in the inner medulla (Figure 7B).
Figure 6.
Effect of clopidogrel on Li-induced alterations in urinary parameters. (A) Urine AVP; (B) urine sodium; (C) urine total nitrates (NO3) and nitrites (NO2); and (D) urine 8-isoprostane. bw, body weight; CNT, control group; CLPD, clopidogral group; LI, Li alone; LI+CLPD, combination of Li and CLPD. n=4–6 rats per group. *Significantly different from all other groups by ANOVA followed by Tukey–Kramer multiple comparison test.
Figure 7.
Effect of clopidogrel on Li disposition in the rat. (A) Terminal Li levels in serum (n=6 rats per group). (B) Li accumulation in the inner medulla (n=4–5 rats per group). Statistical analysis by unpaired t test. LI, Li alone; LI+CLPD, combination of Li and CLPD.
Blockade of P2Y12-R Enhanced the Effect of Desmopressin in Rat IMCD
Interestingly, clopidogrel administration per se significantly increased urinary excretion of AVP (0.54±0.09 in controls versus 1.23±0.18 in clopidogrel treated, in µmol/24 h per 200 g body wt, n=4/group, P<0.01). Preliminary studies revealed expression of P2Y12-R mRNA in rat hypothalamus and significant increase in AVP mRNA expression by selective blockade of P2Y12-R by PSB-0739 in cultured rat hypothalamic cells (Supplemental Figure 4). These findings suggest that the observed increase in urinary AVP excretion in clopidogrel-treated rats was apparently mediated through the blockade of P2Y12-R in hypothalamus. In line with this, in the clopidogrel alone treated group, there was a significant increase in AQP2 protein abundances in the renal cortex and inner medulla (Supplemental Figure 5). These effects were apparently mediated by an increase in AVP levels and by potentiation of AVP effects on the CD by the blockade of P2Y12-R (subsequently discussed). However, the effects of clopidogrel were not mediated through UTP/ATP-activated P2Y2-R, because when administered to P2Y2-R knockout and wild-type mice, clopidogrel had identical effects on urinary concentration (Supplemental Figure 6).
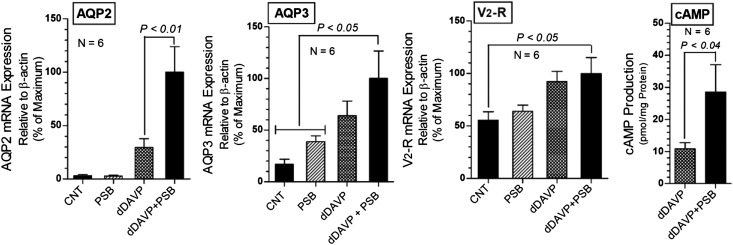
To investigate whether blockade of P2Y12-R had a direct effect on the CD, we performed experiments on primary cultures of rat IMCD cells, where we clamped the concentration of desmopressin (dDAVP; a V2 receptor specific analog of AVP) and used PSB-0739, a selective and reversible antagonist of P2Y12-R. Unlike clopidogrel, PSB-0739 is not a prodrug and therefore is suitable for cell cultures. Blockade of P2Y12-R with PSB-0739 significantly increased dDAVP-induced AQP2 expression by 3.4-fold, but PSB-0739 alone had no effect on AQP2 expression, and enhanced the dDAVP-induced AQP3 expression (Figure 8). PSB-0739 did not augment the increase in dDAVP-induced expression of V2 receptor. However, combination of dDAVP and PSB-0739 significantly increased V2 receptor expression. In parallel cultures, we observed that PSB-0739 significantly enhanced the dDAVP-induced cAMP production (Figure 8). Taken together, these data suggest that blockade of P2Y12-R in IMCD in the absence of dDAVP had little or no effect, but it markedly potentiated the effect of dDAVP on AQP2 and AQP3 expression and on cAMP production. Interestingly, the lack of effect of PSB-0739 alone in CD cells is further supported by our observation that clopidogrel per se had no effect on the urinary concentration in Brattleboro rats that lack AVP (Supplemental Figure 7). Therefore, the observed effect of blockade of P2Y12-R on urinary concentration in Sprague–Dawley rats is dependent on AVP.
