Abstract
Inappropriate activation of the type 1A angiotensin (AT1A) receptor contributes to the pathogenesis of hypertension and its associated complications. To define the role for actions of vascular AT1A receptors in BP regulation and hypertension pathogenesis, we generated mice with cell-specific deletion of AT1A receptors in smooth muscle cells (SMKO mice) using Loxp technology and Cre transgenes with robust expression in both conductance and resistance arteries. We found that elimination of AT1A receptors from vascular smooth muscle cells (VSMCs) caused a modest (approximately 7 mmHg) yet significant reduction in baseline BP and exaggerated sodium sensitivity in mice. Additionally, the severity of angiotensin II (Ang II)–dependent hypertension was dramatically attenuated in SMKO mice, and this protection against hypertension was associated with enhanced urinary excretion of sodium. Despite the lower BP, acute vasoconstrictor responses to Ang II in the systemic vasculature were largely preserved (approximately 80% of control levels) in SMKO mice because of exaggerated activity of the sympathetic nervous system rather than residual actions of AT1B receptors. In contrast, Ang II–dependent responses in the renal circulation were almost completely eliminated in SMKO mice (approximately 5%–10% of control levels). These findings suggest that direct actions of AT1A receptors in VSMCs are essential for regulation of renal blood flow by Ang II and highlight the capacity of Ang II–dependent vascular responses in the kidney to effect natriuresis and BP control.
Keywords: angiotensin, blood pressure, renal hemodynamics, vascular
Dysregulation of the renin angiotensin system (RAS) RAS is a common feature of human hypertension. As such, RAS antagonists lower BP in most patients with hypertension.1,2 The predominant actions of the RAS to influence BP are mediated by angiotensin II (Ang II) acting via the type 1 angiotensin (AT1) receptor, a member of the large family of G-protein coupled receptors. AT1 receptors are the target of Ang receptor blockers, effective and widely used antihypertensive agents. Activation of AT1 receptors can trigger a cascade of physiologic responses, including vasoconstriction, activation of the sympathetic nervous system, and stimulation of sodium reabsorption by the kidney, which can conspire to promote hypertension and end-organ damage.3 However, until recently, it has been difficult to unravel the relative contributions of these distinct AT1 receptor responses to the pathogenesis of hypertension.
Using kidney cross-transplantation, our group previously demonstrated that actions of the major murine AT1 receptor isoform, AT1A, in both the kidney and systemic tissues, are important for maintenance of basal BP,4 whereas AT1A receptors in the kidney play the predominant role in the development of Ang II–dependent hypertension and cardiac hypertrophy.5 The precise cell lineages responsible for these effects are now being elucidated. For example, we and others have shown that AT1A receptors in the renal proximal tubule play an important role in Ang II–dependent hypertension by augmenting renal sodium reabsorption through activation of key sodium transporter, including NHE3.6,7 However, eliminating AT1A receptors in renal epithelia only partially recapitulated the actions of Ang receptor blockers to abrogate hypertension. Along with kidney epithelia, AT1 receptors are broadly expressed on the renal vasculature. Previous studies have demonstrated that inappropriate activation of signaling pathways in the vasculature can potentiate chronic hypertension,8–10 and it has been suggested that vascular dysfunction alone may lead to BP dysregulation.9 For example, Guilluy et al. demonstrated that eliminating Arhgef1, a Rho-exchange factor linked to AT1 receptor signaling, from vascular smooth muscle cells (VSMCs) caused dramatic abrogation of hypertension.8 These authors concluded that activation of Arhgef1 triggered by AT1 receptors in VSMCs caused systemic vasoconstriction, leading to increased systemic vascular resistance and hypertension. On the other hand, vasoconstriction in the kidney circulation can also effect BP control; changes in renal blood flow (RBF) affect sodium reabsorption along the nephron through changes in Starling forces and physical factors.11 Moreover, because approximately 25% of the arterial blood flow enters the renal circulation, vasoconstriction in the kidney may raise BP through effects on systemic vascular resistance. Therefore, to define the precise contribution of actions of vascular AT1A receptors to BP control in vivo, we used Cre-LoxP technology to generate mice with cell-specific deletion of AT1A receptors from smooth muscle cell lineages in vasculature beds throughout the body, including the kidney. We found that AT1A receptors in VSMCs maintain basal BP, augment salt sensitivity, and contribute to the pathogenesis of Ang II–induced hypertension by reducing RBF, thereby enhancing sodium retention.
Results
Generation of Mice with Cell-Specific Deletion of AT1 Receptors in VSMCs
We generated mice with cell-specific deletion of the AT1A receptor from smooth muscle cell lineages by using the KISm22α-Cre mouse line in which Cre-recombinase has been knocked in to the Sm22α gene locus such that its expression is regulated by endogenous regulatory elements of the Sm22α gene. We carried out successive intercrosses between the KISm22α-Cre mouse line and mice homozygous for the conditional floxed Agtr1a allele (Agtr1aflox/flox)7 to generate KISm22α Cre+-Agtr1aflox/flox (SMKO) and littermate Cre-negative control mice (control) for experiments. Using the mTmG dual-reporter mouse line,12 we have previously demonstrated robust green fluorescent protein fluorescence in the media of the aorta and throughout glomerular afferent arterioles in KISm22α-Cre-mTmG mice, confirming robust expression of Cre-recombinase in large arteries and resistance vessels.13
To document efficient elimination of AT1A receptors, we measured levels for AT1A receptor mRNA by real-time quantitiative RT-PCR. Segments of the aorta were isolated from SMKO and control mice. As shown in Supplemental Figure 1, mRNA for the AT1A receptor was easily detected in aortae from control mice (n=9), but not from SMKOs (P=0.001, n=9). Furthermore, mesenteric vessels were isolated (Supplemental Figure 1) from SMKO (n=5) and control mice (n=5) and demonstrated an approximate 97% reduction in AT1A receptor mRNA (control, 1±0.2 versus SMKO, 0.03±0.01 AT1A to GAPDH mRNA, arbitrary units; P=0.004). AT1A receptor mRNA expression was not affected in liver, adrenal gland, or brain stem. Therefore, in SMKOs, AT1A receptor mRNA expression was efficiently and specifically extinguished from VSMCs.
