Abstract
Renal fibrosis is a final common manifestation of CKD resulting in progressive loss of kidney function. Bone marrow–derived fibroblast precursors contribute significantly to the pathogenesis of renal fibrosis. However, the signaling mechanisms underlying the activation of bone marrow–derived fibroblast precursors in the kidney are not fully understood. In this study, we investigated the role of the Janus kinase 3 (JAK3)/signal transducer and activator of transcription (STAT6) signaling pathway in the activation of bone marrow–derived fibroblasts. In cultured mouse monocytes, IL-4 or IL-13 activated STAT6 and induced expression of α-smooth muscle actin and extracellular matrix proteins (fibronectin and collagen I), which was abolished by a JAK3 inhibitor (CP690,550) in a dose-dependent manner or blocked in the absence of STAT6. In vivo, STAT6 was activated in interstitial cells of the obstructed kidney, an effect that was abolished by CP690,550. Mice treated with CP690,550 accumulated fewer bone marrow–derived fibroblasts in the obstructed kidneys compared with vehicle-treated mice. Treatment with CP690,550 also significantly reduced myofibroblast transformation, matrix protein expression, fibrosis development, and apoptosis in obstructed kidneys. Furthermore, STAT6-deficient mice accumulated fewer bone marrow–derived fibroblasts in the obstructed kidneys, produced less extracellular matrix protein, and developed much less fibrosis. Finally, wild-type mice engrafted with STAT6−/− bone marrow cells displayed fewer bone marrow–derived fibroblasts in the obstructed kidneys and showed less severe renal fibrosis compared with wild-type mice engrafted with STAT6+/+ bone marrow cells. Our results demonstrate that JAK3/STAT6 has an important role in bone marrow–derived fibroblast activation, extracellular matrix production, and interstitial fibrosis development.
Keywords: renal fibrosis, fibroblast, extracellular matrix, cell signaling, cytokines
CKD is a serious public health burden and an independent risk factor for cardiovascular disease. It is estimated that about 11% populations in the United States are affected by CKD.1 Fibroblast activation and extracellular matrix (ECM) deposition are pathologic features of CKD.2 The activated fibroblasts produce ECM, which helps tissue repair and regeneration during the wound healing phase but leads to scar formation if the process persists.3 Renal fibrosis leads to the destruction of renal parenchyma and progressive loss of kidney function.4
Accumulating evidence indicates that bone marrow–derived fibroblast precursors termed fibrocytes contribute significantly to the development of renal fibrosis.5–11 These cells express both hematopoietic cell markers such as CD45 and CD11b and mesenchymal cell markers such as collagen I and vimentin.12 We recently showed that bone marrow–derived fibroblast precursors are recruited into the kidney and significantly contribute to the development of renal fibrosis.5,6,10 However, the molecular mechanisms underlying activation of these cells are not fully understood.
Activation of fibroblasts is dependent on cytokines produced in the local microenvironment.13 Profibrotic Th2 cytokines IL-4 and IL-13 promote myeloid fibroblast differentiation, whereas antifibrotic Th1 cytokines IFN-γ and IL-12 inhibit its differentiation.14,15 The signaling mechanisms underlying Th2 cytokine–induced myeloid fibroblast activation are currently not known. In macrophages, downstream intracellular signaling of IL-4 and IL-13 involves activation of Janus kinase 3 (JAK3) and phosphorylation of signal transducer and activator of transcription (STAT6). Phosphorylated STAT6 then translocates into the nucleus and promotes IL-4– and IL-13–responsive gene transcription.13,16
In this study, we examined the role of JAK3/STAT6 signaling in the activation of bone marrow–derived fibroblast precursors in culture and in the kidney in a murine model of tubulointerstitial fibrosis induced by unilateral ureteral obstruction (UUO). Our results show that pharmacological inhibition of JAK3 or genetic disruption of STAT6 suppresses myeloid fibroblast activation and attenuates interstitial fibrosis development. These results establish a critical role of JAK3/STAT6 signaling in the activation of myeloid fibroblast precursors and development of renal fibrosis.
Results
CP690,550 Suppresses Th2 Cytokine–Induced STAT6 Activation in Bone Marrow–Derived Monocytes
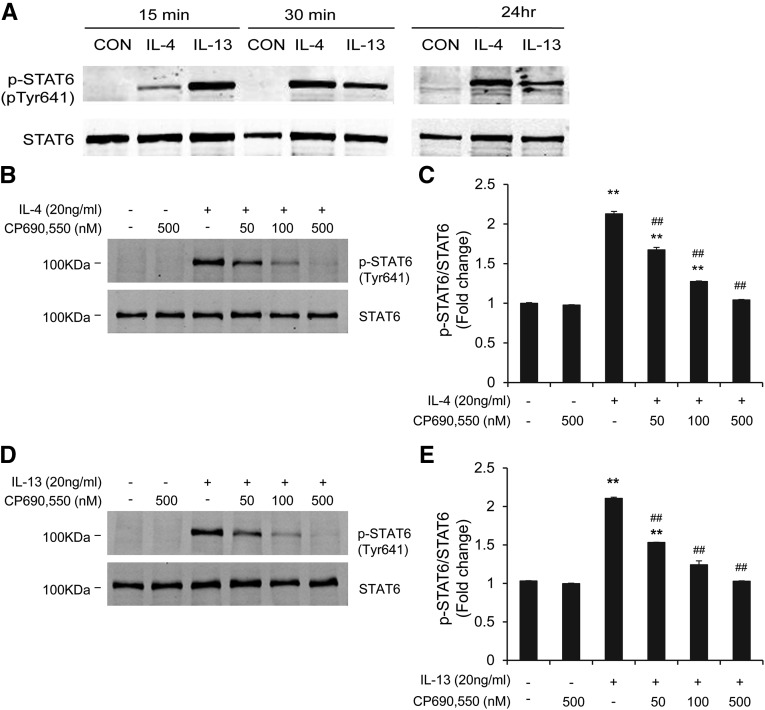
To examine the effect of CP690,550 on Th2 cytokine–induced STAT6 activation, mouse bone marrow–derived monocytes were treated with IL-4 or IL-13 for different time periods. IL-4 or IL-13 treatment activated STAT6 identified as phosphorylated STAT6, which occurred as early as 15 minutes and persisted for at least 24 hours, whereas there were no detectable changes in the levels of total STAT6 (Figure 1A). Pretreatment with CP690,550 dose-dependently suppressed phosphorylation of STAT6 induced by IL-4 or IL-13 (Figure 1, B–E). These results indicate that IL-4/IL-13–induced STAT6 activation is mediated by JAK3 in bone marrow–derived monocytes.
Figure 1.
