Abstract
Progress in long-term renal allograft survival continues to lag behind the progress in short-term transplant outcomes. Dendritic cells are the most efficient antigen-presenting cells, but surprisingly little attention has been paid to their presence in transplanted kidneys. We used dendritic cell–specific intercellular adhesion molecule-3–grabbing nonintegrin as a marker of dendritic cells in 105 allograft biopsy samples from 105 kidney transplant recipients. High dendritic cell density was associated with poor allograft survival independent of clinical variables. Moreover, high dendritic cell density correlated with greater T cell proliferation and poor outcomes in patients with high total inflammation scores, including inflammation in areas of tubular atrophy. We then explored the association between dendritic cells and histologic variables associated with poor prognosis. Multivariate analysis revealed an independent association between the densities of dendritic cells and T cells. In biopsy samples with high dendritic cell density, electron microscopy showed direct physical contact between infiltrating lymphocytes and cells that have the ultrastructural morphologic characteristics of dendritic cells. The origin of graft dendritic cells was sought in nine sex-mismatched recipients using XY fluorescence in situ hybridization. Whereas donor dendritic cells predominated initially, the majority of dendritic cells in late allograft biopsy samples were of recipient origin. Our data highlight the prognostic value of dendritic cell density in allograft biopsy samples, suggest a new role for these cells in shaping graft inflammation, and provide a rationale for targeting dendritic cell recruitment to promote long-term allograft survival.
Keywords: transplant pathology, chronic inflammation, chronic graft deterioration
Dendritic cells (DCs) are the most potent of antigen-presenting cells and are crucial players in the development of tolerance and inflammation.1,2 DCs constitute an abundant population of leukocytes within the kidney.3,4 Although traditionally labeled as macrophages due to their expression of macrophage markers, recent studies have shown that such cells are of DC origin by gene expression microarray5 and in vivo genetic tracing6 studies.
The majority of DCs in the human kidney are myeloid leukocytes, which express DC-SIGN (CD209; a member of the type II C-type lectin family), BDCA-1, HLA-DR, as well as macrophage markers (e.g., CD68 and CX3CR1).7–10 The extensive work by Kaissling and colleages11–13 revealed that DCs within the kidney can be differentiated from macrophages and fibroblasts using transmission electron microscopy without the need of immunostaining due to their specific ultrastructural features, namely their lysosome-poor pericaryon and electron-light cytoplasmic protrusions lacking abundant ribosomes (Supplemental Figure 1).
Although none of the DC immune markers has perfect sensitivity or specificity, DC-SIGN, which participates in T cell stimulation,14–17 is considered reliable in distinguishing myeloid DCs from macrophages.18,19 In kidney biopsies, DC-SIGN+ cells stain with other known kidney DC markers (BDCA-1,8 HLA-DR,8 CD68,7,8 and CX3CR110), show an activated but not fully mature DC phenotype, and are associated with high T cell stimulation capability.20
Our knowledge of the role of kidney DCs in human allografts is limited to three studies8,9,21 (Table 1). These studies have shown that compared with the pretransplant baseline, rejecting allografts display increased number of DCs, which are associated with inflammation, atrophy, and subsequent allograft dysfunction. Although these studies have enhanced our appreciation of the potential role of graft DCs in kidney rejection, they have some limitations. First, immunolabeling of kidney DCs and histologic evaluation were performed on different samples (frozen versus formalin-fixed paraffin-embedded [FFPE], respectively). This is a potentially important drawback due to histologic variability and the focal nature of allograft inflammation.22,23 Second, these studies concentrated on specific allograft disease entities (e.g., rejection, delayed graft function) rather than a broad spectrum of histologic changes. Third, the only study to tackle the correlation of graft DCs and atrophy9 was performed on very early biopsies (median 15 days after transplantation), where atrophy is likely attributed to preexisting donor disease. This study also treated atrophy as a binary, rather than quantitative, variable. Finally, the above three studies did not address the source of graft DCs (donor versus recipient) or their association with graft survival, infiltrating lymphocytes, and total inflammation including inflammation in areas of tubular atrophy, which may represent a form of T cell–mediated rejection (TCMR) not currently recognizable by the Banff classification.24–26
Table 1.
Kidney DC studies in allograft biopsies
| Woltman et al. (2007)8 | Loverre et al. (2007)21 | Zuidwijk et al. (2012)9 | |
|---|---|---|---|
| Country | The Netherlands | Italy | The Netherlands |
| Tissue stained | Frozen tissuea | Frozen tissuea | Frozen tissuea |
| Quantification | Digital image analysis (pixels/areas) | Counted by a pathologist | Digital image analysis (pixels/areas) |
| Types of studied allograft biopsies | Acute rejection | Delayed graft function, calcineurin inhibitor toxicity, and acute rejection | Acute rejection |
| No. of biopsies | 6 | 27 | 102 |
| Association with Inflammation | NA | NA | Yes |
| Fibrosis/atrophy | NA | NA | Yesb |
| T cells | NA | NA | NA |
| Allograft dysfunction | NA | NA | Yesc |
| Allograft failure | NA | NA | NAc |
| Conclusions | DC-SIGN+ cells increased in acute rejection versus pretransplant biopsies and IgA nephropathy | BDCA-1+ and BDCA-3+ cells increased in delayed graft function and acute rejection versus normal kidney and pretransplant biopsies (DC-SIGN was not assessed) | DC-SIGN+, BDCA-1+, and BDCA-2+ cells increased in acute rejection versus pretransplant biopsies and correlated with atrophy; DC-SIGN+ cell density in acute rejection was an independent risk factor for subsequent allograft dysfunction |
Immunolabeling of kidney DCs was performed on a frozen tissue samples, whereas histologic evaluation was performed on a different sample (FFPE tissue).
Fibrosis and atrophy were assessed as binary variables (present or absent based on involving >5% and >0% of biopsy, respectively).
Subsequent allograft dysfunction was assessed by a later decrease of eGFR. However, allograft survival was not assessed.
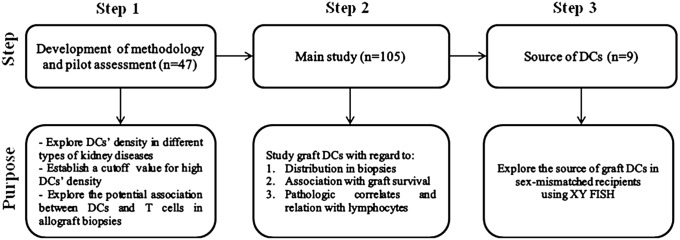
Because DC-SIGN appears to be a reliable marker of kidney DCs, we optimized DC-SIGN staining on FFPE samples so that the light microscopy and immunohistochemical studies were performed on the same tissue. A detailed clinicopathologic study was performed to characterize the significance of DCs in allograft biopsies addressing all of the aforementioned issues (Figure 1). We showed that graft DCs correlated with poor allograft survival, infiltrating lymphocytes, and allograft inflammation, especially inflammation in areas of tubular atrophy. We also found these cells to be of recipient origin in late allograft biopsies.
Figure 1.
Diagram of the study design.