Elimination of AT1A Receptors from VSMCs Reduces Basal BP and Contributes to Salt Sensitivity
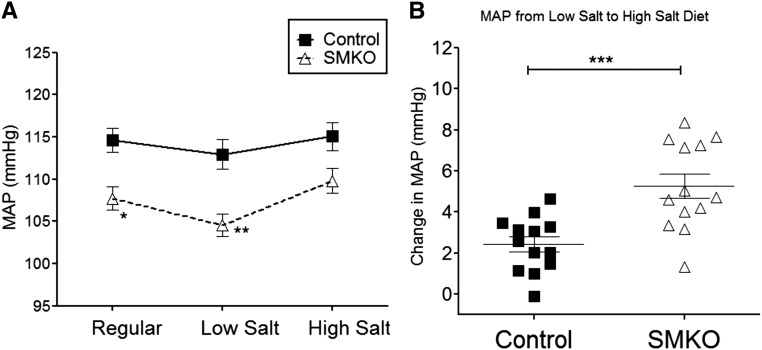
To define the effect of AT1A receptors in the vasculature on BP control, radiotelemetry units were implanted into 8- to 12-week-old male SMKO (n=13) and control (n=13) mice to measure intra-arterial pressure continuously in the conscious, unrestrained state. As shown in Figure 1, mean arterial pressures (MAPs) during normal sodium (0.4% Na+) feeding were approximately 7 mmHg lower in SMKO mice than controls (SMKO, 108±1 versus control, 115±1 mmHg; P<0.002), identifying a role for AT1A receptor activation in the vasculature to maintain normal BP homeostasis. This was accompanied by a qualitative increase in renin (Ren1) mRNA expression in the renal cortex in SMKO mice compared with controls at baseline during normal sodium diet (Supplemental Figure 2). The pattern of diurnal variation of BP was preserved (Supplemental Figure 3). We have previously shown that global deficiency of AT1A receptors enhances the extent of BP changes in response to altered dietary sodium intake (i.e., sodium sensitivity).14 Accordingly, SMKO and control mice were sequentially fed diets of normal (0.4% Na+), low (<0.02% Na+), and high (6% Na+) sodium content. During low Na+ feedings, the difference in MAP between SMKO and control mice was further exaggerated (SMKO, 104±1 versus control, 113±2; P=0.001). In contrast, during high Na+ diet the difference in BP narrowed and statistical significance was lost (SMKO, 110±2 versus control, 115±2; P=0.03) (Figure 1). In humans, sodium sensitivity is defined as the difference in BP between low- and high-sodium states.15 Accordingly, we compared the change in BP between low- and high-salt feeding in the two groups. As shown in Figure 1B, the BP change from low-sodium to high-sodium diet was significantly greater in SMKOs than control mice (SMKO, +5±1 versus control +2±1 mmHg; P=0.001), indicating enhanced sodium sensitivity in the SMKOs.
Figure 1.
Lack of AT1A receptors in VSMCs reduces basal BP and contributes to salt sensitivity. (A) BP was measured via radiotelemetry in control (closed boxes) and SMKO (open triangles) mice. During normal sodium (0.4% Na+) feeding, SMKO mice had lower MAPs than control mice (SMKO, 108±1 versus control, 115±1 mmHg; *P<0.002). Control and SMKO mice were sequentially fed diets of normal (0.4% Na+), low (<0.02% Na+), and high (6% Na+) sodium and BPs were averaged over 5 days respectively. During low-sodium feeding, SMKOs had lower MAPs than controls (SMKO, 104±1 versus control, 113±2 mmHg; **P<0.001). During high-sodium feeding, MAPs increased in both groups, but the difference between SMKO mice and control mice was not significant (SMKO, 110±2 versus control, 115±2 mmHg; P=0.03). Analysis was performed with one-way ANOVA (overall P<0.001) and unpaired t test with Bonferroni adjustment for multiple comparisons (significance for P value <0.02). (B) The change in MAP from a low-sodium to high-sodium diet was significantly greater in SMKO mice compared with control mice (SMKO, +5±1 versus control, +2±1 mmHg, change in MAP; ***P=0.001). Analysis was performed with an unpaired t test.