CP690,550 blocks STAT6 activation in bone marrow–derived monocytes. (A) Representative Western blots show the activation of STAT6 in monocytes at different time points by IL-4 or IL-13. Cultured bone marrow–derived monocytes are treated with IL-4 (100 ng/ml) or IL-13 (100 ng/ml) for the indicated time period. Sterile water is used as the control. Cell lysates are subjected to immunoblot analysis using antibodies against phosphorylated-STAT6 (pTyr641) and STAT6. GAPDH is used as internal loading control. (B and D) Representative Western blots show the effect of CP690,550 on STAT6 phosphorylation. CP690,550 is given 30 minutes before IL-4 or IL-13 treatment. The cells are then treated with IL-4 or IL-13 for 15 minutes. (C and E) Quantitative analysis of STAT6 phosphorylation in response to IL-4 or IL-13 in the presence or absence of CP690,550. **P<0.01 compared with the control group; ##P<0.01 compared with the IL-4 or IL-13 group (n=5 per group). CON, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
CP690,550 Inhibits the Expression of α-Smooth Muscle Actin and ECM Proteins in Bone Marrow–Derived Monocytes
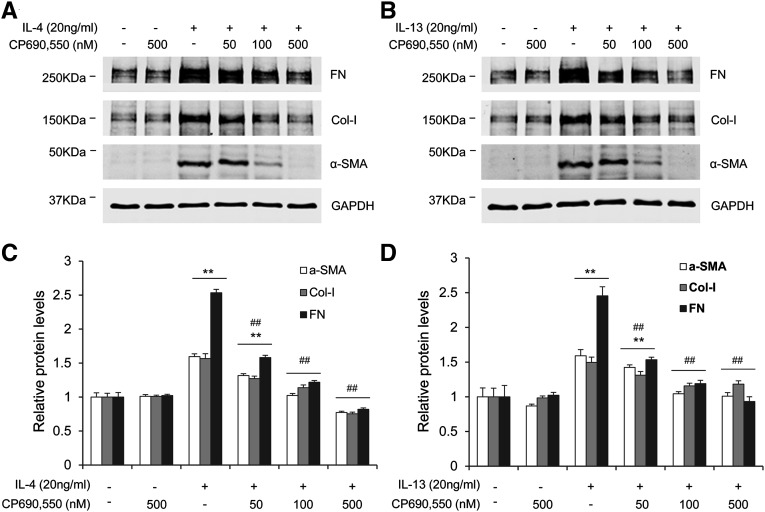
We then examined the effect of CP690,550 on Th2 cytokine–induced monocyte-to-fibroblast transition and ECM protein production in vitro. Mouse bone marrow–derived monocytes were treated with IL-4 or IL-13 for 24 hours after pretreatment with CP690,550. IL-4 or IL-13 promoted the expression of α-smooth muscle actin (α-SMA), fibronectin, and collagen I in bone marrow–derived monocytes, which demonstrated the profibrotic effect of these two cytokines. Pretreatment with CP690,550 at 500 nM dose-dependently blocked IL-4– or IL-13–induced expression of these proteins (Figure 2). These data suggest that JAK3 signaling mediates IL-4– or IL-13–induced activation of myeloid fibroblasts and production of ECM proteins.
Figure 2.
CP-690,550 inhibits IL-4/IL-13–induced α-SMA and ECM protein expression in bone marrow–derived monocytes. (A and B) Representative Western blots show the expression of fibronectin, collagen I, and α-SMA in monocytes after IL-4 (A) or IL-13 treatment (B). Cultured bone marrow–derived monocytes are pretreated with CP690,550 (500 nM) for 30 minutes, followed by either IL-4 (100 ng/ml) or IL-13 (100 ng/ml) treatment for 24 hours. (C and D) Quantitative analysis of the expression of fibronectin, collagen I, and α-SMA in response to IL-4 or IL-13 treatment with or without CP690,550. **P<0.01 compared with the control group; ##P<0.01 compared with the IL-4 or IL-13 group (n=5 per group). Col-I, collagen I; FN, fibronectin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Because TGF-β1 is an important profibrotic cytokine and plays an important role in activating bone marrow–derived fibroblasts,17 we examined the effect of CP690,550 on TGF-β1–induced α-SMA, fibronectin, and collagen I expression in normal rat kidney fibroblasts. Our results showed that CP690,550 did not affect TGF-β1–stimulated α-SMA, fibronectin, and collagen I expression (Supplemental Figure 1), indicating that the effect of CP690,550 is independent of TGF-β1 signaling.
STAT6 Deficiency Inhibits Myeloid Fibroblast Activation In Vitro
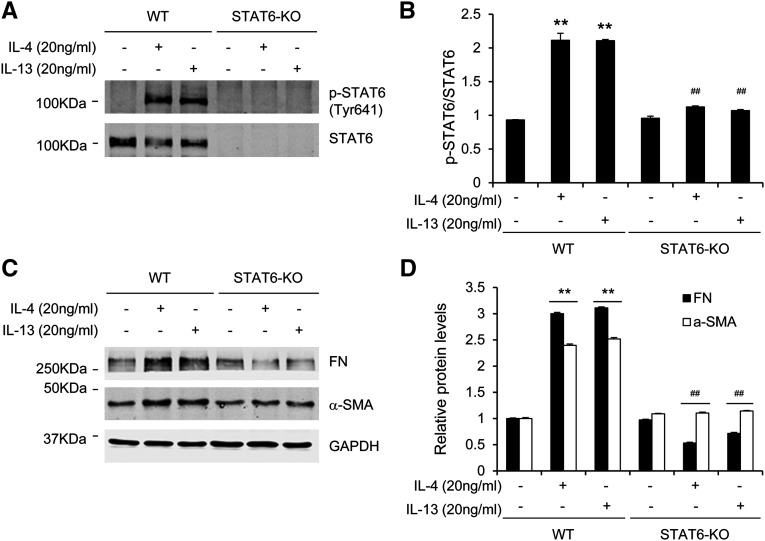
To determine whether STAT6 signaling regulates monocyte-to-fibroblast transition in vitro, we treated bone marrow–derived monocytes from wild-type (WT) and STAT6 knockout (KO) mice with Th2 cytokines. Treatment of STAT6+/+ monocytes with Th2 cytokines induced phosphorylation of STAT6 as well as expression of fibronectin and α-SMA. By contrast, treatment of STAT6−/− monocytes with Th2 cytokines did not induce expression of fibronectin and α-SMA (Figure 3). These data suggest that an important role of STAT6 in Th2 cytokine-induced myeloid fibroblast activation and ECM protein production. Of note, STAT6 was not detected in STAT6−/− monocytes, indicating the specificity of STAT6 antibody.
Figure 3.
STAT6 deficiency abolishes IL-4/IL-13–induced STAT6 activation and α-SMA and ECM protein expression in bone marrow–derived monocytes. (A and B) Western blot analysis shows the effect of STAT6 deficiency on Th2 cytokine–induced STAT6 activation in bone marrow–derived monocytes. (C and D) Western blot analysis shows the effect of STAT6 deficiency on Th2 cytokine–induced α-SMA and fibronectin in bone marrow–derived monocytes. **P<0.01 compared with the control group; ##P<0.01 compared with the IL-4 or IL-13 group (n=5 per group). FN, fibronectin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To evaluate the role of Th2 cytokines in resident kidney fibroblast activation, normal mouse kidney fibroblasts from WT mice and normal rat kidney fibroblasts (NRK-49F; ATTC) were treated with Th2 cytokines for 24 hours. The results showed that Th2 cytokines had no effect on the activation of these fibroblasts (Supplemental Figure 2). These results indicate that Th2 cytokines did not affect resident kidney fibroblast activation.
CP690,550 Inhibits STAT6 Activation in a UUO Model
To determine whether STAT6 is active in the kidney, WT mice were subjected to UUO for 5 days. Freshly isolated kidney cells were stained for CD11b, collagen I, and p-STAT6. Flow cytometric analysis showed that myeloid fibroblast identified as CD11b and collagen I dual-positive cells account for about 50% of total p-STAT6–positive cells (Supplemental Figure 3).
