Abstract
Expression of thioredoxin-interacting protein (TxNIP), an endogenous inhibitor of the thiol oxidoreductase thioredoxin, is augmented by high glucose (HG) and promotes oxidative stress. We previously reported that TxNIP-deficient mesangial cells showed protection from HG-induced reactive oxygen species, mitogen-activated protein kinase phosphorylation, and collagen expression. Here, we investigated the potential role of TxNIP in the pathogenesis of diabetic nephropathy (DN) in vivo. Wild-type (WT) control, TxNIP−/−, and TxNIP+/− mice were rendered equally diabetic with low-dose streptozotocin. In contrast to effects in WT mice, diabetes did not increase albuminuria, proteinuria, serum cystatin C, or serum creatinine levels in TxNIP−/− mice. Whereas morphometric studies of kidneys revealed a thickened glomerular basement membrane and effaced podocytes in the diabetic WT mice, these changes were absent in the diabetic TxNIP−/− mice. Immunohistochemical analysis revealed significant increases in the levels of glomerular TGF-β1, collagen IV, and fibrosis only in WT diabetic mice. Additionally, only WT diabetic mice showed significant increases in oxidative stress (nitrotyrosine, urinary 8-hydroxy-2-deoxy-guanosine) and inflammation (IL-1β mRNA, F4/80 immunohistochemistry). Expression levels of Nox4-encoded mRNA and protein increased only in the diabetic WT animals. A significant loss of podocytes, assessed by Wilms’ tumor 1 and nephrin staining and urinary nephrin concentration, was found in diabetic WT but not TxNIP−/− mice. Furthermore, in cultured human podocytes exposed to HG, TxNIP knockdown with siRNA abolished the increased mitochondrial O2− generation and apoptosis. These data indicate that TxNIP has a critical role in the progression of DN and may be a promising therapeutic target.
Keywords: diabetes mellitus, diabetic nephropathy, oxidative stress
Diabetic nephropathy (DN), a microvascular complication of diabetes mellitus, is the most frequent cause of ESRD in developed countries.1–3 In DN, hyperglycemia contributes to the morphologic alterations of the glomerular filtration barrier and loss of podocytes, ultimately leading to impairment of renal filtration.1,2 Although chronic hyperglycemia is the major cause, the detailed molecular pathogenesis of DN remains unclear.3
Among many pathogenic factors, an excessive production of reactive oxygen species (ROS) is thought to be a major contributor.1,4–6 The presence of excess ROS leads to the oxidation of proteins, DNA, and lipids, consequently deranging their configuration and function.7,8 In addition, by inhibiting glycolytic flux at the level of glyceraldehyde phosphate dehydrogenase, increased oxidative stress drives glucose metabolic pathways; these pathways upregulate signals triggered by advanced glycation endproducts, protein kinase C, and O-GlcNAcylation, which induce the pathologic changes seen in DN.6,8
One protein relevant to the regulation of ROS, which is markedly upregulated by high glucose (HG) in various cell types, is thioredoxin-interacting protein (TxNIP), also known as vitamin D upregulated protein 1 or thioredoxin binding protein.9–13 Under HG conditions, TxNIP has been implicated in impairing ROS scavenging by binding to and attenuating the antioxidant action of thioredoxin, a ubiquitous oxidoreductase.14 In addition, we recently reported that TxNIP enhances HG-induced mitochondria-derived O2− (superoxide) as well as cellular ROS, at least in part, by augmenting the mitochondrial and cytosolic NADPH oxidase isoform, Nox4, in cultured mesangial cells.15 Thus, by increasing the generation and decreasing the degradation of ROS, HG-induced TxNIP may contribute to glucotoxicity, as observed by others and our group in pancreatic β cells.13,16,17
To elucidate the role of TxNIP in the pathogenesis of DN in vivo, we explored the action of TxNIP in the diabetic kidney of wild-type (WT) and TxNIP−/− (knockout [KO]) mice. In streptozotocin (STZ)-induced diabetic mice we found that, unlike in WT, indicators of renal injury and dysfunction such as albuminuria, proteinuria, serum cystatin C, and serum creatinine levels were not increased in diabetic TxNIP KO mice. Furthermore, histopathologic features of DN, such as effacement of podocyte foot processes and reduction in number of podocytes, thickening of the glomerular basement membrane (GBM), expansion of the mesangial matrix, and glomerulosclerosis, were all markedly attenuated in the diabetic KO, as were markers of ROS and inflammation. These data indicate that TxNIP deficiency protects against the renal complications of diabetes.
Results
TxNIP Deficiency Prevents Albuminuria in STZ-Induced Diabetes and Loss of Renal Function
To investigate the role of TxNIP in DN, we induced type 1 diabetes in TxNIP+/+ (WT) and TxNIP−/− (KO) mice for 24 weeks. Results of the blood profile, fluid balance, renal measures, and BP are shown in Tables 1 and 2. Equivalent degrees of hyperglycemia, assessed by glucose concentrations and hemoglobin A1c, were achieved (Table 1). Furthermore, water consumption and urinary fluid excretion were similarly elevated (Table 2). Serum insulin levels were significantly decreased by diabetes but not different between WT and TxNIP KO. Body weights tended to be higher in TxNIP−/− mice but were lower in both diabetic groups (P=NS). Kidney weight/body weight ratios tended to be increased in the diabetic state.
Table 1.
Blood profile of WT and KO nondiabetic or STZ-induced diabetic mice 24 weeks after the first set of STZ injections
| Blood Profile | TxNIP- WT | TxNIP- KO | ||
|---|---|---|---|---|
| Nondiabetic | Diabetic | Nondiabetic | Diabetic | |
| Blood glucose (mmol/L) | 6.47±0.38 | 21.76±1.40a | 5.99±0.32 | 20.33±1.73b |
| Plasma glucose (mmol/L) | 9.68±0.64 | 21.68±1.49a | 8.5±1.09 | 20.83±1.60b |
| HbA1c (%) | 4.53±0.06 | 6.93±0.40c | 4.2±0.15 | 6.62±0.58d |
| Hb (g/L) | 143±11.67 | 145±3.49 | 133.5±11.72 | 133±7.43 |
| RBC (1012 cells/L) | 9.24±0.69 | 9.21±0.33 | 8.51±0.76 | 8.32±0.50 |
| WBC (109 cells/L) | 5.32±0.81 | 10.28±0.57e | 6.97±1.07 | 9.57±2.96 |
| Serum ketone bodies: β-hydroxybutyrate (nmol) | 0.12±0.02 | 0.2±0.036 | 0.28±0.06 | 0.30±0.01 |
| Serum insulin (ng/ml) | 0.25±0.04 | 0.16±0.01f | 0.21±0.03 | 0.12±0.01f |
| Serum lactate (nmol/L) | 6.67±0.86 | 8.85±0.82 | 9.25±1.44 | 17.4±1.53g |
Values are expressed as mean±SEM. HbA1c, hemoglobin A1c; Hb, hemoglobin; RBC, red blood cell; WBC, white blood cell.
P<0.0001 versus WT nondiabetic mice.
P<0.0001 versus KO nondiabetic mice.
P<0.001 versus WT nondiabetic mice.
P<0.01 versus KO nondiabetic mice.
P<0.01 versus WT nondiabetic mice.
P<0.05 versus nondiabetic WT mice.
P<0.05 diabetic KO mice versus all other groups.
Table 2.
