Abstract
Kidney aging is associated with an increasing proportion of globally scarred glomeruli, decreasing renal function, and exponentially increasing ESRD prevalence. In model systems, podocyte depletion causes glomerulosclerosis, suggesting age-associated glomerulosclerosis could be caused by a similar mechanism. We measured podocyte number, size, density, and glomerular volume in 89 normal kidney samples from living and deceased kidney donors and normal poles of nephrectomies. Podocyte nuclear density decreased with age due to a combination of decreased podocyte number per glomerulus and increased glomerular volume. Compensatory podocyte cell hypertrophy prevented a change in the proportion of tuft volume occupied by podocytes. Young kidneys had high podocyte reserve (podocyte density >300 per 106 µm3), but by 70–80 years of age, average podocyte nuclear density decreased to, <100 per 106 µm3, with corresponding podocyte hypertrophy. In older age podocyte detachment rate (urine podocin mRNA-to-creatinine ratio) was higher than at younger ages and podocytes were stressed (increased urine podocin-to-nephrin mRNA ratio). Moreover, in older kidneys, proteinaceous material accumulated in the Bowman space of glomeruli with low podocyte density. In a subset of these glomeruli, mass podocyte detachment events occurred in association with podocytes becoming binucleate (mitotic podocyte catastrophe) and subsequent wrinkling of glomerular capillaries, tuft collapse, and periglomerular fibrosis. In kidneys of young patients with underlying glomerular diseases similar pathologic events were identified in association with focal global glomerulosclerosis. Podocyte density reduction with age may therefore directly lead to focal global glomerulosclerosis, and all progressive glomerular diseases can be considered superimposed accelerators of this underlying process.
Keywords: aging, podocyte, glomerulosclerosis
Biologic aging, the process by which time-associated changes take place in living cells and tissues, is amenable to scientific analysis. With such analysis it can be broken down into its constituent elements and manipulated to change its rate and, thereby, the aging process itself. Chronologic age, on the other hand, is a fixed time dimension that for practical purposes cannot be altered.
Aging-associated structural changes in the kidney and glomerulus have been repeatedly noted for nearly 100 years.1–5 Glassock and Rule provide a comprehensive contemporary review.6 The prevalence of glomerulosclerosis increases with age.2–6 Renal pathologists use an equation ([age/2]−10) as a guide to determine whether the proportion of sclerotic glomeruli present in a biopsy exceeds a “normal” expected range.4 As glomeruli become increasingly sclerosed, downstream tubules atrophy/involute so that glomerular density (number per remaining volume of cortex) increases, interstitial fibrosis accumulates, and kidneys eventually lose mass.3,6 In 1949, Davies and Shock reported that kidney function decreased with age,7 as has been confirmed by most subsequent studies.5,8,9 The eGFR decreases with age at an average rate of about 0.8% per year detectable after age 30 years.5–9 With the advent of RRTs in combination with increasing life expectancy, the true effect of the aging kidney has become all too obvious. ESRD prevalence increases exponentially with age, and age is the strongest known risk factor for ESRD by far.10,11 This age-associated structure-function-outcome triad implies a direct biologic effect of aging on the kidney, particularly on the glomerulus, whose underlying mechanism(s) are not well understood.
It has become clear from model systems that depletion of the long-lived postmitotic neuron-like cell of the kidney glomerulus, the podocyte, causes glomerulosclerosis in direct proportion to the degree of depletion.12–15 FSGS resulting from podocyte injury and loss can occur via a series of pathologic steps defined by Kriz and colleagues.16–18 Glomerular tuft podocyte density (number per volume), the determinant of whether glomerulosclerosis occurs, can become reduced through (1) loss of podocytes per se (via detachment or cell death), (2) glomerular tuft enlargement (so that each podocyte becomes responsible for an increased area of filtration surface), and (3) podocyte dysfunction or phenotype switch (wherein the podocyte no longer performs its normal functions).19 Observational studies in human glomerular diseases also demonstrate an association between podocyte density decrease and outcome in diabetic glomerulosclerosis, IgA nephropathy, hypertensive glomerulosclerosis, and transplant glomerulopathy.20–27
We previously reported age-related reduction in podocyte density (shown as increased glomerular volume per podocyte) associated with podocyte hypertrophic stress and failure leading to glomerulosclerosis in Fischer344 rats.28 Prevention of age-associated glomerular enlargement achieved by reduced calorie intake prevented development of glomerulosclerosis, thereby demonstrating that age-related glomerulosclerosis could potentially be modulated.28 To determine whether simple podocyte depletion per se could cause glomerulosclerosis, we developed the hDTR transgenic rat, in which a predetermined number of podocytes could be selectively eliminated at a predetermined time.13 Using this model we proved that podocyte depletion per se quantitatively caused all features of glomerulosclerosis with an appearance similar to that seen in humans.13 To form an alternative way of causing relative podocyte depletion that would be more analogous to aging, we developed the podocin promoter-driven AA-4EBP1 transgenic rat. In this model, podocytes are selectively prevented from undergoing normal hypertrophy while all other cells can undergo hypertrophy normally.15 Using this model we proved that glomerular enlargement itself drives glomerulosclerosis in a podocyte-dependent manner.15 These model studies therefore tell us that glomerulosclerosis can be caused by podocyte depletion initiated by apparently completely different mechanisms that do not involve the immune system and that have in common reduced podocyte density and increased podocyte stress. In the rat these processes appear to drive aging-associated glomerulosclerosis.28
Because reduced podocyte density causes glomerulosclerosis in models and is associated with glomerulosclerosis in human glomerular diseases, the question arises as to whether glomerular aging in humans might also be associated with decreased podocyte density. A newly developed morphometric approach to measure podocyte parameters (“podometrics”) applicable to archival kidney biopsy material offers the opportunity to test this hypothesis.29
Results
Figure 1 illustrates methods used to estimate podocyte density, number, size, glomerular volume, and other morphometric variables. To span the human age range, we analyzed 89 kidneys that were within normal pathologic limits identified by conventional light microscopic pathologic analysis. The cohort was composed of deceased kidney transplant donors age 4–18 years (n=20) and age 48–64 years (n=11), living kidney transplant donors age 21–53 years (n=29), and the normal pole of nephrectomies for cancer age 43–85 years (n=29).
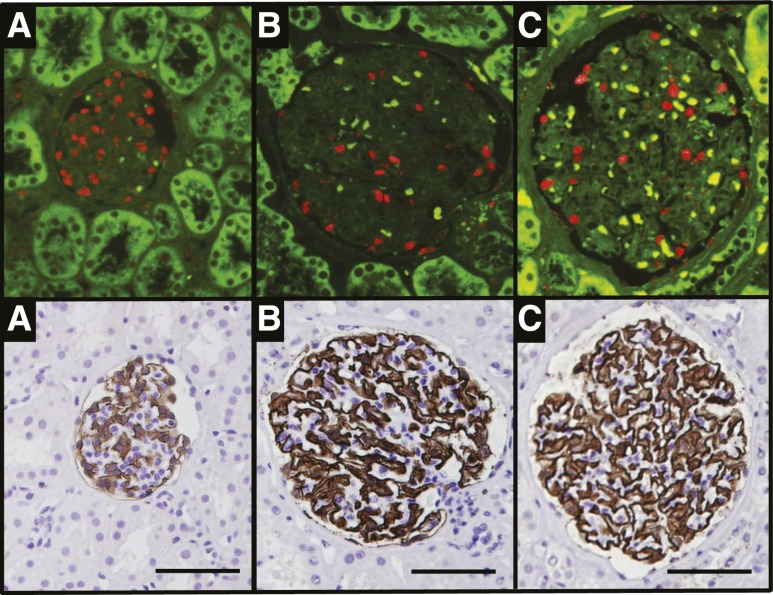
Figure 1.
Podometric methodology. The upper panels show TLE4-red fluorescent podocyte nuclei (red) in glomerular profiles from young (A), middle-aged (B), and older (C) people. The lower panels show the same sections for which the coverslip has been removed and peroxidase immunocytochemistry performed to delineate the Glepp1 peroxidase–positive area (brown) occupied by podocytes. Calibrated photomicrographs of the TLE4 immunofluorescent sections are used to measure podocyte nuclear mean caliper diameter. Photomicrographs of the Glepp1-stained sections are used to measure glomerular tuft area and the percentage of the tuft area occupied by podocytes. These primary data measured in the histologic section are then used to estimate podocyte nuclear density, glomerular volume, number of podocyte nuclei per tuft, Glepp1-positive tuft volume, Glepp1-negative tuft volume, and podocyte cell volume. The bar shows 100 μm.
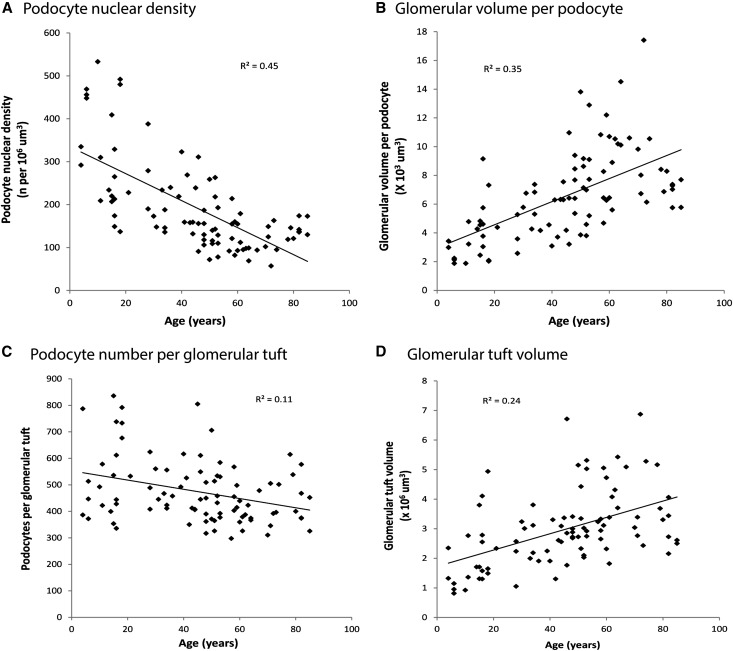
Podocyte nuclear density decreased about 0.9% per year from >300 per 106 μm3 at <20 years of age to <100 106 μm3 by 70–80 years of age (P<0.001) (Figure 2A). Glomerular volume per podocyte, the average territory each podocyte is responsible for managing, increased by 2.7% per year (P<0.001) (Figure 2B). These changes with aging resulted from a combination of (1) reduced podocyte nuclear number per glomerulus (slope, −0.34% per year; P=0.002) (Figure 2C) and (2) increased glomerular tuft volume (slope, 1.6% per year; P<0.001) (Figure 2D). Table 1 shows slope data for these variables. Table 2 shows data by age group across all cohorts to emphasize the continuous change with aging and to provide ranges. Table 3 shows that excluding the normal kidney pole data, the data from older deceased donors, or both of these groups did not significantly change the podocyte density slope or correlation.