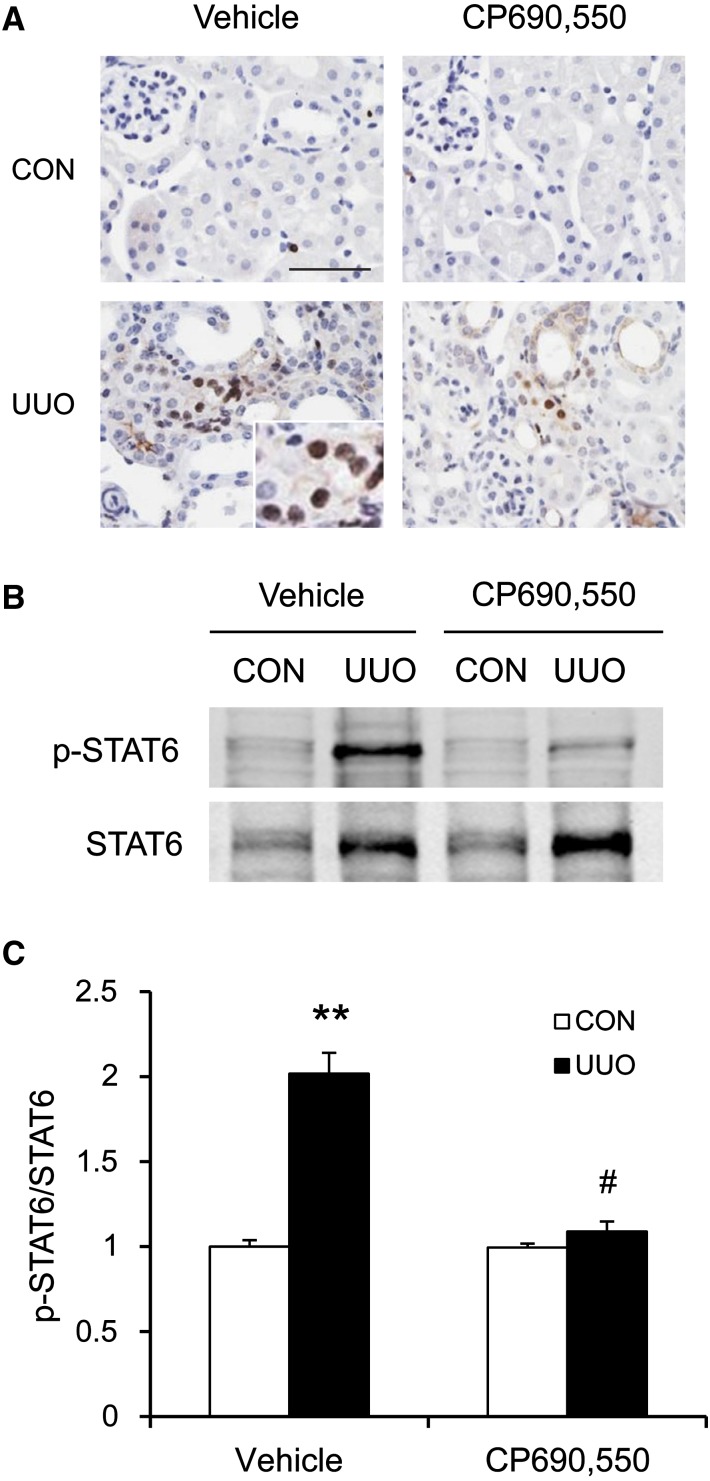
We next examined the effect of CP690,550 on STAT6 activation in the kidney in a mouse model of tubulointerstitial fibrosis induced by UUO. WT mice were administered either vehicle or CP690,550 orally after UUO surgery. The contralateral kidneys were used as controls. Kidney sections were stained with a phospho-STAT6 (Tyr641) antibody. Prominent nuclear staining of phosphorylated STAT6 was mainly detected in interstitial cells after UUO in the vehicle group. CP690,550 significantly inhibited phospho-STAT6–positive staining in the obstructed kidneys (Figure 4A). Consistent with these findings, Western blot analysis showed that CP690,550 suppressed STAT6 activation in response to UUO (Figure 4, B and C).
Figure 4.
CP690,550 inhibits STAT6 activation in the UUO kidneys. (A) Representative photomicrographs of kidney sections stained for phosphorylated STAT6 (brown) and counterstained with hematoxylin (blue). (B) Representative Western blots show the effect of CP690,550 on STAT6 activation in UUO kidneys. (C) Quantitative analysis of STAT6 phosphorylation. **P<0.01 compared with the vehicle control group; #P<0.01 compared with the vehicle UUO group (n=7 per group). CON, control. Bar, 50 μm.
CP690,550 Attenuates Bone Marrow–Derived Fibroblast Accumulation and Myofibroblast Transformation
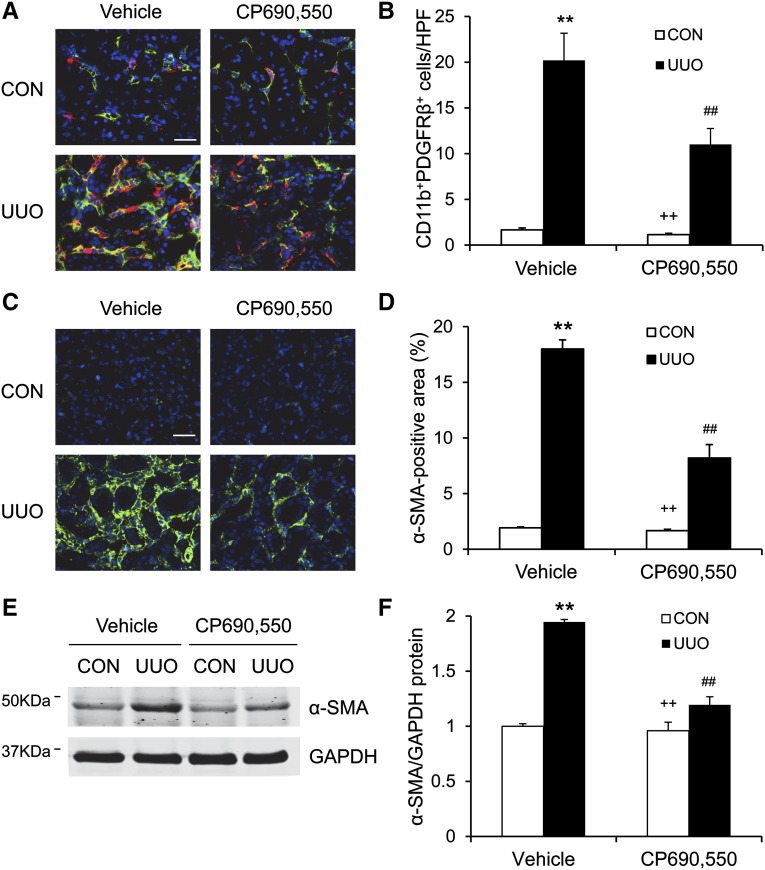
Bone marrow–derived fibroblasts contribute significantly to the pathogenesis of renal fibrosis. To examine the role of JAK3 in the activation of bone marrow–derived fibroblasts in response to obstructive injury, we performed immunofluorescence staining on frozen sections from both vehicle- and CP690,550-treated mice. The bone marrow–derived fibroblasts were identified using double staining for both CD11b, a hematopoietic marker, and platelet-derived growth factor receptor β (PDGFR-β), a mesenchymal marker. The number of CD11b+ and PDGFR-β+ cells was markedly increased in injured kidneys in the vehicle-treated mice. By contrast, the number of CD11b+ and PDGFR-β+ cells was significantly reduced in obstructed kidneys of CP690,550-treated mice (Figure 5, A and B). These data indicate that JAK3 mediates the activation of bone marrow–derived fibroblast precursors in the UUO kidneys.
Figure 5.
CP690,550 suppresses bone marrow–derived fibroblast accumulation and myofibroblast activation. (A) Representative photomicrographs of kidney sections stained for CD11b (red), PDGFR-β (green), and DAPI (blue). (B) Quantitative analysis of CD11b+ and PDGFR-β+ fibroblasts in the kidneys. (C) Representative photomicrographs of kidney sections stained for α-SMA (green) and DAPI (blue). (D) Quantitative analysis of α-SMA staining in the kidneys. (E) Representative Western blots show the protein levels of α-SMA in the kidneys. (F) Quantitative analysis of α-SMA protein expression in the kidneys. **P<0.01 compared with vehicle control; ##P<0.01 compared with vehicle UUO; ++P<0.01 compared with CP690,550 UUO (n=7 per group). CON, control; DAPI, 4',6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HPF, high-power field. Bar, 25 μm.
We next determined whether the JAK3 signaling pathway regulates the development of myofibroblasts in the kidneys. The number of α-SMA+ myofibroblasts was significantly increased in the vehicle-treated mice after UUO. CP690,550 treatment led to a significant reduction in the number of myofibroblasts in the obstructed kidneys (Figure 5, C and D). Consistent with these findings, the expression levels of α-SMA protein were markedly reduced by CP690,550 in the obstructed kidneys (Figure 5, E and F). These results indicate that JAK3 signaling promotes the development of myofibroblasts in the kidneys.