Figure 8.
Effect of blockade of P2Y12-R on dDAVP effect in primary cultures of rat IMCD cells. Effect of PSB-0739 (100 nM), a selective antagonist of P2Y12-R on dDAVP (50 nM) induced expression of AQP2 and AQP3 water channels and vasopressin V2 receptor mRNA and cAMP production. PSB-0739 was added 24 hours before the addition of dDAVP, and the incubation was continued for another 24 hours. Results of two independent experiments were pooled to obtain n=6 for each condition (mean±SEM). For gene expression, before pooling the data, the values were normalized as percent maximum response for each set of culture. Statistical significance shown above the bars is derived by ANOVA followed by Tukey–Kramer multiple comparison test (AQP2, AQP3, and V2-R) or unpaired t test (cAMP). CNT, controls; dDAVP, desmopressin; PSB, PSB-0739.
Discussion
We demonstrated that (1) P2Y12-R is expressed in rat kidney and is localized in the CD cells; (2) administration of clopidogrel, a selective and irreversible antagonist of P2Y12-R ameliorated Li-induced NDI in rats, without decreasing Li levels in the blood or in the renal inner medulla; and (3) selective blockade of P2Y12-R by PSB-0739 in primary cultures of rat IMCD cells potentiated the expression of AQP2 and AQP3 and cAMP production induced by dDAVP. In parallel, we documented that administration of clopidogrel alone increased urinary concentrating ability and AQP2 protein abundance in rat kidney in an AVP-dependent manner. Our observations define a new role of P2Y12-R in the kidney and suggest that pharmacologic blockade of P2Y12-R can be a new strategy for the development of a new class of drugs for the treatment of conditions associated with AVP resistance.
The finding that PSB-0739 augmented the effect of dDAVP on gene expression of AQP2 and AQP3 and enhanced cAMP production provides direct evidence that blockade of P2Y12-R per se has marked effect on CD and the observed in vivo effect of clopidogrel in rats is not just caused by increased levels of AVP. It is possible that under basal conditions P2Y12-R through Gi may exert tonic inhibitory effect on the AC in the CD. When P2Y12-R is blocked by clopidogrel/PSB-0739, the tonic inhibition is relieved; therefore, the effect of AVP/dDAVP is markedly enhanced. The subdued activity of phospholipase C during the blockade of P2Y12-R may also contribute to the potentiation of AVP/dDAVP effect on CD. However, in the absence of AVP, PSB-0739 alone has no effect on AQP2 gene expression in the cultured IMCD cells, which is consistent with our finding of lack of effect of clopidogrel in Brattleboro mutant rats.
Clopidogrel not only prevented Li-induced polyuria but reversed the significant increase in electrolyte-free water excretion, which suggest that clopidogrel is acting on CD. The latter is in turn supported by the improved abundance and cellular disposition of AQP2 protein in the renal medulla and CD, respectively. It is also clear that the ameliorating effect of clopidogrel was not caused by impaired absorption of Li in the intestines, resulting in lower blood Li levels or reduced accumulation of Li in the renal medulla. Although administration of clopidogrel augmented Li induced increase in urinary AVP excretion, one cannot attribute this effect of clopidogrel per se to its ability to ameliorate Li-induced NDI because Li-induced NDI is caused by the resistance of the kidney to circulating AVP levels, which are often high. Because we showed that in primary cultures of rat IMCD cells blockade of P2Y12-R potentiates dDAVP effect on AQP2 water channel expression and cAMP production, it is reasonable to suggest that blockade of P2Y12-R by clopidogrel might have sensitized the CD during Li treatment and therefore relieved the NDI.