Attenuated Hypertensive Response to Chronic Infusions of Ang II in SMKO Mice
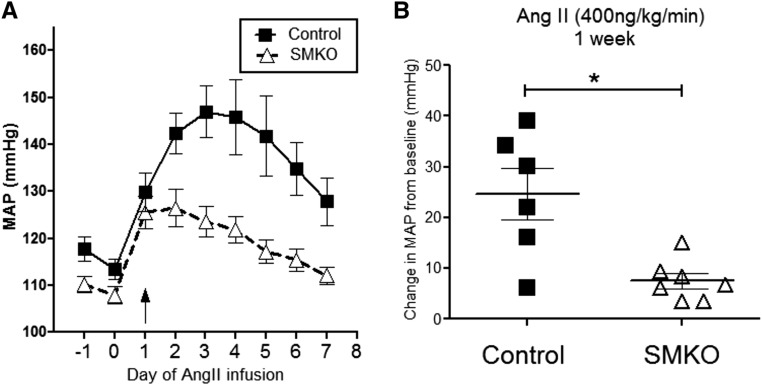
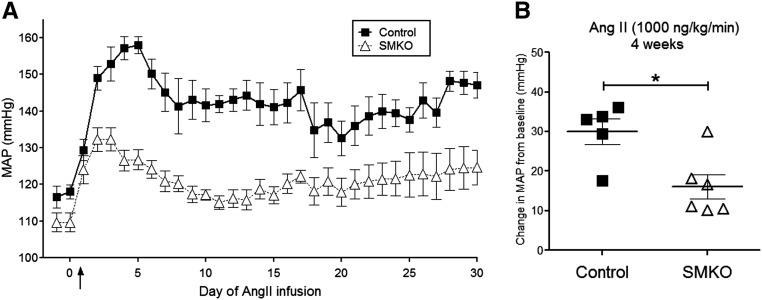
To determine the contribution of vascular AT1A receptors to the development of hypertension, osmotic minipumps were implanted into SMKO mice and controls to infuse Ang II for 4 weeks, and intra-arterial pressures were measured via radiotelemetry. We carried out two independent experiments infusing low (400 ng/kg per min) or high (1000 ng/kg per min) concentrations of Ang II. It has been suggested that the lower concentration of Ang II induces a slower onset of hypertension that may be more dependent on neural and/or renal mechanisms.16 As shown in Figure 2, control mice (n=6) infused with the lower concentration of Ang II (400 ng/kg per min) developed robust hypertension with an increase in MAP of 25±5 mmHg during the first week. By comparison, the increase in MAP was significantly attenuated in SMKOs (8±2 mmHg; P<0.01; n=7). At the higher dose of Ang II (1000 ng/kg per min), MAP increased by 30±3 mmHg in control mice (n=5) and was likewise attenuated in the SMKO mice (16±4 mmHg; P<0.02; n=6) (Figure 3). These findings suggest that AT1A receptors in VSMCs play a key role in the pathogenesis of Ang II–dependent hypertension.
Figure 2.
AT1A receptors in VSMCs promote the hypertensive response to low-dose Ang II–dependent hypertension. (A) Daily radiotelemetry tracings from control mice (closed squares) and SMKO mice (open triangles) infused with chronic low-dose Ang II (400 ng/kg per min) over 1 week. (B) Control mice developed robust hypertension with an increase in MAP of 25±5 mmHg from baseline during the first week of Ang II administration. The increase in MAP was significantly attenuated in SMKO mice (8±2 mmHg; *P<0.01, open triangles, unpaired t test).
Figure 3.
AT1A receptors in VSMCs promote the hypertensive response to high-dose Ang II–dependent hypertension. (A) Daily radiotelemetry tracings from control mice (closed squares) and SMKO mice (open triangles) infused with chronic high-dose Ang II (1000 ng/kg per min) over 4 weeks. (B) Control mice developed hypertension with an increase in MAP of 30±3 mmHg. However, the hypertensive response in SMKO mice was significantly attenuated (16±4 mmHg; *P<0.02 versus control, unpaired t test).
Enhanced Natriuresis in SMKOs during the Development of Hypertension
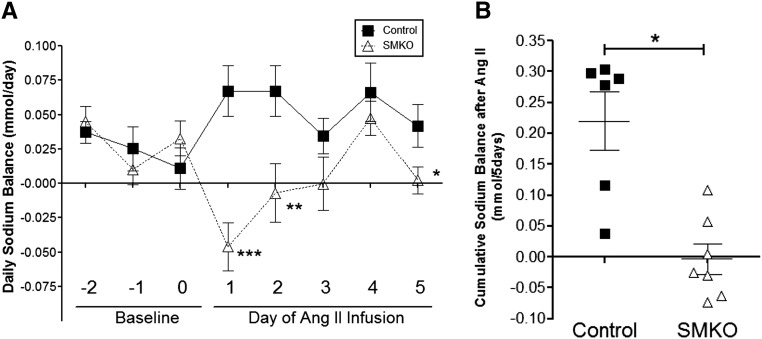
To determine whether alterations in renal sodium handling may have contributed to the attenuated hypertensive response to Ang II in the SMKOs, we measured urinary sodium excretion during the first 5 days of the Ang II infusion, whereas sodium intake was clamped at similar levels between the experimental groups. As shown in Figure 4, we found that cumulative sodium balance in the controls was positive (0.22±0.05 mmol Na+/5 d; n=6), representing net retention of sodium during the initiation of hypertension. By contrast, sodium balance remained effectively neutral in SMKOs (–0.003±0.02 mmol Na+/5 d; P=0.001 versus controls; n=7). Therefore, attenuated hypertension in SMKOs was associated with preserved renal sodium excretion and a shift of the pressure-natriuresis relationship, indicating that AT1A receptors in VSMCs promote BP elevation by stimulating renal sodium reabsorption.
Figure 4.
Enhanced natriuresis in SMKO mice during the development of hypertension. (A) Daily sodium balance in control (closed squares) and SMKO (open triangles) mice for 3 days before and 5 days after high-dose Ang II–induced hypertension (1000 ng/kg per min). Significant differences in sodium balance were seen at days 1, 2, and 5 after Ang II infusion between control and SMKO mice (day 1 of Ang II: 0.067±0.018 versus –0.046±0.017 mmol Na+/d at day 1, ***P=0.001; day 2 of Ang II: 0.067±0.02 versus –0.0069±0.02, **P=0.02; day 5 of Ang II, 0.042±0.02 versus 0.0024±0.01, *P=0.05, unpaired t test.). B. Cumulative sodium balance was significantly lower in the SMKOs (n=7) than controls (n=7), (Control: 0.22±0.05 versus SMKO: -0.003±0.02 mmol, Na+/5 days **P=0.001, unpaired Student’s t test) during the first 5 days of Ang II infusion. Error bars represent SEM.