JAK3 Inhibitor Reduces Renal Fibrosis and ECM Protein Production
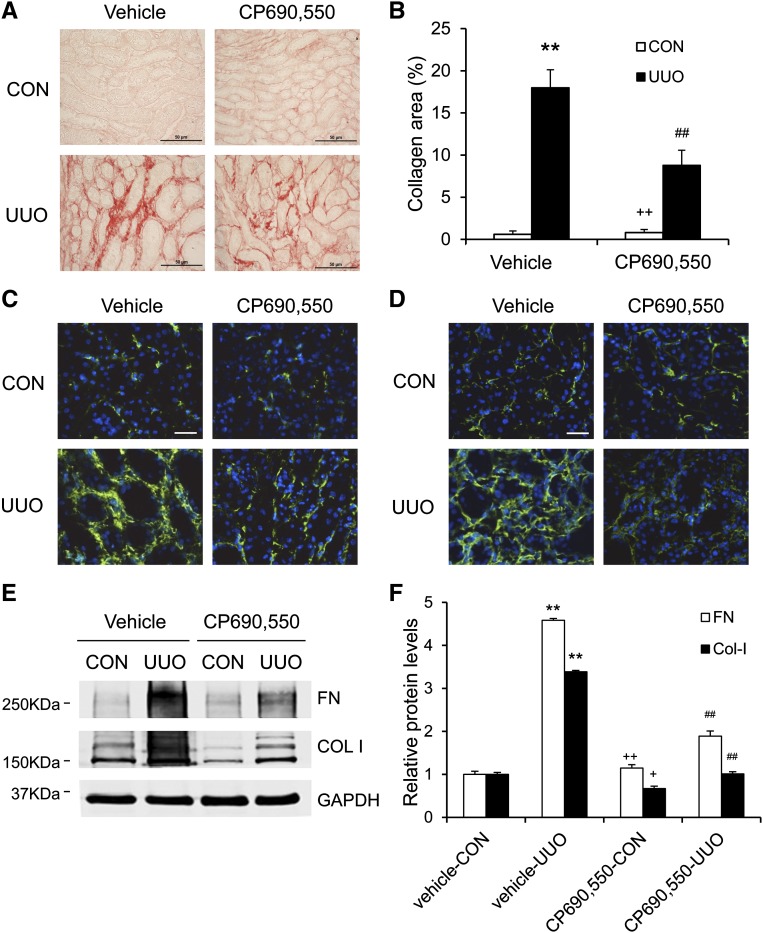
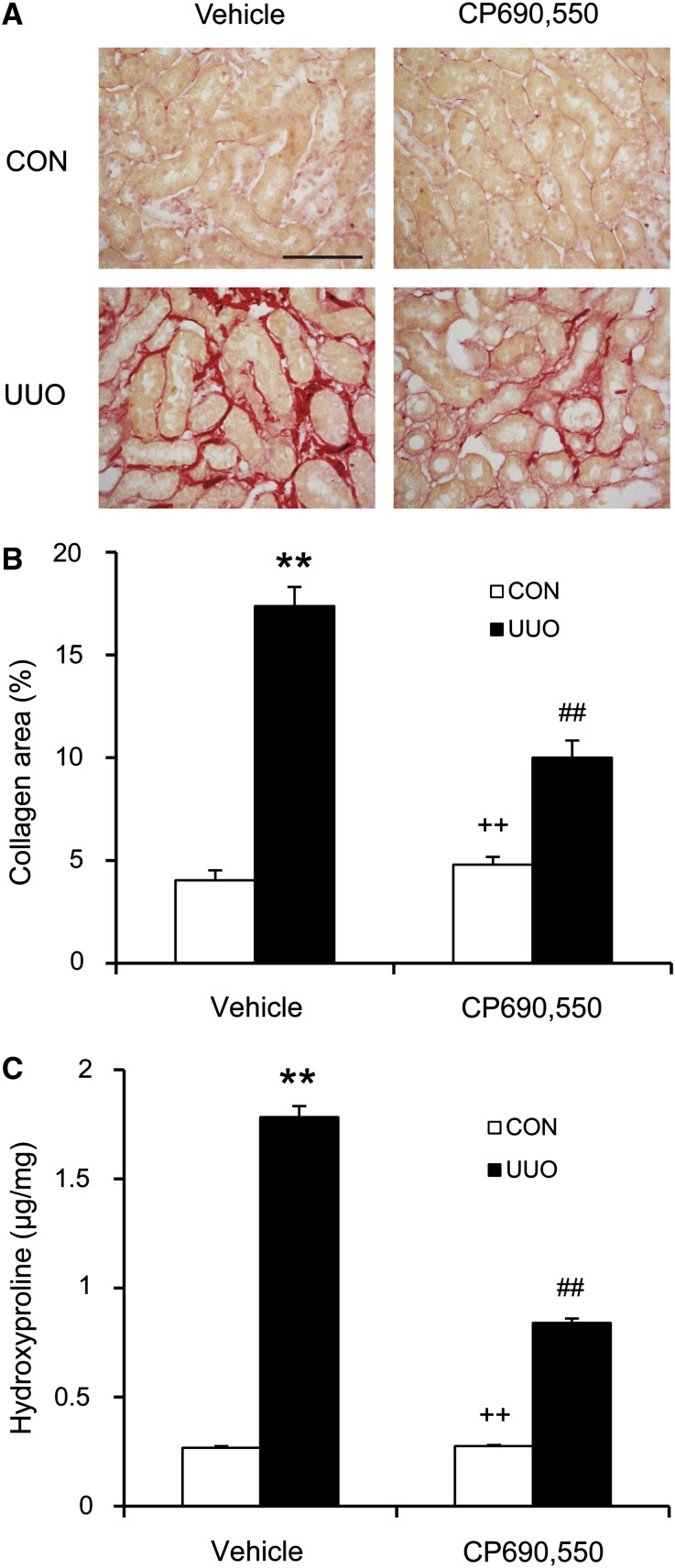
We then examined the effect of the JAK3 inhibitor on the development of renal fibrosis. Vehicle-treated mice developed significant interstitial collagen deposition in the obstructed kidneys as demonstrated by picrosirius red staining, whereas these fibrotic responses were significantly attenuated in the obstructed kidneys of mice treated with CP690,550 (Figures 6, A and B, and 7, A and B).
Figure 6.
CP690,550 attenuates renal fibrosis and ECM deposition. (A) Representative photomicrographs of kidney sections stained with picrosirius red for assessment of total collagen deposition in the kidneys 1 week after UUO. (B) Quantitative analysis of renal interstitial collagen deposition in the kidneys 1 week after UUO. (C) Representative photomicrographs of kidney sections stained for fibronectin (green) and counterstained with DAPI (blue). (D) Representative photomicrographs of kidney sections stained for collagen I (green) and counterstained with DAPI (blue). (E) Representative Western blots show protein expression of fibronectin and collagen I in the kidneys. (F) Quantitative analysis of protein levels of fibronectin and collagen I in the kidneys. **P<0.01 compared with vehicle control; ##P<0.01 compared with vehicle UUO; +P<0.05 compared with CP690,550 UUO; ++P<0.01 compared with CP690,550 UUO (n=7 per group). COL I, collagen 1; Col-I, collagen I; CON, control; DAPI, 4',6-diamidino-2-phenylindole FN, fibronectin. Bar, 50 μm in A; 25 μm in C and D.
Figure 7.
CP690,550 attenuates renal fibrosis and collagen deposition. (A) Representative photomicrographs of kidney sections stained with picrosirius red for assessment of total collagen deposition in the kidneys 2 week after UUO. (B) Quantitative analysis of renal interstitial collagen deposition in the kidneys 2 week after UUO. (C) Hydroxyproline assay demonstrates that CP690,550 leads to a significant reduction in collagen contents in the kidneys 2 weeks after UUO. **P<0.01 compared with vehicle control; ##P<0.01 compared with vehicle UUO; ++P<0.01 compared with CP690,550 UUO (n=8 per group). CON, control. Bar, 50 μm.
We further tested the effect of the JAK3 inhibitor on the expression of fibronectin and collagen I in the UUO kidneys. Immunofluorescence staining and Western blot analysis showed that CP690,550 treatment resulted in a significant reduction in the protein levels of fibronectin and collagen I in the obstructed kidneys (Figure 6, C–F). Moreover, CP690,550 treatment led to a significant reduction in collagen contents in the kidneys after UUO (Figure 7C). These data indicate that JAK3 signaling plays an important role in the production and deposition of ECM proteins.
