Abstract
Inflammation has an integral role in the pathophysiology of AKI. We investigated the associations of two biomarkers of inflammation, plasma IL-6 and IL-10, with AKI and mortality in adults undergoing cardiac surgery. Patients were enrolled at six academic centers (n=960). AKI was defined as a ≥50% or ≥0.3-mg/dl increase in serum creatinine from baseline. Pre- and postoperative IL-6 and IL-10 concentrations were categorized into tertiles and evaluated for associations with outcomes of in-hospital AKI or postdischarge all-cause mortality at a median of 3 years after surgery. Preoperative concentrations of IL-6 and IL-10 were not significantly associated with AKI or mortality. Elevated first postoperative IL-6 concentration was significantly associated with higher risk of AKI, and the risk increased in a dose-dependent manner (second tertile adjusted odds ratio [OR], 1.61 [95% confidence interval (95% CI), 1.10 to 2.36]; third tertile adjusted OR, 2.13 [95% CI, 1.45 to 3.13]). First postoperative IL-6 concentration was not associated with risk of mortality; however, the second tertile of peak IL-6 concentration was significantly associated with lower risk of mortality (adjusted hazard ratio, 0.75 [95% CI, 0.57 to 0.99]). Elevated first postoperative IL-10 concentration was significantly associated with higher risk of AKI (adjusted OR, 1.57 [95% CI, 1.04 to 2.38]) and lower risk of mortality (adjusted HR, 0.72 [95% CI, 0.56 to 0.93]). There was a significant interaction between the concentration of neutrophil gelatinase-associated lipocalin, an established AKI biomarker, and the association of IL-10 concentration with mortality (P=0.01). These findings suggest plasma IL-6 and IL-10 may serve as biomarkers for perioperative outcomes.
Keywords: cytokines, mortality, Epidemiology and outcomes, clinical epidemiology
AKI is a common complication of cardiac surgery, leading to increased morbidity and mortality.1 Current paradigms of diagnosis and treatment are based on serum creatinine and are thus insensitive. These limitations have spurred the search for novel proteins that are involved in the process of kidney injury.2,3 While AKI is traditionally recognized and diagnosed by renal functional impairment and structural damage, it is also an inflammatory condition. Understanding and measuring the processes of inflammation in AKI may provide unique tools for its assessment and prediction.
To this end, we developed an ancillary study to our large, multicenter cohort study of adult patients undergoing cardiac surgery to investigate the association of two biomarkers of inflammation, IL-6 and IL-10, with in-hospital AKI and postdischarge, long-term mortality. IL-6 is a major proinflammatory mediator well characterized in the orchestration of the inflammatory response following acute renal insult4,5 and has been shown to be a superior marker in renal patients compared with other proinflammatory candidates, such as the systemic inflammatory marker C-reactive protein.6–9 On the other hand, IL-10, while not as established in kidney injury, is appreciated to be a prototypical anti-inflammatory cytokine that carries out the critical function of modulating inflammation.10,11 Accordingly, IL-6 has been suggested to exacerbate renal injury,12,13 while IL-10 has been shown to confer protective effects.14–19 We hypothesized that our cohort would also show these trends. Notably, a preclinical study20 has demonstrated that IL-10 protects against renal ischemia through the induction of neutrophil gelatinase-associated lipocalin (NGAL), an established AKI biomarker that our group has previously studied. Thus, we evaluated whether elevated NGAL modified any observed protective effects of IL-10 in humans.
We used cardiac surgery, a well characterized trigger of sterile inflammation resulting largely from the extracorporeal circulation, as a clinical model of inflammatory AKI.21 This study is one of the first large-scale validations of these two inflammatory cytokines from the early human studies in the setting of cardiac surgery,22–25 and it elucidates the state and contributions of inflammation via the cytokine response and balance of pro- and anti-inflammatory forces in AKI.
Results
Patient Characteristics
Baseline characteristics for the overall population are presented in Table 1. The original cohort included 1219 adults who underwent cardiac surgery between July 2007 and December 2009. Our analyses included 960 patients after exclusion of 20 who died in the hospital and 239 who did not have biomarker measurements because of inadequate samples. The average age was 71.5 years, and 68.2% were men. A majority of surgeries were elective (80.5%), and most used cardiopulmonary bypass (91%). The mean preoperative eGFR was 68 ml/min per 1.73 m2. Three hundred thirty of the 960 participants (34.4%) developed AKI (defined as change in serum creatinine of ≥50% or ≥0.3 mg/dl), and 37 (3.9%) developed severe AKI (defined as change in serum creatinine ≥100%). Nine patients received acute dialysis (0.9%). During a median follow-up of 2.92 years (interquartile range, 2.23–3.48), 104 participants died (10.8%).
Table 1.