Preserved Systemic Vascular Responses to Ang II in SMKO Mice
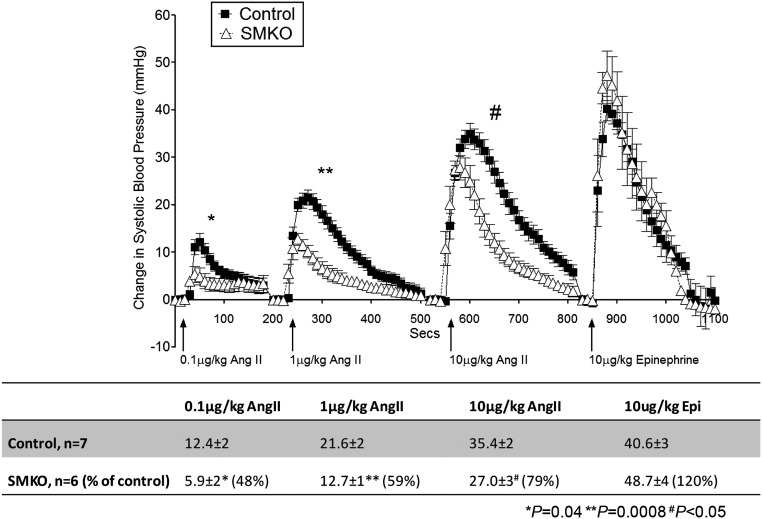
We next examined the consequences of removing AT1A receptors from VSMC on acute BP responses to Ang II measured via the carotid artery. As shown in Figure 5, bolus injection of Ang II caused acute vasoconstriction in control mice (n=7) with an immediate, marked increase in MAP that gradually returned to baseline within approximately 5 minutes. In response to doses ranging from 0.1 to 10 µg/kg Ang II, there was a dose-dependent increase in the peak and area under the curve. This systemic vasoconstrictor response was largely preserved in the SMKOs (n=6), with a residual peak increase in MAP that was approximately 80% of control values (Figure 5). Therefore, despite the significant difference in BP between SMKOs and controls, acute systemic vasoconstrictor responses to Ang II were only modestly attenuated. As expected, vasoconstrictor responses to epinephrine were unaffected in SMKOs.
Figure 5.
Preserved systemic vascular responses to Ang II in SMKO mice. BP tracing averaged every 10 seconds after acute administration of Ang II or epinephrine in anesthetized mice. The maximal increases in systolic BP compared to baseline in response to bolus infusions of Ang II (0.1, 1, and 10 μg/kg) were present but significantly lower in SMKO mice (open triangles, n=6) versus control mice (closed squares, n=7). No differences in vasoconstriction were detected with epinephrine (10 μg/kg) between control and SMKO mice. Error bars represent SEM.
Residual Systemic Vasoconstriction in SMKOs Is Not Mediated by AT1B Receptors
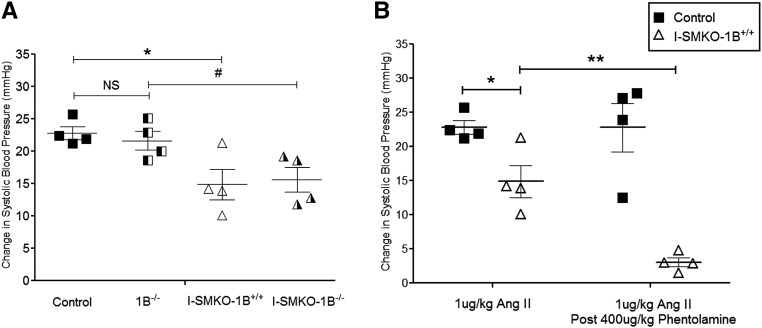
The vasoconstrictor response to Ang II is substantially attenuated in mice with complete deficiency of AT1A receptors17; therefore, the finding of preserved systemic vasoconstriction after Ang II in SMKOs (Figure 5) was unexpected. Because AT1B receptors are expressed in the vasculature and have the capacity to induce vasoconstriction,18 we considered the possibility that AT1B receptors might be responsible for residual Ang II–dependent vasoconstriction in SMKOs. To test this, we generated mice with smooth muscle cell–specific deletion of AT1A receptors on an AT1B-null background (I-SMKO-1B−/−). In this case, we used a tamoxifen-inducible Cre transgene driven by the smooth muscle myosin heavy chain promoter (SMMHC-ERT2-Cre) to allow deletion of AT1A receptors from VSMCs in adult mice and obviate potential confounding effects of the absence of VSMC AT1 receptors during fetal development. After administration of tamoxifen, VSMCs in I-SMKO-1B−/− mice will be deficient in both AT1A and AT1B receptors.
We first verified appropriate expression of Cre recombinase in both aorta and small vessels of the kidney in SMMHC-ERT2-Cre-mTmG mice (Supplemental Figure 4). In addition, we found significant reductions in AT1A receptor mRNA levels verified by quantitative RT-PCR of aortae and mesenteric vessels in I-SMKO mice (Supplemental Figure 5). As shown in Figure 6A, we compared the intensity of acute Ang II–dependent vasoconstriction in the following four experimental groups: control, 1B−/−, I-SMKO-1B+/+, and I-SMKO-1B−/− mice. Compared with control mice, the absence of AT1B receptors alone in 1B−/− mice did not significantly affect the systemic vasoconstrictor response to 1 µg/kg of Ang II (22.8±1 versus 21.7±2 mmHg peak increase in systolic BP from baseline, P=NS, n=4). As in the SMKOs, this response was modestly diminished in the I-SMKO-1B+/+ animals relative to controls mice (14.9±2 versus 22.8±1 mmHg peak increase in systolic BP from baseline, P=0.02, n=4). Finally, the extent of vasoconstriction was virtually identical in the I-SMKO-1B+/+ (14.9±2 mmHg) and I-SMKO-1B−/− (15.6±2 mmHg, P=NS, n=4) groups, indicating that AT1B receptors do not mediate the persistent systemic vasoconstrictor response to Ang II in SMKOs.