Figure 2.
Aging-associated changes in podocyte density resulting from changes in podocyte nuclear number per tuft and glomerular volume. (A) Podocyte nuclear density (number per tuft volume). (B) Reciprocal glomerular volume per podocyte. The underlying causes of the density change are a reduction in podocyte number per glomerular tuft (C) and an increase in glomerular volume (D). Table 1 provides slopes and statistical information for these data using bivariate linear regression models.
Table 1.
Glomerular morphometric variables by age
| Variable | c at Age = 0 | m | Error of Estimate | P Value |
|---|---|---|---|---|
| Podocyte density (n per 106 μm3) | 338 | −3.17 | 79.4 | <0.001 |
| Glomerular volume per podocyte (×103) | 2.93 | 0.08 | 2.51 | <0.001 |
| Podocytes per glomerular tuft (n) | 554 | −1.76 | 116 | 0.002 |
| Glomerular volume (×106 μm3) | 1.72 | 0.028 | 1.13 | <0.001 |
| Glepp1-positive tuft area (%) | 43.3 | 0.028 | 5.6 | 0.30 |
| Glepp1-positive tuft volume (×106 μm3) | 0.71 | 0.013 | 0.46 | <0.001 |
| Glepp1-negative tuft volume (×106 μm3) | 1.00 | 0.014 | 0.73 | <0.001 |
| Mean podocyte cell volume (×103 μm3) | 1.18 | 0.038 | 0.10 | <0.001 |
| Mean podocyte nuclear volume (×103 μm3) | 124 | 2.5 | 53.8 | <0.001 |
Data shown as the linear relationship with age for each measured parameter to allow a normal range for any parameter at any age to be calculated according to y=mx+c, where y is the parameter of interest, x is the age, m is the coefficient of x, and c is the intercept at age=0. The error of the estimate is shown.
Table 2.
Glomerular morphometric characteristics by age group
| Variable | Age ≤18 yr (Column A) | Comparison | Age 19–45 yr (Column B) | Comparison | Age 46–60 yr (Column C) | Comparison | Age >60 yr (Column D) | Comparison | All Ages |
|---|---|---|---|---|---|---|---|---|---|
| Average age (yr) | 12.6±4.9 | 35.5±6.8 | 52.4±4.6 | 72.2±9.1 | 45.0±22.6 | ||||
| Patients (n) | 20 | 19 | 27 | 23 | 89 | ||||
| Podocyte density (n per 106 μm3) | |||||||||
| Mean | 318±127 | B,a C,b Db | 211±69 | A,a C,a Db | 151±60 | A,b B,a Dc | 121±34 | A,b B,b Cc | 194±106 |
| Range | 137–533 | 132–388 | 72–311 | 57–179 | 57–533 | ||||
| Glomerular volume per podocyte (×103) | 6.6±3.1 | ||||||||
| Mean | 3.8±1.9 | B, C,b Db | 5.2±1.5 | A, C,b Db | 7.5±2.7 | A,b Bb | 9.0±2.9 | A,b Bb | 9.0±2.9 |
| Range | 1.9–9.2 | 2.6–7.5 | 3.2–13.8 | 5.6–17.4 | 1.9–17.4 | ||||
| Podocytes per glomerular tuft (n) | |||||||||
| Mean | 544±164 | C,c Da | 494±107 | Da | 454±99 | Ac | 422±82 | A,a Bc | 477±121 |
| Range | 336–836 | 350–805 | 298–706 | 310–615 | 298–836 | ||||
| Glomerular volume (×106 μm3) | |||||||||
| Mean | 2.0±1.1 | C,b Db | 2.5±0.7 | C,a Db | 3.3±1.2 | A,b Ba | 3.8±1.3 | A,b Bb | 3.0±1.3 |
| Range | 0.8–4.9 | 1.1–3.8 | 1.8–6.7 | 1.8–6.9 | 0.8–6.9 | ||||
| Glepp1-positive tuft area (%) | |||||||||
| Mean | 44.0±5.0 | 43.9±5.5 | 45.8±5.2 | 44.2±6.6 | |||||
| Range | 35.5–53.0 | 32.8–52.6 | 35.5–54.5 | 32.5–54.9 | 32.5–54.9 | ||||
| Glepp1-positive tuft volume (×106 μm3) | |||||||||
| Mean | 0.9±0.4 | C,b Db | 1.1±0.3 | C,a Db | 1.5±0.5 | A,b Ba | 1.6±0.5 | A,b Bb | 1.3±0.5 |
| Range | 0.3–1.8 | 0.5–1.6 | 0.8–2.8 | 0.9–2.8 | 0.3–2.8 | ||||
| Glepp1-negative tuft volume (×106 μm3) | |||||||||
| Mean | 1.2±0.7 | C,c Db | 1.4±0.5 | C,c Db | 1.8±0.7 | A,a Bc | 2.1±0.8 | A,b Ba | 1.7–0.8 |
| Range | 0.5–3.2 | 0.6–2.5 | 1.0–3.9 | 1.0–4.5 | 0.5–4.5 | ||||
| Mean podocyte cell volume (×103 μm3) | |||||||||
| Mean | 1.6±0.6 | B,a C,b Db | 2.3±0.7 | A,a C,b Db | 3.4±1.2 | A,b B,b Db | 3.9±1.0 | A,b Bb | 2.9±1.3 |
| Range | 0.9–2.6 | 1.2–3.7 | 1.4–6.7 | 2.4–6.0 | 0.9–6.7 | ||||
| Mean podocyte nuclear volume (×103 μm3) | |||||||||
| Mean | 167±40 | C,b Db | 193±62 | C,b Db | 259±66 | A,b B,b Da | 307±50 | A,b B,b Ca | 237±78 |
| Range | 115–272 | 100–363 | 131–416 | 210–397 | 100–416 |
Data are shown as the mean±1 SD and range. ANOVA with Bonferroni correction for multiple comparisons was applied to compare means. Statistical comparisons are shown by column designation (below) where each age group is compared with all other age groups. The average number of glomerular tufts evaluated per biopsy was 24.2±9.3 (total of 2154 glomeruli).
P<0.01.
P<0.001.
P<0.05.
Table 3.
Podocyte density decrease with age persists in subgroup analysis
| Group designation | Samples (n) | Slope | Correlation r | P Value |
|---|---|---|---|---|
| All samples | 89 | y=337–3.2x | −0.67 | <0.001 |
| All: nephrectomy samples | 60 | y=369–4.1x | −0.66 | <0.001 |
| All: older deceased donor kidneys | 78 | y=339–3.0x | −0.67 | <0.001 |
| All: both nephrectomy and older deceased donor | 49 | y=360–3.6x | −0.53 | <0.001 |
Changes in podocyte density with age persist after elimination from analysis either the nephrectomy normal pole samples, which may have been affected by kidney cancer–related effects (All: nephrectomy samples), or by elimination of the older deceased donor samples, which might have been affected by vascular disease or other factors (All: older deceased donor kidneys), or both of these groups (All: both nephrectomy and older decreased donors) from the analysis. Slope data are shown in a y=mx+c format where y is the podocyte density per 106 μm3 and x is the age in years. The Pearson correlation r is shown.
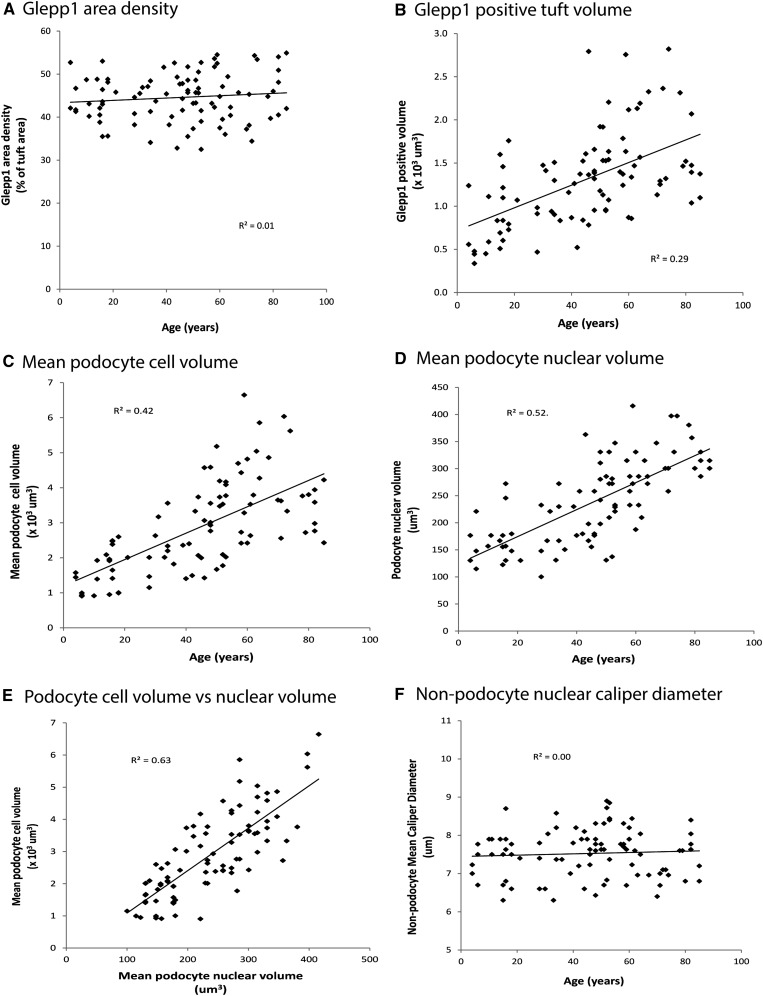
Although podocyte nuclear density decreased with age, the Glepp1-positive podocyte cytoplasmic area expressed as percentage of the tuft area occupied by podocytes did not change with age (Figure 3A). Furthermore, when glomerular volume is considered, the total podocyte cell volume (mass) significantly increased with age at a rate of 1.8% per year (P<0.001) (Figure 3B). The increased total podocyte cell mass and decreased podocyte nuclear number per glomerulus mean that the average podocyte cell volume increased with age (increase of 3.2% per year; P<0.001) (Figure 3C).