Baseline characteristics of study participants (n=960)
| Variable | P Value |
|---|---|
| Demographic characteristics | |
| Age at the time of surgery (yr) | 71.5±10.0 |
| Men, n (%) | 655 (68.2) |
| White race, n (%) | 897 (93.4) |
| Medical history (time of surgery) | |
| Diabetes, n (%) | 379 (39.5) |
| Hypertension, n (%) | 759 (79.1) |
| Congestive heart failure, n (%) | 230 (24.0) |
| LVEF <40%, n (%) | 98 (10.2) |
| Previous myocardial infarction, n (%) | 250 (26.0) |
| eGFR (ml/min per 1.73 m2) | 68±19.46 |
| eGFR, n (%) | |
| eGFR>60 ml/min per 1.73 m2 | 633 (65.9) |
| eGFR 30–60 ml/min per 1.73 m2 | 296 (30.8) |
| eGFR<30 ml/min per 1.73 m2 | 31 (3.2) |
| Median serum creatinine (mg/dl) | 1 (1–1) |
| Surgical characteristics | |
| Elective surgery, n (%) | 773 (80.5) |
| CABG or valve surgery, n (%) | 752 (78.3) |
| Off-pump, n (%) | 87 (9.1) |
| Re-do surgery, n (%) | 18 (1.9) |
| Perfusion time (min) | 112.39±58.45 |
| Cross-clamp time (min) | 76.69±43.93 |
| No. of diseased coronary vessels, n (%) | |
| 0 | 251 (26.1) |
| 1 | 125 (13.0) |
| 2 | 397 (41.4) |
| 3 | 182 (19.0) |
| Left main disease ≥50%, n (%) | 348 (36.9) |
| Postoperative complications | |
| Clinical AKI | |
| Increase in SCr≥50% or ≥0.3 mg/dl, n (%) | 330 (34.4) |
| Increase in SCr≥100%, n (%) | 37 (3.9) |
| Acute dialysis, n (%) | 9 (0.9) |
| Oliguria in first day, n (%) | 11 (1.1) |
| Increase in peak serum creatinine (mg/dl) | 0.20 (0.38) |
| No. of nonrenal complications, n (%) | |
| 0 | 591 (61.6) |
| 1–2 | 289 (30.1) |
| >2 | 80 (8.3) |
| Ventilator >48 hr, n (%) | 34 (3.5) |
| Median ICU LOS (d) | 2 (1–3) |
| Median hospital LOS (d) | 6 (5–8) |
| Death following discharge, n (%) | 104 (10.8) |
| Median duration of follow-up (yr) | 2.92 (2.23, 3.48) |
Values expressed with a plus/minus sign are the mean±SD. Median values are accompanied by interquartile ranges in parentheses. Small cell counts are only presented for data collected by Translational Research Investigating Biomarker Endpoints in AKI and not from Institute for Clinical Evaluative Sciences data holdings. LEVF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; SCr, serum creatinine; ICU, intensive care unit; LOS, length of stay.
Perioperative Plasma IL-6 and IL-10 Levels
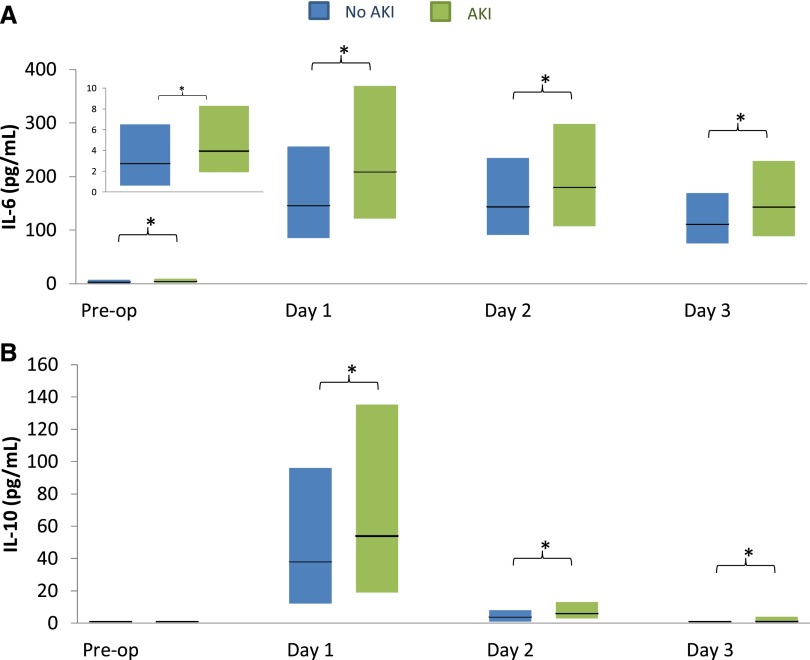
Preoperative IL-6 levels were significantly higher in patients who developed AKI than in those who did not (median, 3.9 versus 2.7 pg/ml; P=0.001) (Figure 1, Supplemental Table 1A). Levels of IL-6 were elevated in both AKI and non-AKI groups at all postoperative time points, including the first postoperative day (day 1, 0–6 hours after surgery), day 2 (48 hours after surgery), and day 3 (72 hours after surgery). Further, IL-6 levels were significantly higher at each of these three time points in patients who developed AKI than in those who did not (medians of 208 versus 146 pg/ml; 180 versus 144 pg/ml; and 143 versus 111, respectively; P<0.001 for all time points). IL-6 levels peaked between 0 and 6 hours after surgery.
Figure 1.
Inflammatory biomarkers, plasma IL-6 and IL-10, significantly increase in AKI patients after cardiac surgery. In patients with AKI, plasma IL-6 levels (A) were significantly increased both pre- and postoperatively, while plasma IL-10 levels (B) were significantly increased only at all postoperative time points. Each bar represents the interquartile range (25th percentile to 75th percentile), and the horizontal black line represents the median. *P<0.05. Day 1 refers to postoperative time 0–6 hours after surgery, day 2 corresponds to 48 hours after surgery, and day 3 corresponds to 72 hours after surgery.
Preoperative IL-10 levels did not significantly differ between patients who did and did not develop AKI (Figure 1, Supplemental Table 1A). Levels of IL-10 were also increased and differed between those who did and did not develop AKI at all three postoperative time points. IL-10 levels also peaked within 6 hours, although the magnitude of increase was less than that of IL-6; by day 3, median levels of IL-10 in the AKI group had returned to baseline medians.
The correlations between IL-6 and IL-10 at preoperative, first postoperative, and peak levels were weak (r=0.25, 0.13, and 0.06, respectively). The correlations were also weak (r<0.1) between the baseline and first postoperative values for individual biomarkers.