Figure 6.
Exaggerated sympathetic responses rather than AT1B receptors mediate residual vasoconstriction to Ang II in SMKO mice. (A) The absence of AT1B receptors alone in 1B−/− mice did not significantly affect the systemic vasoconstrictor response to 1 µg/kg of Ang II (22.8±1 versus 21.7±2 mmHg change in systolic BP from baseline, P=NS, unpaired t test). This vasoconstrictor response was diminished by approximately 35% in the I-SMKO-1B+/+ mice relative to control mice (controls, 22.8±1 versus I-SMKO-1B+/+, 14.9±2 mmHg change in systolic BP from baseline; *P=0.02, unpaired t test) and diminished by approximately 29% in the I-SMKO-1B−/− mice compared with 1B−/− mice (1B−/−, 21.7±1 versus SMKO-1B−/−, 15.6±2 mmHg, #P=0.04). The peak vasoconstrictor response was similar in the I-SMKO-1B+/+ (14.9±2 mmHg) and I-SMKO-1B−/− (15.6±2 mmHg, P=NS, unpaired t test). (B) After administration of phentolamine (400 µg/kg), the magnitude of Ang II–dependent vasoconstriction in controls was not significantly affected (22.8±1 versus 22.8±4 mmHg, P=NS, unpaired t test). This response was significantly diminished in SMKO mice after phentolamine administration (14.9±2 versus 3±1 mmHg, **P=0.003, unpaired t test). Error bars represent SEM.
α-Adrenergic Blockade Extinguishes Ang II–Dependent Vasoconstriction in SMKOs
Central administration of Ang II induces rapid peripheral vasoconstriction mediated via activation of sympathetic outflow by AT1A receptors in the central nervous system.19,20 To determine whether sympathetic stimulation contributes to the residual Ang II–dependent systemic vasoconstriction in SMKOs, we compared the acute vasoconstrictor responses to Ang II in SMKOs and controls before and after administration of the α-adrenergic blocker phentolamine. Before administration of phentolamine, the acute pressor response to Ang II was reduced by approximately 35% in I-SMKOs compared with controls (Figure 6B). Administration of phentolamine had no effect on the magnitude of Ang II–dependent vasoconstriction in controls (22.8±1 versus 22.8±4 mmHg, P=NS, n=4) (Figure 6B). By contrast, this response was almost completely abrogated in SMKOs after phentolamine (14.9±2 versus 3±1 mmHg, P=0.003, n=4) (Figure 6B), suggesting that residual vasoconstriction in I-SMKO mice requires α-adrenergic stimulation.
Increased Urinary Catecholamine Excretion in SMKO Mice
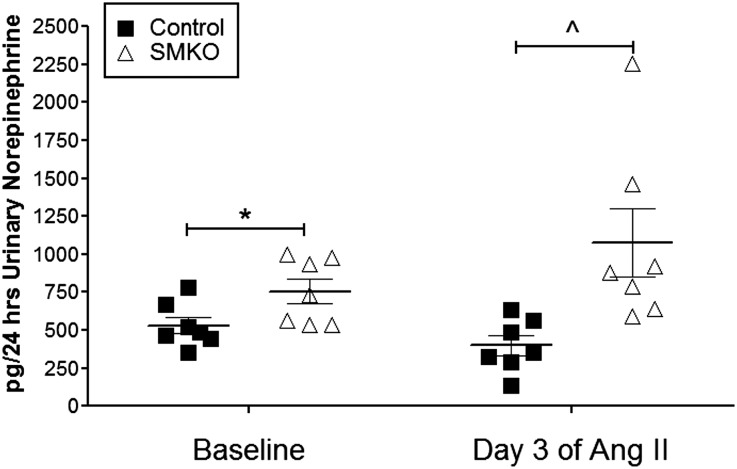
The enhanced contribution of α-adrenergic output to Ang II–dependent vasoconstriction in SMKOs suggests altered activation of the sympathetic nervous system. As an assessment of adrenergic activity, we measured urinary norepinephrine excretion in controls and SMKOs at baseline and after 3 days of chronic Ang II administration (1000 ng/kg per min). As shown in Figure 7, baseline urinary norepinephrine excretion was significantly higher in SMKOs than in controls (754±81 versus 530±55 pg/24 h, P=0.04, n=7 in both groups). After 3 days of Ang II infusion, there was a tendency toward suppression of norepinephrine excretion in the controls (from 530±55 to 398±66 pg/24 h, P=0.07, n=7), whereas Ang II caused a further qualitative increase in norepinephrine levels in SMKOs (from 754±81 to 1076±224 pg/24 h, P=0.16, n=7 in both groups), such that the difference in norepinephrine excretion between SMKOs and controls was magnified by chronic Ang II infusion (1076±224 versus 398±66 pg/24 h, P=0.01, n=7 in both groups). These findings suggest that deletion of AT1A receptors from VSMCs is associated with enhancement of sympathetic nervous system activity. We also measured catecholamine biosynthetic enzyme gene products (tyrosine hydroxylase, dopamine β-hydroxylase, and phenylethanolamine-N-methyltransferase) from the adrenal gland at baseline in both control and SMKO mice and did not detect a difference (Supplemental Figure 6).
Figure 7.