Results
Development of Methodology and Pilot Assessment
DC-SIGN/CD3 double immunostaining was performed in 47 native and allograft kidney biopsies including nephrectomies with no significant histologic abnormality (n=5), native atrophic biopsies (n=17), native biopsies with interstitial nephritis (n=5), allografts with conventional TCMR (n=11), and inflamed atrophic allografts (n=9). As previously described,7 we found that DCs were invariably identified in kidney biopsies and were largely limited to the interstitial compartment. We first defined the cutoff value of abnormally high DC-SIGN+ cell density as >3.8 cells/high-power field (hpf) (>2 SDs from the mean DC-SIGN+ cell density of normal nephrectomy samples; see the Concise Methods). Our pilot assessment revealed that inflamed atrophic allograft biopsies displayed higher DC-SIGN+ cell density compared with nephrectomies and native atrophic biopsies (Supplemental Figure 2A). When each cellular high-power field was separately assessed, DC-SIGN/CD3 clustering was not significant in native nephrectomies, was weak in each of native atrophic biopsies and allografts with TCMR, and was strong in each of native biopsies with interstitial nephritis and inflamed atrophic allograft biopsies (Supplemental Figure 2, B–E). This pilot assessment revealed that inflamed atrophic allografts have increased density of DCs, which cluster with infiltrating T cells.
We then moved to study the significance of DCs in allograft biopsies from an independent cohort of 105 kidney transplant recipients, who underwent “for-cause” allograft biopsies. Demographic, clinical, and pathologic characteristics of the cohort are presented in Table 2.
Table 2.
Patient demographic, clinical, and histologic characteristics
| Characteristic | Value (n=105) |
|---|---|
| Demographic and clinical | |
| Age (yr) | 52 (44, 62) |
| Women | 43/105 (41) |
| African-American race | 7/105 (7) |
| Deceased donors | 40/105 (38) |
| Post-transplant time (d) | 702 (81, 2190) |
| eGFR at biopsy (ml/min per 1.73 m2) | 26 (15, 38) |
| Proteinuria (dipstick 0–3) | 1.4±1.5 |
| Circulating DSAsa | 40/105 (38) |
| Histology | |
| Main diagnosis | |
| Mixed rejectionb | 22/105 (21) |
| TCMR (including borderline changes)c | 24/105 (23) |
| AMRd | 7/105 (7) |
| GNe | 8/105 (8) |
| ATI | 16/105 (15) |
| IFTA | 17/105 (16) |
| NSA | 2/105 (2) |
| Othersf | 9/105 (8) |
| C4d score (0–3) | 0.6±1.2 |
| Peritubular capillaritis (ptc) score (0–3) | 0.9±1.0 |
| Glomerulitis (g) score (0–3) | 0.5±0.8 |
| Interstitial inflammation (i) score (0–3) | 0.8±1.0 |
| Tubulitis (t) score (0–3) | 0.9±1.1 |
| Intimal arteritis (v) score (0–3) | 0.9±1.0 |
| Chronic transplant glomerulopathy (cg) score (0–3) | 0.2±0.5 |
| Interstitial fibrosis (ci) score (0–3) | 1.4±1.1 |
| Tubular atrophy (ct) score (0–3) | 1.4±1.1 |
| Arteriolar hyalinosis (ah) score (0–3) | 1.4±1.0 |
| Arterial fibrointimal (cv) score (0–3) | 1.5±1.0 |
| Inflammation in area of tubular atrophy (iatr) score (0–3) | 1.7±1.4 |
| Total inflammation (ti) score (0–3) | 1.4±1.1 |
Data are presented as the median (interquartile range), n (%), or mean±SD. AMR, antibody-mediated rejection; ATI, acute tubular injury; NSA, no significant abnormalities.
Although DSAs were assessed in 73 patients, the vast majority of patients for whom DSAs were not assessed had negative C4d and no histologic signs suggestive of AMR; see the Concise Methods).
Six of 22 (27%) patients had C4d− AMR, whereas the remaining had C4d+ AMR; 8 of 22 (36%) patients had borderline changes, whereas the remaining had TCMR grades ≥1A.
Fourteen of 24 (58%) patients had borderline changes, whereas the remaining had TCMR grades ≥1A.
Three of seven (43%) patients had C4d− AMR, whereas the remaining had C4d+ AMR.
Two of eight (25%) patients had recurrent GN.
Patients whose biopsies were labeled as “others” included vascular sclerosis (n=3), polyomavirus nephropathy (n=2), pyelonephritis (n=1), granulomatous interstitial nephritis (n=1), diabetic nephropathy (n=1), and interstitial inflammation without tubulitis (n=1).
DC-SIGN+ Cells Are Abundant in the Interstitium of Inflamed Allografts
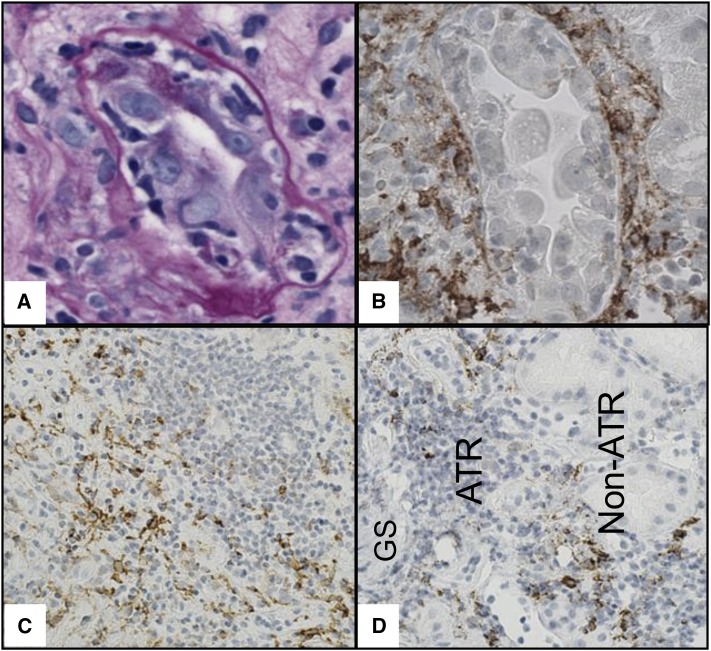
The anatomic distribution of different leukocytes within allograft microcompartments was assessed in a subset of 40 renal allograft biopsies. CD68+ cells were frequently encountered in all microcompartments. CD68+ cells present in glomerular capillaries, peritubular capillaries, tubules, and arterial walls did not show cytoplasmic protrusions. Prior reports revealed that these cells lack DC-SIGN expression and are likely to represent macrophages.7 On the other hand, many interstitial CD68+ cells displayed cytoplasmic protrusions and were expected to be DC-SIGN+ DCs as previously demonstrated.8 DC-SIGN labeled stellate-shaped cells with dendritic appearance, which were largely present in the interstitium especially around inflamed tubules and at the periphery of lymphoid aggregates spilling into the noninvolved parenchyma (Figure 2, Supplemental Figure 3). These cells were presumed to be DCs as previously reported.7,8 As an indirect way to assess pure macrophages (CD68+ DC-SIGN− cells), we subtracted DC-SIGN+ from CD68+ cell densities in each biopsy (CD68+−DC-SIGN+). Quantitative data revealed that CD68+–DC-SIGN+ cells were seen in all microcompartments (Supplemental Figure 4). Although CD3+ cells were also found in all microcompartments, they were most abundant within the interstitium. Quantitative data also confirmed that DC-SIGN+ cells were frequently observed in the interstitium, were occasionally encountered in the peritubular capillaries, and were almost nonexistent in the tubules, glomeruli, or arteries (Supplemental Figure 4). We therefore focused on the interstitium as the microcompartment of interest to further assess the significance of graft DCs.
Figure 2.