Perioperative Biomarkers and Risk of AKI
The unadjusted and adjusted associations of preoperative IL-6 and IL-10 with AKI are presented in Table 2. Neither preoperative IL-6 nor IL-10 levels were significantly associated with the outcome of postoperative AKI. While the highest tertile of preoperative IL-6 was associated with higher risk of AKI compared with the lowest tertile (unadjusted odds ratio [OR], 1.81; 95% confidence interval [95% CI, 1.29 to 2.53]), the association was attenuated following multivariable adjustment (adjusted OR, 1.44; 95% CI, 0.97 to 2.14).
Table 2.
Tertiles of inflammatory biomarkers and risk of AKI
| Pre-op | Post-op | ||||||
|---|---|---|---|---|---|---|---|
| Tertile (Range) | % AKI | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | Tertile (Range) | % AKI | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
| IL-6 (pg/mL) | |||||||
| T1 (<1.9) | 27.2% | 1.00 (referent) | 1.00 (referent) | T1 (<111) | 23.0% | 1.00 (referent) | 1.00 (referent) |
| T2 (2.0–5.2) | 33.9% | 1.37 (0.97, 1.94) | 1.29 (0.88, 1.87) | T2 (111–239) | 34.5% | 1.77 (1.25, 2.50) | 1.61 (1.10, 2.36) |
| T3 (>5.2) | 40.3% | 1.81 (1.29, 2.53) | 1.44 (0.97, 2.14) | T3 (>239) | 45.3% | 2.78 (1.97, 3.91) | 2.13 (1.45, 3.13) |
| IL-10 (pg/mL) | |||||||
| T1 (0.9) | 33.3% | 1.00 (referent) | 1.00 (referent) | T1 (<21.7) | 27.7% | 1.00 (referent) | 1.00 (referent) |
| T2 (>0.9) | 36.2% | 1.14 (0.73, 1.77) | 1.02 (0.62, 1.68) | T2 (21.8–79.5) | 34.5% | 1.38 (0.98, 1.93) | 1.23 (0.84, 1.81) |
| – | – | – | – | T3(>79.5) | 40.6% | 1.78 (1.28, 2.49) | 1.57 (1.04, 2.38) |
AKI defined as an increase in serum creatinine >50% or >0.3 mg/dL or dialysis during hospitalization.
Small cell counts are only presented for data collected by TRIBE-AKI and not from ICES data holdings. OR=odds ratio, CI=confidence interval.
Pre-op Models: Adjusted for age, sex, white race, non-elective surgery, pre-op eGFR, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery. Number of patients per tertile: IL-6 T1 n=309, T2 n=307 T3 n=308; IL-10 T1 n=814, T2 n=94.
Post-op Models: Adjusted for age, sex, white race, non-elective surgery, pre-op eGFR, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery. Number of patients per tertile: IL-6 T1 n=318, T2 n=319 T3 n=318; IL-10 T1 n=318, T2 n=319, T3 n=318.
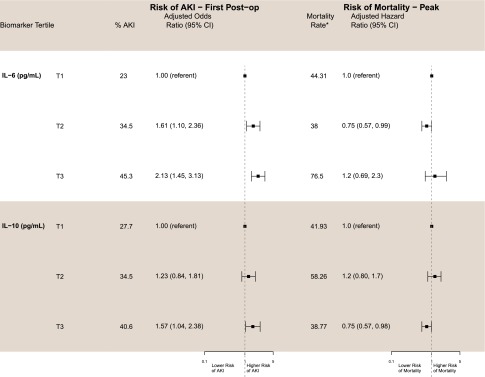
Clinical AKI as measured by serum creatinine was diagnosable at a median of 3 days following surgery (interquartile range, 2–4 days), and thus, first postoperative biomarker measurements (day 1, 0–6 hours after surgery) can assist with early detection of AKI. These first postoperative measurements and their associated risks of AKI are also presented in Table 2. The second and third tertiles of first postoperative IL-6 were associated with higher risks of AKI both before (unadjusted OR, 1.77 [95% CI, 1.25 to 2.50]; unadjusted OR, 2.78 [95% CI, 1.97 to 3.91], respectively) and after(adjusted OR, 1.61 [95% CI, 1.10 to 2.36]; adjusted OR, 2.13 [95% CI, 1.45 to 3.13], respectively) multivariable adjustment compared with the lowest tertile, and the risk of AKI increased in a dose-dependent manner (Figure 2). The highest tertile of first postoperative IL-10 was also associated with higher risk of AKI both before and after multivariable adjustment compared with the lowest tertile (unadjusted OR, 1.78 [95% CI, 1.28 to 2.49]; adjusted OR, 1.57 [95% CI, 1.04 to 2.38], respectively).
Figure 2.
High plasma IL-6 and IL-10 are associated with increased risk of AKI, and high plasma IL-10 is associated with decreased 3-year mortality after cardiac surgery. Adjusted for age (per year), sex, white race, cardiopulmonary bypass time >120 minutes, nonelective surgery, preoperative eGFR, diabetes, hypertension, site, congestive heart failure, myocardial infarction, preoperative urinary albumin-to-creatinine ratio, increase in serum creatinine, and type of surgery. *P<0.05. Mortality rate per 1000 patient-years adjusted for site.
Perioperative Biomarkers and Risk of Mortality
The unadjusted and adjusted associations of preoperative IL-6 and IL-10 with all-cause mortality are presented in Table 3. Neither preoperative IL-6 nor IL-10 measurements were associated with mortality. First postoperative IL-6 levels were also not associated with risk of mortality; however, the second tertile of peak IL-6 was significantly associated with an approximately 25% lower risk of mortality compared with the lowest tertile (adjusted hazard ratio [HR], 0.75 [95% CI, 0.57 to 0.99]).