Elevated urinary norepinephrine in mice lacking AT1A receptors in VSMCs. Baseline urinary norepinephrine excretion was significantly higher in SMKO mice than control mice (754±81 versus 530±55 pg/24 h, *P=0.04, unpaired t test). After 3 days of Ang II infusion, norepinephrine excretion was decreased in control mice compared with baseline (530±55 baseline versus 398±66 pg/24 h at day 3 of Ang II, P=0.07, paired t test). Ang II infusion led to a further nonsignificant increase in norepinephrine levels in SMKO mice (754±81 baseline versus 1076±224 pg/24 h at day 3 of Ang II, P=0.16, paired t test). However, the difference in norepinephrine excretion between SMKO mice and control mice was magnified by chronic Ang II infusion (1076±224 versus 398±66 pg/24 h, ^P=0.01, unpaired t test). Error bars represent SEM.
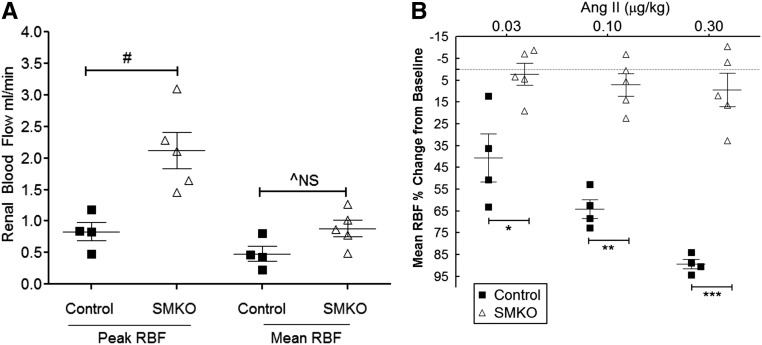
Increased Baseline RBF and Attenuated Renal Vasoconstrictor Responses to Ang II in SMKO Mice
Given the blunted hypertensive response and exaggerated natriuresis in the SMKO cohort, we hypothesized that deletion of AT1A receptors from VSMCs might attenuate the sensitivity of the renal circulation to Ang II. To test this possibility, we measured RBF using an ultrasound probe placed over the renal artery. At baseline, RBF during systole was significantly higher in SMKO mice (n=5) than control (n=4) mice (2.1±0.3 versus 0.83±0.1 ml/min, P=0.01), and there was a suggestive increase in mean RBF (0.88±0.1 versus 0.48±0.1 ml/min) that approached statistical significance (P=0.06) (Figure 8A). Furthermore, in control mice, there was a dose-dependent reduction in mean RBF induced by Ang II (–42.1%±15% at 0.03 µg/kg Ang II, –64.7%±6% at 0.1 µg/kg Ang II, –87.8%±2% at 0.3 µg/kg Ang II decrease in mean RBF from baseline) (Figure 8B). In contrast, the renal vasoconstrictor response to Ang II was substantially blunted in SMKO mice (–2.2%±5% at 0.03 µg/kg Ang II, –7.1%±5% at 0.1 µg/kg Ang II, and –7.5%±9% at 0.3 µg/kg Ang II decrease in mean RBF from baseline; P=0.02, P=0.001, and P=0.001 versus control at each dose, respectively, n=5) (Figure 8B). The failure of Ang II to induce renal vasoconstriction in SMKOs is similar to that seen in mice with global deletion of AT1A receptors.21 Therefore, the elimination of AT1A receptors from VSMCs yields enhanced RBF and discrepant vascular responses to Ang II in the systemic and renal circulations. Lack of AT1A receptors from VSMCs leads to diminished but preserved systemic vascular responses in the systemic circulation as measured in the carotid artery, but dramatically attenuated vasoconstriction in the renal circulation. We suggest that this preservation of RBF represents a potential mechanism explaining the diminished severity of hypertension in mice lacking vascular AT1A receptors.
Figure 8.
Increased Baseline RBF and Attenuated Renal Vasoconstrictor Responses to Ang II in SMKO Mice. RBF was measured in anesthetized mice using an ultrasound flow probe over the renal artery. (A) Peak RBF during systole was significantly increased in SMKO mice (n=5) compared with control mice (n=4) (SMKO, 2.1±0.3 versus control, 0.83±0.1 ml/min; #P=0.01 unpaired t test). Mean RBF also tended to be higher in SMKO mice than control mice, but this did not reach statistical significance (SMKO, 0.88±0.1 versus control, 0.48±0.1 ml/min; ^P=0.06, NS, unpaired t test). (B) Reduction in mean RBF was compared with baseline values. RBF was significantly diminished after Ang II administration in control mice; however, no change in RBF was observed in SMKO mice. Control mice had a decrease in RBF by –42.1%±15% at 0.03 µg/kg Ang II, –64.7%±6% at 0.1 µg/kg Ang II, and –87.8%±2% at 0.3 µg/kg Ang II from baseline. However, SMKO mice had only minimal changes in RBF by –2.2%±5% at 0.03 µg/kg Ang II, –7.1%±5% at 0.1 µg/kg Ang II, and –7.5%±9% at 0.3 µg/kg Ang II from baseline. *P=0.02, **P=0.001, ***P=0.001 versus control at each dose, unpaired t test. Error bars represent SEM.
Discussion
Vasoconstriction is the signature physiologic action of Ang II and was the basis for the bioactivity assay used in its original discovery >70 years ago.22,23 Ang II elicits vasoconstriction by activating AT1 receptors in VSMCs, triggering Gαq-dependent signaling pathways with consequent increases in intracellular calcium concentration.24,25 Because the primary determinants of BP are cardiac output and systemic vascular resistance, it has been widely presumed that these vascular actions of Ang II explain its role in hypertension pathogenesis. In this regard, compelling arguments have been made for the importance of systemic vasoconstriction in various forms of hypertension, including Ang II–dependent hypertension.26,27 On the other hand, Guyton has argued for the primacy of pathways controlling sodium excretion by the kidney in long-term BP control, suggesting that the capacity of the kidney to excrete sodium provides a compensatory system with virtually infinite gain to oppose increases in BP by other mechanisms, including systemic vasoconstriction.28 Our study bridges these two perspectives and offers a mechanism for vascular effects to effect sodium handling by the kidney.