Representative images of the association of DC-SIGN+ cells with allograft inflammation and atrophy. (A) An inflamed tubule showing relatively thickened and wrinkled tubular basement membrane suggestive of an undergoing atrophy (periodic acid–Schiff staining). (B) On a different level, several DC-SIGN+ cells (brown) are distributed around the same inflamed tubule. (C) DC-SIGN+ cells (brown) are present at the periphery rather than the center of a lymphocytic aggregate. (D) Such DC-SIGN+ cells (brown) spill toward nonatrophic kidney allograft parenchyma. (B–D) DC-SIGN immunohistochemical staining. ATR, atrophic parenchyma; GS, a globally sclerosed glomerulus; Non-ATR, nonatrophic parenchyma. Original magnification ×600 in A and B; ×200 in C; ×400 in D.
DC-SIGN+ Cell Density Is Adversely Associated with Allograft Function and Survival
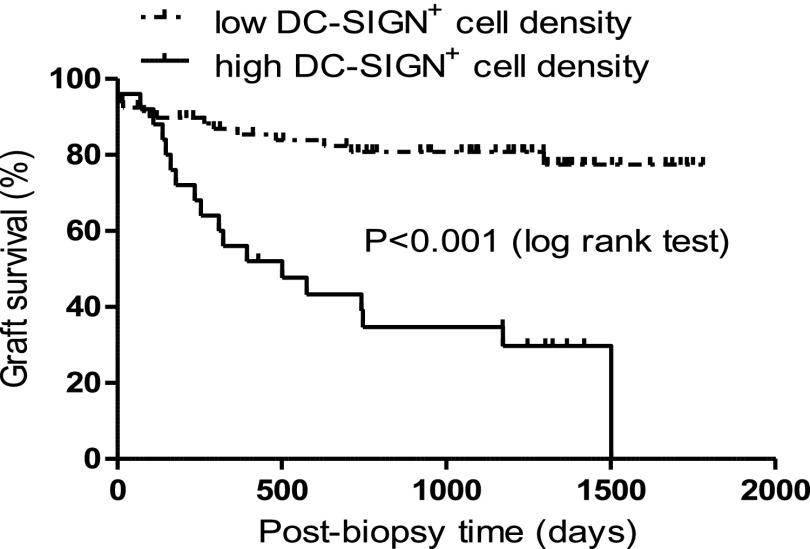
DC-SIGN+ cell density showed a negative correlation with eGFR at 1 year (r=−0.29, P=0.004) and 2 years (r=−0.36, P<0.001) after biopsy. In this cohort in which 33 graft losses were recorded during the follow-up period, patients with high DC-SIGN+ cell density (>3.8/hpf) had worse allograft survival than patients with low DC-SIGN+ cell density (<3.8/hpf, P<0.001) (Figure 3). We explored whether immunosuppressive treatment may have confounded DC effects on allograft outcome especially in patients with interstitial fibrosis/tubular atrophy (IFTA). Postbiopsy treatment was assessed in patients without IFTA, with IFTA/low DC-SIGN+ cell density, and with IFTA/high DC-SIGN+ cell density (Supplemental Table 1). There was no difference in the proportion of patients who received postbiopsy immunosuppression treatment among these groups. Similarly, prednisone maintenance did not have an effect on graft survival or DC-SIGN density (Supplemental Figure 5).
Figure 3.
Kaplan–Meier survival curves for postbiopsy cumulative kidney allograft survival in patients stratified according to DC-SIGN+ cell density (low DC-SIGN+ cell density [< 3.8/hpf; n=80] versus high DC-SIGN+ cell density [>3.8/hpf; n=25]).
We then investigated whether DC-SIGN+ cell density could independently predict poor allograft survival. Univariate analyses of demographic and clinical data identified recipient age, post-transplant time, eGFR at biopsy, proteinuria, and circulating donor-specific antibodies (DSAs) as clinical variables associated with suboptimal allograft survival (Table 3). Among the histologic variables, pathologic scores of peritubular capillaritis, chronic transplant glomerulopathy, interstitial fibrosis, tubular atrophy, arteriolar hyalinosis, arterial fibrointimal thickening, inflammation in areas of tubular atrophy, and total inflammation, as well as each of DC-SIGN+, CD68+−DC-SIGN+, and CD3+ cell densities, were associated with poor allograft survival (Table 3). Multivariate analyses revealed that DC-SIGN+ cell density, when included with clinical variables, but not with pathologic variables, was an independent predictor of allograft loss (Table 3). To examine whether IFTA by itself, rather than DC density, contributed to graft outcomes, we assessed an additional model adjusted for the five-aforementioned clinical variables that categorized patients in one of the following three groups: IFTA absent (reference group), IFTA/low DC-SIGN+ cell density, and IFTA/high DC-SIGN+ cell density (Table 4). The percentages of graft failure for participants within these three groups were 8%, 26%, and 70%, respectively. The data showed that there was clearly an independent risk of graft failure in patients with IFTA/high DC-SIGN+ cell density compared with IFTA absent (Table 4). In addition, the pairwise comparison of IFTA/low DC-SIGN+ cell density versus IFTA/high DC-SIGN+ cell density demonstrated a significant increased risk in the latter group (hazard ratio, 2.43; 95% confidence interval, 1.13 to 5.20; P=0.02).
Table 3.
Univariate analyses of the association of different variables with allograft survival followed by multivariate analyses of the associations of graft DC-SIGN+ cell density with graft survival after adjustment for other clinical or pathologic variables
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Univariate analyses (n=105) | ||
| Demography and clinical | ||
| Age (yr) | 0.96 (0.94 to 0.99) | 0.02 |
| Female sex | 1.10 (0.54 to 2.20) | 0.80 |
| African-American race | 0.86 (0.23 to 3.27) | 0.85 |
| Deceased donors | 0.68 (0.33 to 1.41) | 0.30 |
| Post-transplant time (mo) | 1.01 (1.00 to 1.01) | <0.001 |
| eGFR at biopsy (ml/min per 1.73 m2) | 0.94 (0.91 to 0.97) | <0.001 |
| Proteinuria (dipstick 0–3) | 2.11 (1.47 to 3.04) | <0.001 |
| Circulating DSAsa | 3.70 (1.21 to 11.30) | <0.001 |
| Histology | ||
| C4d score (0–3) | 1.02 (0.76 to 1.37) | 0.87 |
| Peritubular capillaritis (ptc) score (0–3) | 1.83 (1.29 to 2.59) | <0.001 |
| Glomerulitis (g) score (0–3) | 1.14 (0.76 to 1.70) | 0.52 |
| Interstitial inflammation (i) score (0–3) | 1.28 (0.92 to 1.78) | 0.15 |
| Tubulitis (t) score (0–3) | 1.16 (0.85 to 1.56) | 0.35 |
| Intimal arteritis (v) score (0–3) | 1.58 (0.89 to 2.79) | 0.12 |
| Chronic transplant glomerulopathy (cg) score (0–3) | 1.86 (1.30 to 2.67) | 0.001 |
| Interstitial fibrosis (ci) score (0–3) | 3.12 (2.09 to 4.66) | <0.001 |
| Tubular atrophy (ct) score (0–3) | 3.18 (2.12‒ 4.77) | <0.001 |
| Arteriolar hyalinosis (ah) score (0–3) | 2.44 (1.66 to 3.58) | <0.001 |
| Arterial fibrointimal (cv) score (0–3) | 1.56 (1.09 to 2.23) | 0.02 |
| Inflammation in area of tubular atrophy (iatr) score (0–3) | 1.88 (1.34 to 2.65) | <0.001 |
| Total inflammation (ti) score (0–3) | 3.01 (2.02 to 4.47) | <0.001 |
| DC-SIGN+ cell density (average cells/hpf) | 1.33 (1.15 to 1.55) | <0.001 |
| CD68+–DC-SIGN+ cell density (average cells/hpf) | 1.06 (1.01‒ 1.13) | 0.03 |
| CD3+ cell density (average cells/hpf) | 1.05 (1.03 to 1.07) | <0.001 |
| Multivariate analyses (n=105) | ||
| DC-SIGN+ cell density adjusted for clinical variablesb | 1.28 (1.07 to 1.53) | 0.01 |
| DC-SIGN+ cell density adjusted for histology variablesc | 0.97 (0.78 to 1.21) | 0.83 |
Thirty-three graft failures were recorded during the follow-up period.