In addition to its effect on the CD, the blockade of P2Y12-R had other beneficial effects on the kidney that might contribute to amelioration of Li-induced NDI. These are the reversal of Li-induced increase in nitric oxide production and oxidative stress. Endogenously derived nitric oxide exerts tonic inhibition on the expression of sodium transporters,21 which in turn may contribute to impaired urinary concentrating ability in Li-induced NDI. Oxidative stress is known to repress expression of several genes, including transcription factors22; however, less is known about its significance in relation to renal transport functions.23
The significance of predominant expression of P2Y12-R on the brush border of proximal tubules is not known at this stage. However, P2Y12-R does not seem to have an effect on the bulk absorption of sodium because urinary sodium levels were not altered by the administration of clopidogrel alone or in combination with Li. A parallel study conducted in a mouse model, where we analyzed the sodium transporter/channel/exchanger profile, also did not support a major role for the proximal tubules.24
Li-induced NDI is associated with CD remodeling,25 with a decreased number of principal cells and an increased number of intercalated cells.26,27 Hence, there is a possibility that the observed effect of clopidogrel administration on Li-induced decrease in AQP2 protein may be related to its ability to interfere in CD remodeling. However, the IF data in clopidogrel- and/or Li-treated rats do not suggest that possibility in our short-term model.
Finally, previous reports showed that P2Y12-R may contribute to early mesangial cell transformation and renal vessel hypertrophy28 or altered renal autoregulation,29 both in the setting of angiotensin II–induced hypertension. Recent studies by Morel et al.30,31 also demonstrated an impaired platelet P2Y12-R inhibition in CKD, which appears to be related to the effect of pathophysiologic milieu of CKD on platelet activity. Furthermore, most clinical studies on clopidogrel bisulfate were designed with the criteria of evaluation on the basis of cardio- or cerebrovascular events, but not renal effects, and therefore were prone to miss the findings documented in our study.
Concise Methods
Animal Experiments
Animal procedures were approved by the Institutional Animal Care and Use Committees of the VA Salt Lake City Health Care System and the University of Utah. Male Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Brattleboro mutant rats that lack AVP (HsdBlu:BRAT-Avpdi) were obtained from the Rat Resource & Research Center, University of Missouri (Columbia, MO). All animals had free access to drinking water and standard food during the experimental period. NDI was induced by feeding Li-added food (40 mmol LiCl/kg food; MP Biomedicals, Solon, OH) to rats for 13 days. Clopidogrel bisulfate was administered orally by mixing finely powdered clopidogrel bisulfate tablets (Bristol-Myers Squibb, New York, NY) in drinking water. The concentration of the drug in the drinking water was adjusted daily on the basis of water consumption of the animals on the previous day. Sprague–Dawley rats received 20 mg clopidogrel/kg body wt per day, whereas the Brattleboro rats received double this dose (40 mg/kg body wt per day). The rationale for the doses of clopidogrel used is provided in the Supplemental Material. Twenty-four hour urine samples were collected by placing the animals in plastic metabolic cages (1 rat per cage). Blood and kidney tissues were collected at necropsy. Cortical, outer, and inner medullary regions of the kidneys were dissected out and processed for laboratory analysis. In some untreated Sprague–Dawley rats, samples of cerebral cortex and whole hypothalamic tissue were collected and processed.