In previous studies using kidney cross-transplantation, we identified a major contribution of AT1A receptors inside the kidney to the development of Ang II–dependent hypertension.5 The mechanism of this effect involved stimulation of renal sodium reabsorption and modification of pressure-natriuresis responses.7 However, the exact cell lineages mediating these effects could not be precisely distinguished because AT1A receptors are expressed in a number of regions in the kidney, including glomeruli, tubules, and renal vasculature. Accordingly, in the experiments described here, we directly assessed the role of AT1A receptors in VSMCs by generating male mice with cell-specific deletion of AT1A receptors from smooth muscle (SMKOs). Elimination of AT1A receptors from VSMCs caused a modest and statistically significant reduction in baseline BP, indicating a key role for vascular actions of Ang II in determining the normal level of BP. In addition, SMKOs manifested augmented fluctuations in their BPs between low- to high-sodium feeding (i.e., sodium sensitivity29), which we have previously observed in mice with global deficiency of AT1A receptors.14 Furthermore, the difference in BP between SMKOs and controls was abrogated during high-salt feeding, suggesting a primary disturbance in total body fluid volume. By contrast, in our previous studies of mice with cell-specific deletion of AT1A receptors from renal epithelial cell populations in the proximal tubule7 or collecting duct (J. Stegbauer et al. submitted manuscript), this phenotype of sodium sensitivity is not recapitulated. This indicates an important role for vascular actions of AT1A receptors in homeostatic response to variations in dietary sodium intake.
Along with reduced BPs at baseline, the severity of Ang II–dependent hypertension was markedly diminished in the SMKOs; MAP was reduced by >60% of control values during the entire 4-week infusion period. This protection against hypertension was associated with enhanced urinary sodium excretion in SMKO compared with control mice, consistent with an alteration of the pressure-natriuresis response wherein urinary excretion of sodium was triggered at lower levels of BP in the SMKOs. Despite the dramatic attenuation of hypertension, there was only a modest diminution of systemic vasoconstrictor responses to Ang II in SMKOs (approximately 20% reduction from control levels). On the other hand, their acute vasoconstrictor response to Ang II in the renal circulation was almost completely extinguished. We suggest that the absence of Ang II–dependent renal vasoconstriction and the consequent preservation of RBF are a critical mechanism facilitating natriuresis and protection against hypertension in this setting.
The influence of renal hemodynamics on sodium handling by the kidney has been long recognized.30,31 For example, classic studies from Martino and Earley demonstrated that hydrostatic and osmotic pressures in peritubular capillaries had a significant effect on sodium reabsorption by the proximal tubule.32,33 Later, Cowley and Roman suggested that reduction in renal medullary blood flow was a key component of hypertension pathogenesis, promoting enhanced sodium reabsorption, volume expansion, and increases in BP.34 Conversely, infusion of vasodilators into the renal artery causes natriuresis.35 Furthermore, resistance to hypertension mediated by autocrine and paracrine vasodilators, such as prostanoids, has been correlated with increased RBF and enhanced natriuresis.36 Such a direct connection between kidney hemodynamics and control of urinary sodium excretion provides an attractive mechanism to explain the pressure-natriuresis responses proposed by Guyton.28 Accordingly, we suggest that impaired renal vasoconstriction explains the enhanced natriuretic response and abrogation of Ang II–dependent hypertension in the SMKO mice.
In the classic paradigm, Ang II induces vasoconstriction directly by activating AT1 receptors in VSMCs, triggering a complex series of intracellular events resulting in cellular contraction.37 Therefore, we were surprised that despite dramatic reductions in expression of AT1A mRNA in both large and small arteries in the SMKOs, their acute vasoconstrictor responses to Ang II were largely preserved with the exception of the renal vasculature. Although expression of the AT1B receptor is negligible in most tissues, we and others have shown that its effects may be more pronounced in the absence of the major AT1A receptor isoform and that it is capable of mediating Ang II–dependent vasoconstriction in vivo18,38 and in isolated vessels ex vivo.39 Therefore, we considered the possibility that the minor murine AT1 receptor, AT1B, might be responsible for this preserved response. However, eliminating AT1B receptors genetically from the SMKO background did not diminish their acute vasoconstrictor response to Ang II (Figure 6A).
Ang II can also cause vasoconstriction indirectly, through stimulation of AT1 receptors in the central nervous system, triggering sympathetic outflow and peripheral vasoconstriction.40 For example, intraventricular injection of Ang II causes acute vasoconstriction, and this is dependent on central AT1A receptors.19,20 In addition, brain selective AT1A receptor overexpression causes enhanced systemic pressor response to central Ang II administration.41 Therefore, we tested whether the preserved peripheral vasoconstrictor response we observed in the SMKOs might be related to accentuation of these central responses. Indeed, we found that the residual vasoconstrictor response in SMKOs was completely extinguished by prior administration of the α-sympathetic blocker phentolamine, confirming that exaggerated sympathetic tone maintained a relatively normal systemic vasoconstrictor response in the SMKOs. Furthermore, urinary excretion of norepinephrine was increased in the SMKOs, indicating a general increase in sympathetic activity in these animals. An increase in urinary norepinephrine content at baseline has previously been reported in global AT1A receptor knockout mice.42 Although the increase in urinary norepinephrine content at baseline in SMKOs may be a compensatory response to their lower BPs, the exaggerated response during chronic Ang II infusion could reflect a generalized alteration in central responsiveness to Ang II. Whatever the mechanism, this heightened sympathetic activity is not sufficient to return baseline BP to normal or restore the full hypertensive response to Ang II. Moreover, this enhanced sympathetic response did not augment Ang II–dependent vasoconstriction in the renal circulation, which was virtually absent in the SMKOs (Figure 8B). The reason for the differential response in renal versus systemic vasculature is not clear, but these findings indicate that direct actions of AT1 receptors in VSMCs primarily control Ang II–dependent vasoconstriction in the kidney.