95% CI, 95% confidence interval; HR, hazard ratio.
DSAs were assessed in 73 patients. For the purpose of the multivariate analysis, DSAs were considered negative in patients where DSAs were not assessed (the vast majority of these patients had negative C4d and no histologic signs suggestive of antibody-mediated rejection; see the Concise Methods).
Adjusted for age, post-transplant time, eGFR at biopsy, proteinuria, and DSAs.
Adjusted for ptc, cg, ci, ah, cv, iatr, and ti scores and for each of CD68+−DC-SIGN+ and CD3+ cell densities; ct scores were not included in the multivariate analysis because of collinearity with ci scores.
Table 4.
Multivariate analysis of the association between IFTA, DC-SIGN+ cell density, and allograft survival adjusted for the significant clinical variables
| Group | Patients (n) | Graft Failure (n) | Graft Failure (%) | HR (95% CI) | P Value |
|---|---|---|---|---|---|
| IFTA absent | 24 | 2 | 8 | Reference | Reference |
| IFTA and low DC-SIGN+ cell density | 58 | 15 | 26 | 2.90 (0.62 to 13.66) | 0.18 |
| IFTA and high DC-SIGN+ cell density | 23 | 16 | 70 | 7.06 (1.48 to 33.55) | 0.01 |
The analysis was adjusted for the same demographic and clinical variables as in Table 3 (age, post-transplant time, eGFR at biopsy, proteinuria, and circulating DSAs).
95% CI, 95% confidence interval; HR, hazard ratio.
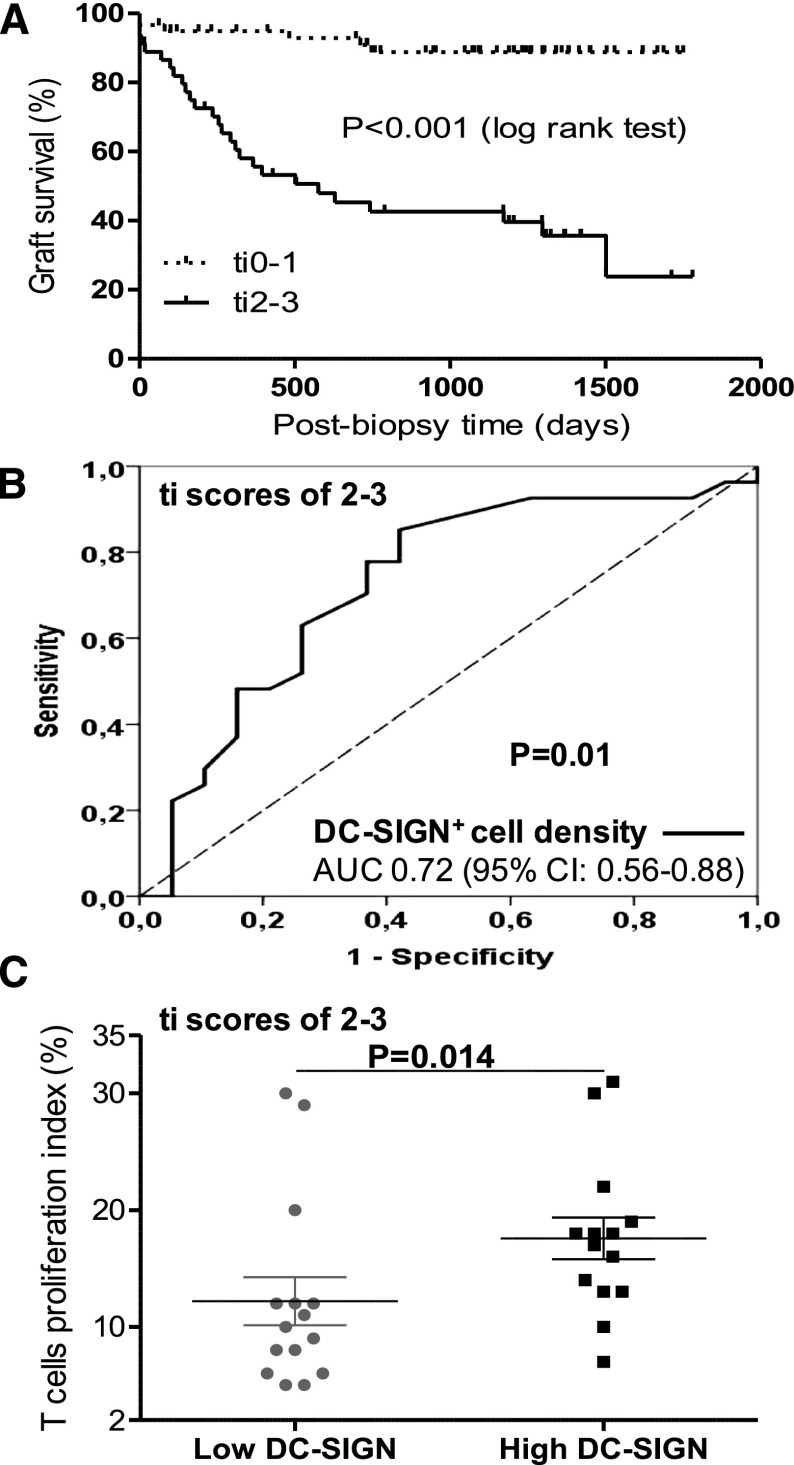
DC-SIGN+ Cell Density Predicts Poor Allograft Survival in Patients with High Total Inflammation Scores and Is Associated with T Cell Proliferation
Total inflammation, which includes inflammation in areas of tubular atrophy, may potentially represent a broader spectrum of TCMR than what is currently recognized by Banff.25,26 Therefore, we were interested in studying the potential significance of DCs in biopsies with a high burden of total inflammation. We first confirmed the findings of Mengel et al.,25 namely that high total inflammation scores (ti2–ti3) portend worse allograft survival compared with low total inflammation scores (ti0–ti1) (Figure 4A). In addition, using the receiver operating characteristic curve, we showed that DC-SIGN+ cell density was a significant predictor of allograft loss in patients with high total inflammation scores (Figure 4B). To explore the association of graft DCs with T cell proliferation, biopsies with high total inflammation scores that had residual tissue (n=29) were double immunostained for Ki67 and CD3. Biopsies with high DC-SIGN+ cell density had higher labeling indices of Ki-67 in infiltrating CD3+ cells (Figure 4C), which also correlated significantly with DC-SIGN+ cell density (r=0.45, P=0.01).
Figure 4.
Total inflammation scores and graft survival followed by the association of DC-SIGN+ cell density with graft survival and T cell proliferation in biopsies with high total inflammation score. (A) Kaplan–Meier survival curves for postbiopsy cumulative kidney allograft survival in patients stratified according to total inflammation scores (low scores [ti0–ti1; n=59] versus high scores [ti2–ti3; n=46]). (B) Receiver operating characteristic DC-SIGN curve in patients with high total inflammation scores (ti2–ti3) showing the effect of DC-SIGN+ cell density on postbiopsy allograft survival. (C) T cell proliferation indices in biopsies with high versus low DC-SIGN+ density, which have residual material to perform immunohistochemical staining and analysis (n=29) (17.5% [13.3, 18.8], n=14; versus 10% [7, 12], n=15; P=0.01). 95% CI, 95% confidence interval; AUC, area under the curve.