Renal phenotype and metabolic measures of WT and KO nondiabetic or STZ-induced diabetic mice 24 weeks after the first set of STZ injections
| Characteristic | TxNIP-WT | TxNIP-KO | ||
|---|---|---|---|---|
| Non-diabetic | Diabetic | Non-diabetic | Diabetic | |
| Body weight (g) | 28.54±1.33 | 24.87±0.43 | 33.34±0.66 | 28.26±1.76 |
| Left kidney weight (g) | 0.199±0.011 | 0.265±0.005a | 0.242±0.007 | 0.275±0.001b |
| Left kidney weight/body weight | 0.007±0.0001 | 0.012±0.0008 | 0.007±0.0003 | 0.010±0.001 |
| Glomerular volume (×103/μm3) | 327.80±9.64 | 499.96±19.62c | 252.24±14.74 | 256.41±9.87 |
| Aortic systolic BP (mmHg) | 113.4±3.8 | 102.9±2.5a | 125.6±3.5 | 104.4±2.5d |
| Aortic diastolic BP (mmHg) | 80.8±2.5 | 74.9±2.1 | 85.0±2.0 | 69.8±2.5d |
Values are expressed as mean±SEM.
P<0.05 versus WT nondiabetic mice.
P<0.05 versus KO nondiabetic mice.
P<0.01 versus WT nondiabetic mice.
P<0.001 versus KO nondiabetic mice.
Serum lactate was elevated in the KO mice in the diabetic state, consistent with impaired mitochondrial glucose metabolism.15 BPs were lower in both diabetic groups, likely reflecting a degree of volume contraction due to fluid loss (Table 2).
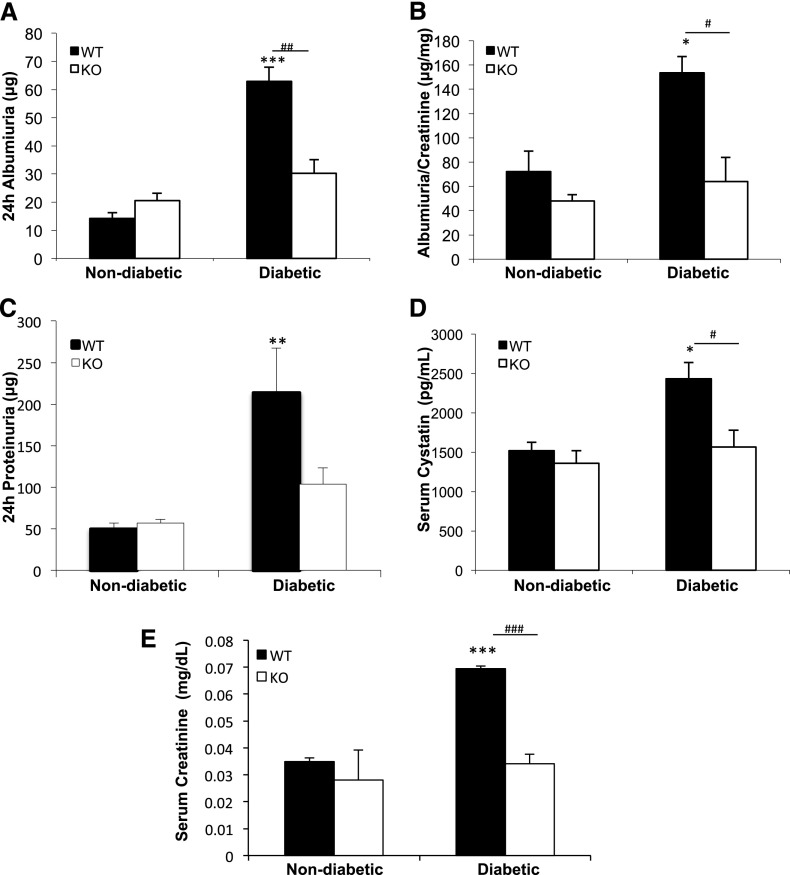
Renal measures were assessed 1 week before euthanasia (23 weeks). Albumin, albumin-to-creatinine ratio, and protein increased significantly in the urine of diabetic TxNIP WT mice but not in the TxNIP KO mice (Figure 1, A–C). Similarly, serum cystatin C, a marker of GFR that is not influenced by extrarenal factors, such as muscle mass, dietary protein, sex, and age (Figure 1D),18 and serum creatinine (Figure 1E) were significantly augmented only in the diabetic TxNIP WT mice. These findings suggest that a lack of TxNIP protects the kidneys from the glomerular manifestations of DN.
Figure 1.
Diabetic TxNIP−/− mice are protected from glomerular filtration barrier dysfunction and renal impairment. Renal measures in nondiabetic and diabetic TxNIP WT and KO mice. (A) Twenty-four-hour albuminuria (μg) and (B) albumin-to-creatinine (μg/mg) ratio were determined using kits from Exocell. (C) Twenty-four-hour urinary protein levels (μg) were detected by the Bradford protein assay. (D) Serum cystatin C (pg/ml), a biomarker of GFR, was measured with an ELISA kit from R&D Systems. (E) Serum creatinine (mg/dl) was measured by HPLC. Results are expressed as mean±SEM (n=6–7 mice/condition). *P<0.05; **P<0.01; ***P<0.001; #P<0.05; ##P<0.01; and ###P<0.001 diabetic WT mice versus nondiabetic WT and diabetic TxNIP KO mice.
We performed similar experiments in TxNIP-deficient Hcb-19 mice (Supplemental Table 1). In the nondiabetic state, these Hcb-19 mice manifested increased body weight along with an absolute increase in kidney weight (but not kidney weight-to-body weight ratio) and an increased albumin-to-creatinine ratio (Supplemental Table 1). However, while relative kidney weight was increased by diabetes in both groups, the TxNIP-deficient Hcb-19 mice showed no increase in albuminuria from baseline in contrast to C3H controls (Supplemental Table 1). Of interest, diabetic TxNIP-HET (TxNIP+/−) mice were also protected from diabetes-induced albuminuria (Supplemental Figure 1). While TxNIP mRNA in the diabetic TxNIP-HET mice was only approximately 50% that of diabetic WT, this still represented a marked increase compared with nondiabetic controls or TxNIP-HET mice (Supplemental Figure 2).