Table 3.
Tertiles of inflammatory biomarkers and all-cause mortality
| Pre-op | Day 1 0-6 Hours | Peak | ||||||
|---|---|---|---|---|---|---|---|---|
| Tertile | Mortality Rate * | Adjusted Hazard Ratio (95% CI) | Tertile | Mortality Rate * | Adjusted Hazard Ratio (95% CI) | Tertile | Mortality Rate * | Adjusted Hazard Ratio (95% CI) |
| IL-6 (pg/mL) | ||||||||
| T1 (<1.9) | 29.69 | 1.0 (referent) | T1 (<111) | 40.07 | 1.0 (referent) | T1 (<176) | 44.31 | 1.0 (referent) |
| T2 (2.0–5.2) | 40.91 | 1.2 (0.9, 1.7) | T2 (111–239) | 44.28 | 0.97 (0.76,1.24) | T2 (176–323) | 38 | 0.75 (0.57, 0.99) |
| T3 (>5.2) | 56.97 | 1.3 (0.9, 2.1) | T3 (>239) | 74.61 | 1.4 (0.65,2.9) | T3 (>323) | 76.5 | 1.2 (0.69, 2.3) |
| IL-10 (pg/mL) | ||||||||
| T1 (0.9) | 45.5 | 1.0 (referent) | T1 (<21.7) | 43.31 | 1.0 (referent) | T1 (<24.1) | 41.93 | 1.0 (referent) |
| T2 (>0.9) | 43.49 | 1.1 (0.8, 1.4) | T2 (21.8–79.5) | 56.47 | 1.1 (0.72,1.6) | T2 (24.2–79.5) | 58.26 | 1.2 (0.80, 1.7) |
| – | – | – | T3 (>79.5) | 38.38 | 0.72 (0.56, 0.93) | T3 (>79.5) | 38.77 | 0.75 (0.57, 0.98) |
Mortality rate per 1000 patient years adjusted for site.
Adjusted for age (per year), sex, white race, CPB time > 120 minutes, non-elective surgery, pre-op eGFR, diabetes, hypertension, site, congestive heart failure, myocardial infarction, pre-op UACR, delta serum creatinine and type of surgery. Models with preoperative biomarkers were not adjusted for CPB time > 120 minutes and delta serum creatinine.
In addition, the highest tertiles of IL-10 at both the first postoperative time point and peak levels were significantly associated with an approximately 25% to 30% lower risk of all-cause mortality following multivariable adjustment (adjusted HR, 0.72 [95% CI, 0.56 to 0.93]; adjusted HR, 0.75 [95% CI, 0.57 to 0.98], respectively) (Figure 2). The highest tertiles of IL-10 remained significantly associated with mortality when IL-6 was added to the multivariable models (data not shown). There was no significant interaction between IL-10 and IL-6 at both the first postoperative time point and peak levels (Supplemental Table 3).
First Postoperative Biomarkers and Interaction with Plasma and Urine NGAL
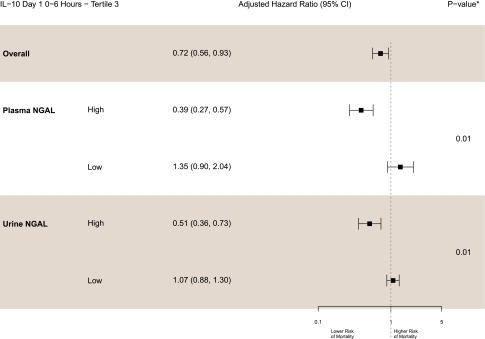
First postoperative levels of both plasma and urine NGAL were stratified at their median values into high and low categories (Figure 3). In the high NGAL groups, the highest tertile of IL-10 was strongly and independently associated with decreased mortality; effect sizes were larger than in the overall cohort (plasma NGAL greater than median third tertile of IL-10: adjusted HR, 0.39 [95% CI, 0.27 to 0.57], P for interaction=0.01; urine NGAL greater than median third tertile of IL-10: adjusted HR, 0.51 [95% CI, 0.36 to 0.73], P for interaction=0.01). In contrast, in the low NGAL groups, the highest IL-10 tertile was not associated with mortality risk because the point estimates were >1.0 (adjusted HR, 1.35 [95% CI, 0.9 to 2.04]; adjusted HR, 1.07 [95% CI, 0.88 to 1.3]). The highest IL-6 tertile had no association with mortality in the high or low NGAL strata, except for the second tertile of IL-6, which demonstrated significant interaction with urine NGAL (Supplemental Table 4).
Figure 3.
NGAL modulates the protective effects of IL-10. The protective effect of those with the highest levels of postoperative IL-10 was observed only with NGAL levels above the mean (P=0.01 for both plasma and urine NGAL). High NGAL levels were defined as greater than the median value, and low NGAL was defined as less than or equal to the median value. Adjusted for age (per year), sex, white race, cardiopulmonary bypass time >120 minutes, nonelective surgery, preoperative eGFR, diabetes, hypertension, site, congestive heart failure, myocardial infarction, preoperative urinary albumin-to-creatinine ratio, increase in serum creatinine, and type of surgery. *P value for the interaction between the third tertile of IL-10 and NGAL (high/low).