JAK3 Inhibitor Attenuates Apoptotic Cell Death
Because obstructive injury led to apoptotic cell death in the kidney18 and caspase 3 is the final effector caspase that mediates apoptotic cell death,19 we then examined the effect of CP690,550 on caspase 3 activation in the kidney. Western blot analysis using an antibody against active caspase 3 demonstrated that the levels of active caspase 3 were significantly higher in the obstructed kidneys of vehicle-treated mice. CP690,550 treatment significantly reduced caspase 3 activation in the kidney after UUO (Supplemental Figure 4). These data indicate that CP690,550 inhibits caspase 3 activation and apoptotic cell death in the kidney after obstructive injury.
STAT6 Deficiency Suppresses Bone Marrow–Derived Fibroblast Accumulation
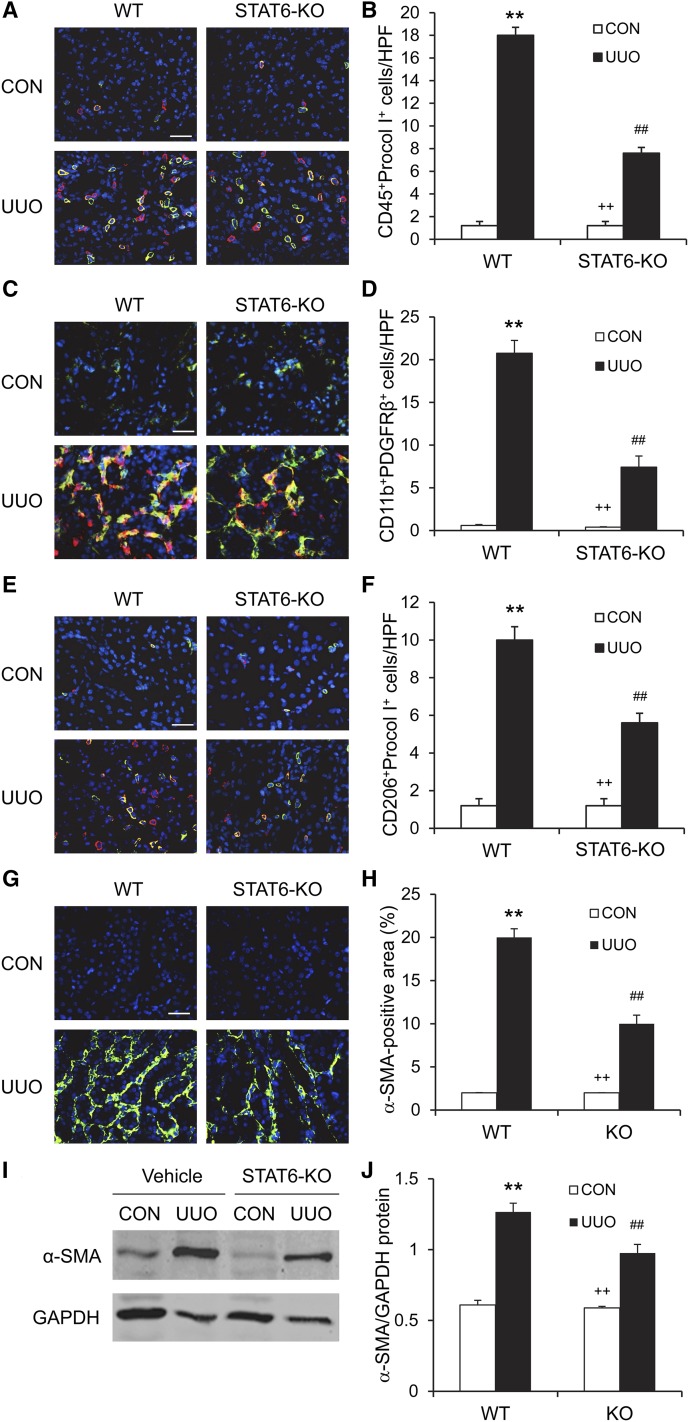
To explore the role of STAT6 signaling in bone marrow–derived fibroblast accumulation, WT and STAT6 KO mice were subjected to obstructive injury for 7 days. Kidney sections were stained for CD45 and procollagen I or CD11b and PDGFR-β and examined with a fluorescence microscope. The number of bone marrow–derived fibroblasts that were dual positive for CD45 and procollagen I or CD11b and PDGFR-β was significantly reduced in the kidneys of STAT6 KO mice compared with WT mice (Figure 8, A–D). These data indicate that STAT6 has an important role in recruiting bone marrow–derived fibroblast precursors into the kidney in response to obstructive injury.
Figure 8.
STAT6 deficiency suppresses bone marrow–derived fibroblast accumulation, macrophage polarization, and myofibroblast transformation. (A) Representative photomicrographs of kidney sections stained for CD45 (red), procollagen I (green), and DAPI (blue). (B) Quantitative analysis of CD45+ and procollagen I+ fibroblasts in the kidneys. (C) Representative photomicrographs of kidney sections stained for CD11b (red), PDGFR-β (green), and DAPI (blue). (D) Quantitative analysis of CD11b+ and PDGFR-β+ fibroblasts in the kidneys. (E) Representative photomicrographs of kidney sections stained for CD206 (red), PDGFR-β (green), and DAPI (blue). (F) Quantitative analysis of CD206+ and PDGFR-β+ fibroblasts in the kidneys. (G) Representative photomicrographs of kidney sections stained for α-SMA (green) and DAPI (blue). (H) Quantitative analysis of α-SMA staining in the kidneys. (I) Representative Western blots show the protein levels of α-SMA in the kidneys. (J) Quantitative analysis of α-SMA protein expression in the kidneys. **P<0.01 compared with WT control; ##P<0.01 compared with WT UUO; ++P<0.01 compared with KO UUO (n=6 per group). CON, control; DAPI, 4',6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HPF, high-power field. Bar, 25 μm in A, C, E, and G.
We recently showed that myeloid fibroblasts are derived from monocytes through M2 macrophage polarization.7 We next determined whether STAT6 signaling pathway affected macrophage polarization and myeloid fibroblast transformation. Kidney sections were stained for CD206, a marker for M2 macrophages, and procollagen I. Our results revealed that the number of CD206 and procollagen I dual-positive cells was increased in obstructed kidneys of vehicle-treated mice, whereas the number of CD206 and procollagen I dual-positive cells was significantly reduced in obstructed kidneys of STAT6 KO mice (Figure 8, E and F). These results support our notion that myeloid fibroblasts are derived from monocytes through M2 macrophage polarization and this process is regulated by STAT6 signaling pathway.
STAT6 Deficiency Suppresses Myofibroblast Transformation
To determine the effect of STAT6 deficiency on the myofibroblast formation, WT and STAT6 KO mice were subjected to UUO for 14 days. Kidney sections were stained for α-SMA and examined with a fluorescence microscope. STAT6 deficiency resulted in a significant reduction in the number of α-SMA+ myofibroblasts in obstructed kidneys compared with those of WT mice (Figure 8, G and H). Consistent with these findings, Western blot analysis showed that STAT6 deficiency significantly reduced the protein expression level of α-SMA in obstructed kidneys compared with WT mice (Figure 8, I and J).
We next examined the role of STAT6 on the T cell infiltration into the kidney. Kidney sections were stained for CD3, a marker for T cells. The results showed that STAT6 deficiency had no effect on T cell infiltration into the kidney (Supplemental Figure 5).