Figure 3.
Podocyte cell hypertrophy compensates for reduction in podocyte density with age. Glepp1 area density does not change with successful aging (A). However, the volume occupied by podocytes (Glepp1 percentage area×glomerular volume) does increase with aging (B). The average podocyte cell volume (Glepp1 volume/podocyte nuclear number per tuft) increases with age (C). Average podocyte nuclear volume also increases with aging (D). Podocyte cell volume and nuclear volume are highly correlated (E). In contrast, average glomerular nonpodocyte nuclear volumes do not change with aging (F). Table 1 provides slopes and statistical information for these data. The data shown in Figures 2 and 3 are for male and female patients combined, using linear regression modeling. Analysis by sex showed no statistical difference between sexes (or trends toward differences) for any of the preceding variables (data not shown).
Podocyte nuclear volume also increased with age at a rate of 2% per year (P<0.001) (Figure 3D), being highly correlated with podocyte cell volume (R2=0.63) (Figure 3E). This relationship between cell volume and nuclear volume is well known as the karyoplasmic ratio30,31 and independently confirms the age-related changes noted above. Podocyte nuclear size is therefore a surrogate for cell size. In contrast nonpodocyte cell nuclei did not increase in size with age (Figure 3F) reflecting the fact that glomerular endothelial cells and mesangial cells proliferate in response to hypertrophic stress whereas podocytes have to hypertrophy.
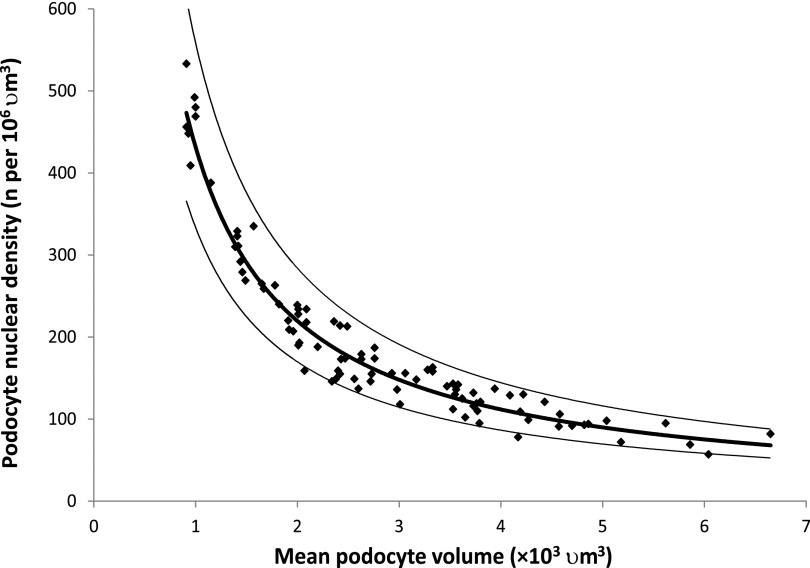
Figure 4 shows how as podocyte nuclear density decreases from >300 to <100 per 106 μm3, podocyte cell size must increase by as much as 6-fold. At low podocyte density, small further decrements require larger and ever-increasing podocyte hypertrophic responses that would be projected to eventually result in podocyte hypertrophic stress.
Figure 4.
Podocyte density/size plot providing a mechanistic explanation for podocyte hypertrophic failure associated with aging. A plot of podocyte density against cell size with the mean and 95% confidence limits demonstrates how a reduction in podocyte density with aging necessitates an exponential (inversely proportional) increase in cell size to maintain the filtration barrier. At high podocyte densities (younger age), a density decrease is easily accommodated by small increases in podocyte size. However, at a density <100 per 106 μm3 (by older age), small decrements in podocyte density require large and ever-increasing compensatory changes in cell size, thereby driving hypertrophic stress and detachment. Best fit curve estimation revealed a “power curve” with R2=0.93.
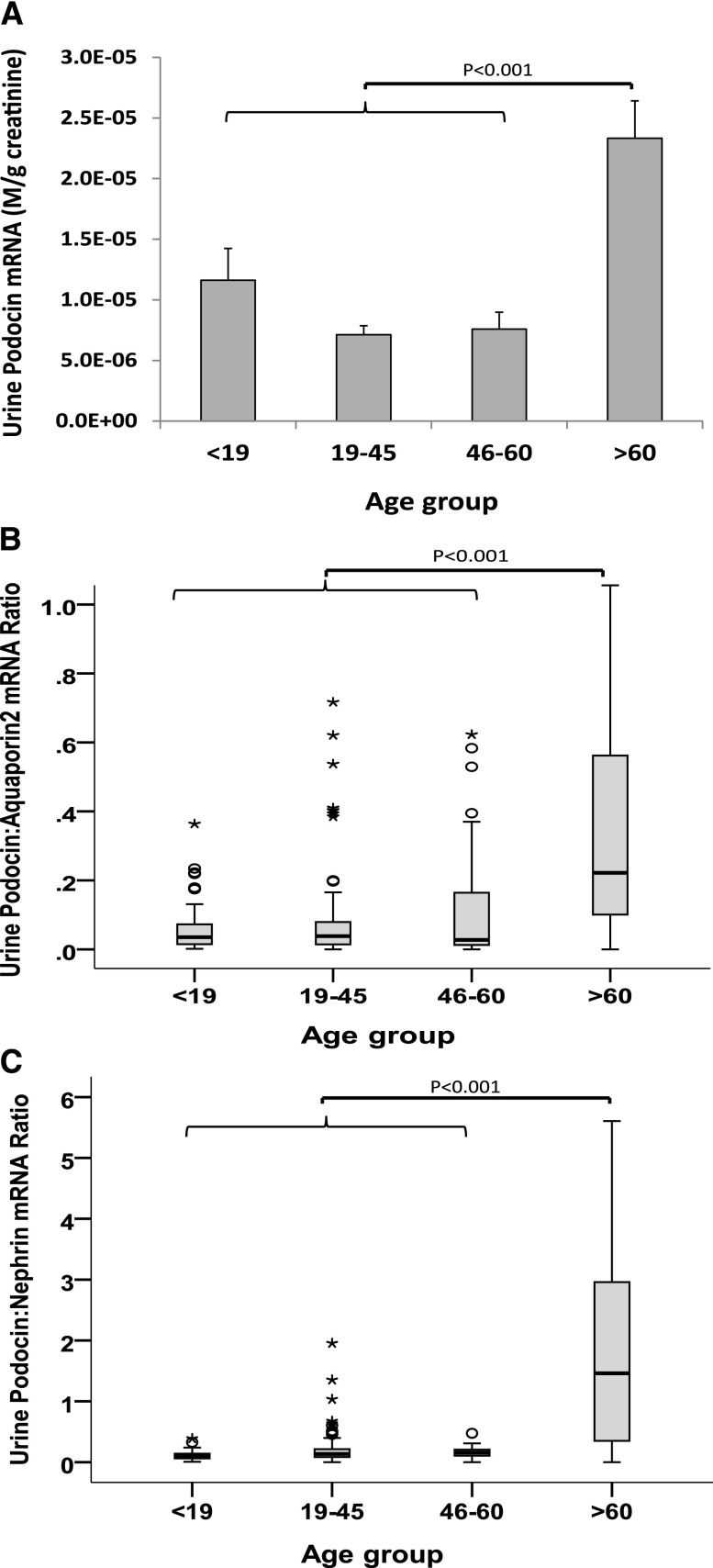
To test this projection, we measured the amount of podocyte detachment in urine pellet samples from 277 people age 3–92 years with normal urine/kidney function and urine protein-to-creatinine ratio ≤0.18 (Figure 5). The group older than age 60 years had significantly higher urine pellet podocin mRNA-to-creatinine ratio (3.3-fold; P<0.001). The urine podocin-to-aquaporin2 mRNA ratio was also higher in this age group, demonstrating preferential podocyte loss versus distal nephron cells (expressing aquaporin2). Therefore, the increased podocin mRNA is the result of preferential podocyte detachment rather than overall nephron loss in older age. Furthermore, podocyte stress was also increased in the older group, as assessed by comparing two mRNA species from the same cell that we have previously reported as reflecting podocyte stress (the urine podocin-to-nephrin mRNA ratio).32 Thus, in older age, podocytes are more stressed and podocytes are detaching in greater amounts.
Figure 5.