Inflammatory Biomarkers and Risk of severe AKI
Plasma IL-6 and IL-10 levels stratified by severe AKI demonstrated trends similar to those stratified by AKI, except that first postoperative IL-10 levels were not significantly different between those who developed severe AKI and those who did not (Supplemental Figure 1, Supplemental Table 1B). Preoperative IL-6 was not associated with severe AKI (Supplemental Figure 2). The highest tertile of first postoperative IL-6 was associated with higher risk of severe AKI compared with the lowest tertile (unadjusted OR, 6.4 [95% CI, 2.2 to 18.7]), but the association was attenuated following multivariable adjustment (adjusted OR, 3.0 [95% CI, 0.94 to 9.5]). Neither pre- nor postoperative IL-10 was associated with severe AKI.
Discussion
This study presents one of the first large-scale validations and comprehensive assessments of IL-6 and IL-10 as biomarkers of inflammation in the perioperative setting. Following cardiac surgery, the levels of both inflammatory biomarkers rose in all patients, even in those without clinically apparent AKI. The range of cytokine increases observed in the inflammatory response highlights the significance of individual patient proinflammatory states that lead to the heterogeneity of inflammatory responses in perioperative outcomes. Before surgery, for example, we had already observed significant differences in IL-6 among patients who went on to develop both AKI and severe AKI. Our findings build on our previous work that has provided both diagnostic and prognostic information for clinical prediction models26–34 and demonstrates that these cytokines may serve as prognostic biomarkers for perioperative outcomes.
Strikingly, we found higher IL-10 to be associated with decreased risk of all-cause mortality following cardiac surgery after adjustment for clinical and demographic factors, as well as renal function. Previous studies have reported the influence of IL-10 in attenuating inflammation and kidney injury in animal models of acute GN,17,18 CKD,35 cisplatin nephrotoxicity,14,36,37 and ischemic injury.14 Further, animal studies in a variety of other disease settings have also demonstrated similar protective properties of IL-10 and have even reported that the cytokine creates an environment conducive for regenerative adult wound healing.38–42 This is the first perioperative clinical study, to our knowledge, that has found a significantly protective association of a cytokine on all-cause mortality after discharge.
Jung et al. demonstrated that IL-10 may exert protective effects through the induction of lipocalin-2, also known as NGAL, in a rat model of renal ischemia.20 Overexpressing IL-10 via adoptive transfer reduced inflammation and kidney injury dependent on increased expression of NGAL, its receptor, and a supply of intracellular iron. Our study extends this finding by demonstrating in a postoperative setting that the protective effects of high IL-10 may be modified by NGAL. The highest tertiles of IL-10 were independently associated with decreased mortality only in the high NGAL groups, while those in the low NGAL groups were not protected; this finding suggests that IL-10 may be protective only when NGAL is induced. The interaction of IL-10 with NGAL, a biomarker of kidney injury,26,33,43 links IL-10 with a downstream target that has already been demonstrated to predict renal dysfunction and associated outcomes. Thus, the mortality captured by IL-10 in this study may reflect the inflammatory subset of the mortality that is captured by the more general kidney injury marker NGAL from our previous work.26
The biologic functions and temporal profiles of the two inflammatory cytokines as prototypical representatives of the inflammatory response are consistent with and help explain our findings. Injury spurs necessary inflammation, regulated by proinflammatory mediators, such as IL-6, which is suppressed by anti-inflammatory forces, such as IL-10.44,45 This temporal trajectory—of IL-6 functioning before, and eventually spurring, the production of IL-10—may provide perspective and a potential rationale to our findings that IL-6 was more strongly associated with the earlier outcome of AKI in a dose-dependent manner, while IL-10 was more strongly associated with the long-term outcome of mortality. In addition, the proinflammatory functionality of IL-6 lends credibility to the predictive potential of the cytokine to predict AKI, while the anti-inflammatory properties of IL-10 are consistent with the ability of IL-10 levels to predict protection against mortally. While first postoperative IL-6 only bordered on a significant association with severe AKI following multivariable adjustment, this loss of significance may be due to low number of severe AKI cases (n=37). Despite this limitation, these analyses point out the trend that an increase in disease severity leads to an increase in association because the point estimates notably increased for severe AKI compared with those for AKI (adjusted OR, 3.0 versus 2.1, respectively), corroborating IL-6 as a marker for this disease state.
Of note, the second tertile of peak IL-6 was protective, associated with lower risk of mortality (adjusted OR, 0.75; 95% CI, 0.57 to 0.99). Although this finding may represent the ideal balance of proinflammatory stimuli, we believe it may be due to chance and needs to be confirmed in future studies. In addition, the highest tertile of first postoperative IL-10 was significantly associated with higher risk of AKI, which may seem to conflict with the concurring significant association with lower mortality risk; however, this finding may highlight the long-term mechanism that underlies the protective effects of IL-10 via NGAL induction. A study preceding that of Jung et al. from the same group demonstrated that renal regeneration, prompted by mediators including IL-10, occurs only following, and not during, ischemia/reperfusion injury.46 This segregation of the two inflammatory arms, with respect to the inflammatory milieu, is consistent with our understanding of the transition in macrophages, which secretes both IL-6 and IL-10, from a state that promotes injury to one that initiates repair.47
Our study also expands on the literature of these cytokines in the perioperative setting, which has generally been conducted with small cohorts, in single-center settings, and with lack of defined hard outcomes. While some larger studies have reported inconsistent results,48,49 a majority of these were not undertaken in the perioperative setting. The discrepancies may primarily be due to the complications by other underlying abnormalities in the patient cohorts, such as sepsis or other critical illnesses. These conditions produce underlying inflammation and, in some cases, may directly lead to inflammatory dysfunction, thus disrupting the natural progression of inflammatory events proximal to renal injury. Such issues can interfere with cytokine measurement within the natural course of inflammation, and we believe that the perioperative cardiac surgery setting addresses some of these issues and is a strength of our study.