Synthesis and Characterization of PSB-0739
PSB-0739 (1-amino-4-[4-phenylamino-3-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate) is a highly potent, selective, reversible non-nucleotide antagonist of P2Y12-R that is not toxic to the cells.32,33 Unlike clopidogrel, PSB-0739 does not require bioactivation; therefore, it is suitable for use in cell cultures. PSB-0739 was designed, synthesized, purified, and characterized in the laboratory of Dr. Christa Müller at the University of Bonn, Bonn, Germany.32,34
Blood, Urine, and Tissue Analysis
Osmolalities of clear urine samples were measured by vapor pressure method (Wescor, Logan, UT). Sodium, potassium, and Li levels in serum and/or urine were determined on EasyElectrolytes (Medica, Bedford, MA). On the basis of serum and urine analysis, electrolyte-free water clearance [CH2O(e)] was computed using the formula CH2O(e)=V(1–UNa+UK)/PNa, where V=urine volume, UNa and UK are urine Na and K concentrations, and PNa is plasma Na concentration, respectively. Li levels in the inner medullary tissue homogenates were assayed by inductively coupled plasma-mass spectrometry (Exova, Santa Fe Springs, CA) and were normalized to their respective protein contents. Inductively coupled plasma-mass spectrometry has a detection limit of 0.06 parts per million for Li, with recovery of 91% in spiked samples. Li was not detectable in the homogenization medium (0.5% SDS and 0.02% sodium azide in ultrapure water). Commercial enzyme immunoassay/ELISA or colorimetric assay kits were used to quantify urinary excretion of AVP (Enzo Life Sciences, Farmingdale, NY), total nitrates and nitrites (NO3/NO2) (Cayman Chemical, Ann Arbor, MI), and 8-isoprostane (Oxford Biomedical Research, Oxford, MI) as per the manufacturers’ instructions and published in our previous study.35
Detection of mRNA by Real-Time RT-PCR Assay
This was performed essentially as described previously.16,35,36 Briefly, total RNA was extracted from samples of kidney cortex, outer and inner medullas, cerebral cortex, and whole hypothalamus. cDNA was synthesized by SuperScript Reverse transcription (Invitrogen, Carlsbad, CA). Using gene-specific primers (Supplemental Table 2), real-time PCR amplifications were carried out on cDNA samples in Applied Biosystems 7500 Real-Time PCR System (Foster City, CA), with AmpliTaq Gold and SYBR Green for detection. Expression of target genes was normalized to that of expression of the housekeeping gene β-actin.
Generation and Characterization of P2Y12-R Antibody
We designed and generated a peptide-derived rabbit polyclonal antibody for a 16-amino acid C-terminus sequence (KKKGQEGGEPSEETPM) of mouse P2Y12-R (accession no. Q9CPV9), which differs from the corresponding rat sequence by one amino acid residue. This peptide has no sequence homology with related proteins, such as P2Y13, P2Y14, GPR34, GPR82, GPR87, or GPR171 (Supplemental Table 1). Peptide synthesis and purification, coupling to keyhole limpet hemocyanin, immunization of the rabbits, and determination of antibody titers by ELISA were contracted to Thermo Fisher Scientific (Rockford, IL). Affinity purification of the antibody and isolation of preimmune IgG fractions and characterization of the specificity of the antibody were done in our laboratories as described previously.36,37
P2Y12-R Knockdown and Overexpression
The specificity of the antibody was evaluated using knockdown and overexpression approaches in cells. IMCD3 cells, a mouse inner medullary CD cell line (ATCC, Manassas, VA), were maintained in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and F-12 (Life Technologies, Grand Island, NY), supplemented with 10% FBS (Life Technologies) at 37°C with 5% CO2. For transfection via electroporation, IMCD3 cells were grown to 80%–90% confluence, trypsinized, and resuspended in warm complete medium. Cells were pelleted, suspended in Nucleofector solution (Lonza Cell Transfection System), and transfected with mouse P2ry12 SureSilencing shRNA, GFP plasmids (Qiagen, Valencia, CA). DNA from the four individual P2Y12 shRNA plasmids (1.5 µg of each plasmid, totaling 6 µg) were mixed with the suspended cells (0.5×106 cells). Cells were transfected using the program A24 on the transfection device (Lonza Amax Biosystems Nucleofector I). Each transfection was plated in an individual well of a 12-well plate. Two days after transfection, the cells were processed for either immunoblotting or IF microscopy. For immunoblotting, cells were washed with PBS and collected into 1× blue loading buffer containing DTT (Cell Signaling, Danvers, MA). Lysed cells were homogenized using TissueLyser (Qiagen) for 10 minutes and heated at 95°C for 5 minutes. The samples were processed for Western blotting. For IF microscopy, cells growing on chamber slides were washed briefly with PBS and then fixed in 2% paraformaldehyde for 20 minutes and permeabilized in 0.2% Triton X-100. P2Y12-R antibody (which we generated) was used in combination with Cy5 anti-rabbit secondary antibody (Thermo Fisher Scientific, Pittsburgh, PA) to detect the receptor protein in the transected IMCD3 cells. Propidium iodide (Sigma-Aldrich, St. Louis, MO) was used to stain the nuclei. Cells were visualized in Personal Confocal Microscope PCM-2000 (Nikon).