Lack of TxNIP Protects Mice from Diabetes-Induced Renal Fibrosis and Extracellular Matrix Accumulation
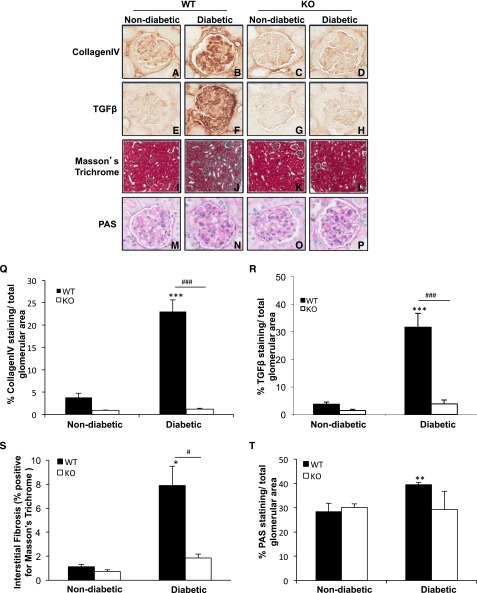
The pathologic characteristics associated with DN in glomeruli were examined. Glomerular collagen IV (Figure 2, A–D) and TGF-β (Figure 2, E–H), key pathologic hallmarks, were significantly elevated (6- and 8.5- fold, respectively) in the diabetic WT mice compared with nondiabetic controls (Figure 2, Q and R). While the staining of these proteins tended to be lower in the nondiabetic TxNIP KO mice compared with WT mice (P=NS), there was a marked protection from the effects of diabetes in the KO mice with minimal increases (P=NS) in collagen IV (Figure 2, C, D, O) and TGF-β (Figure 2, G, H, R). Similarly, the degree of interstitial fibrosis, detected by Masson trichrome staining, was markedly augmented in the diabetic WT mice (Figure 2, I, J, S) but not altered in diabetic TxNIP KO mice (Figure 2, K, L, S). Glomerular extracellular matrix expansion, assessed by periodic acid-Schiff (PAS)–positive area (Figure 2, M, N, T) and glomerular volume (Table 2), were significantly increased only in the diabetic WT mice (Figure 2, O, P, T, and Table 2). The results in our studies of C3H (TxNIP+/+) and Hcb-19 (TxNIP-deficient) mice were consistent with those in TxNIP KO mice: namely, that diabetes increased glomerular PAS, collagen IV, and TGF-β1 staining in C3H mice but that these outcomes were attenuated in the Hcb-19 mice (Supplemental Figure 3, A–F). We also noted that partial TxNIP deficiency in the TxNIP-HET mice protected against the diabetes-induced renal profibrotic state (Supplemental Figure 4). These results support our15 and Kobayashi and colleagues’19 observations that high glucose-stimulated collagen accumulation in cultured mesangial cells requires TxNIP and indicate that TxNIP is necessary for the induction of extracellular matrix deposition and renal fibrosis in diabetes.
Figure 2.
Diabetic TxNIP−/− mice do not develop mesangial matrix expansion and renal fibrosis. Renal fibrosis and mesangial matrix expansion in nondiabetic and diabetic TxNIP WT and KO mice. Representative images of renal fibrosis assessed by collagen IV (A–D) and TGF-β (E–H), as well as renal interstitial fibrosis (I–L; blue-green color detected by Masson trichrome staining). Mesangial matrix expansion was detected using PAS staining (M–P). Quantitation, determined by Visiomorph software (MSH), is shown in the graphs in the bottom panels (Q–T). Results are mean±SEM (n=5 mice/condition). *P<0.05; **P<0.01; and ***P<0.001 diabetic WT mice versus nondiabetic WT; #P<0.05 and ###P<0.001 diabetic WT versus diabetic TxNIP KO mice. Original magnifciation, ×400.
Reduction in Podocyte Number and Increased Urinary Nephrin Is Not Observed in Diabetic TxNIP KO Mice
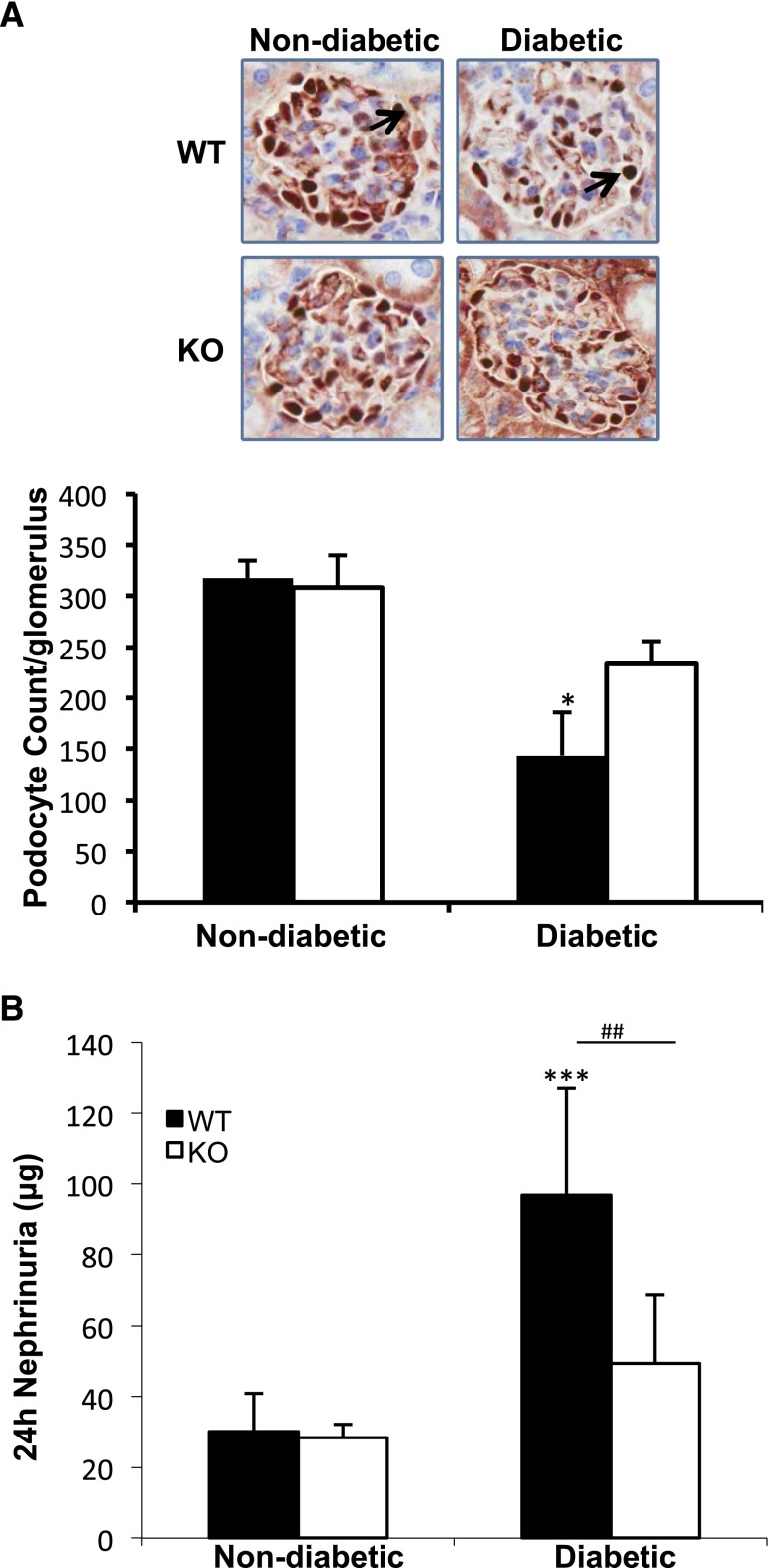
Podocytes function as part of the normal glomerular filtration barrier.20 Podocyte numbers decline in DN, and this decrease signals progression to advanced disease.1,2,21–23 Thus, the numbers of podocytes were determined by staining for (Wilms’ tumor antigen-1 (WT-1), a transcription factor restricted to podocytes.24 The podocyte number per glomerulus was determined as described in the Concise Methods section to correct for changes in glomerular volume. The number of podocytes decreased significantly in diabetic WT mice, while in the TxNIP KO diabetes caused a minimal decrease (P=NS) (Figure 3A). These data were confirmed by staining for nephrin, another marker of podocytes (Supplemental Figure 5). Podocyte loss, associated with apoptosis, has been accompanied by increased urinary excretion of nephrin, a signal transducing and adhesion protein localized to the slit diaphragm.24 Total 24-hour urinary nephrin showed a marked increase caused by diabetes in WT but no significant change in TxNIP KO mice (Figure 3B).