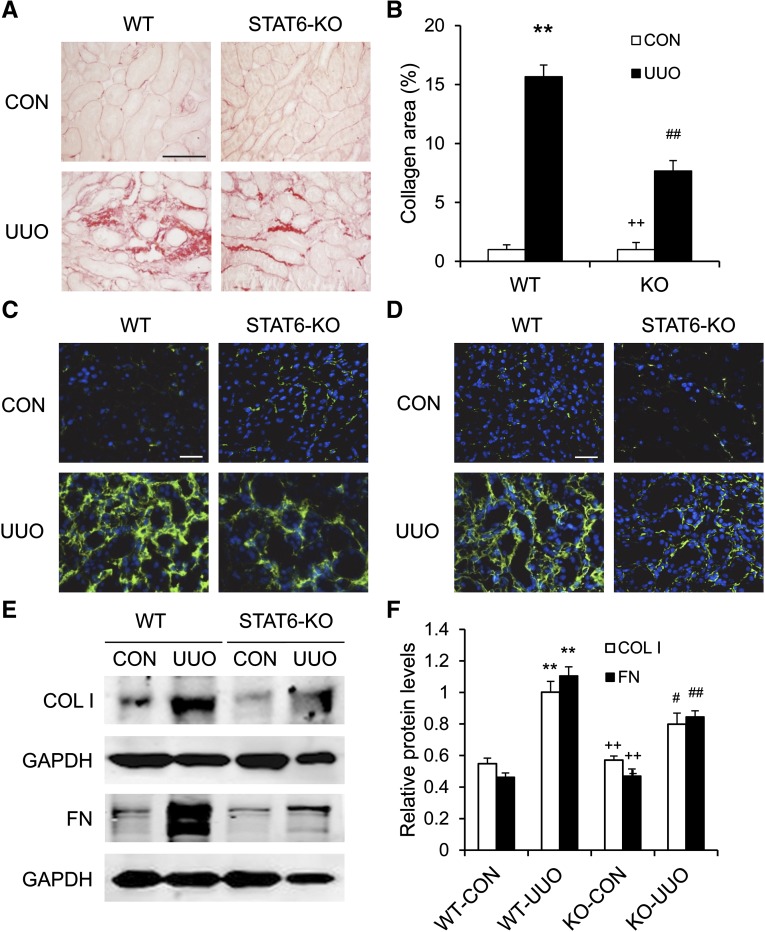
STAT6 Deficiency Inhibits Renal Fibrosis and ECM Protein Production
To evaluate the effect to STAT6 deficiency on the development of renal fibrosis, kidney sections were stained with picrosirius red. Total collagen deposition was significantly reduced in the obstructed kidneys of STAT6 KO mice compared with WT mice (Figure 9, A and B).
Figure 9.
STAT6 deficiency reduces renal fibrosis and ECM deposition. (A) Representative photomicrographs of kidney sections stained with picrosirius red for assessment of total collagen deposition in the kidneys. (B) Quantitative analysis of renal interstitial collagen deposition in the kidneys. (C) Representative photomicrographs of kidney sections stained for fibronectin (green) and counterstained with DAPI (blue). (D) Representative photomicrographs of kidney sections stained for collagen I (green) and counterstained with DAPI (blue). (E) Representative Western blots show protein expression of fibronectin and collagen I in the kidneys. (F) Quantitative analysis of protein levels of fibronectin and collagen I in the kidneys. **P<0.01 compared with WT control; #P<0.05 compared with WT UUO; ##P<0.01 compared with WT UUO; ++P<0.01 compared with KO UUO (n=6 per group). COL I, collagen I; CON, control; DAPI, 4',6-diamidino-2-phenylindole; FN, fibronectin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Bar, 50 μm in A; 25 μm in C and D.
We next examined the effect of STAT6 deficiency on the expression and accumulation of collagen I and fibronectin, two major components of ECM. Immunofluorescence staining and Western blot analysis demonstrated that loss of STAT6 attenuated the upregulation of collagen I and fibronectin in the kidneys after 2 weeks of obstructive injury (Figure 9, C–F). These data indicate that STAT6 deficiency inhibits collagen deposition and ECM protein production in response to UUO.
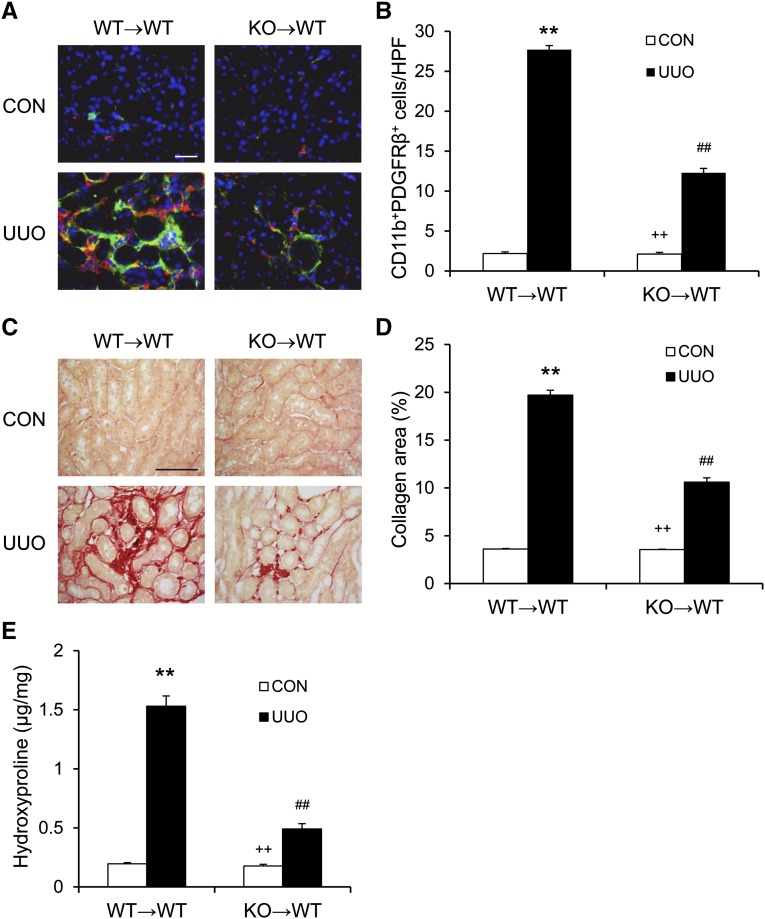
STAT6 Deficiency in Bone Marrow–Derived Fibroblasts Inhibits Renal Fibrosis
To dissect the role of STAT6 in bone marrow–derived cells in the development of renal fibrosis in vivo, we performed bone marrow transplantation of WT mice with STAT6+/+ or STAT6−/− bone marrow cells. Seven to 8 weeks after bone marrow transplantation, chimeric mice were subjected to UUO for 10 days. The genotype of bone marrow–derived cells from the chimeric mice was confirmed by PCR of DNA extracted from peripheral blood cells (Supplemental Figure 6). Compared with WT mice transplanted with STAT6+/+ bone marrow cells, WT mice transplanted with STAT6−/− bone marrow cells accumulated fewer bone marrow–derived fibroblasts and displayed a lesser degree of renal fibrosis (Figure 10). These data indicate that STAT6 signaling in bone marrow–derived cells is important for activation of myeloid fibroblasts and development of renal interstitial fibrosis.
Figure 10.
STAT6 deficiency in bone marrow–derived cells inhibits myeloid fibroblast accumulation and collagen deposition. (A) Representative photomicrographs of kidney sections stained for CD11b (red), PDGFR-β (green), and DAPI (blue). (B) Quantitative analysis of CD11b+ and PDGFR-β+ fibroblasts in the kidneys. (C) Representative photomicrographs of kidney sections stained with picrosirius red for assessment of total collagen deposition in the kidneys. (D) Quantitative analysis of renal interstitial collagen deposition in the kidneys. (E) Hydroxyproline assay demonstrates STAT6 deficiency causes a significant reduction in collagen contents in the kidneys 10 days after UUO. **P<0.01 compared with WT→WT control; ##P<0.01 compared with WT→WT UUO; ++P<0.01 compared with KO→WT UUO (n=9–10 per group). CON, control; DAPI, 4',6-diamidino-2-phenylindole; HPF, high-power field. Bar, 25 μm in A; 50 μm in C.