Urine pellet podocyte mRNA markers by age showing accelerated detachment and podocyte stress in the group older than age 60 years. (A) The urine podocin mRNA-to-creatinine ratio is a measure of the rate of podocyte detachment relative to urine creatinine excretion analogous to the urine protein-to-creatinine ratio. The urine podocin mRNA-to-creatinine ratio in the >60-year-old group is increased 3.3-fold above the mean of younger people (<60 years; P<0.001). (B) The urine podocin-to-aquaporin2 mRNA ratio is a measure of the rate of podocyte detachment relative to detachment of another kidney cell type (distal tubule and collecting duct cells that express aquaporin2 mRNA) demonstrating that podocytes are lost preferentially to other kidney cells in the >60-year-old age group. (C) The urine pellet podocin-to-nephrin mRNA ratio is a ratio of two podocyte-specific markers that we have previously reported as a measure of podocyte stress.32 Distribution of male and female patients did not significantly differ in the various age groups. The box plots show median and interquartile ranges, and the corresponding means were compared using ANOVA with Bonferroni correction. Outliers outside the mean±2SD range are shown as circles and those outside the mean±3SD range are shown as asterisks.
We used Glepp1 peroxidase-stained histologic sections to search for putative short-lived intermediates between normal glomeruli and globally sclerosed glomeruli that from the preceding data would be expected to be present in old but not young kidneys. Several potential intermediates were identified (Figure 6, Table 4). Proteinaceous matrix in the Bowman space (PMBS) was present in a proportion of >60-year-old glomeruli (Figures 6A and 7B) and rarely present in young glomeruli. This suggests the possibility that glomeruli with hypertrophic stressed podocytes might be leaking protein into the Bowman space. To test this concept, we measured the podocyte nuclear density in kidneys with a high proportion of glomeruli with PMBS and found that the average density was significantly lower in these kidneys compared with other old kidneys (Figure 8A). The average podocyte nuclear volume in glomeruli containing PMBS was also significantly larger than in neighboring glomeruli in the same kidney section that did not contain PMBS (Figure 8B). These data are compatible with PMBS reflecting higher level podocyte hypertrophy and stress in the subset of glomeruli with leaky glomeruli.
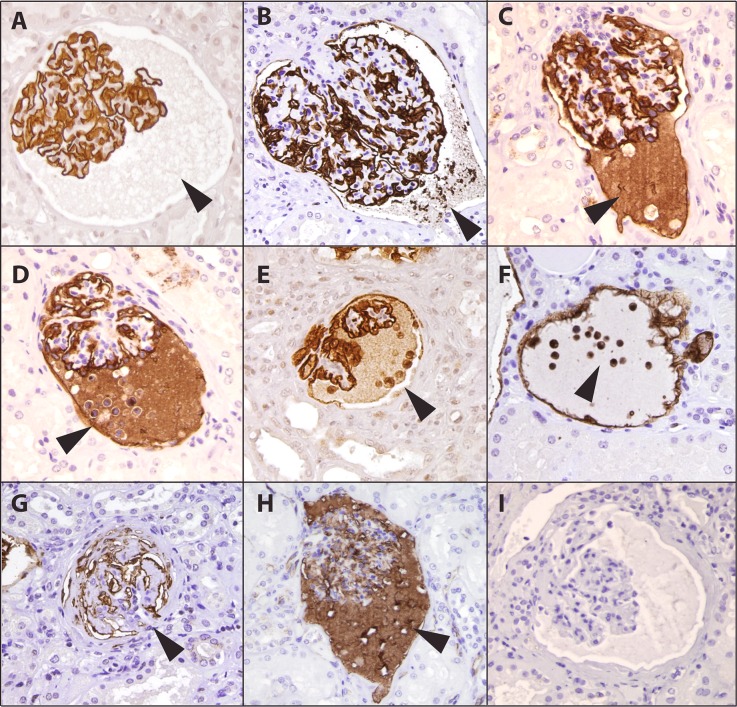
Figure 6.
Old kidneys show stages of podocyte stress and detachment by Glepp1 and podocalyxin immunoperoxidase histochemistry. Images from >60-year-old kidneys. (A) A subset of old glomeruli develop Glepp1-negative proteinaceous material in the Bowman space (arrowhead). (B) Glepp1-positive subcellular cell particles detach from podocytes and are present within the proteinaceous matrix in the Bowman space (arrowhead). (C) The proteinaceous matrix in the Bowman space becomes Glepp1 positive, suggesting loss of glycocalyx material from podocytes. (D–F) Glepp1-positive podocytes detach from the glomerulus and appear within proteinaceous matrix material in the Bowman space. (G) Glomeruli become globally scarred but still contain remnants of Glepp1. (H) Similar staining is present for podocalyxin, as confirmation that two independent podocyte antibodies show the same patterns. (I) Control immunoperoxidase staining showing that the observed peroxidase product was specific.
Table 4.
Proportion of globally sclerotic and transitional glomeruli per age group observed in Glepp1-peroxidase sections (n=89)
| Variable | Age <19 yr (Column A) | Age 19–45 yr (Column B) | Age 46–60 yr (Column C) | Age >60 yr (Column D) |
|---|---|---|---|---|
| Glomeruli evaluated (n) | 419 | 648 | 1682 | 2788 |
| FGGS (%) | 0.4±1.2a | 0.0+0.0b | 1.8±3.9b | 5.7±6.2c |
| Transitional glomeruli (%) | 0.5±1.6a | 0.7±1.7a | 3.8±5.0d | 5.5±7.0d |
| Podocyte mass detachment events (%) | 0.0±0.0 | 0.0±0.0 | 0.3±1.1 | 0.2±0.4 |
| Podocyte glycocalyx detachment (%) | 0.0±0.0 | 0.0±0.0 | 0.7±1.8 | 0.8±1.2 |
Values expressed with a plus/minus sign are the mean±SD. FGGS was defined according to Figure 6I. Transitional glomeruli were defined according to Figure 6, B–F. Transitional glomeruli were any glomerulus that had proteinaceous material and/or detached cells in the Bowman space. Podocyte mass detachment events contained at least three detached Glepp1-positive cells in the Bowman space. Podocyte glycocalyx detachment events contained definitely Glepp1-positive material in the proteinaceous cast in the Bowman space, as shown in Figure 7.
P<0.05 vs. columns C and D.
P<0.05 vs. column D.
P<0.05 vs. columns A, B, and C.
P<0.05 vs. columns A and B.
Figure 7.
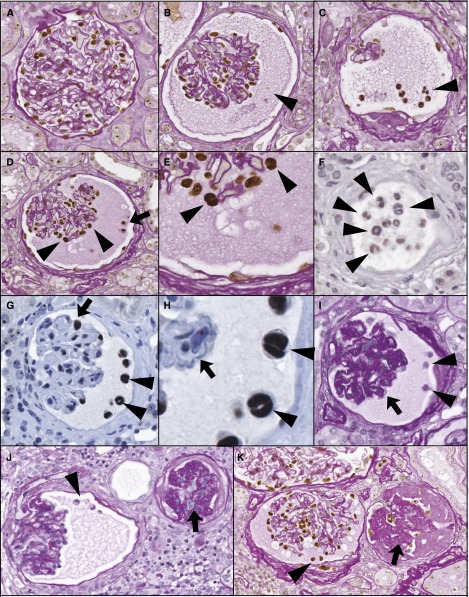
Detaching podocytes are multinucleate and associated with wrinkling of the GBM, glomerular collapse, and periglomerular fibrosis. (A) Normal PAS-stained old glomerulus with peroxidase-stained WT1-positive podocyte nuclei. (B) Proteinaceous material in the Bowman space is PAS positive (arrowhead) and the glomerulus shows a wrinkled appearance with early periglomerular layering. (C) WT1-positive nuclei present within the PAS-positive proteinaceous material in the Bowman space (arrowhead). (D and E) WT1-positive podocyte nuclei within the Bowman space (arrowhead). Podocyte nuclei of cells remaining attached to the glomerular tuft (arrowheads) are shown at higher power to be binucleate (E, arrowheads). (F) Detached podocytyes are binucleate (arrowheads). (G) Glomerulus with binucleate detached WT1-positive cells are present in the Bowman space (G, arrowhead) and remain attached to the glomerular tuft (H, arrowheads). (H) High-power view of panel G to illustrate binucleate cells (arrowheads) and wrinkled glomerular capillary loops (arrow). (I) Glomerulus with detached podocytes in the Bowman space (shown by arrowheads) that is wrinkled and collapsed (arrow). (J) PAS-stained section showing a glomerulus at an earlier stage with detached podocytes in the Bowman space (arrowheads) at left and a glomerulus with major tuft collapse (arrow) at right. (K) PAS-stained section with WT1 peroxidase again showing three glomeruli. The upper glomerulus appears normal. The left glomerulus shows detached podocytes (arrowhead), proteinaceous material in the Bowman space, and periglomerular layering. The right glomerulus shows major glomerular tuft collapse and global glomerulosclerosis. The average glomerular tuft diameter is 137 micrometers.