Figure 3.
Diabetic TxNIP−/− mice are protected from podocyte loss. Podocyte loss and nephrinuria in nondiabetic and diabetic TxNIP WT and KO mice. (A) Representative images of WT-1 staining (3-μm section, IHC); arrows indicate glomerular WT-1 stain. The data in the graph (bottom panel) from n=3–5 mice/condition expressed as podocyte/glomerulus (mean±SEM) were obtained from 3-μm and 9-μm sections followed by calculation as described in the Concise Methods. (B) Twenty-four-hour urinary nephrin excretion (μg) was assessed using an ELISA kit from Exocell. Results are expressed as mean±SEM (n=6–7mice/condition). *P<0.05 diabetic WT mice versus nondiabetic WT mice; **P<0.01 and ***P<0.001 diabetic WT mice versus non-diabetic TxNIP KO mice; and ##P<0.01 diabetic WT versus diabetic TxNIP KO mice.
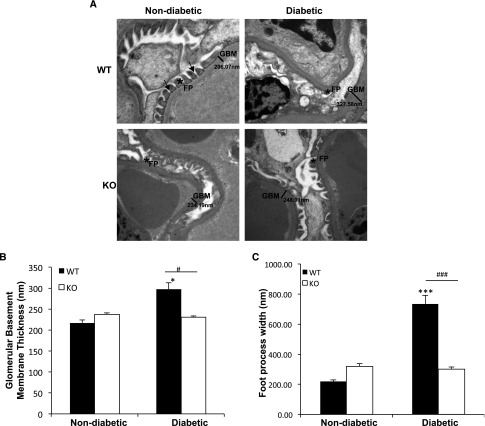
Podocyte Foot Process Effacement and Increased Glomerular Basement Membrane Thickness Are Observed in Diabetic WT but Not in TxNIP KO Mice
Thickening of the GBM and effacement of foot processes are major early ultrastructural changes observed by electron microscopy in DN.1,2 As shown in Figure 4, A and B, GBM thickness increased significantly in the diabetic WT mice but did not change in the diabetic TxNIP KO. Similarly, effacement of the podocyte foot processes was observed only in the diabetic WT mice (Figure 4, A and C). In the TxNIP-HET mice, while we did not observe a significant effect of diabetes on podocyte foot process effacement, GBM thickening increased significantly (P<0.01) (Supplemental Figure 6). Taken together, these results demonstrate that TxNIP contributes to the structural damage of the glomerular filtration barrier during DN.
Figure 4.
Diabetic TxNIP−/− mice do not develop podocyte foot process effacement and glomerular basement membrane thickening. Podocyte foot process effacement and GBM thickness in nondiabetic and diabetic TxNIP WT and KO mice. (A) Representative electron microscopic images (original magnification, approximately ×15,000) of glomeruli from control and diabetic TxNIP WT and KO mice. Arrows indicate slit diaphragm. FP, foot process. (B) Quantification of GBM thickness and (C) podocyte foot process effacement determined as described in the Concise Methods from nine different fields (n=3 mice/condition). Results are mean±SEM. *P<0.05 and ***P<0.001 diabetic WT mice versus nondiabetic WT; #P<0.05 and ###P<0.001 diabetic WT versus diabetic TxNIP KO mice.
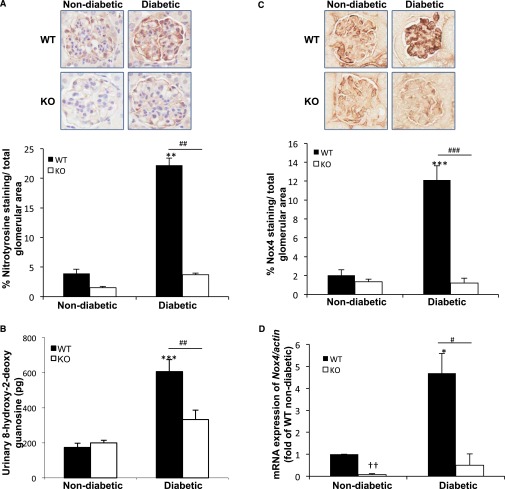
TxNIP Mediates the Augmented Renal ROS/Reactive Nitrogen Species and Inflammation in Diabetic Mice
ROS, reactive nitrogen species, and inflammation are reported to contribute to the progression of DN.25 We previously reported that TxNIP-deficient cultured mesangial cells are resistant to HG-induced stimulation of ROS.15 Therefore, we explored the involvement of TxNIP in the elevation of ROS, marked by urinary (8-hydroxy-2-deoxy guanosine [8-OhdG]), reactive nitrogen species (nitrotyrosine staining), and inflammation in vivo. Expression of nitrotyrosine was significantly increased in the glomeruli of diabetic WT mice (5.6-fold), but no change was observed between the nondiabetic and diabetic KO mice (Figure 5A). Similarly, urinary 8-OHdG levels, an indicator of oxidative DNA damage, was significantly increased in the WT diabetic mice and unchanged in the diabetic KO mice (Figure 5B).
Figure 5.
TxNIP−/− mice do not demonstrate increased ROS/reactive nitrogen species and Nox4 expression in response to hyperglycemia. Evidence of ROS/reactive nitrogen species and Nox4 expression in nondiabetic and diabetic TxNIP WT and KO mice. (A) Representative images of glomerular reactive nitrogen species as indicated by nitrotyrosine staining and quantification (bottom panel) (n=5 mice/condition). (B) Urinary 8-OhdG levels (pg), reflective of ROS, in 24-hour urine collections from 6 to 7 mice/condition. Results are mean±SEM. **P<0.001 and ***P<0.0001 diabetic WT mice versus nondiabetic and diabetic TxNIP KO mice. (C) Representative images and quantitation of glomerular Nox4 protein in (n=5 mice/condition). (D) Expression of Nox4 mRNA, corrected for the housekeeping gene actb and normalized to nondiabetic WT in renal cortex. Results are mean±SEM (n=5 mice/condition). *P<0.05 diabetic WT mice versus nondiabetic WT and diabetic KO; **P<0.01 nondiabetic TxNIP KO mice versus nondiabetic WT; ***P<0.001 diabetic WT mice versus nondiabetic WT; ††P<0.001 nondiabetic KO versus nondiabetic WT, and #P<0.05; ##P<0.001; and ###P<0.0001 diabetic WT versus diabetic TxNIP KO mice.
Of interest, while urinary 8-OHdG was not altered by diabetes in the TxNIP-HET mice, nitrotyrosine staining was significantly increased (Supplemental Figure 7), suggesting that hyperglycemia can induce a degree of oxidative/nitrosative stress in the presence of lower amounts of TxNIP.
Because we found that TxNIP contributes to the increased expression of the NADPH oxidase isoform Nox4 in cultured mesangial cells,15 we examined this in our in vivo model. Both Nox4 protein (glomerular) and Nox4 mRNA (renal cortex) were significantly elevated only in the diabetic WT mice (Figure 5, C and D), supporting our in vitro findings. This was confirmed in the diabetic C3H (control) and Hcb-19 (TxNIP-deficient) mice (Supplemental Figure 3, G and H). Of interest, the oxidative/nitrosative stress and induction of Nox4 was also attenuated in the tubules of the diabetic KO mice (Supplemental Figure 8).