Discussion
Bone marrow–derived fibroblasts have been shown to contribute significantly to the production of ECM proteins and development of renal fibrosis.6–9,17,20,21 Recruitment of these circulating fibroblast precursors depends on locally produced chemokines. We previously showed that chemokine CXCL16 and its receptor CXCR6 play a critical role in recruiting these cells from the circulation to the injured kidney after obstructive injury, ischemia-reperfusion, and angiotensin II–induced hypertension.5,6,10,11 The activation of myeloid fibroblast precursors is regulated by locally produced cytokines.7,8,17 Profibrotic cytokines IL-4 and IL-13 promote fibrocyte differentiation, whereas antifibrotic cytokines IFN-γ and IL-12 inhibit its differentiation, suggesting that a complex interplay in the inflamed milieu determines the fate of bone marrow–derived fibroblast precursors.14,15 Naive CD4+ T cells can differentiate into two major distinct phenotypes, Th1 and Th2 cells, which are characterized by specific cytokine expression patterns.13 Th2 cells produce IL-4 and IL-13, which induce alternative activation of macrophages and promote monocyte-to-fibroblast transition.13 However, the molecular signaling mechanisms by which Th2 cytokines promote bone marrow–derived fibroblast activation are not defined. IL-4 and IL-13 have been shown to activate JAK3/STAT6 signaling pathway in hematopoietic cells.16,22,23 Once these cytokines bind to their cognate receptors, the associated JAK3 kinase is activated, which phosphorylates STAT6 at Y-641.24 Once phosphorylated, STAT6 forms homodimers that translocate to the nucleus, bind to cognate DNA elements, and regulate gene expression.16 In this study, we identified an important role of JAK3/STAT6 signaling in activation of bone marrow–derived fibroblast precursors and development of renal fibrosis.
CP690,550 (tofacitinib) is a novel small-molecule inhibitor of JAK3. It was originally developed as an immunosuppressive agent for organ transplantation.25 Recent studies have shown its efficacy for the treatment of rheumatoid arthritis,26 psoriasis,27 and ulcerative colitis.28 In this study, our results show that CP690,550 inhibits the phosphorylation of STAT6 and production of ECM proteins in bone marrow–derived monocytes induced by IL-4 and IL-13. Furthermore, CP690,550-treated mice accumulate fewer myeloid fibroblasts and myofibroblasts and exhibit fewer fibrotic changes in the kidney after UUO. These data indicate that JAK3 mediates IL-4– and IL-13–induced STAT6 phosphorylation, myeloid fibroblast activation, and ECM protein production in vitro and in vivo.
STAT6 is activated by IL-4 and IL-13 and plays an important role in the immune system.16 However, accumulating evidence indicates that STAT6 may function in other organ systems.29 Using genetic mutant mice, the important role of STAT6 activation in the pathogenesis of fibrosis has been investigated. In a tight-skin mutation mouse model, STAT6 KO mice develop less severe skin fibrosis.30 In a mouse pulmonary fibrosis model, STAT6 gene expression was markedly increased in the lung. Furthermore, the expression of Found In Inflammatory Zone 1 protein was decreased in STAT6-deficient mice, suggesting its role in promoting lung fibrosis and tissue remodeling.31 In this study, we have shown that STAT6 is activated in mouse bone marrow–derived monocytes in response to IL-4 or IL-13 and in the interstitial cells of the kidney after obstructive injury. STAT6 deficiency inhibits bone marrow–derived fibroblast accumulation, myofibroblast formation, ECM protein expression, and collagen deposition in the obstructed kidneys. Furthermore, the results of bone marrow chimeric experiments provide strong evidence that STAT6 in bone marrow–derived cells mediates myeloid fibroblast activation and renal fibrosis development after obstructive injury.
There are at least two strains of STAT6 KO mice. One strain of STAT6 KO mice was generated by deleting the first coding exon of STAT6, resulting in a truncated form of STAT6.32 Another strain of STAT6 KO mice was generated by deletion of amino acids 505–584 encoding the SH2 domain of STAT6.33 These two strains of STAT6 knockout mice may exhibit different phenotypes.34 Mice with a deletion of the first coding exon of STAT6 induce Th2 cell differentiation and are resistant to experimental autoimmune encephalomyelitis induction. By contrast, STAT6−/− mice generated by deletion of the SH2 domain of STAT6 are defective in Th2 cell differentiation and develop severe experimental autoimmune encephalomyelitis. In our study, we utilized mice with genetic deletion of the SH2 domain of STAT6. Our results show that deletion of the SH2 domain of STAT6 suppresses myeloid fibroblast activation both in vitro and in vivo and inhibits ECM protein production in the kidney after obstructive injury. Yukawa et al. used mice with deletion of the first coding exon of STAT6 and showed that these mice had more apoptotic cells and F4/80+ cells, exhibited less severe renal fibrosis, and accumulated less collagen I in the obstructed kidneys.35
Macrophages play an important role in the regulation of fibrosis.36 Recent evidence indicates that macrophages are heterogeneous and display a remarkable plasticity.37 Classically activated macrophages promote inflammation, whereas alternatively activated macrophages or M2 macrophages stimulate ECM production.38 F4/80 and CD68 are thought to be macrophage-specific markers. Recent studies have shown that bone marrow–derived fibroblasts also express F4/80 and CD68.39–41 In this study, our results demonstrate that disruption of STAT6 impairs M2 macrophage polarization and myeloid fibroblast transformation, which is consistent with the notion that myeloid fibroblasts are derived from monocytes via M2 macrophage polarization.7,14 Nevertheless, the optimal marker for macrophage subtypes in vivo and the potential involvement of JAK3/STAT6 in the regulation of inflammatory macrophages require further investigation.
In conclusion, our study demonstrates that JAK3/STAT6 signaling plays a critical role in the pathogenesis of renal fibrosis. In response to obstructive injury, JAK3/STAT6 signaling activated by Th2 cytokines promotes bone marrow–derived fibroblast activation, ECM protein production, and development of renal interstitial fibrosis. Therefore, inhibition of JAK3/STAT6 signaling could be a novel therapeutic target for the treatment of CKD.
Concise Methods
Animals and UUO Surgery
Animal experiments were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. The investigation conforms to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The STAT6 KO mice on a background of C57BL/6J were generated as described.33 WT C57BL/6 and STAT6 KO mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Male WT or STAT6 KO mice aged 8–10 weeks (weighing 20–30 g) were used for the studies. The UUO procedure was performed as described.6,7,17,42,43
Bone Marrow Transplantation
Bone marrow transplantation was performed as previously described.6,10,17,42,44 Briefly, bone marrow cells (5×106) from WT or STAT6 KO mice were transferred to lethally irradiated WT mice. Chimeric mice were allowed to recuperate for 7–8 weeks before UUO surgery.
CP690,550 Administration
CP690,550 was purchased from LC Laboratories (Woburn, MA) and dissolved in 0.5% methylcellulose for in vivo study or in DMSO for in vitro use. Either vehicle or CP-690,550 (15 mg/kg) in a volume of 200 μl was given to the mice through a feeding needle twice daily from days 1 to 7. The doses of CP690,550 used in our studies are well established in previous reports.45 A dosage of 30 mg/kg per day was selected based on our preliminary studies showing that 15 mg/kg per day had slight effect on renal fibrosis. The UUO surgery was performed 4 hours after the first dose of either vehicle or CP690,550 on day 1.
Bone Marrow Monocyte Culture and Treatment
Mouse bone marrow monocytes were isolated and cultured as previously described.7 Briefly, bone marrow monocytes were isolated from the femur and tibia of 8- to 10-week-old mice, passed through a 40-μm cell strainer (Corning, Tewksbury, MA), and cultured in RPMI medium containing 10% FBS, 10% L929-conditioned medium, 1% glutamine, 1% MEM vitamins, and 1% penicillin/streptomycin in a humid incubator at 37°C and 5% CO2. Fresh medium was replaced every 2–3 days. For IL-4 or IL-13 treatment, cells were starved with RPMI 1640 containing 2% FBS and 2% L929-conditioned medium for 24 hours and then exposed to vehicle, IL-4, or IL-13 (PeproTech, Rocky Hill, NJ) in the absence or presence of CP690,550 (LC Laboratories).
Immunofluorescence
Immunofluorescence staining was performed as described.5–7,10,11 For double immunofluorescence, kidney sections were incubated with primary antibodies, followed by appropriate secondary antibodies. Fluorescence intensity was visualized using a microscope equipped with a digital camera (Nikon, Melville, NY). Quantitative evaluation of sections was performed using NIS-Elements Br 3.0 software.