In another experiment, lysates of HEK293T cells overexpressing human P2Y12-R and control lysates were purchased from the Novus Biologicals. The overexpression cells were created using plasmid ID RC205005 and on the basis of accession number NM_022788. The cells were lysed in modified RIPA buffer containing protease cocktail mixture by the Novus and were shipped to us. The control and overexpressing cell lysates were processed for immunoblotting using our P2Y12-R antibody.
Immunoblotting
Processing of the kidney tissue samples and immunoblotting were performed as described previously.13,16 Negative controls for P2Y12-R antibody were generated by preadsorbing the affinity purified antibody with a molar excess of the immunizing peptide or by substituting the antibody with preimmune IgG fraction. Generation and characterization of rabbit polyclonal antibody (GN-762) to AQP2 was described previously.37 Rabbit polyclonal antibody to AQP1 was a kind gift from Dr. Janet Klein (Emory University, Atlanta, GA), and β-actin rabbit polyclonal antibody was purchased from Biolegend (San Diego, CA). Where applicable, protein band densities were determined, and differences in protein loading were corrected by normalizing the band densities of the target proteins to that of β-actin.
Localization of P2Y12-R Protein by Immunoperoxidase Labeling
Formalin-fixed and paraffin-embedded kidney sections (5 µm) were processed for immunoperoxidase labeling as described previously,36 except for the following modifications. Dako target retrieval solution (Dako North America, Carpinteria, CA) was used for antigen retrieval. Negative controls for labeling were generated by incubating the sections with P2Y12-R antibody preadsorbed with a molar excess of the immunizing peptide or preimmune IgG fraction in lieu of the affinity purified P2Y12-R antibody. Sections were counterstained with methyl green.
Localization of P2Y12-R with AQP2 Protein by Confocal IF
This was performed essentially as described previously.36 Sections were probed with P2Y12-R rabbit polyclonal antibody overnight followed by incubation with secondary Alexa Fluor 594-conjugated goat anti-rabbit antibody (Invitrogen) for 1 hour. Some sections were double labeled first by incubation with AQP2 mouse monoclonal antibody, followed by incubation for 1 hour with goat anti-mouse secondary Alexa Fluor 488-conjugated antibody. DAPI (4′,6-diamino-2-pheylindole) was used to stain the nuclei.
Primary Cultures of Rat Inner Medullary CD Cells
Primary cultures of rat IMCD cells were prepared as described previously38 with the following modifications. Freshly obtained inner medullas were cut into small pieces and digested in PBS containing 1 mg/ml collagenase B (Roche Applied Sciences, Indianapolis, IN) and 0.1 mg/ml DNase (Sigma-Aldrich) at 37°C for 1 hour. After that, non-IMCD cells were eliminated by osmotic shock by adding two volumes of sterile water. The suspension was filtered through 100-µm cell strainer and centrifuged at 1000 rpm for 5 minutes. The pellet was washed with 10% BSA in PBS, suspended in modified defined medium (DMEM/Ham’s F12; composition given in the Supplemental Material) containing 10% FBS, and seeded into Transwell semipermeable support inserts (Corning Costar, Cambridge, MA). Cells were incubated at 37°C in an atmosphere of 5% CO2. The medium was replaced every 2 days, and when the cells became 70%–80% confluent, the medium was changed to the starvation one (composition given in the Supplemental Material). After 24 hours the test agents, namely dDAVP (desmopressin; Sigma-Aldrich) and/or PSB-0739, were added, and the incubation continued for another 48 hours. After removing the medium, cells were collected into Trizol reagent for total RNA extraction. For cAMP assay, the medium was gently aspirated an 200 µl of 0.1 M HCl was added to each insert and incubated for 20 minutes at room temperature. Then cells were scrapped off the semipermeable support and lysed by pipetting up and down. The cell lysate was centrifuged at 1000 xg for 10 minutes. cAMP concentration in the supernatant was determined by an EIA Kit (Cayman Chemical) and normalized to the protein content (Pierce BCA Protein Assay Kit; Rockford, IL).