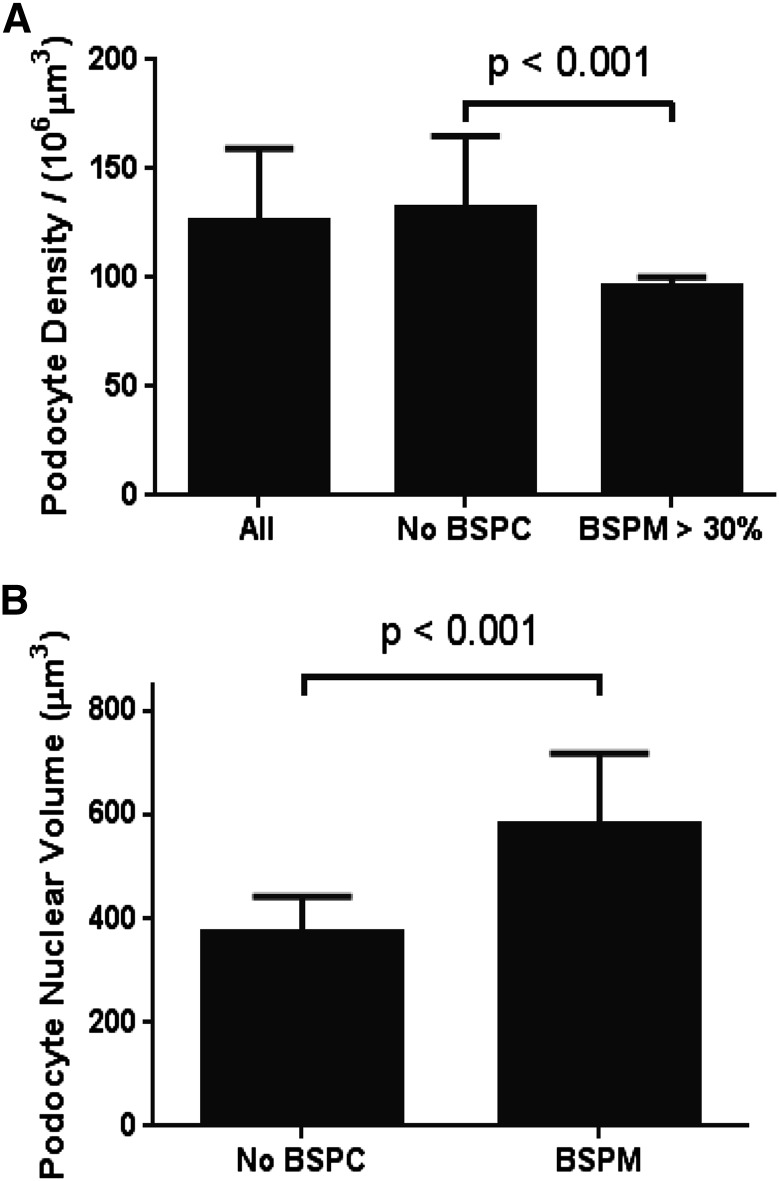
Figure 8.
Glomeruli with proteinaceous material in the Bowman space have reduced podocyte density and larger podocyte nuclei. (A) Podocyte density. Kidney sections in which a large proportion of glomeruli had PMBS (n=5) (PMBS >30%) had significantly lower podocyte density than glomeruli that did not have a large proportion of glomerular tufts with protein in the Bowman space (n=15). (B) Podocyte nuclear volume as a surrogate for podocyte cell volume: WT1-positive podocyte nuclear volume was measured in glomeruli that contained Bowman space proteinaceous cast material (PMBS) versus neighboring glomeruli in the same kidney section that did not have PMBS. The average podocyte nuclear volume in glomeruli containing PMBS was larger (582±137 μm3; n=609) than in neighboring glomeruli in the same kidney sections that did not contain PMBS (372±72 μm3; n=554) (P<0.01), compatible with greater podocyte hypertrophy in the subset of glomeruli associated with PMBS.
Some glomeruli with PMBS also had podocyte-derived subcellular fragments trapped within the proteinaceous matrix identified by being Glepp1 positive (Figure 6B). A subset of these glomeruli also had podocyte-derived homogeneous periodic acid–Schiff (PAS)–positive glycocalyx material associated with the PMBS that was both Glepp1 and podocalyxin positive by immunoperoxidase (Figure 6, C–E). A further subset of glomeruli with PMBS had multiple detached podocytes present within the PMBS (mass podocyte detachment events [MPDEs]) as demonstrated by the cells being Glepp1 and podocalyxin positive and containing WT1-positive nuclei (Figures 6, H–F, and 7, C–H). On average, 50% of these detached podocytes were binucleate or multinucleate. This result is compatible with a “catastrophic” mitosis-driven podocyte detachment process as suggested by Lasagni and colleagues.33
Evaluation of PMBS glomeruli undergoing mass podocyte detachment events showed that glomerular capillaries had become wrinkled and appeared to be in various stages of tuft collapse in association with development of periglomerular fibrosis (Figure 9, C–K).
Figure 9.
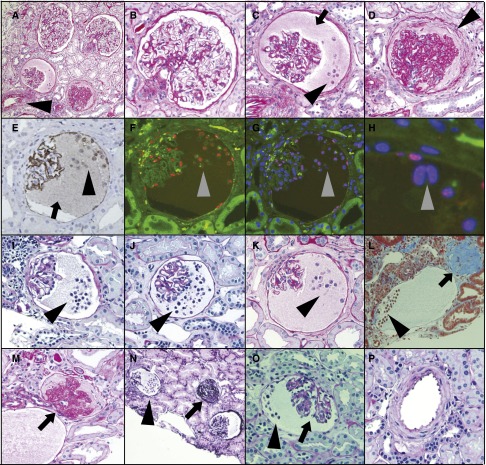
Glomeruli from diverse forms of glomerular injury not associated with old age also show mass podocyte detachment events, multinucleate podocytes in the Bowman space, wrinkled GBM, glomerular collapse, and FGGS in the absence of arteriosclerosis. See Tables 5 and 6 for clinical information. (A) PAS-stained kidney biopsy from a 52-year-old kidney showing two normal glomeruli, one glomerulus with proteinaceous material and cells in the Bowman space, and one glomerulus with a collapsed tuft, global sclerosis, and periglomerular fibrosis. (B–D) Higher-power views of glomeruli from panel A. Note a normal glomerulus (panel A), proteinaceous material (arrow) and detached cells (arrowhead) in the Bowman space of a glomerulus with wrinkled GBM (panel C) and a collapsed glomerulus with periglomerular fibrosis (arrowhead) in panel D. (E) Glepp1-peroxidase showing that both cells (arrowhead) space and proteionaceous material (arrow) in Bowman space contain Glepp1. (F–H) Immunofluorescent images using TLE4 to identify podocyte nuclei (red in panel F) and DAPI to identify nuclei (blue with overlap between TLE4 and DAPI showing as purple). Note that detached cells contain nuclei staining for TLE4 and some nuclei are binucleate (gray arrowheads). Parietal podocytes are present. (I–K) PAS-stained sections showing detached cells in the Bowman space in biopsy specimens from 9-, 16-, and 31-year old kidneys, respectively (arrowheads). (L) Masson trichrome–stained section from a 38-year-old showing detached podocytes (arrowhead) and globally sclerosed glomerulus at right (arrow). (M) PAS staining of the same glomerulus as shown in panel L showing an apparently patent arteriole feeding the collapsed glomerulus (arrow). (N–P) Sections from a 9-year-old kidney. (N) Three glomeruli, including one glomerulus with PMBS, one glomerulus with numerous detached cells in the Bowman space (arrowhead), and one collapsed globally sclerotic glomerulus (arrow) (silver stain). (O) Collapsing glomerulus (arrow) associated with cells in the Bowman space (arrowhead). (P) Normal artery in this biopsy specimen (PAS). The average glomerular tuft diameter is 137 micrometers.
Five kidney biopsy specimens from younger people with diverse underlying clinical conditions in which we had also noted MPDEs were reviewed (Tables 5 and 6). All specimens showed low podocyte density and binucleate podocytes in the Bowman space (Figure 9, Table 6), together with wrinkled GBM, glomerular tuft collapse, and focal global glomerulosclerosis (FGGS), but not FSGS. None of the biopsy specimens showed arteriosclerosis (Figure 9, A and P). Therefore, the same pattern of glomerular injury occurred in these nonaging biopsy specimens in association with MPDEs.
Table 5.
Clinical characteristics associated with MPDEs
| Patient | Kidney Type | Kidney Age (yr) | Patient Age (yr) | Sex | Clinical Diagnosis | eGFR (ml/min per 1.73 m2) | UPCR |
|---|---|---|---|---|---|---|---|
| 1 | Native | 16 | 16 | M | SDNS | 125 | 1.4/11.4 |
| 2 | Native | 9 | 9 | M | SRNS | >100 | 0.06/11.6 |
| 3 | TP | 31 | 51 | F | Diabetes | 26 | 0.8 |
| 4 | TP | 52 | 64 | M | ANCA/diabetes | 38 | 0.4 |
| 5 | TP | 38 | 16 | M | FSGS | 45 | 15 |
| Mean±SD | 29.2±17.2 | 31.2±24.6 | 58.5±45.0 | 5.4±8.3 |
The two sets of values shown in patients 1 and 2 for UPCR are variations present at the time of biopsy when patients were in relapse and shortly afterwards when in remission after treatment with calcineurin. inhibitors. UPCR, urinary protein-to-creatinine ratio; M, male; TP, transplant; SDNS, steroid-dependent nephrotic syndrome; SRNS, steroid resistant nephrotic syndrome.
Table 6.
Pathologic characteristics associated with MPDEs
| Patient | Foot Process Effacement | Podocyte Density | Glomerular Number | FGGS (%) | FSGS (%) | MPDE (%) | PGDE (%) | Ischemic (%) | |
|---|---|---|---|---|---|---|---|---|---|
| per 106 μm3 | Normal (%) | ||||||||
| 1 | Focal | 153 | 48 | 22 | 31.8 | 0.0 | 13.6 | 0.0 | 0.0 |
| 2 | Partial artial | 142 | 45 | 26 | 19.2 | 0.0 | 3.8 | 3.8 | 0.0 |
| 3 | ND | 129 | 61 | 24 | 50.0 | 0.0 | 12.5 | 0.0 | 0.0 |
| 4 | ND | 82 | 54 | 33 | 9.1 | 0.0 | 6.1 | 0.0 | 0.0 |
| 5 | >90% | 153 | 48 | 27 | 18.5 | 0.0 | 7.4 | 11.1 | 0.0 |
| Mean±SD | 131.8±29.5 | 51.2±6.4 | 26.4±4.2 | 25.9±21.4 | 0.0 | 8.7±3.4 | 3.7±6.4 | 0.0 | |
In all five biopsy specimens, the Bowman space contained multinucleate podocytes as judged by TLE4 immunostaining with DAPI counterstain to identify nuclei (Figure 9). PGDE, podocyte glycocalyx detachment event; ND, not done.