We also acknowledge that our study possesses important limitations. As in our previous studies, the findings are specific for white patients at high risk for AKI following cardiac surgery and thus may not be readily generalizable to diverse populations. We did not collect height and weight information and thus could not adjust for obesity, a recently identified risk factor for AKI.50 The cause of death and long-term kidney function were also not available. Studies in the settings of ESRD and CKD have shown that IL-10 is an antiatherosclerotic cytokine and may prevent CVD-related mortality, while IL-6 is proatherogenic, suggesting that CVD resulting from inflammation and kidney injury may be a mechanism leading to mortality.45,51,52 Logistically, plasma samples were stored for 3–4 days before measurement for this ancillary study, and the literature indicates that extended storage may introduce considerable degradation and thus compromise our findings.53,54 Specifically, there are caveats to the interpretation of plasma cytokine measurements, particularly IL-10, which may act locally in a paracrine manner and may not be fully detected in circulation. IL-6 is appreciated to act more systemically and thus may more accurately reflect circulating levels; however, adipose tissue may contribute a considerable amount of systemic IL-6 and may not completely reflect response to inflammation.55,56 While we attempted to select prototypical pro- and anti-inflammatory cytokines most relevant to renal abnormality for our study, we ultimately examined only one proinflammatory and one anti-inflammatory biomarker; other investigators have assessed larger panels. Our group traditionally examines biomarkers in relevant pathophysiologic panels and plans to further explore other inflammatory markers in future studies.
In conclusion, we have demonstrated that inflammation measured by plasma IL-6 and IL-10 is associated with short- and long-term outcomes in a large prospective cohort of adults who underwent cardiac surgery. The early AKI detection potential and prognostic information for all-cause mortality provided by these inflammatory biomarkers offers a unique perspective to evaluate perioperative outcomes and may also prove beneficial in other inflammatory diseases.
Concise Methods
Patient Cohorts and Samples
The detailed methods of the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) cardiac surgery cohort, including sample collection and processing, have been described in detail previously.26 We prospectively enrolled 1219 adults undergoing cardiac surgery (coronary artery bypass grafting or valve surgery) who were at high risk for AKI at six academic medical centers in North America between July 2007 and December 2009. Day 1 was the day of surgery, and day 2 corresponded to the first day after surgery. In brief, we collected plasma specimens preoperatively and daily up to day 5. We collected first postoperative samples soon after admission to the intensive care unit within 6 hours after surgery (0- to 6-hour sample). Subsequently, we obtained daily blood samples at the time of routine morning blood collection done for clinical care. Some patients presented insufficient sample volume preoperatively, which resulted in missing preoperative values. These patients, however, were still included in analyses because they had at least one preoperative biomarker measurement. Patients who died during the index hospitalization for surgery (n=20) and those who did not have biomarkers measured due to inadequate sample (n=239) were excluded from this analysis.
Study Variables
Our study had two co-primary outcomes. The first was the development of AKI, defined as a ≥50% or ≥0.3-mg/dl increase in serum creatinine concentration from baseline preoperative concentration consistent with Acute Kidney Injury Network stage 1 AKI classification and Kidney Disease Improving Global Outcomes stage 1 AKI classification.57,58 We collected preoperative characteristics, operative details, and postoperative complications using definitions of the Society of Thoracic Surgeons.
The other primary outcome was all-cause mortality. We obtained vital status after discharge through various mechanisms (and cross-referenced when possible). For patients living in the United States, we telephoned patients’ homes, searched the National Death Index, and reviewed hospital records. For Canadian participants (those enrolled into the TRIBE-AKI study in London, Ontario), we also telephoned the patients' homes and analyzed data held at the Institute for Clinical Evaluative Sciences to acquire vital status. These datasets were linked using unique, encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences. Vital status and date of death were recorded through the last follow-up date of February 21, 2012. There was 100% ascertainment of vital status on the cohort.
The secondary outcome was severe AKI, defined by a doubling in serum creatinine concentration from baseline preoperative concentration consistent with the injury classification of the Risk, Injury, Failure, Loss of Function, ESRD (RIFLE) criteria and Acute Kidney Injury Network stage 2 AKI classification, Kidney Disease Improving Global Outcomes stage 2 AKI classification or receiving acute dialysis during the hospital stay.57–59
Biomarker Assays
Following one freeze-thaw (storage at −80°C), plasma IL-6 and IL-10 samples were analyzed on the Randox Evidence Investigator using a Randox-developed custom cytokine array (Randox Laboratories Ltd.). The detection ranges are 0.6–790.0 pg/ml for IL-6 and 0.9–840 pg/ml for IL-10. The intra-assay coefficients of variation for IL-6 and IL-10 are 7% and 6%, respectively, and the interassay coefficients of variation are 6% and 17%, respectively. We blinded the personnel measuring the biomarkers to clinical outcomes, and samples were analyzed according to manufacturer specifications.
Statistical Analyses
We compared continuous variables with a two-sample t test or Wilcoxon rank-sum test and dichotomous variables with the chi-squared test or Fisher exact test. We divided each population into tertiles using the value of each biomarker, with the lowest tertile as the reference group. To evaluate the association between each biomarker and AKI, we used logistic regression models. We used Cox proportional hazards regression with robust sandwich variance estimators (accounting for clustering within centers) to examine the association between biomarkers and time to death from the date of surgery excluding in-hospital deaths. We used Schoenfeld residuals to confirm the proportional-hazards assumption.