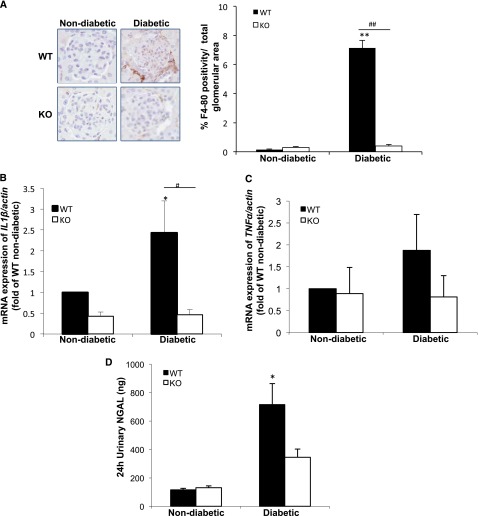
To assess the degree of glomerular macrophage infiltration and inflammation, we stained for the macrophage marker F4/80. F4/80 staining was markedly increased in the glomeruli of WT diabetic mice compared with nondiabetic controls (7.13%±0.52% versus 0.16%±0.03% of the total glomerular area) (Figure 6A). In addition, the mRNA expression of IL1β was significantly increased in the renal tissue of diabetic WT animals, while no significant change was observed in the diabetic KO (Figure 6B); this finding is consistent with reports that TxNIP, in association with the NLRP3 inflammasome, contributes to the production of the mature form of IL-1β.26,27 In the case of TNF-α expression, only a modest increase occurred in the diabetic WT mice, but no change was seen in the diabetic KO (Figure 6C). Of interest, the urinary neutrophil gelatinase-associated lipocalin (NGAL or lipocalin-2) level, an early biomarker of DN and acute renal injury,28 was significantly augmented only in the diabetic WT (Figure 6D). The TxNIP-HET mice showed a lesser but significant increase in inflammatory cell infiltration in the presence of diabetes, manifested by increased F4/80 staining (Supplemental Figure 9).
Figure 6.
TxNIP−/− mice lack a renal inflammatory response in response to chronic hyperglycemia. Inflammatory markers in the renal tissues from nondiabetic and diabetic TxNIP WT and KO mice. (A) Representative images of F4/80 (marker for macrophages)-positive staining in the glomeruli and quantification from n=5 mice/condition. Expression of cytokine mRNA, (B) IL-1β, and (C) TNF-α, corrected for actb and normalized to nondiabetic WT in renal cortex (n=5 mice/condition). (D) Twenty-four-hour urinary NGAL levels (ng) from 6 to 7 mice/condition. Results are mean±SEM. *P<0.05 and **P<0.01 diabetic WT mice versus nondiabetic WT; and #P<0.05 and ##P<0.01 diabetic WT versus diabetic TxNIP KO mice.
TxNIP Promotes HG-Induced Mitochondrial O2− and Apoptosis in Cultured Podocytes
Our observations of protection from diabetes-mediated podocyte loss in TxNIP KO mice and the recently reported elevated levels of TxNIP in podocytes in DN29 suggest cell autonomous effects. HG increased the protein levels of TxNIP by 2.4±0.26-fold (P<0.001) (Figure 7E) in cultured human podocytes. HG also significantly stimulated mitochondrial O2− generation (Figure 7A) and the mitochondrial membrane potential (Figure 7B) determined by MitoSox and JC-1, respectively. TxNIP knockdown in HG markedly inhibited these effects, associated with a concomitant increase in lactate production (Figure 7C); this is consistent with decreased mitochondrial TCA cycle flux and increased glycolysis. Of note, HG-induced cultured podocyte apoptosis was also blocked by TxNIP small interfering RNA (siRNA) (Figure 7, D and E).
Figure 7.
TxNIP knockdown in cultured human podocytes exposed to high glucose inhibits the increased mitochondrial O2−, membrane potential, and apoptosis. Knockdown of TxNIP attenuates mitochondrial O2−, and membrane potential and apoptosis in cultured human podocytes. Podocytes were transfected with 50 nM TxNIP-specific siRNA (siTxNIP) or universal negative control siRNA (scrambled, scr) for 24 hours and then incubated in 5 mM normal glucose (NG) or 25 mM HG for 48 hours. (A and B) Mitochondrial superoxide formation and mitochondrial membrane potential were assessed by confocal microscopy using MitoSox and JC-1 dyes, respectively (n=3), with the quantification illustrated as graphs in the bottom panel. (C) Lactate concentration in the cell culture media (n=6). Apoptosis in podocytes treated with NG/HG and scr/siTxNIP was assessed with (D) TUNEL assay and (E) cleaved caspase-3 protein expression in three independent experiments. Results are mean±SEM. *P<0.05; **P<0.01; and ***P<0.001 versus scr NG; #P<0.05 and ###P<0.001 versus scr 48-hour HG.
Discussion
DN accounts for almost one third of all cases of ESRD and, with the global “epidemic” of diabetes, has become a major health burden.2,30,31 Current attempts to normalize glycemia, control BP, and maintain blockade of the renin-angiotensin system have not eliminated DN or the need for dialysis and/or renal transplantation.2,30,31 Thus, it is essential to identify novel drug targets and more effective therapy.
Several studies have implicated the protein TxNIP as having a potential role in pathogenesis.15,19,32–35 First, we and others observed that TxNIP is a mediator of the increased collagen accumulation induced by HG in cultured mesangial cells.15–19 Second, TxNIP is increased in human diabetic kidney.34 Third, a correlation between protection from DN by the antifibrotic agent tranilast33 and the angiotensin receptor blocker telmisartan35 were correlated with a decrease in renal TxNIP in rodents. However, none of these reports demonstrated a clear pathogenic role for TxNIP in DN in vivo.
In the present study, we used two mouse models of TxNIP deficiency, Hcb-19, which harbors a spontaneous mutation, and TxNIP−/− (KO), with a targeted gene deletion.36 Both TxNIP-deficient mouse strains showed significant protection from DN. There was no or minimal increase in diabetes-induced albuminuria or in the pathologic changes of mesangial expansion, expression of TGF-β, collagen IV, and interstitial fibrosis.
Although the nondiabetic Hcb-19 mice manifested albuminuria in the absence of the morphologic changes observed in the diabetic state, the nondiabetic TxNIP−/− mice were identical to controls in all renal measures studied. The cause of the baseline albuminuria in Hcb-19 mice is not clear, but this did not mask the protection from the diabetes-induced changes measured. Electron microscopic analysis revealed a significant attenuation of podocyte foot process effacement and GBM thickening in the diabetic TxNIP−/− mice. These are well characterized findings of early DN in mice.1,2,20,21,23,37 At the same time, glycemia and BP were similar in WT and TxNIP−/− diabetic mice. While TxNIP deficiency has been reported to protect against STZ-induced diabetes by our group13 and others,17 we developed a two-course STZ injection protocol to generate equivalent hyperglycemia.13 Thus, differences in glycemia or BP do not explain protection from DN.