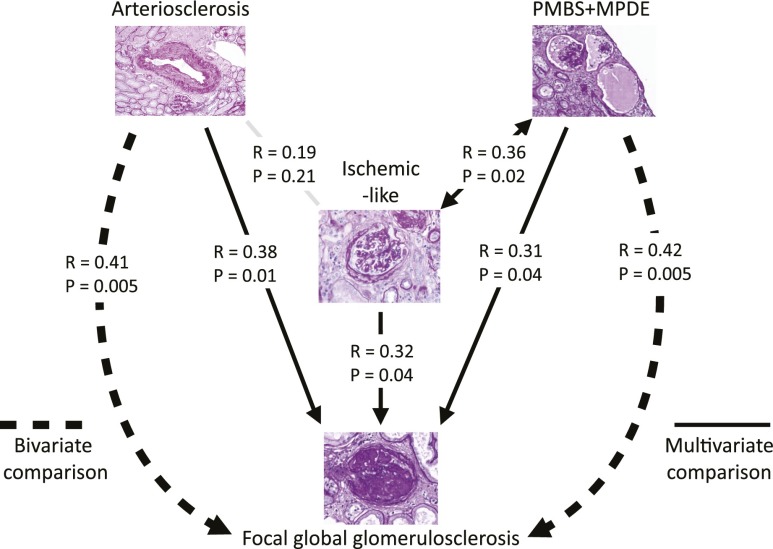
The term “benign nephrosclerosis” has historically been used to describe pathologic changes associated with aging and culminating in FGGS.3,34 The mechanism behind this process has been thought to be ischemic and is generally assumed to be secondary to arteriosclerosis. To better define these relationships, we therefore evaluated PAS-stained sections from 44 nephrectomy samples aged >60 years containing 8570 glomerular profiles. Sections were scored for arteriosclerosis, FGGS, ischemic-like glomerulopathy (see Concise Methods), and podocyte stress/detachment events (PMBS and MPDEs). Multivariate analysis (Figure 10) showed that the ischemic-like glomerulopathy phenotype did not correlate with arteriosclerosis but did correlate significantly with both podocyte detachment events and FGGS. This result supports the concept developed from two independent data sets shown above (Figures 8 and 9) that podocyte detachment events are mechanistically related to glomerulopathy with wrinkled GBM, collapsing tufts, and periglomerular fibrosis, which, in turn, results in FGGS.
Figure 10.
Multivariate regression analysis shows that ischemic-like glomeruli correlate with podocyte detachment events but not arteriosclerosis. PAS-stained sections from 44 nephrectomies (average age, 71.1±6.9 years) containing 8570 glomerular profiles were scored for glomeruli with FGGS (average, 9.5%±7.9%), PMBS (average, 1.0%±1.3%), MPDEs containing at least three cells in the Bowman space (0.2%±0.6%), ischemic-like glomerular tufts (average, 2.8%±3.7%), and arteriosclerosis score (average, 2.4±1.0; range, 0–5). Partial correlation coefficients with the corresponding P values are shown where the net relationship between two variables is a product of multivariate linear regression model after adjustment with the effect of other covariates. Note that podocyte stress/detachment events (PMBS+MPDE) correlated with ischemic-like lesions (P=0.02) while arteriosclerosis did not (P=0.21). This finding suggests that the ischemic-like lesions are not directly related to arteriosclerosis. However, all three variables (arteriosclerosis, ischemic-like lesions, and podocyte stress/detachment) independently correlated with FGGS. Bivariate comparison correlating arteriosclerosis or podocyte detachment events with FGGS showed independent correlations of approximately equal weight (R=0.4; P<0.01). This result suggests that arteriosclerosis-associated processes can independently amplify the rate of development of FGGS being caused by other mechanisms (e.g., podocyte depletion) or cause FGGS by different de novo mechanisms.
Figure 10 also shows that arteriosclerosis correlates significantly with FGGS independent of podocyte detachment events. From bivariate analysis shown in Figure 10, we conclude that in this older kidney cohort the relative contributions of arteriosclerosis and podocyte depletion to net FGGS were approximately equal.
Discussion
This report shows that as normal human glomeruli age, the average podocyte (nuclear) density progressively decreases from >300 per 106 μm3 at young age to <100 per 106 μm3 by about 70–80 years of age because of the combined effects of reduced podocyte number per glomerulus and increasing glomerular volume. As podocyte density decreases, further density decrements mandate larger and ever-increasing podocyte hypertrophic responses. For a cell as complex as the podocyte, with its requirement to maintain contiguous interdigitating foot processes with its neighbors to completely cover the filtration surface area, this represents an important logistic challenge that at some point will result in podocyte hypertrophic stress. Consistent with this concept, the amount of podocyte detachment increased in older age. This increase was not merely due to loss of whole nephrons because the urine podocin-to-aquaporin2 mRNA ratio was also increased, thereby demonstrating that podocytes were being preferentially lost relative to distal tubule and collecting duct cells that contain aquaporin2 mRNA. Furthermore, the urine pellet podocin-to-nephrin mRNA ratio of two podocyte-specific markers from the same cell, which we have previously reported as an index of podocyte stress,32 was also increased in older kidneys. This represents preferential downregulation of nephrin mRNA expression previously documented to occur in aging rat glomeruli and in association with hypertrophic podocyte stress.14,28
Microscopic examination of old kidneys (but not young kidneys) showed candidates for direct evidence for podocyte stress and accelerated detachment in glomeruli. A subset of glomeruli could be identified with PMBS to form an apparently semi-stable matrix. Kidneys with large numbers of PBMS glomeruli had lower podocyte density, and within these kidneys the PMBS glomeruli had larger podocyte nuclei than neighboring glomeruli without PMBS. This finding suggests that compensatory podocyte hypertrophy had reached especially high levels in these leaky glomeruli. Some PMBS glomeruli also showed detached podocyte-derived subcellular particles (containing the apical podocyte markers podocalyxin and Glepp1) trapped within the proteinaceous cast, suggesting that stressed podocytes can detach subcellular elements, as has previously been identified in glomerular disease urine.35,36 Additionally, podocytes themselves detached and were retained in the proteinaceous matrix. Some glomeruli exhibited large numbers of detached podocytes (MPDEs) wherein about 50% of the detached podocytes were binucleate. In many glomeruli binucleate podocytes were also present in glomeruli, potentially accounting for the increase in average podocyte nuclear size. Prior reports have also identified binucleate/multinucleate podocytes in glomerular disease urine.37,38 Glomeruli that were undergoing MPDEs also had wrinkled GBM and collapsing glomerular tufts associated with periglomerular fibrosis, compatible with the concept that mass podocyte detachment itself resulted in glomerular tuft collapse.
In a small cohort of younger kidneys with diverse underlying glomerular diseases we similarly identified MPDEs associated with low podocyte density, mitotic cells in the Bowman space, glomerular tuft collapse, and the FGGS phenotype (but not the FSGS phenotype). Therefore, glomerular tuft collapse appears to result from podocyte depletion occurring (1) mass podocyte detachment (as described above) or (2) as a result of a changed podocyte phenotype. In the latter scenario, HIV infection (or other mechanisms) causes podocytes to lose their normal markers and foot processes and to become proliferative cells that cannot serve normal podocyte functions (collapsing FSGS variant).39,40 Tuft collapse is therefore a common downstream event caused by loss of normal podocytes resulting from podocyte detachment (detachment-associated collapse) or proliferation (proliferation-associated collapse).
These data are consistent with the concept that within a glomerular population at any one time, a subset of glomeruli can develop critically low podocyte density as a consequence of aging or glomerular injury. As podocytes become hypertrophically stressed, they leak protein across the filter to form a matrix-like structure in the Bowman space, detach subcellular fragments, and/or attempt to divide. Lasagni and colleagues33 have suggested that the rearrangement of the actin cytoskeleton required for podocyte cell division is incompatible with podocytes maintaining their normal function and adhesion to the GBM. Mitotic podocytes therefore detach (catastrophic mitotic podocyte detachment).33 Cho and colleagues used a theoretical construct to predict that detachment of one podocyte would result in sequential detachment of neighboring podocytes through a cooperative mechanism.41 Support for this concept is provided by experimental models from Ichikawa and colleagues (podocyte damage damages podocytes) and Cui and colleagues (innocent bystander hypothesis) and by our studies showing that once a critical proportion of podocytes are lost the remaining podocytes autonomously detach in an angiotensin II–dependent manner leading to global glomerulosclerosis.14,42,43
The mechanism of FGGS associated with aging (so-called benign nephrosclerosis) and its relationship to BP has been controversial.3,34,44 The concept that glomerular lesions were ischemic was not supported by the India ink perfusion studies of autopsied kidneys performed by Shapiro; these studies found that ischemic glomeruli were particularly well perfused, thereby proving that obstructive arteriopathy could not be the cause of ischemic changes.45 However, age-associated glomerular lesions are commonly called “ischemic,” with a presumed arteriosclerotic association,34 although the precise mechanism by which ischemia occurs is not well understood. To evaluate these concepts we scored 8570 glomeruli from 44 old kidneys and found that the presumptive ischemic-like glomeruli did not correlate with arteriosclerosis, as would have been expected if the lesions were caused by underlying arterial disease. Instead, the ischemia-like phenotype correlated with podocyte detachment events. The relationship between podocyte detachment and collapsing FGGS is supported in three ways from these data: (1) glomeruli with detaching podocytes showed wrinkling of glomerular tufts and collapsing glomerulopathy; (2) in a subset of younger kidneys we identified the same association of mass podocyte detachment and collapsing glomerulopathy in the absence of arterial disease; and (3) by multivariate analysis we showed that arteriosclerosis did not correlate with the ischemic-like phenotype.