Statistical Analyses
All quantitative data were expressed as mean±SEM. Comparisons among the means of multiple groups were made by ANOVA, followed by the assessment of statistical significance by Tukey–Kramer multiple comparison test or Student–Newman–Keuls multiple comparison test of Bonferroni test. Differences between the means of two groups were determined by unpaired t test or where applicable, by Mann–Whitney nonparametric test. P values <0.05 were considered significant. GraphPad Instat (GraphPad Software, La Jolla, CA) was used for statistical analyses.
Disclosures
The use of P2Y12 receptor selective antagonists for the treatment of NDI is proprietary to the US Department of Veterans Affairs and the University of Utah Research Foundation and is protected by a pending patent application with the US Patent and Trademark Office.
Supplementary Material
Acknowledgments
We thank Dr. Janet D. Klein and Dr. Andrew Weyrich lab for generously providing AQP1 antibody and human platelet lysates, respectively, and Linda Schmidt for expert technical assistance. Dr. Beverly Koller kindly supplied breeders of P2Y2-R knockout mice.
Supported by grants from the US Department of Veterans Affairs Merit Review Program (to B.K.K.) and the National Kidney Foundation of Utah and Idaho (to B.K.K.) and resources and facilities at the Veterans Affairs Salt Lake City Health Care System (Salt Lake City, UT). J.P.-P. and D.E.K. were supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant nos. DK-64324, DK-100944 and DK-097007, respectively).
Parts of the data were presented at the American Society of Nephrology’s Kidney Week 2011; November 8–13, 2011; Philadelphia, Pennsylvania; and at the American Society of Nephrology’s Kidney Week; November 5–10, 2013; Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010118/-/DCSupplemental.
References
- 1.Brown D, Bouley R, Păunescu TG, Breton S, Lu HA: New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells. Am J Physiol Cell Physiol 302: C1421–C1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE: P2Y(2) receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallon V, Rieg T: Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radin MJ, Yu MJ, Stoedkilde L, Miller RL, Hoffert JD, Frokiaer J, Pisitkun T, Knepper MA: Aquaporin-2 regulation in health and disease. Vet Clin Pathol 41: 455–470, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olesen ET, Fenton RA: Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol 24: 169–178, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Trepiccione F, Christensen BM: Lithium-induced nephrogenic diabetes insipidus: New clinical and experimental findings. J Nephrol 23[Suppl 16]: S43–S48, 2010 [PubMed] [Google Scholar]
- 7.Grünfeld JP, Rossier BC: Lithium nephrotoxicity revisited. Nat Rev Nephrol 5: 270–276, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Sands JM, Bichet DG, American College of Physicians. American Physiological Society : Nephrogenic diabetes insipidus. Ann Intern Med 144: 186–194, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Finley PR, Warner MD, Peabody CA: Clinical relevance of drug interactions with lithium. Clin Pharmacokinet 29: 172–191, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Murray MD, Brater DC: Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol 33: 435–465, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Phelan KM, Mosholder AD, Lu S: Lithium interaction with the cyclooxygenase 2 inhibitors rofecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs. J Clin Psychiatry 64: 1328–1334, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK: Potential role of purinergic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296: F1194–F1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kohan DE, Nelson RD, Carlson NG, Kishore BK: Potential involvement of P2Y2 receptor in diuresis of postobstructive uropathy in rats. Am J Physiol Renal Physiol 298: F634–F642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V: Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK: Potential role of purinergic signaling in urinary concentration in inner medulla: Insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715–F1724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Pop