Immunohistochemistry
Immunohistochemical staining was performed on paraffin sections. Antigen retrieval was performed with antigen unmasking solution (Vector Laboratories, Burlingame, CA). Endogenous peroxidase activity was quenched with 3% H2O2. A rabbit Vectastain ABC-peroxidase kit (Vector Laboratories) was used for the staining for phosphorylated-STAT6. After blocking, slides were incubated with rabbit anti–phosphorylated STAT6 antibody (Abcam, Inc., Cambridge, CA) in a humidified chamber overnight. After washing, slides were incubated with appropriate secondary antibody and ABC solution sequentially according to the kit protocol. The reaction was visualized by incubation with 3,3′-Diaminobenzidine substrate solution (Vector Laboratories). Slides were then counterstained with hematoxylin. The images were acquired with a Nikon microscope image system (Nikon).
Western Blot Analyses
Protein was extracted using radioimmunoprecipitation buffer containing cocktail proteinase inhibitors (Thermo Fisher Scientific, Rockford, IL). Equal amounts of protein were separated on SDS-polyacrylamide gels in a Tris/glycine buffer system, transferred onto nitrocellulose membranes, and blotted according to standard procedures with primary antibodies overnight followed by incubation with appropriate fluorescence-conjugated secondary antibodies. The proteins of interest were analyzed using an Odyssey IR scanner, and signal intensities were quantified using NIH ImageJ software.
Hydroxyproline Assay
Hydroxyproline content was determined using a colorimetric kit (Sigma-Aldrich, St. Louis, MO). Tissue samples were hydrolyzed in 6 N HCl (final concentration) at 120°C for 3 hours. The hydroxyproline content was assayed according to the manufacturer’s protocol. Values were expressed as micrograms per milligram of tissue.
Statistical Analyses
All data were expressed as mean±SEM. Multiple group comparisons were performed by ANOVA followed by the Bonferroni procedure for comparison of means. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Xiaogao Jin and Mr. Joel M. Sederstrom for technical assistance with flow cytometry.
This work was supported by grants from the NIH (K08-HL92958 and R01-DK95835 to Y.W., training grant T32-DK62706 to Ji.Y., and R37-DK37175 to W.E.M.) and the American Heart Association (11BGIA7840054 to Y.W.). The Cytometry and Cell Sorting Core at Baylor College of Medicine was supported by grants from the NIH (AI036211, CA125123, and RR024574).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “T Helper 2 Cytokine Signaling in Bone Marrow–Derived Fibroblasts: A Target for Renal Fibrosis,” on pages 2896–2898.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070717/-/DCSupplemental.
References
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Farris AB, Colvin RB: Renal interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol Hypertens 21: 289–300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Yan J, Jin X, Entman ML, Wang Y: The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney Int 86: 327–337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Lin SC, Chen J, He L, Dong F, Xu J, Han S, Du J, Entman ML, Wang Y: CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J Am Soc Nephrol 22: 1876–1886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Lin SC, Chen G, He L, Hu Z, Chan L, Trial J, Entman ML, Wang Y: Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol 24: 1644–1659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Göbel N, Talke Y, Schweda F, Mack M: CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A 106: 17892–17897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S: Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 103: 14098–14103, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Y, Jin X, Yan J, Entman ML, Wang Y: CXCR6 plays a critical role in angiotensin II-induced renal injury and fibrosis. Arterioscler Thromb Vasc Biol 34: 1422–1428, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Y, Entman ML, Wang Y: Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension 62: 1129–1137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blakaj A, Bucala R: Fibrocytes in health and disease. Fibrogenesis Tissue Repair 5[Suppl 1]: S6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn TA: Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4: 583–594, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D: Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol 83: 1323–1333, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML: Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol 50: 248–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wurster AL, Tanaka T, Grusby MJ: The biology of Stat4 and Stat6. Oncogene 19: 2577–2584, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Xia Y, Lin X, Feng XH, Wang Y: Smad3 signaling activates bone marrow-derived fibroblasts in renal fibrosis. Lab Invest 94: 545–556, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truong LD, Petrusevska G, Yang G, Gurpinar T, Shappell S, Lechago J, Rouse D, Suki WN: Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int 50: 200–207, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Thornberry NA, Lazebnik Y: Caspases: Enemies within. Science 281: 1312–1316, 1998 [DOI] [PubMed] [Google Scholar]
- 20.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich B, Schmidbauer K, Rodriguez Gomez M, Johannes Hermann F, Göbel N, Brühl H, Ketelsen I, Talke Y, Mack M: Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int 84: 78–89, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Maier E, Duschl A, Horejs-Hoeck J: STAT6-dependent and -independent mechanisms in Th2 polarization. Eur J Immunol 42: 2827–2833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang YS, Kim HA, Park SR, Lee MR, Park JB, Kim PH: IL-4 stimulates mouse macrophages to express APRIL through p38MAPK and two different downstream molecules, CREB and Stat6. Cytokine 47: 43–47, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Mikita T, Campbell D, Wu P, Williamson K, Schindler U: Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol 16: 5811–5820, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des Etages SG, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si MS, Paniagua R, Higgins J, Holm B, Reitz B, Zhou YJ, Morris RE, O’Shea JJ, Borie DC: Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302: 875–878, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators : Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 63: 1150–1158, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Mamolo C, Harness J, Tan H, Menter A: Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis [published online ahead of print January 7, 2013]. J Eur Acad Dermatol Venereol 10.1111/jdv.12081 [DOI] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W, Study A3921063 Investigators : Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 367: 616–624, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Goenka S, Kaplan MH: Transcriptional regulation by STAT6. Immunol Res 50: 87–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong CJ, Ip S, Teh SJ, Wong C, Jirik FR, Grusby MJ, Teh HS: A role for T helper 2 cells in mediating skin fibrosis in tight-skin mice. Cell Immunol 196: 60–68, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH: Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: Role of IL-4/IL-13 and mediation via STAT-6. J Immunol 173: 3425–3431, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN: Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380: 630–633, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan MH, Schindler U, Smiley ST, Grusby MJ: Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4: 313–319, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Evans JT, Rodriguez F, Fields P, Mueller C, Chitnis T, Khoury SJ, Bynoe MS: A tale of two STAT6 knock out mice in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol 206: 76–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yukawa K, Kishino M, Goda M, Liang XM, Kimura A, Tanaka T, Bai T, Owada-Makabe K, Tsubota Y, Ueyama T, Ichinose M, Maeda M, Takeda K, Akira S: STAT6 deficiency inhibits tubulointerstitial fibrosis in obstructive nephropathy. Int J Mol Med 15: 225–230, 2005 [PubMed] [Google Scholar]
- 36.Wynn TA, Barron L: Macrophages: Master regulators of inflammation and fibrosis. Semin Liver Dis 30: 245–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon S, Plüddemann A, Martinez Estrada F: Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol Rev 262: 36–55, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S, Martinez FO: Alternative activation of macrophages: Mechanism and functions. Immunity 32: 593–604, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Pilling D, Fan T, Huang D, Kaul B, Gomer RH: Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 4: e7475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldrich A, Kielian T: Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am J Pathol 179: 2952–2962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kisseleva T, von Köckritz-Blickwede M, Reichart D, McGillvray SM, Wingender G, Kronenberg M, Glass CK, Nizet V, Brenner DA: Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection. J Mol Med (Berl) 89: 997–1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia Y, Entman ML, Wang Y: CCR2 regulates the uptake of bone marrow-derived fibroblasts in renal fibrosis. PLoS ONE 8: e77493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Chen J, Yan J, Zhang L, Chen G, He L, Wang Y: Effect of interleukin 6 deficiency on renal interstitial fibrosis. PLoS ONE 7: e52415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin X, Chen J, Hu Z, Chan L, Wang Y: Genetic deficiency of adiponectin protects against acute kidney injury. Kidney Int 83: 604–614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migita K, Miyashita T, Izumi Y, Koga T, Komori A, Maeda Y, Jiuchi Y, Aiba Y, Yamasaki S, Kawakami A, Nakamura M, Ishibashi H: Inhibitory effects of the JAK inhibitor CP690,550 on human CD4(+) T lymphocyte cytokine production. BMC Immunol 12: 51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.