The Effect of Graft DCs on Allograft Loss Is Likely the Result of an In Situ Immune Response
Because DC-SIGN was identified as one of the histologic variables associated with allograft loss, we aimed to understand the relation between DC-SIGN+ cells and the other histologic variables associated with poor prognosis. Multivariate linear regression identified CD3+ cell density as the single histologic variable significantly associated with DC-SIGN+ cell density independent of other histologic variables (Table 5).
Table 5.
Multivariate associations between DC-SIGN+ cell density and other histologic variables that affect graft survival
| Variable | B | P Value |
|---|---|---|
| Peritubular capillaritis (ptc) score (0–3) | 0.12 | 0.44 |
| Arterial fibrointimal (cv) score (0–3) | −0.04 | 0.88 |
| Chronic transplant glomerulopathy (cg) score (0–3) | 0.21 | 0.32 |
| Interstitial fibrosis (ci) score (0–3) | 0.01 | 0.96 |
| Arteriolar hyalinosis (ah) score (0–3) | −0.65 | 0.49 |
| Inflammation in area of tubular atrophy (iatr) score (0–3) | 0.01 | 0.98 |
| Total inflammation (ti) score (0–3) | 0.31 | 0.09 |
| CD68–DC-SIGN cells (average cells/hpf) | −0.02 | 0.39 |
| CD3+ cells (average cells/hpf) | 0.04 | 0.01 |
ct scores were not included in the multivariate analysis because of collinearity with ci scores.
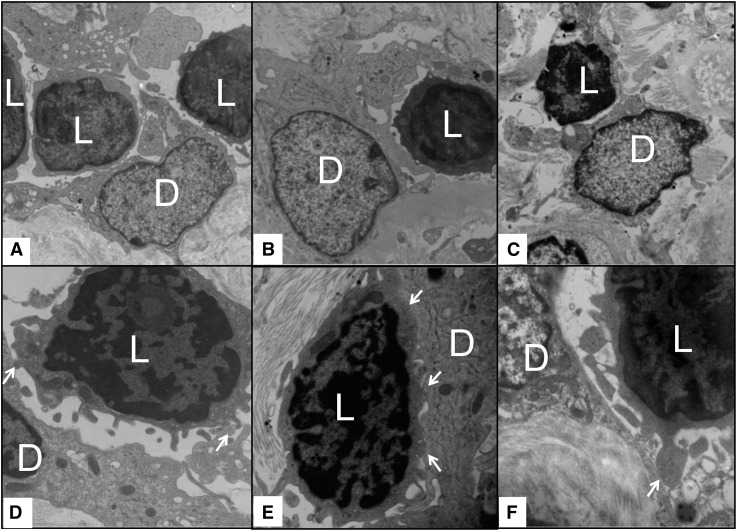
To further dissect the relation between lymphocytes and DCs, biopsies with high DC-SIGN+ cell density that had material submitted for ultrastructural studies were reassessed for lymphocytes and DCs. Despite the small size of the tissue portions submitted for electron microscopy evaluation, 12 of 16 (75%) of these samples showed direct physical contact between infiltrating lymphocytes and cells that have morphologic characteristics of kidney DCs (Figure 5). Taken together, these data suggest that graft DC effects on allograft survival might be mediated by an in situ stimulation of T cells.
Figure 5.
Representative electron microscopy images from six different biopsies with high DC-SIGN+ cell density (>3.8/hpf) showing physical association between lymphocytes, which are characterized by their small size and very small rim of cytoplasm around the nuclei and cells that have morphologic characteristics of kidney DCs with round nuclei, light-appearing cytoplasmic projections, and lack of abundant ribosomes or lysosomes. (A–C) In the upper panels, relatively lower power images show cytoplasmic projections of the presumed DCs surrounding and touching infiltrating lymphocytes. (D–F) The lower panels illustrate close-up views of the physical association (arrows) between lymphocytes and cells with morphologic characteristics of dendritic cells. D, dendritic cell; L, lymphocyte. Original magnification, ×10,000 in A–C; ×20,000 in D–F.
Pathologic Correlates of DC-SIGN+ Cells
After showing that graft DC density is a poor prognostic factor associated with T cell infiltration and proliferation, we moved our attention to study the pathologic correlates of graft DCs with regard to chronic changes, rejection, and nonalloimmune conditions. DC-SIGN+ cell density showed weak correlation with Banff scores of most of chronic changes, peritubular capillaritis, interstitial inflammation, tubulitis, and intimal arteritis (Table 6). The last three are characteristics of TCMR. Notably, DC-SIGN+ cell density also correlated moderately with scores for inflammation in areas of tubular atrophy (r=0.47, P<0.001). In samples with inflammation in areas of tubular atrophy (n=65), TCMR tended to be more frequent in biopsies with high compared with low DC-SIGN+ cell density (14 of 22 [64%] versus 17 of 43 [40%], P=0.07). With regard to antibody-mediated rejection, DC-SIGN+ cell density did not correlate with C4d scores (Table 6) but tended to be higher in patients with detectable DSAs compared with these with negative or nonstudied DSAs (3.1 [2.1, 4] versus 2.6 [1.6, 3.3] cells/hpf; P=0.05). In patients with biopsies showing nonalloimmune inflammatory conditions (polyomavirus nephropathy [n=2], pyelonephritis [n=1], GN [n=2; lupus nephritis and IgA nephropathy]), only the patient with recurrent IgA nephropathy had a high DC-SIGN+ cell density. Interestingly, this was the only patient in this small subset who developed graft failure during follow-up. Finally, we turned our attention to the single case of granulomatous interstitial nephritis in our cohort. This case had low DC-SIGN+ but very high CD68+ cell densities (1.8 and 24.9 cells/hpf, respectively), supporting the notion that the DC-SIGN marker is fairly specific for DCs and does not stain macrophages.
Table 6.