The pathogenesis of diabetes complications, including DN, has been proposed to be initiated by excess generation of ROS by mitochondria stimulated by HG.4–6 The consequent change in cellular redox state is thought to inhibit glyceraldehyde-3-phosphate dehydrogenase, a redox-sensitive enzyme upstream of pyruvate synthesis that results in increased glucose metabolism via several different pathways, which mediate its “toxicity.” These include formation of advanced glycation endproducts, activation of protein kinase C, and increased flux through the polyol and hexosamine biosynthesis pathways.4–6
We and others have implicated TxNIP in this initiating event. In cardiomyocytes38 and mesangial cells from TxNIP-deficient mice,15 decreased mitochondrial glucose metabolism concomitant with increased glycolysis and lactate production has been observed. Furthermore, HG did not stimulate mesangial cell mitochondrial membrane potential or ROS generation in the TxNIP-deficient state, while adenoviral mediated overexpression of TxNIP restored these responses.15 These data are consistent with the modestly elevated circulating lactate levels in TxNIP−/− mice and the marked increase observed in the diabetic state (Table 1).
The mechanism by which TxNIP regulates mitochondrial metabolism is not clear, but it has been proposed that TxNIP may regulate pyruvate dehydrogenase, thus promoting TCA cycle flux and oxidative phosphorylation.15,38 Evidence for a defect in ROS generation in vivo was manifested by the lack of increase in urinary 8-OHdG in the TxNIP−/− diabetic mice in contrast to diabetic WT (Figure 5B). This was supported by the reduced mitochondrial membrane potential and ROS production in TxNIP-silenced cultured podocytes exposed to HG (Figure 7, A–C). In addition, glomerular and tubular staining for nitrotyrosine, which is closely associated with oxidative stress,39 was increased by diabetes only in WT mice (Figure 5A, Supplemental Figure 8A, and Supplemental 8C). Apart from mitochondria, elevated ROS in the hyperglycemic/diabetic state results from augmented NADPH oxidase activity.40–42 At least three NADPH oxidase isoforms have been found in the kidney, Nox1, Nox2 and Nox4, and there is some uncertainty as to which one(s) contribute to ROS in diabetes.41 There is recent evidence for Nox4,43–45 and we reported that in TxNIP-deficient cultured mesangial cells there is a defect in Nox4 upregulation by HG.15 The present in vivo data support these observations, showing that TxNIP−/− diabetic mice, in contrast to WT, did not display an increase in kidney cortex Nox4 mRNA or glomerular and tubular staining for Nox4 protein (Figure 5, C and D, Supplemental Figure 8, A and B).
Several measures predictive of progression of DN were assessed. Thus, podocyte loss, increased 24-hour urinary nephrin and NGAL excretion were observed in the diabetic WT but not in the TxNIP−/− mice. Protection from podocyte loss in vivo was supported by inhibition of HG-induced apoptosis observed in the TxNIP-silenced cultured human podocytes (Figure 7, D and E). In addition, evidence for a decrease in GFR and renal function after 6 months of diabetes was demonstrated by increases in serum cystatin C and serum creatinine in the diabetic WT mice. However, these variables were not altered by diabetes in the TxNIP−/− mice. These findings are consistent with an important role of TxNIP in the initiation and progression of DN.
TxNIP has also been implicated in the innate immune response, associated with NLRP3 complex signaling to caspase-1-mediated cleavage and release of IL-1β, activation of NF-κB, and increased cytokine expression.10,26,27 Increased IL-1β expression has also been reported to be stimulated by TxNIP.27 Furthermore, infiltration of macrophages and inflammation has been documented in DN.7,25 In TxNIP−/− mice, glomerular macrophage infiltration in the diabetic state was decreased compared with WT (Figure 6A), and kidney cortex IL-1β mRNA was increased only in diabetic WT (Figure 6B).
Diabetic heterozygous TxNIP+/− (HET) mice showed a partial protection from several of the preceding endpoints of DN (Supplemental Figures 1, 4, 6, 7, and 9). These mice had a significant increase in renal TxNIP mRNA induced by diabetes, which was less than that in WT mice but clearly far greater than in the nondiabetic state (Supplemental Figure 2). We noted that the inhibition of pathologic changes associated with fibrosis in the diabetic HET mice was, in general, greater than the partial inhibition of oxidative stress and inflammation. Thus, whether the lower level of TxNIP inhibits progression from early to more advanced DN or significantly delays (but does not completely block) progression requires further study. Taken together, these data in diabetic TxNIP−/−, TxNIP+/−, and Hcb-19 mice indicate that TxNIP is a key mediator of oxidative stress and the pathologic markers of DN. Because these mouse models lack TxNIP in all cells and tissues, it is not possible to completely exclude a contribution of systemic effects (e.g., decreased inflammation) to protection from DN. However, our data in podocytes and in mesangial cells,15 as well as in cardiomyocytes,38,46 β cells,13,17 and, recently, renal proximal tubular cells,47 indicate major cell autonomous protective effects of TxNIP deficiency. The precise contribution and role of TxNIP in various cell types in the kidney to DN (i.e., endothelial, mesangial, epithelial [podocytes] and tubular cells) remain to be documented, along with tissue-specific KO mouse models.
In summary, to our knowledge this is the first in vivo study of DN in TxNIP-deficient animal models. The results indicate that a lack of TxNIP protects against DN and support in vitro data in various cell types that upregulation of TxNIP by HG is a key mediator of early oxidative stress and a trigger for the development and progression of DN. The downregulation of TxNIP and/or interference with its function may be an effective therapeutic strategy. The data in the diabetic heterozygous TxNIP+/− mice suggest that TxNIP would have to be maintained at or lowered to near basal levels to achieve complete protection. Further work in different diabetic mouse models and in specific renal cell types is warranted.
Concise Methods
Mice, Reagents, and Metabolic Studies
TxNIP WT (TxNIP+/+), TxNIP KO (TxNIP−/−), and TxNIP HET (TxNIP +/−) mice (kindly provided by R.T. Lee, Harvard University),46 as well as Hcb-19 mice (lacking TxNIP because of a spontaneous mutation) and its control C3H/DiSnA (kindly provided by the late R.A. Davis, San Diego State University),36 were housed under standard conditions and provided chow and water ad libitum at the Animal Resource Center, University Health Network (UHN, Toronto, ON, Canada). All the experimental procedures were approved by the Animal Care Committee of the UHN and were followed according to the guidelines of the Canadian Council of Animal Care. Diabetes was induced in 10 mice per group at 6–8 weeks of age by intraperitoneal injections of STZ (40 mg/kg in fresh 0.1 M sodium citrate buffer, pH 4.5) daily for 5 days (http://www.diacomp.org), followed 5 weeks later by a second round of five STZ injections. Control (nondiabetic) groups received citrate buffer. Blood glucose levels were monitored with a glucometer (FreeStyle Lite, Alameda, CA) monthly throughout the experimental period.
Twenty-three weeks after STZ injections, the animals were individually placed in Tecniplast metabolic chambers for 24 hours to determine the volume of urine excretion and water consumption. After measurement of urine volume, urine was centrifuged at 1000 rpm for 1 minute to remove any debris and then frozen at −80°C for further analysis.
At 24 weeks mice were subjected to BP, and the remaining mice were euthanized for renal tissues and blood as described below.