Analyses were adjusted for the following variables: age (per year), sex, white race, cardiopulmonary bypass time >120 minutes, nonelective surgery, preoperative eGFR (as determined with the CKD-Epidemiology Collaboration formula), diabetes, hypertension, center, congestive heart failure, myocardial infarction, preoperative urine albumin-to-creatinine ratio, and type of surgery. We adjusted for important covariates that predict AKI in the cardiac surgery setting.60 To examine the relationship between IL-10 and IL-6, we categorized each biomarker by the median value and examined the interaction term in the model. The effect of NGAL on the association between IL-10 and mortality was assessed by adding interaction terms to the model. Small cell counts are presented only for data collected by TRIBE-AKI and not from Institute for Clinical Evaluative Sciences data holdings. Analyses were performed with SAS software, version 9.3 (SAS Institute Inc., Cary, NC), and R 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria).
Study Approval
The institutional review boards from each participating site approved the study and protocols. All participants provided written informed consent.
Disclosures
P.K. has received grants/honorariums/consultant/advisor fees/from Abbott Laboratories, Abbott Point of Care, Beckman Coulter, Ortho Clinical Diagnostics, Randox Laboratories, Roche Diagnostics, and the Canadian Agency for Drugs and Technologies in Health. He is listed as an inventor on patents filed by McMaster University related to laboratory testing in acute cardiac care.
Supplementary Material
Acknowledgments
Members of TRIBE-AKI Consortium are as follows: Dr. Prasad Devarajan (University of Cincinnati Children’s Hospital), Dr. Charles Edelstein (University of Colorado), Dr. Cary Passik (Danbury Hospital), Dr. Madhav Swaminathan and Dr. Uptal Patel (Duke University), Dr. Michael Zappitelli (Montreal Children’s Hospital), and Dr. Isabel Butrymowicz (Yale-New Haven Hospital).
The Evidence Investigator Cytokine Custom Array 4 kits were donated by Randox Laboratories Ltd.
The granting agencies, Randox Laboratories Ltd., did not participate in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
This study was supported by the National Institutes of Health (NIH) (R01-HL085757 to C.R.P.) to fund the TRIBE-AKI Consortium to study novel biomarkers of AKI in cardiac surgery. S.G.C. has been supported by an NIH Career Development Award (K23-DK080132). C.R.P. is supported by the NIH (K24-DK090203) and P30-DK079310-07 O'Brien Center Grant. S.G.C., A.X.G., and C.R.P. are also members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Consortium (U01-DK082185).
The results presented in this article have not been published previously in whole or part, except in abstract form.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080764/-/DCSupplemental.
References
- 1.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Westenfelder C: Earlier diagnosis of acute kidney injury awaits effective therapy. Kidney Int 79: 1159–1161, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Soni SS, Ronco C, Katz N, Cruz DN: Early diagnosis of acute kidney injury: The promise of novel biomarkers. Blood Purif 28: 165–174, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH: IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones SA, Fraser DJ, Fielding CA, Jones GW: Interleukin-6 in renal disease and therapy [published online ahead of print July 10, 2014]. Nephrol Dial Transplant 10.1093/ndt/gfu233 [DOI] [PubMed] [Google Scholar]
- 6.Kavsak PA, Ko DT, Newman AM, Palomaki GE, Lustig V, MacRae AR, Jaffe AS: Risk stratification for heart failure and death in an acute coronary syndrome population using inflammatory cytokines and N-terminal pro-brain natriuretic peptide. Clin Chem 53: 2112–2118, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ortiz A, Massy ZA, Fliser D, Lindholm B, Wiecek A, Martínez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM, Zoccali C: Clinical usefulness of novel prognostic biomarkers in patients on hemodialysis. Nat Rev Nephrol 8: 141–150, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Stenvinkel P, Lindholm B: C-reactive protein in end-stage renal disease: Are there reasons to measure it? Blood Purif 23: 72–78, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Sinuani I, Beberashvili I, Averbukh Z, Sandbank J: Role of IL-10 in the progression of kidney disease. World J Transplant 3: 91–98, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, de Vries JE: Interleukin-10. Curr Opin Immunol 4: 314–320, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C: Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther 312: 1170–1178, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA: Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118–2128, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Liu R, Xie J, Xiong H, He JC, Chen N: Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest 93:801–811, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Huang XR, Kitching AR, Tipping PG, Holdsworth SR: Interleukin-10 inhibits macrophage-induced glomerular injury. J Am Soc Nephrol 11: 262–269, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Kitching AR, Katerelos M, Mudge SJ, Tipping PG, Power DA, Holdsworth SR: Interleukin-10 inhibits experimental mesangial proliferative glomerulonephritis. Clin Exp Immunol 128: 36–43, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi YK, Kim YJ, Park HS, Choi K, Paik SG, Lee YI, Park JG: Suppression of glomerulosclerosis by adenovirus-mediated IL-10 expression in the kidney. Gene Ther 10: 559–568, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR: Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int 57: 518–525, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Jung M, Sola A, Hughes J, Kluth DC, Vinuesa E, Viñas JL, Pérez-Ladaga A, Hotter G: Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int 81: 969–982, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Kaya K: Inflammatory response in cardiovascular surgery. In: Inflammatory Response in Cardiovascular Surgery, edited by Gabriel EA, Gabriel SA, London, Springer, 2013, pp 275–280 [Google Scholar]
- 22.Gueret G, Lion F, Guriec N, Arvieux J, Dovergne A, Guennegan C, Bezon E, Baron R, Carre J-L, Arvieux C: Acute renal dysfunction after cardiac surgery with cardiopulmonary bypass is associated with plasmatic IL6 increase. Cytokine 45: 92–98, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Musleh GS, Datta SS, Yonan NN, Grotte GJ, Prendergast BA, Hasan RI, Deyrania AK: Association of IL6 and IL10 with renal dysfunction and the use of haemofiltration during cardiopulmonary bypass. Eur J Cardiothorac Surg 35: 511–514, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S: Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case-control study. Crit Care 13: R104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikłaszewska M, Korohoda P, Zachwieja K, Mroczek T, Drożdż D, Sztefko K, Moczulska A, Pietrzyk JA: Serum interleukin 6 levels as an early marker of acute kidney injury on children after cardiac surgery. Adv Clin Exp Med 22: 377–386, 2013 [PubMed] [Google Scholar]