Univariate pathologic correlates of DC-SIGN+ cell density
| Variable | Spearman’s r | P Value |
|---|---|---|
| C4d score (0–3) | 0.13 | 0.18 |
| Peritubular capillaritis (ptc) score (0–3) | 0.38 | <0.001 |
| Glomerulitis (g) score (0–3) | 0.16 | 0.10 |
| Interstitial inflammation (i) score (0–3) | 0.37 | <0.001 |
| Tubulitis (t) score (0–3) | 0.31 | 0.002 |
| Intimal arteritis (v) score (0–3) | 0.29 | 0.003 |
| Chronic transplant glomerulopathy (cg) score (0–3) | 0.31 | 0.001 |
| Interstitial fibrosis (ci) score (0–3) | 0.38 | <0.001 |
| Tubular atrophy (ct) score (0–3) | 0.35 | <0.001 |
| Arteriolar hyalinosis (ah) score (0–3) | 0.30 | 0.002 |
| Arterial fibrointimal (cv) score (0–3) | 0.24 | 0.01 |
| Inflammation in area of tubular atrophy (iatr) score (0–3) | 0.47 | <0.001 |
| Total inflammation (ti) score (0–3) | 0.54 | <0.001 |
| CD3+ cells (average cells/hpf) | 0.62 | <0.001 |
In Late Allograft Biopsies, DC-SIGN+ Cells Are Largely of Recipient Origin
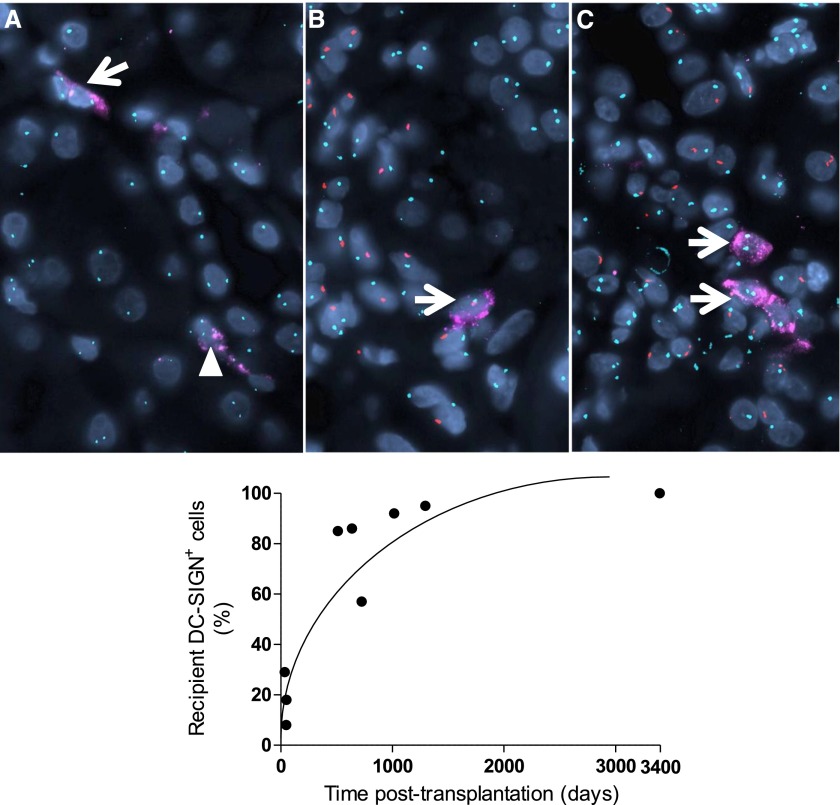
To explore the source of graft DCs, DC-SIGN staining and fluorescence in situ hybridization (FISH) for XY chromosomes were performed on nine biopsies from sex-mismatched recipients. Whereas donor DC-SIGN+ cells predominated in early and noninflamed biopsies, DC-SIGN+ cells, which clustered with inflammatory cells, were largely of recipient origin especially at later time points (Figure 6, Supplemental Table 2).
Figure 6.
Representative immunofluorescence images and a graph summarizing DC source in sex-mismatched allograft biopsies. The upper panels show representative images of DC-SIGN immunostaining and XY FISH. In these images, DC-SIGN is labeled in pink, centromeres of X and Y chromosomes are labeled in green and red, respectively, whereas 4′,6-diamidino-2-phenylindole nuclear stain is blue. (A) This biopsy is performed 50 days after transplantation and shows no significant pathologic changes. The donor is female and the recipient is male. Tubular epithelial cells are stained for XX (donor origin). In this field, one DC-SIGN+ cell stains for XX (donor origin, arrowhead), whereas the other stains for XY (recipient origin, arrow). (B) This biopsy is performed 1295 days after transplantation and shows interstitial fibrosis and tubular atrophy according to Banff criteria with inflammation observed within the foci of atrophy. The donor is female and the recipient is male. Tubular epithelial cells are stained for XX (donor origin). The DC-SIGN+ cell in this field stains for XY (recipient origin), and is surrounded by infiltrating XY recipient leukocytes. (C) This biopsy is performed 3395 days after transplantation and shows TCMR with additional inflammation seen within the foci of atrophy. In this case, the donor is male and the recipient is female. Tubular epithelial cells are stained for XY (donor origin). DC-SIGN+ cells in this field stain for XX (donor origin) and cluster with infiltrative XX recipient leukocytes (original magnifications ×600). The lower panel graph demonstrates the percentage of recipient DC-SIGN+ cells plotted against time after transplantation. The detailed characteristics of the patients are presented in Supplemental Table 2. Original magnification, ×600.
Discussion
DCs are professional antigen-presenting cells, which are well positioned to orchestrate inflammatory responses by linking the innate and the adaptive arms of the immune system.27,28 In humans, kidney DCs can be either myeloid or plasmacytoid; the former are more abundant in steady-state as well as in inflammatory conditions, including rejection.8,9 A potential role of myeloid kidney DCs in shaping renal inflammation and/or prognosis has been proposed in several kidney diseases including ANCA-associated GN,29 IgA nephropathy,30 lupus nephritis,31 and other proliferative GN.7 In the transplant setting, our understanding of the significance of DCs in kidney injury is further limited to a few studies.8,9,21
As such, a systematic and detailed assessment of myeloid DCs in the kidney allograft biopsies would have the potential to enhance our understanding of the pathophysiologic as well as the prognostic aspects of allograft inflammation. In kidney, DC-SIGN is a protein that labels activated myeloid DCs with high T cell stimulation capability.20 By performing double immunostaining for DC-SIGN/BDCA-1 in kidney frozen samples, Woltman et al.8 confirmed the reliability of DC-SIGN as a marker of myeloid kidney DCs by showing that all DC-SIGN+ cells expressed BDCA-1. In this report, we observed DC-SIGN staining in elongated cells with cytoplasmic protrusions that were mainly present in the interstitium. DCs density and clustering with T cells were first assessed in 47 allograft and native kidney biopsies. Compared with native atrophic biopsies, we found inflamed atrophic allograft biopsies to have higher density of DCs, which showed stronger correlation with infiltrating T cells in each high-power field. We then assessed graft DCs in allograft biopsies from an independent cohort of 105 kidney transplant recipients who had relatively long-term follow-up. Although the effect of kidney DCs on allograft survival has not been assessed before, a prior study has shown that DCs density in rejected allograft biopsies could predict subsequent allograft dysfunction.9 We found that DCs density was able to predict poor allograft survival independent of clinical variables.
Total inflammation of the allograft, which includes inflammation in areas of tubular atrophy, has similar proinflammatory transcription profiles but worse prognosis than conventional TCMR, which ignores inflammation in nonatrophic areas.25 In addition to confirming the worse prognostic significance of increased total inflammation, we have shown that DC density could predict allograft loss in biopsies with high total inflammation scores. Our results suggest that, in addition to the total burden of allograft inflammation, assessing the amount of DCs may further add to the prognostic data that can be extracted from evaluating allograft biopsies.
To further understand the histologic reasons behind the association of graft DCs with poor prognosis, a multivariate analysis was performed. CD3+ cell density was identified as the single histologic variable significantly associated with DC density (Table 3). Furthermore, DC density correlated with the T cell proliferation index, whereas electron microscopy evaluation showed physical contact of lymphocytes with cells that have the morphologic characteristics of kidney DCs. Taken together, our data suggest that the poor prognostic effects of graft DCs may be related to an interaction between DCs and T cells within the graft to promote inflammation and atrophy. Additional studies are needed to confirm these observations and to further dissect the association between graft DCs and each of CD4+ and CD8+ cells. Notably, recent experimental studies have proved that alloreactive naive T cells can be primed within the lung allograft, likely through antigen presentation by DCs.32,33 Moreover, investigators have observed that DCs cluster with infiltrating T lymphocytes in allograft biopsies from heart transplant patients.34
In addition to the correlation with T cells, DCs density correlated weakly with Banff scores for TCMR but moderately with scores for inflammation in areas of tubular atrophy. The latter finding, together with the observation that conventional TCMR tended to be more frequent in biopsies with inflammation in areas of tubular atrophy and high versus low DCs density support that inflammation in areas of tubular atrophy may represent an unrecognized form of TCMR especially in patients with high DC density. Such areas of inflammation and atrophy may not be quiescent, but rather immunologically dynamic milieu that triggers persistent cycles of lymphocyte stimulation.