Blood Profile and Urinalysis
At the time of euthanasia, blood was removed via cardiac puncture. Samples of whole blood and plasma and serum were provided to the Pathology and Lab Medicine (PLM) Department, Mount Sinai Hospital (MSH), Toronto for the following determinations: blood glucose, plasma glucose, Hemoglobin A1c, hemoglobin, red blood cell count, and white blood cell count (Table 1). The instruments used to assess these measures were Roche Modular, Cobas Integra 400, and Sysmex XE-2100 Automated Hematology System. Serum insulin (ELISA kit from EMD Millipore), serum lactate and ketones (kits from Abcam, Inc.), and serum cystatin C (Quantikine ELISA kit from R&D Systems) were measured according to the manufacturer’s guidelines and assessed using a Bio-Tek microplate reader and Gen5 software. Serum creatinine was measured using HPLC by the Hormone Assay and Analytical Services Core, Vanderbilt University School of Medicine (Nashville, TN).
Urinary assays for albumin, creatinine, and nephrin (kits from Exocell), NGAL (Alpco Diagnostics), and 8-OHdG (StressMarq Biosciences) were assessed according to the manufacturer’s guidelines. Protein concentration was measured using the Bio-Rad Protein Assay.
Invasive Hemodynamics
Hemodynamics were performed on mice under 1% isoflurane anesthesia using a 1.4-F Millar pressure-transducing catheter introduced through the right common carotid artery. Data were collected and analyzed using AcqKnowledge (version 2.8.1; BIOPAC Systems, Montreal, QC, Canada) as previously described.48 All measurements were performed in triplicate and averaged for each animal (n=3 for HET and n=6 for WT and KO groups).
Tissue Histology and Immunohistochemistry
Kidneys were immediately harvested, fixed in 10% buffered formalin, and embedded in paraffin; 3-μm sections were subjected to PAS staining; Masson trichrome staining; and WT-1 (1:500; Santa Cruz Biotechnology), F4/80 (1:500; Serotec), and Nitrotyrosine (1:800; Chemicon) staining. Staining of sections using the antibodies directed against collagen IV (1:2500; Rockland), TGF-β (1:2500, Acris-antibodies), nephrin (1:1000; Abnova), and Nox4 (1:2500; Santa Cruz Biotechnology) was performed by following the procedures previously described.49 Quantitative analysis was performed on 50 glomeruli per kidney section with the aid of Visiomorph software (PLM Department, MSH, Toronto, ON).
Glomerular Volume
Glomerular volume was calculated using the formula GV=(β/κ)(GA)3/2, where β=1.38 (the shape coefficient of spheres as assumed model glomeruli) and κ=1.10, the size distribution coefficient.50 Glomerular area was determined using Visiomorph software.
The number of WT-1–positive cells per glomerulus (i.e., glomerular cell volume) was counted as previously described by the Animal Models of Diabetic Complications Consortium (http://www.diacomp.org) and Sanden et al. (2003).50 Briefly, WT-1–positive nuclei (counted manually) and glomerular area (Visiomorph software) were assessed in 50 random glomeruli (outer to inner cortex) in two kidney sections of different thickness (3 and 9 μm). From the data, the average podocyte number/glomerular area (P/GA) was calculated for both sections and the difference, P/GA Δ, was determined. The average glomerular volume/podocyte, GV/P, was calculated by dividing the difference in section thickness, 6.3, by the P/GA Δ. Then, using the Weibel formula, the mean glomerular volume was calculated. The podocyte number per glomerulus was obtained by dividing the mean glomerular volume by the glomerular volume/podocyte (GV/P).
Cell Culture
Immortalized human podocytes (a kind gift of R. Coward and M. Saleem, University of Bristol) transfected with the temperature-sensitive simian vacuolating virus 40 (SV40) gene were grown in RPMI 1640 media supplemented with 10% FBS and 1% insulin-transferrin-selenium at 33°C until they were 70%–80% confluent. They were then thermo-switched to 38°C to silence the SV40 gene and allowed to differentiate for 14–20 days before experiments. Transfection was conducted with 50 nM of universal negative control (scrambled siRNA) or TxNIP siRNA (catalog no. TXNIPHSS173789, Invitrogen) for 24 hours, followed by 48 hours of HG treatment in 0.2% FBS-containing medium. After this period, the cells were harvested for Western blot or stained for immunofluorescence studies.
MitoSox and JC-1 Immunofluorescence
Studies on the transfected podocytes with MitoSox and JC-1 were carried out as previously described.15
Lactate Assay
Lactate in the cell culture medium was assessed using a lactate colorimetric assay kit from Abcam, Inc. as previously described.15
Terminal Deoxynucleotidyl Transferase-Mediated Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Immunofluorescence
Terminal deoxynucleotidyl transferase-mediated transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assays were performed to detect apoptotic cells after various treatments. The DeadEnd Fluorometric TUNEL System (Promega) was used according to the manufacturer’s instructions. Briefly, cells were cultured on glass coverslips and differentiated at 38°C for 14 days. After treatment, they were fixed in 4% paraformaldehyde for 25 minutes at room temperature. They were subsequently permeabilized with 0.2% Triton X-100 in PBS, equilibrated in EQUILIBRIUM BUFFER, and incubated with 50 μl of TUNEL reaction mixture for 1 hour in a humidified chamber at 37°C. Saline-sodium citrate (2×) was used to stop the reaction, and 300 nM DAPI was added to stain for nuclei. Coverslips were then mounted on microscope slides and viewed under a fluorescence microscope.
Immunoblotting
Western blot was performed as previously described.15 Briefly, protein concentrations were determined with the modified Lowry microassay (Bio-Rad). After the samples were boiled in 4× sample buffer, 20 μg of protein was separated by 10%–15% SDS-PAGE, transferred onto nitrocellulose membranes, and blocked with 5% milk/Tris-buffer saline with 0.1% Tween 20. The primary antibodies used were cleaved caspase-3 (1:500; Cell Signaling Technology), TxNIP (1:1000; MBL), and β-actin (1:10,000; Santa Cruz Biotechnology), and the secondary antibodies (1:4000) were anti-rabbit IgG horseradish peroxidase conjugate (Bio-Rad) and peroxidase-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories). Immunoblots were visualized by the ECL detection system (KPL Mandel Scientific) and the densitometric analyses were conducted with ImageJ software (National Institutes of Health).
Electron Microscopy
Transmission electron microscopy was performed on renal cortical tissues from three mice per group (PLM Department, MSH). Nine fields per experimental condition were examined for GBM thickness and podocyte foot process width as previously described.49,51
Quantitative Real-Time PCR
Fifty milligrams of frozen renal cortex was sheared to powder using liquid nitrogen and then homogenized in 1 ml TRIzol reagent (Invitrogen) for RNA extraction. Purity and concentration of RNA were checked with a Nanodrop spectrophotometer (Thermo Fisher Scientific). cDNA synthesis was performed using M-MLV reverse transcription kit (Invitrogen). The resulting cDNA was then used for real-time PCR analysis using the Taqman Gene Expression System (Applied Biosystems). The gene-specific primers for mouse obtained from Applied Biosystems were as follows: mouse TxNIP (Mm00452393_m1), mouse nox4 (Mm00479246_m1), mouse IL-1β (Mm00434228_m1), mouse TNF-α (Mm00443260_g1), and the housekeeping gene–mouse actin (Mm00607939_s1). A standard curve was generated with diluted cDNA from the control group. A PCR reaction mix (25 μl/well) was prepared containing 10 μl of 300 ng/μl cDNA, 1.25 μl of double-distilled water, 1.25 μl of Taqman Gene Expression Primers, and 12.5 μl of Taqman Gene Expression Master Mix. The samples were loaded into an ABI Prism 96-well reaction plate and then run on an ABI Prism 7900HT sequence detection system. The values obtained were determined using SDS 2.3 software (ABI Prism 7900 HT) and then normalized to the expression of actin for each sample. The final values are expressed as fold of nondiabetic WT (control) group.