IL, Carlson NG, Kishore BK: Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302: F70–F77, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK: Attenuation of lithium-induced natriuresis and kaliuresis in P2Y2 receptor knockout mice. Am J Physiol Renal Physiol 305: F407–F416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schöneberg T, Hermsdorf T, Engemaier E, Engel K, Liebscher I, Thor D, Zierau K, Römpler H, Schulz A: Structural and functional evolution of the P2Y(12)-like receptor group. Purinergic Signal 3: 255–268, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalantzi KI, Tsoumani ME, Goudevenos IA, Tselepis AD: Pharmacodynamic properties of antiplatelet agents: Current knowledge and future perspectives. Expert Rev Clin Pharmacol 5: 319–336, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Hervé C, Uzabiaga MF, Pereillo JM, Culouscou JM, Bono F, Ferrara P, Herbert JM: The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci U S A 103: 11069–11074, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JS, Choi KC, Jeong MH, Kim SW, Oh YW, Lee JU: Increased expression of sodium transporters in rats chronically inhibited of nitric oxide synthesis. J Korean Med Sci 21: 1–4, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel Y, Barouki R: Repression of gene expression by oxidative stress. Biochem J 342: 481–496, 1999 [PMC free article] [PubMed] [Google Scholar]
- 23.Palm F, Nordquist L: Renal oxidative stress, oxygenation, and hypertension. Am J Physiol Regul Integr Comp Physiol 301: R1229–R1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Carlson NG, Kishore BK, Ecelbarger CM: Pharmacological blockade of P2Y12 receptor reverses lithium-induced downregulation of NKCC2, NCC and α-ENaC protein abundances in mice [Abstract]. J Am Soc Nephrol 24: 537A, 2013. 23334390 [Google Scholar]
- 25.Kishore BK, Ecelbarger CM: Lithium: A versatile tool for understanding renal physiology. Am J Physiol Renal Physiol 304: F1139–F1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Christensen BM, Marples D, Kim YH, Wang W, Frøkiaer J, Nielsen S: Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG: Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161–F169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osmond DA, Zhang S, Pollock JS, Yamamoto T, De Miguel C, Inscho EW: Clopidogrel preserves whole kidney autoregulatory behavior in ANG II-induced hypertension. Am J Physiol Renal Physiol 306: F619–F628, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, Caillard S, Campia U, Moulin B, Gachet C, Ohlmann P: Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol 57: 399–408, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Morel O, Muller C, Jesel L, Moulin B, Hannedouche T: Impaired platelet P2Y12 inhibition by thienopyridines in chronic kidney disease: Mechanisms, clinical relevance and pharmacological options. Nephrol Dial Transplant 28: 1994–2002, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Baqi Y, Atzler K, Köse M, Glänzel M, Müller CE: High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem 52: 3784–3793, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann K, Baqi Y, Morena MS, Glänzel M, Müller CE, von Kügelgen I: Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther 331: 648–655, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Baqi Y, Müller CE: Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. Nat Protoc 5: 945–953, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK: Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol 300: F657–F668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishore BK, Zhang Y, Gevorgyan H, Kohan DE, Schiedel AC, Müller CE, Peti-Peterdi J: Cellular localization of adenine receptors in the rat kidney and their functional significance in the inner medullary collecting duct. Am J Physiol Renal Physiol 305: F1298–F1305, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA: Cellular localization of P2Y(2) purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Strait KA.Stricklett PK, Kohan JL, Miller MB, Kohan DE: Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol 293: F601–F606, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.