Although classic studies stressed the importance of donor and recipient DCs in early versus late rejection, respectively,35–37 recent studies showed that donor DCs have an extended life span after transplantation38 and may even provoke chronic rejection.39 With regard to kidney DCs, very little is known about their life span; 10 weeks after kidney transplantation in the rat, donor kidney DCs could still identified.40 To underscore the source of graft DCs, XY FISH was performed on DC-SIGN–stained biopsies from nine sex-mismatched recipients. Although occasional donor DCs remained present in the allograft a long time after transplantation, the majority of DCs that clustered with infiltrating recipient leukocytes were of recipient origin. Our preliminary assessment in this small sample suggests that recruited recipient DCs might play an important role in shaping chronic allograft inflammation, which may be via indirect or probably via semidirect presentation, in which recipient DCs can acquire donor MHC.41,42
Limitations of this study include its retrospective nature and the lack of mechanistic studies to confirm our findings. However, our data highlight the prognostic significance of kidney DCs in allograft biopsies and suggest an important role for recruited DCs in shaping allograft inflammation, especially within foci of atrophy. Diagnostically, our results would support further extension of the current Banff definition of TCMR to include inflammation in areas of tubular atrophy especially in the presence of high DCs density. Therapeutically, this work could provoke research to target DCs influx to the kidney in an attempt to improve long-term allograft survival.
Concise Methods
This retrospective multistep study (Figure 1) was performed under the approved guidelines of the institutional review board. For the purpose of this study, kidney DCs were defined by circumferential DC-SIGN staining or characteristic electron microscopy features.
Development of Methodology and Pilot Study
To define the cutoff value of biopsies with high DC-SIGN+ cell density and explore the potential association of graft DCs and T cells, a pilot analysis was conducted on 47 nonstudy kidney biopsies (performed in 2013–2014): 5 nephrectomies (non-neoplastic areas of kidneys resected for renal neoplasms of which histologic assessment revealed no significant changes), 17 severely atrophic native biopsies (vascular disease or diabetic nephropathy with >50% parenchymal atrophy), 5 native biopsies with interstitial nephritis, 11 allografts with conventional TCMR, and 9 severely atrophic allografts with inflammation (>50% parenchymal atrophy). Immunohistochemical double staining for DC-SIGN and CD3 was performed on FFPE tissue.
Main Cohort
We then studied all patients who underwent “for-cause” kidney allograft biopsies obtained more than 15 days after transplantation between February 2009 and October 2010 at the Brigham and Women’s Hospital and had sufficient remnant biopsy tissue to perform immunohistochemical analysis. When multiple biopsies from a single patient were performed, only the first biopsy “index biopsy” was analyzed. Biopsies were assessed by light microscopy, immunofluorescence, immunohistochemistry, and, when available, by electron microscopy.
The study included 105 biopsies from 105 kidney transplant recipients, the majority of whom received induction therapy with thymoglobulin and were maintained on calcineurin inhibitors and antimetabolite therapy with or without prednisone. After the biopsy, patients were followed for a median of 32 months (interquartile range 1 and 3 quartiles, 8.5, 43 months) and were either event-censored (allograft failure) or censored at the end of the follow-up. eGFR was calculated using the four-value Modification of Diet in Renal Disease equation.43 Around the time of biopsy, DSAs were assessed for 73 patients using the Luminex single-antigen bead assay (One Lambda Inc., Canoga Park, CA). The patients for whom DSAs were not assessed (n=32) did not show histologic signs suspicious for antibody-mediated rejection, with the exception of one biopsy that showed focal C4d and two biopsies with significant microvascular inflammation (glomerulitis score [g]+peritubular capillaritis score [ptc]≥2).
Histologic parameters were evaluated according to the Banff criteria for renal allograft pathology.26,44–46 TCMR was defined as the presence borderline changes or above (IA, IB, IIA, IIB, or III TCMR) according to Banff criteria. The scores for inflammation in areas of tubular atrophy were assessed per Mannon et al.24 and a score of zero was given for nonatrophic biopsies. Total inflammation scores were assessed per Mengel et al.25 IFTA was categorized as absence when no interstitial fibrosis or tubular atrophy were seen (tubular atrophy score [ct]+interstitial fibrosis score [ci]=0).
C4d was assessed in all allograft biopsies by immunofluorescence microscopy (n=103) or rarely by immunohistochemical studies (n=2; one showed diffuse staining and the other was completely negative). Full panel immunofluorescence staining and electron microscopy evaluation were performed on 101 (96%) and 71 (68%) of the biopsies, respectively. Biopsies with high DC-SIGN+ cell density that had available material submitted for electron microscopy studies (n=16) were rescoped to evaluate the ultrastructural association of kidney DCs and lymphocytes. Immunohistochemical DC-SIGN staining and CD68/CD3 double staining were performed on all biopsies (n=105) and the average number of positively stained cells in the allograft cortex were determined (see the Supplemental Material for detailed methods). Histopathologic and immunohistochemical evaluation of the biopsies was done by one observer (I.B.) to eliminate reproducibility as a confounding factor in this study.
Exploring the Source of Graft DCs
To explore the source of DCs in the allograft, DC-SIGN immunohistochemical staining followed by XY FISH was performed on nine allograft biopsies from sex-mismatched cases (see the Supplemental Material for detailed methods).
Statistical Analyses
Statistical analysis was performed using Stata 11 (StataCorp., College Station, TX) and Prism 5 2007 (GraphPad Inc., San Diego CA) software. Semiquantitative values were presented as the mean±SD, whereas continuous data were presented as the median and interquartile range (25th–75th percentiles). To obtain a stringent cutoff for abnormally high DC-SIGN+ cell density, the cutoff value of >2 SDs from the mean of DC-SIGN+ cell density in nephrectomy samples was used.
Categorical variables were compared using chi-squared or Fisher’s exact tests, as appropriate. Continuous variables were compared using Mann–Whitney U or ANOVA tests followed by Tukey’s multiple comparison tests, as appropriate. Correlations were performed using Spearman correlation, where r values of 0.2–0.39, 0.4–0.59, or ≥0.6 were considered weak, moderate, or strong correlations, respectively. Multivariate linear regression models were performed to evaluate the adjusted association between DC-SIGN+ cell density and other histologic variables.
Graft survival was assessed by Kaplan–Meier and comparisons were performed using log-rank test and proportional hazards models. Because of the large number of correlated covariates of interest, we performed univariate proportional hazards for several clinical and pathologic variables to guide the selection of variables for multivariate analysis. Area under the curve was also used to evaluate the ability of DC-SIGN+ cell density to discriminate patients with graft loss during follow-up. P values <0.05 with a two-sided hypothesis testing were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Terri Woo and the Immunohistochemical Laboratories at the Brigham and Women’s Hospital for the excellent technical assistance.
This work was supported in part by a American Society of Transplantation Clinical Science Fellowship Award and Pathological Society Small Grant Scheme (to I.B.) and by a grant from the Clinical Trials in Organ Transplantation cooperative research program through the National Institutes of Health (U01-AI 063623 to N.N.). S.A.D.S. is the recipient of a Kidney Research Scientist Core Education and National Training Program New Investigator Award from Canadian Institutes of Health Research (KRI123890) and holds a scholarship from the Fonds de recherche du Québec–Santé. T.U. is a recipient of the American Heart Association Scientist Development Grant (11SDG5150000). TMN is the recipient of National Institutes of Health grant (R01-DK099507).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080804/-/DCSupplemental.