In our analysis arteriosclerosis appeared to independently contribute to the FGGS phenotype. We speculate that NFκB signaling (which we previously identified as a major pathway in old glomeruli and is known to be a driver of atherosclerosis) could contribute to net FGGS, but further studies are required to evaluate this possibility.44,46 This would be analogous to Alzheimer disease and vascular disease as the two major contributors to dementia often working in parallel but contributing variable amounts to net brain dysfunction.47 Kidney aging must also involve other vascular, genetic, epigenetic, and environmental processes as outlined by Striker.48
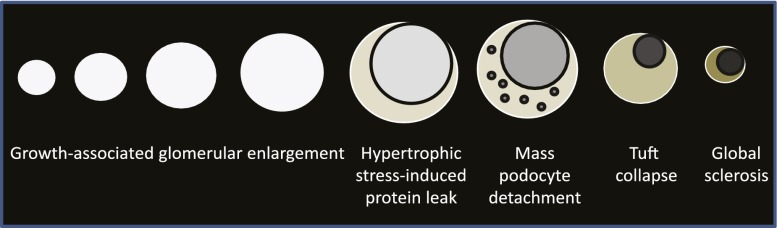
The data suggest that glomeruli can be considered to have a life cycle (Figure 11). During normal life, if an individual glomerulus density decreases enough (because of podocyte loss or glomerular volume increase), it eventually reaches a critical stage at which podocytes are forced into mitosis and thereby undergo podocyte detachment followed by glomerular tuft collapse, resulting in FGGS. In normal kidneys the MPDE intermediate is rare and short-lived (observable in only 0.2% of 11,358 old glomeruli in the two old kidney cohorts examined by different methods, and probably much rarer in younger glomeruli), but in pathologic conditions can be much higher (mean of 8.7% in five biopsy cases with low podocyte density shown in Table 5). The rare MPDE in a normal kidney cortex is therefore analogous to a supernova in the life cycle of stars. It is a rarely observed but very important event toward understanding the star life cycle. MPDEs seem to result in subsequent glomerular tuft collapse through presumed vasoconstrictor mechanisms, which rapidly limit blood flow into a glomerulus and thereby protein loss into the filtrate. In contrast, segmental sclerosis may occur when podocyte depletion occurs at a slower rate, allowing remaining podocytes to migrate together (regroup) to protect regions (capillary loops) of the glomerular filter while other segments are abandoned to sclerosis. This latter scenario occurs in many glomerular diseases commonly affecting younger people, whose podocytes will be less hypertrophied and therefore more agile and adaptable. Although these longer-lived semi-stable intermediates (e.g., diabetic glomerulosclerosis and FSGS variants) do preserve glomerular filtration, their disorganized glomerular structure is unable to efficiently prevent protein leaking from blood into the filtrate.
Figure 11.
Glomerular life cycle hypothesis. The data suggest that a glomerulus can have a life cycle analogous to that of a star. During normal life span, podocyte density decreases. At some point critical podocyte depletion is reached when podocytes are forced into attempting to divide. Mitosis is incompatible with podocytes remaining attached to the GBM, so they detach en masse (catastrophic mitotic detachment) in a manner analogous to a supernova. Rapidly following mass detachment the glomerular tuft vasoconstriction/collapse (possibly to minimize protein loss) periglomerular fibsosis is activated, leading to global glomerulosclerosis and loss of function (analogous to a dwarf star). Under normal conditions a small proportion of glomeruli reach criticality so that the typical mass podocyte detachment events are rarely observed, but under conditions of accelerated reduction in podocyte density (due to podocyte loss and/or glomerular enlargement), this mechanism can become a major driver of FGGS.
Steffes and colleagues also reported an increase in average glomerular volume with age but did not report a decreased podocyte number per tuft.22 The average slope of reduced podocyte density—about 0.9% per year—is similar to the average rate of reduced eGFR reported in apparently normal humans (about 0.8% per year).5–9 Variation in the density slope between individuals would explain differences in aging-associated rates of progression, as has been reported by Fliser and colleagues.9 Huber and colleagues report that podocyte replacement capacity is minimal in the adult mouse.49
Interpretation of the data presented requires the assumption that the kidneys analyzed are representative of the normal population. Potential limitations include the following: (1) Deceased donors do not necessarily reflect a random sample of the population; (2) living donors are carefully selected and therefore do not represent a random population sample; and (3) the unaffected pole of a kidney containing cancer could theoretically be affected by predisposing genetic, environmental, or growth factors that lead to cancer or the cancer milieu itself. Furthermore, these kidneys were selected as lacking major stigmata of kidney disease at the light microscopic level and therefore may not have been representative of a random population sample. Nevertheless, the overall study represents a reasonable approximation of a normal kidney sample over a wide age range, and the density slope was not significantly altered by excluding the normal kidney pole or the older deceased donor groups from the analysis. The association of podocyte detachment events with FGGS was also confirmed in non-nephrectomy samples.
In summary, the data provided support a hypothesis wherein reduction in podocyte density throughout life in humans causes hypertrophic podocyte stress in some glomeruli, eventually resulting in glomerular tuft collapse and FGGS. This FGGS mechanism may be a significant cause of ESRD in both the aging kidney and diverse glomerular diseases. According to this scenario, whether adequate numbers of glomeruli successfully survive for a lifetime will depend on (1) cumulative podocyte loss, (2) glomerular volume increase, and (3) the ability of podocytes to successfully adapt to hypertrophic stress. Each of these variables could potentially be modulated by diet and therapeutic intervention. All glomerular diseases can be seen as superimposed upon this underlying mechanism.
Concise Methods
Use of archival kidney tissues from biopsies from deceased donors, living donors, and the uninvolved pole from nephrectomies for cancer was approved by the University of Michigan Institutional Review Board (HUM00055525, HUM00083116).
Kidney Sample Selection
Pathologic light microscopic analysis was used as the gold standard for inclusion or exclusion of samples. Samples were included if kidney structure in all tissue compartments available for analysis was within normal limits, as assessed by conventional light microscopy in the kidney biopsy for transplants or deidentified kidney sections from the uninvolved poles of kidneys removed for cancer. All kidney transplants were biopsied at the time of implantation. Kidney biopsy specimens from living donors were from people screened and selected to ensure that underlying diseases that could compromise the health of the donor or recipient were absent (age 21–53 years; n=29). To extend the age range above and below the living donor group, we used groups of deceased donors from younger (4–18 years, n=20) and older (48–64 years; n=11) ages. Deceased donors are approved as kidney donors if they had normal kidney function before death (n=31). To extend the older age range further, we used kidney sections obtained from the normal pole of nephrectomies performed for cancer (age range, 45–85 years; n=29). These deidentified kidney samples (for which only age and sex information was available) were selected as having no pathologic stigmata of kidney disease. Additional clinical information about preexisting diseases in this cohort was not available. Therefore, we cannot exclude the possibility that some deidentified samples could have been from people with diabetes or hypertension but without kidney abnormalities. Because the Glepp1 area density did not change with age (as shown in Figure 3, Table 1), we used it to define a lower range of normal for Glepp1 percentage area density (mean−2SD=33.0). One sample fell below this normal range and was excluded from further analysis. One additional biopsy specimen had fewer than eight glomerular profiles present and was therefore excluded on the basis of criteria for adequate estimate of glomerular volume, as outlined below and previously reported.29
Podometric Analysis
The TLE4 immunofluorescent method for estimating podocyte nuclear density29 was combined with a Glepp1 immunoperoxidase method for measuring podocyte cell area density in the same section, as previously reported.27 A total of 2154 glomeruli were evaluated in 89 biopsy specimens (24.5±9.3 per biopsy). For PAS-stained sections (3952016; Sigma-Aldrich, St. Louis, MO), podocyte nulcei were identified by immunoperoxidase using a murine monoclonal anti-WT1 antibody (LS-C105719) from LifeSpan BioSciences Inc. (Seattle, WA).
Podocyte Nuclear Density by TLE4 Immunofluorescence
The method used was as reported elsewhere.27
Glepp1 Positive Percentage Area by Immunoperoxidase
This immunoperoxidase method was used as previously reported27 to identify podocyte cell area. It is piggy-backed onto the TLE4 immunofluorescent method outlined earlier so that podocyte nuclear data from the identical slide can be combined with the peroxidase method for estimation of podocyte cell size. Because the sections are affixed to the slide, no changes in relative structure or size occur between the two measurements, even though sections are in aqueous phase for the immunofluorescent TLE4 measurements and dehydrated for the immunoperoxidase measurements. After imaging of the TLE4 immunofluorescent sections, the coverslip is removed by incubation in xylene. After rehydration, sections were stained for immunoperoxidase using Vectastain Mouse IgG Kit (PK-6102; Vector Laboratories Inc., Burlingame, CA) as previously reported.27
Glomerular Volume Estimation
The area of all glomerular tuft profiles present in the Glepp1-stained section (including every small profile) is measured. The average radius (r) of all tuft profiles is then calculated assuming glomeruli are spherical. The average maximal radius (R) is then calculated as R=r×4/π according to Weibel.50 Glomerular tuft volume is given as 4/3πR3. If fewer than eight tuft profiles were present in the biopsy specimen, then glomerular volume estimates are considered to be insufficient to reliably make the estimate, as previously described.29 Globally sclerotic glomeruli were excluded from analysis.
Mean Podocyte Cell Volume Estimation
The average Glepp1 positive percentage area×glomerular volume estimates average total podocyte volume (mass) per glomerular tuft. Similarly, the Glepp1-negative glomerular tuft volume represents scarred tuft, mesangial matrix expansion, mesangial cells, endothelial cells, and open capillary loops containing blood products. The average total glomerular volume divided by the average number of podocyte nuclei per tuft estimates average individual podocyte volume.