- 26.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG, TRIBE-AKI Consortium : Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8: 1079–1088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel UD, Garg AX, Krumholz HM, Shlipak MG, Coca SG, Sint K, Thiessen-Philbrook H, Koyner JL, Swaminathan M, Passik CS, Parikh CR, Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium : Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgery. Circulation 125: 1347–1355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyner JL, Garg AX, Shlipak MG, Patel UD, Sint K, Hong K, Devarajan P, Edelstein CL, Zappitelli M, Thiessen-Philbrook H, Parikh CR, Translational Research Investigating Biomarker Endpoints in AKI (TRIBE AKI) Consortium : Urinary cystatin C and acute kidney injury after cardiac surgery. Am J Kidney Dis 61: 730–738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, Shlipak MG, Parikh CR, TRIBE-AKI Consortium : Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 25: 1063–1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu JCT, Coca SG, Patel UD, Cantley L, Parikh CR, Translational Research Investigating Biomarkers and Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium : Searching for genes that matter in acute kidney injury: A systematic review. Clin J Am Soc Nephrol 4: 1020–1031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spahillari A, Parikh CR, Sint K, Koyner JL, Patel UD, Edelstein CL, Passik CS, Thiessen-Philbrook H, Swaminathan M, Shlipak MG, TRIBE-AKI Consortium : Serum cystatin C- versus creatinine-based definitions of acute kidney injury following cardiac surgery: A prospective cohort study. Am J Kidney Dis 60: 922–929, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR, TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shlipak MG, Coca SG, Wang Z, Devarajan P, Koyner JL, Patel UD, Thiessen-Philbrook H, Garg AX, Parikh CR, TRIBE-AKI Consortium : Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis 58: 366–373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ: IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol 16: 3651–3660, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Tadagavadi RK, Reeves WB: Endogenous IL-10 attenuates cisplatin nephrotoxicity: Role of dendritic cells. J Immunol 185: 4904–4911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M-G, Yang HN, Kim H-W, Jo S-K, Cho WY, Kim H-K: IL-10 mediates rosiglitazone-induced kidney protection in cisplatin nephrotoxicity. J Korean Med Sci 25: 557–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman MA, Reznikov LL, Raeburn CD, Selzman CH: Interleukin-10 attenuates the response to vascular injury. J Surg Res 121: 206–213, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Yoshidome H, Kato A, Edwards MJ, Lentsch AB: Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: Implications of a central role for nuclear factor kappaB. Hepatology 30: 203–208, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, Lin C-S, Jobin C, Brand S, Sotlar K, Wada K, Katayama K, Nakajima A, Mizuguchi H, Kawasaki K, Nagata K, Müller W, Snapper SB, Schreiber S, Kaser A, Zeissig S, Blumberg RS: Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509: 497–502, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW: IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol 128: 1852–1860, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Ling G-S, Cook HT, Botto M, Lau Y-L, Huang F-P: An essential protective role of IL-10 in the immunological mechanism underlying resistance vs. susceptibility to lupus induction by dendritic cells and dying cells. Rheumatology (Oxford) 50: 1773–1784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Girndt M, Köhler H, Schiedhelm-Weick E, Schlaak JF, Meyer zum Büschenfelde K-H, Fleischer B: Production of interleukin-6, tumor necrosis factor alpha and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. Kidney Int 47: 559–565, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Vinuesa E, Hotter G, Jung M, Herrero-Fresneda I, Torras J, Sola A: Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol 214: 104–113, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi B-S, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, Korpak A, Thompson BT, Chertow GM, Matthay MA, National Heart, Lung, and Blood Institute ARDS Network Clinical Trials Group : Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 35: 2755–2761, 2007 [PMC free article] [PubMed] [Google Scholar]
- 49.Murugan R, Wen X, Shah N, Lee M, Kong L, Pike F, Keener C, Unruh M, Finkel K, Vijayan A, Palevsky PM, Paganini E, Carter M, Elder M, Kellum JA, Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators : Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dial Transplant 29: 1854–1864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Billings FT, 4th, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ: Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol 23: 1221–1228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch W, Kastrati A, Böttiger C, Mehilli J, von Beckerath N, Schömig A: Interleukin-10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis 159: 137–144, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Spoto B, Mattace-Raso F, Sijbrands E, Leonardis D, Testa A, Pisano A, Pizzini P, Cutrupi S, Parlongo RM, D’Arrigo G, Tripepi G, Mallamaci F, Zoccali C: Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clin J Am Soc Nephrol 10: 232–240, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Fragala MS, McElhaney JE, Kuchel GA: Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care 13: 541–547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V: Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol 10: 52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girndt M, Kaul H, Sester U, Ulrich C, Sester M, Georg T, Köhler H: Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int 62: 949–955, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Stenvinkel P, Barany P, Heimbürger O, Pecoits-Filho R, Lindholm B: Mortality, malnutrition, and atherosclerosis in ESRD: What is the role of interleukin-6? Kidney Int Suppl 61: 103–108, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kidney Disease. Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 59.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED, Society of Thoracic Surgeons National Cardiac Surgery Database Investigators : Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 114: 2208–2216, quiz 2208, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.