References
- 1.Womer KL, Kaplan B: Recent developments in kidney transplantation—a critical assessment. Am J Transplant 9: 1265–1271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM: Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol 30: 1–22, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Krüger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Müller-Newen G, Floege J, Kurts C: Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M, Immunological Genome Consortium : Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol 13: 888–899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, Rogers NC, Moncaut N, Carvajal JJ, Reis e Sousa C: Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 154: 843–858, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Segerer S, Heller F, Lindenmeyer MT, Schmid H, Cohen CD, Draganovici D, Mandelbaum J, Nelson PJ, Gröne HJ, Gröne EF, Figel AM, Nössner E, Schlöndorff D: Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int 74: 37–46, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Woltman AM, de Fijter JW, Zuidwijk K, Vlug AG, Bajema IM, van der Kooij SW, van Ham V, van Kooten C: Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int 71: 1001–1008, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zuidwijk K, de Fijter JW, Mallat MJ, Eikmans M, van Groningen MC, Goemaere NN, Bajema IM, van Kooten C: Increased influx of myeloid dendritic cells during acute rejection is associated with interstitial fibrosis and tubular atrophy and predicts poor outcome. Kidney Int 81: 64–75, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann U, Bergler T, Segerer S, Rümmele P, Krüger B, Banas MC, Reinhold S, Banas B, Krämer BK: Impact of chemokine receptor CX3CR1 in human renal allograft rejection. Transpl Immunol 23: 204–208, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Kaissling B, Le Hir M: Characterization and distribution of interstitial cell types in the renal cortex of rats. Kidney Int 45: 709–720, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Kaissling B, Le Hir M: The renal cortical interstitium: Morphological and functional aspects. Histochem Cell Biol 130: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaissling B, Hegyi I, Loffing J, Le Hir M: Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 193: 303–318, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Gijzen K, Tacken PJ, Zimmerman A, Joosten B, de Vries IJ, Figdor CG, Torensma R: Relevance of DC-SIGN in DC-induced T cell proliferation. J Leukoc Biol 81: 729–740, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG: Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 106: 1278–1285, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hesse C, Ginter W, Förg T, Mayer CT, Baru AM, Arnold-Schrauf C, Unger WW, Kalay H, van Kooyk Y, Berod L, Sparwasser T: In vivo targeting of human DC-SIGN drastically enhances CD8⁺ T-cell-mediated protective immunity. Eur J Immunol 43: 2543–2553, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG: Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100: 575–585, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B: Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol 71: 445–457, 2002 [PubMed] [Google Scholar]
- 19.Alberts-Grill N, Denning TL, Rezvan A, Jo H: The role of the vascular dendritic cell network in atherosclerosis. Am J Physiol Cell Physiol 305: C1–C21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figel AM, Brech D, Prinz PU, Lettenmeyer UK, Eckl J, Turqueti-Neves A, Mysliwietz J, Anz D, Rieth N, Muenchmeier N, Buchner A, Porubsky S, Siegert SI, Segerer S, Nelson PJ, Noessner E: Human renal cell carcinoma induces a dendritic cell subset that uses T-cell crosstalk for tumor-permissive milieu alterations. Am J Pathol 179: 436–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loverre A, Capobianco C, Stallone G, Infante B, Schena A, Ditonno P, Palazzo S, Battaglia M, Crovace A, Castellano G, Ranieri E, Schena FP, Gesualdo L, Grandaliano G: Ischemia-reperfusion injury-induced abnormal dendritic cell traffic in the transplanted kidney with delayed graft function. Kidney Int 72: 994–1003, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Randhawa P: The “borderline” renal allograft biopsy in the era of molecular diagnostics: A sampling conundrum? Am J Transplant 12: 11–12, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Drachenberg CB, Papadimitriou JC, Hirsch HH, Wali R, Crowder C, Nogueira J, Cangro CB, Mendley S, Mian A, Ramos E: Histological patterns of polyomavirus nephropathy: Correlation with graft outcome and viral load. Am J Transplant 4: 2082–2092, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D, DeKAF Investigators : Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant 10: 2066–2073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mengel M, Reeve J, Bunnag S, Einecke G, Jhangri GS, Sis B, Famulski K, Guembes-Hidalgo L, Halloran PF: Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant 9: 1859–1867, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Nelson PJ: Renal ischemia-reperfusion injury: Renal dendritic cells loudly sound the alarm. Kidney Int 71: 604–605, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Land WG: Emerging role of innate immunity in organ transplantation part II: Potential of damage-associated molecular patterns to generate immunostimulatory dendritic cells. Transplant Rev (Orlando) 26: 73–87, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Wilde B, van Paassen P, Damoiseaux J, Heerings-Rewinkel P, van Rie H, Witzke O, Tervaert JW: Dendritic cells in renal biopsies of patients with ANCA-associated vasculitis. Nephrol Dial Transplant 24: 2151–2156, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Pei G, Zeng R, Han M, Liao P, Zhou X, Li Y, Zhang Y, Liu P, Zhang C, Liu X, Yao Y, Xu G: Renal interstitial infiltration and tertiary lymphoid organ neogenesis in IgA nephropathy. Clin J Am Soc Nephrol 9: 255–264, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiore N, Castellano G, Blasi A, Capobianco C, Loverre A, Montinaro V, Netti S, Torres D, Manno C, Grandaliano G, Ranieri E, Schena FP, Gesualdo L: Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol Immunol 45: 259–265, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, Kornfeld CG, Kreisel FH, Sugimoto S, Tietjens JR, Dempster J, Patterson GA, Krupnick AS, Miller MJ, Kreisel D: Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol 182: 3969–3973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelman AE, Okazaki M, Sugimoto S, Li W, Kornfeld CG, Lai J, Richardson SB, Kreisel FH, Huang HJ, Tietjens JR, Zinselmeyer BH, Patterson GA, Miller MJ, Krupnick AS, Kreisel D: CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. Am J Transplant 10: 1189–1199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kofler S, Petrakopoulou P, Grimm C, Kaczmarek I, Meiser BM, Weis M: Graft-infiltrating dendritic cells and coronary endothelial dysfunction after human heart transplantation. J Heart Lung Transplant 27: 387–393, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Kelly CM, Benham AM, Sawyer GJ, Dalchau R, Fabre JW: A three-cell cluster hypothesis for noncognate T-B collaboration via direct T cell recognition of allogeneic dendritic cells. Transplantation 61: 1094–1099, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Vella JP, Vos L, Carpenter CB, Sayegh MH: Role of indirect allorecognition in experimental late acute rejection. Transplantation 64: 1823–1828, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Rogers NJ, Lechler RI: Allorecognition. Am J Transplant 1: 97–102, 2001 [PubMed] [Google Scholar]
- 38.Celli S, Albert ML, Bousso P: Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med 17: 744–749, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Jurewicz M, Ueno T, Azzi J, Tanaka K, Murayama T, Yang S, Sayegh MH, Niimi M, Abdi R: Donor antioxidant strategy prolongs cardiac allograft survival by attenuating tissue dendritic cell immunogenicity(†). Am J Transplant 11: 348–355, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Ozaki KS, Kimura S, Nalesnik MA, Sico RM, Zhang M, Ueki S, Ross MA, Stolz DB, Murase N: The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngeneic kidney transplantation. Kidney Int 81: 1015–1025, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI: A novel pathway of alloantigen presentation by dendritic cells. J Immunol 173: 4828–4837, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Afzali B, Lombardi G, Lechler RI: Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant 13: 438–444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff '09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.