Methodologic Assumptions
The correction factor morphometric method allows analysis of archival formalin-fixed biopsy specimens, thereby facilitating unbiased evaluation of all glomeruli per sample (total 2154 glomeruli in 89 biopsy specimens). The glomerular and podocyte morphometric variables obtained are as reported by others.20–26 The method relies on identifying podocyte nuclei by immunostaining for the transcription factor TLE4 rather than the traditional podocyte transcription factor WT1.29,51 TLE4 provides a robust podocyte nuclear signal that allows software to estimate the podocyte nuclear size (mean caliper diameter) and thus quantitate them in tissue sections.29 Under pathologic circumstances, podocytes can sometimes lose their normal transcription factor complement and have duplicated nuclei. We have focused on quantitating podocytes that can perform normal podocyte functions. If podocyte nuclei do not express TLE4 (or WT1) then we assume that they will have changed their phenotype and no longer are functioning as normal podocytes; thus they would not be counted by this method. If two nuclei are present within a single cell, we anticipate that this will reflect the unsuccessful attempt of that podocyte to divide in response to hypertrophic signals. We rationalize that such a cell will be larger than a cell with a single nucleus, and therefore that counting both nuclei will appropriately represent the larger filtration surface area represented by that podocyte. As with any method, assumptions must be understood and critically evaluated within the context of the task at hand.
Urine Sample Analysis
Urine samples were collected (n=291) as previously described from deidentified people age 3–89 years who had no known kidney disease or hypertension.52 Each sample was associated with a short consent form filled out by the donor (or parent) that included information about age, sex, race, medical conditions, and medications. Exclusion criteria were any kidney-related disease, antihypertensive medication, urine dipstick positive for blood or protein, and urine protein-to-creatinine ratio ≤0.18. There were no statistical differences in mRNA levels between those excluded from analysis on the basis of the preceding criteria (n=37) as a group and the samples included in the normal group for subsequent analysis. To obtain additional samples from an older population (age 62–92 years; n=57), patients attending the Geriatric Clinics at the University of Michigan were screened to exclude any of the preceding criteria and to exclude any cases with abnormal kidney function according to Modification of Diet in Renal Disease criteria. Patients signed a consent form allowing examination of their medical records to obtain fuller information. After this examination, five patients were excluded because they were listed as taking angiotensin-converting enzyme inhibitors that reduce the rate of podocyte detachment. We also excluded one additional patient who had a urine protein-to-creatinine ratio that exceeded the normal range of ≤0.18. Of the 51 remaining patients, 17 were listed as having mild hypertension; some of these patients were taking medications (calcium channel blocker, β-blocker, or diuretic). No mRNA measurements differed between patients identified as having hypertension and those not identified as being hypertensive in the clinic record (data not shown). Therefore, all 51 patient urine samples were included for further analysis. A total of 343 urine samples were available for analysis (age 1–10 years, n=58; 11–20 years, n=34; 21–30 years, n=47; 41–50 years, n=67; 51–60 years, n=15; 61–70 years, n=20; 71–80 years, n=25; >81 years, n=22). For analysis the age ranges were divided into <19 years, 19–45 years, 46–60 years, and >60 years. For podocin mRNA assay, data were available for 277 samples because an undetected signal was present in 65 samples (19%).
Urine Pellet mRNA Analysis
The urine pellet was collected, washed in PBS, RNA purified, reverse transcribed, and assayed in duplicate using TaqMan probes (Podocin NPHS2 Hs00922492; nephrin NPHS1 Hs00190446 and Aquaporin2 AQP2 Hs00166640) as previously described.52 cDNA standards were used for each assay and data expressed per g creatinine as previously described.52
Statistical Analyses
A linear regression model was used to quantify the relationship between the continuous variables. Partial correlation coefficients were used to show the relationship between variables in multivariable analysis. ANOVA was used for comparisons between multiple groups using the Bonferroni correction for multiple comparisons. Analyses were performed using SPSS software, version 21 (IBM, Armonk, NY).
Acknowledgments
R.W. is grateful for the support of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grants DK-RO146073 and the University of Michigan O’Brien Kidney Core Center P30-DK081943). J.H. is supported by K08-DK088944 and the NephCure-ASN Foundation. M.B. was supported by 1 R01-DK10044901A1, 5-R03-AG04065102, and a Junior Development Grant in Geriatric Nephrology by the American Society of Nephrology and the Claude Pepper Older Americans Independence Center. F.A. and Y.Y. were supported by National Institutes of Health grant 5T32-DK737834. Funding from D.B.K. through the Robert C. Kelsch Collegiate Professorship is gratefully acknowledged.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Glomerular Effects of Age and APOL1,” on pages 2901–2903.
References
- 1.Councilman WT: The conditions presented in the heart and kidneys of old people. In: Contributions to Medical and Biological Research, Dedicated to Sir William Osler on His 70th Birthday, New York, 1919, pp. 918–928 [Google Scholar]
- 2.Kaplan C, Pasternack B, Shah H, Gallo G: Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 80: 227–234, 1975 [PMC free article] [PubMed] [Google Scholar]
- 3.McLachlan MSF: The ageing kidney. Lancet 2: 143–145, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Hoy WE, Cobb L: Low incidence of glomerulosclerosis in normal kidneys. Arch Pathol Lab Med 113: 1253–1255, 1989 [PubMed] [Google Scholar]
- 5.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD: The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassock RJ, Rule AD: The implications of anatomical and functional changes of the aging kidney: With an emphasis on the glomeruli. Kidney Int 82: 270–277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Fliser D, Franek E, Ritz E: Renal function in the elderly—is the dogma of an inexorable decline of renal function correct? Nephrol Dial Transplant 12: 1553–1555, 1997 [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Diabetes and Digestive and Kidney Diseases: Information Clearinghouse (NKUDIC) Available at: http://www.niddk.nih.gov/KUDiseases/pubs/kustats/#1. Accessed March 17, 2015
- 11.United States Organ Procurement and Transplant Network and Scientific Registry of Transplant Recipients (OPTN/SRTR) Annual Data Report, 2012. Available at http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/2012_SRTR_ADR.pdf. Accessed March 17, 2015
- 12.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Kriz W: Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57: 189–195, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Wiggins RC: The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group : Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
- 23.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: European Study for the Prevention of Renal Disease in Type I diabetes (ESPRIT): Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD: Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P: Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52: 1031–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Lai FM, Kwan BC, Lai KB, Chow KM, Li PK, Szeto CC: Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens 22: 300–306, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Hodgin JB, Afshinnia F, Wang SQ, Wickman L, Chowdhury M, Nishizono R, Kikuchi M, Huang Y, Samaniego M, Wiggins RC: The two kidney to one kidney transition and transplant glomerulopathy: A podocyte perspective [published online ahead of print November 11, 2014]. J Am Soc Nephrol 10.1681/ASN.2014030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggins PA, Wiggins RC: Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol 25: 1118–1129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber MD, Gerace L: The size-wise nucleus: Nuclear volume control in eukaryotes. J Cell Biol 179: 583–584, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalier-Smith T: Economy, speed and size matter: Evolutionary forces driving nuclear genome miniaturization and expansion. Ann Bot (Lond) 95: 147–175, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC: Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant 27: 4079–4087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasagni L, Lazzeri E, Shankland SJ, Anders H-J, Romagnani P: Podocyte mitosis—a catastrophe. Curr Mol Med 13: 13–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmchen U, Wenzel UO: Benign and malignant nephrosclerosis and renovascular disease. In: Renal Pathology. With Clinical and Functional Correlations, Volume II, edited by Tisher CC, Brenner BM, Philadelphia, J.B. Lippincott, 1994, pp. 1201–1236 [Google Scholar]
- 35.Hara M, Yanagihara T, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Kihara I: Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol 41: 1265–1275, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T: Apical cell membranes are shed into urine from injured podocytes: A novel phenomenon of podocyte injury. J Am Soc Nephrol 16: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Hara M, Yanagihara T, Kihara I: Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 89: 342–347, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV: Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 285: F40–F48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes MB, D’Agati VD: Morphologic variants of focal segmental glomerulosclerosis and their significance. Adv Chronic Kidney Dis 21: 400–407, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V: Idiopathic collapsing focal segmental glomerulosclerosis: A clinicopathologic study. Kidney Int 50: 1734–1746, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Cho CR, Lumsden CJ, Whiteside CI: Epithelial cell detachment in the nephrotic glomerulus: A receptor co-operativity model. J Theor Biol 160: 407–426, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa I, Ma J, Motojima M, Matsusaka T: Podocyte damage damages podocytes: Autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens 14: 205–210, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Cui S, Guo G, Li C, Willecke K, Quaggin SE: Innocent bystander theory for progression to glomerulosclerosis in a transgenic mouse model [Abstract]. J Am Soc Nephrol 15: 241A, 2004. 14747370 [Google Scholar]
- 44.Wiggins JE, Patel S, Shedden K, Goyal M, Wharram B, Martini S, Kretzler M, Wiggins RC: NfkappaB promotes inflammation, coagulation, and fibrosisin the aging glomerulus. J Am Soc Nephrol 21: 587–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro P: Malignant nephrosclerosis. Transactions of the Chicago Pathological Society 13: 353–391, 1931 [Google Scholar]
- 46.Pamukcu B, Lip GY, Shantsila E: The nuclear factor—kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res 128: 117–123, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia : Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 42: 2672–2713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Striker G: Introduction to the aging kidney. J Gerontol A Biol Sci Med Sci 67: 1341–1342, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB: Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25: 707–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weibel ER: Stereologic Methods: Practical Methods for Biologic Morphometry, London, Academic Press, 1979, pp. 40–116, 415 [Google Scholar]
- 51.Sharma M, Fopma A, Brantley JG, Vanden Heuvel GB: Coexpression of Cux-1 and Notch signaling pathway components during kidney development. Dev Dyn 231: 828–838, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M, Wiggins J, Escobar GA, Rovin B, Song P, Gipson D, Kershaw D, Wiggins RC: Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol 24: 2081–2095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]