Statistical Analyses
Data are presented as mean±SEM, unless otherwise stated. Statistical significance was calculated using Graphpad Prism software, version 4.0 (Graphpad Prism, San Diego, CA) by one-way ANOVA, followed by the Newman-Keuls post hoc method for multiple comparisons. Comparison between two sets of samples was analyzed by t test. P<0.05 was considered to represent a statistically significant difference.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. R.T. Lee and J. Yoshioka (Harvard Medical School, Boston, MA) for providing mice and helpful discussion.
This work was supported by the Canadian Institutes of Health Research grants MOP 97979 and PCN 49409 (to I.G.F.). A.S. was sponsored in part by a Novo Nordisk Studentship and UHN- Graduate Studentship from the Banting and Best Diabetes Centre, a Canada Graduate Scholarship (NSERC), and an Ontario Graduate Scholarship.
A portion of this work was presented at the 16th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism (CDA/CSEM) Professional Conference (Montreal, Quebec; October 17–20, 2013).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050528/-/DCSupplemental.
References
- 1.Rask-Madsen C, King GL: Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17: 20–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dronavalli S, Duka I, Bakris GL: The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4: 444–452, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Kakehi T, Yabe-Nishimura C: NOX enzymes and diabetic complications. Semin Immunopathol 30: 301–314, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M: The pathobiology of diabetic complications: A unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Giacco F, Brownlee M: Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf G: New insights into the pathophysiology of diabetic nephropathy: From haemodynamics to molecular pathology. Eur J Clin Invest 34: 785–796, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H: Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 14[Suppl 3]: S241–S245, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Qi W, Chen X, Gilbert RE, Zhang Y, Waltham M, Schache M, Kelly DJ, Pollock CA: High glucose-induced thioredoxin-interacting protein in renal proximal tubule cells is independent of transforming growth factor-beta1. Am J Pathol 171: 744–754, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP: Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol 221: 262–272, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Björnholm M, Tornqvist H, Zierath JR, Ridderstråle M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK: TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4: e158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng DW, Jiang Y, Shalev A, Kowluru R, Crook ED, Singh LP: An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem 112: 189–218, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Masson E, Koren S, Razik F, Goldberg H, Kwan EP, Sheu L, Gaisano HY, Fantus IG: High beta-cell mass prevents streptozotocin-induced diabetes in thioredoxin-interacting protein-deficient mice. Am J Physiol Endocrinol Metab 296: E1251–E1261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxena G, Chen J, Shalev A: Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem 285: 3997–4005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah A, Xia L, Goldberg H, Lee KW, Quaggin SE, Fantus IG: Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 288: 6835–6848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A: Thioredoxin-interacting protein: A critical link between glucose toxicity and beta-cell apoptosis. Diabetes 57: 938–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, Lusis AJ, Davis RA, Shalev A: Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J 22: 3581–3594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mussap M, Dalla Vestra M, Fioretto P, Saller A, Varagnolo M, Nosadini R, Plebani M: Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int 61: 1453–1461, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Uehara S, Ikeda T, Itadani H, Kotani H: Vitamin D3 up-regulated protein-1 regulates collagen expression in mesangial cells. Kidney Int 64: 1632–1642, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ly J, Alexander M, Quaggin SE: A podocentric view of nephrology. Curr Opin Nephrol Hypertens 13: 299–305, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE: Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spurney RF, Coffman TM: Stressed-out podocytes in diabetes? J Am Soc Nephrol 19: 2035–2037, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H: Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15: 1379–1383, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Forbes JM, Cooper ME: Mechanisms of diabetic complications. Physiol Rev 93: 137–188, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Schroder K, Zhou R, Tschopp J: The NLRP3 inflammasome: A sensor for metabolic danger? Science 327: 296–300, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J: Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Alter ML, Kretschmer A, Von Websky K, Tsuprykov O, Reichetzeder C, Simon A, Stasch JP, Hocher B: Early urinary and plasma biomarkers for experimental diabetic nephropathy. Clin Lab 58: 659–671, 2012 [PubMed] [Google Scholar]
- 29.Gao P, Meng XF, Su H, He FF, Chen S, Tang H, Tian XJ, Fan D, Wang YM, Liu JS, Zhu ZH, Zhang C: Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim Biophys Acta 1843: 2448–2460, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW, American Diabetes Association : Nephropathy in diabetes. Diabetes Care 27[Suppl 1]: S79–S83, 2004 [DOI] [PubMed] [Google Scholar]
- 31.The Diabetes Control and Complications Trial Research Group : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Hamada Y, Fukagawa M: A possible role of thioredoxin interacting protein in the pathogenesis of streptozotocin-induced diabetic nephropathy. Kobe J Med Sci 53: 53–61, 2007 [PubMed] [Google Scholar]
- 33.Tan SM, Zhang Y, Cox AJ, Kelly DJ, Qi W: Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol Dial Transplant 26: 100–110, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Advani A, Gilbert RE, Thai K, Gow RM, Langham RG, Cox AJ, Connelly KA, Zhang Y, Herzenberg AM, Christensen PK, Pollock CA, Qi W, Tan SM, Parving HH, Kelly DJ: Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol 20: 730–741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Lin H, Liu D, Liu J, Wang N, Mei X, Sun J, Yang G, Zhang X: The protective effect of telmisartan in type 2 diabetes rat kidneys is related to the downregulation of thioredoxin-interacting protein. J Endocrinol Invest 36: 453–459, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Castellani LW, Weinreb A, Bodnar J, Goto AM, Doolittle M, Mehrabian M, Demant P, Lusis AJ: Mapping a gene for combined hyperlipidaemia in a mutant mouse strain. Nat Genet 18: 374–377, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR: Diabetic nephropathy: Mechanisms of renal disease progression. Exp Biol Med (Maywood) 233: 4–11, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Yoshioka J, Chutkow WA, Lee S, Kim JB, Yan J, Tian R, Lindsey ML, Feener EP, Seidman CE, Seidman JG, Lee RT: Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Invest 122: 267–279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dröge W: Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem 17: 4256–4269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorin Y, Block K: Nox as a target for diabetic complications. Clin Sci (Lond) 125: 361–382, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Gill PS, Wilcox CS: NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE: Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Block K, Gorin Y, Abboud HE: Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hébert RL: Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT: Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res 101: 1328–1338, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Huang C, Lin MZ, Cheng D, Braet F, Pollock CA, Chen XM: Thioredoxin-interacting protein mediates dysfunction of tubular autophagy in diabetic kidneys through inhibiting autophagic flux. Lab Invest 94: 309–320, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Zaidi SH, Huang Q, Momen A, Riazi A, Husain M: Growth differentiation factor 5 regulates cardiac repair after myocardial infarction. J Am Coll Cardiol 55: 135–143, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi K, Xia L, Goldberg HJ, Lee KW, Shah A, Stavar L: E, AYM, Momen, A, Shikatani, EA, John, R, Husain, M, Fantus, IG: Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 62: 3874–3886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC: Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms’ tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol 14: 2484–2493, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Mallipattu SK, Liu R, Zhong Y, Chen EY, D’Agati V, Kaufman L, Ma’ayan A, Klotman PE, Chuang PY, He JC: Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int 83: 626–634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.