Our studies have confirmed the importance of vascular responses to Ang II in BP regulation and hypertension pathogenesis. These vascular actions also play an important role in maintaining homeostasis during variation in sodium intake and may affect propensity for salt sensitivity. In particular, our model illustrates the powerful capacity for AT1 receptor actions in the renal circulation to impair natriuresis and thereby promote hypertension. Relief of renal vasoconstriction despite the presence of high levels of circulating Ang II promotes powerful resistance to hypertension.
Concise Methods
Details of Mice
A mouse line with a conditional Agtr1aflox allele was generated using homologous recombination in embryonic stem cells as described previously.7,43 To delete the AT1A receptor from VSMCs, we crossed inbred C57BL/6 transgenic mouse lines expressing Cre recombinase specifically in smooth muscle cells under the control of the Sm22α promoter (KISm22α-Cre, strain name: B6.129S6-Taglntm2(cre)Yec/J, stock number: 006878; The Jackson Laboratory, Bar Harbor, ME) or the inducible SMMHC-ERT2 promoter44 with C57BL/6-Agtr1aflox/flox mice. The Sm22α promoter drives expression of Sm22α protein, one of the earliest markers of a differentiated smooth muscle cells.45 Cell-specific expression of Cre recombinase in smooth muscle cell lineages driven by this promoter has been well documented.46,47 Therefore, we generated two separate mouse models, KISm22α -Cre+Agtr1aflox/flox (SMKO) mice and inducible SMMHC-ERT2-Cre+ Agtr1aflox/flox (I-SMKO), both on inbred C57BL/6 background. For the acute hemodynamic experiments, we bred the I-SMKO mouse line8 with the AT1B global KO mouse line48 (C57BL/6-Agtr1b−/−) for two successive generations to generate C57BL/6-SMMHC-ERT2 Cre+ Agtr1aflox/flox Agtr1b−/− mice (I-SMKO-1B−/−). Cre recombinase activity was induced in I-SMKO and I-SMKO-1B−/− mice by daily intraperitoneal injection of 2 mg of tamoxifen (Sigma-Aldrich, St. Louis, MO) mixed in corn oil for 5 successive days. Experiments were performed at least 1 week after completion of tamoxifen injections. All experiments were performed on 8- to 12-week-old male mice. Only male mice are used in these studies because they are in direct follow-up of past work completed in our laboratory using kidney cross-transplantation,4 which can only be performed in male mice because of technical constraints. Therefore, these results may not be generalizable to female mice.
All experimental mice were bred in an Association for Assessment and Accreditation of Laboratory Animal Care international accredited animal facility at the Duke University and Durham Veterans Affairs medical centers under National Institutes of Health guidelines for care and use of laboratory animals and housed with free access to standard rodent food and water unless otherwise specified.
BP Measurements in Conscious Mice
BPs were measured in conscious, unrestrained 8- to 12-week-old male SMKO and control mice using radiotelemetry (PA-C10) as described previously.5 Arterial BP was collected, stored, and analyzed using Dataquest A.R.T software (version 4.0; Data Sciences International, St. Paul, MN). Mice were allowed to recover for 7 days after telemetry implantation to re-established normal circadian rhythms.49 After recovery, telemetry data were collected continuously with sampling every 5 minutes for 10-second intervals. Baseline measurements were recorded for 7 consecutive days while mice ingested a normal sodium diet (0.4% Na+). On day 8, mice started ingesting a low-sodium diet (<0.02% Na+; Harlan Teklad, Indianapolis, IN) for 1 week and then high-sodium diet (6% Na+; Harlan Teklad) for 1 week. At the end of the salt challenge and while on a normal sodium diet, an osmotic minipump (Alzet, Cupertino, CA) infusing Ang II (Sigma-Aldrich) at a rate of 400 or 1000 ng/kg per min were implanted subcutaneously, and BP measurements continued for 7 and 30 days, respectively.
Assessment of Acute Vasoconstrictor Responses
We examined acute pressor responses to Ang II, epinephrine, and phentolamine (all from Sigma-Aldrich, reconstituted in sterile saline) in mice anesthetized with 2% isoflurane as described previously.13 A catheter (pulled PE-50) was inserted into the left jugular vein for the administration of basal fluids and vasoconstrictors. A second catheter (Mikro-Tip 1.4F; Millar, Houston, TX) was placed into the right carotid artery. Intra-arterial BP was recorded continuously through the carotid catheter using the PowerLab data acquisition system and LabChart software (ADInstruments, Colorado Springs, CO). At 5-minutes intervals, increasing doses (0.1, 1, and 10 µg/kg) of Ang II, 10 µg/kg of epinephrine, and 400 µg/kg of phentolamine were injected intravenously into the internal jugular vein at a volume of 1 µl/g body wt (25–30 µl total volume) followed immediately by a 30 µl bolus of saline. Before the injection of vasoactive agents, each mouse received an equivalent volume (55–60 µl, 2 ul/g body wt of saline as a vehicle control. Intra-arterial pressures were continuously monitored.
Assessment of RBF
We examined acute RBF responses to Ang II in mice anesthetized with 2% isoflurane as previously described.13 A catheter (pulled down PE-50) was inserted into the left jugular vein for the administration of basal fluids and vasoconstrictors. A small incision was made on the right flank to expose the kidney and a noncannulating ultrasonic flowmeter interfaced with a 5 mm V-shaped probe was placed around the right renal artery (MA0.5PSB and TS420 Flowmeter; Transonic Systems, Ithaca, NY). Mice were allowed to stabilize for 30 minutes before measurements were started. Vascular reactivity of the renal circulation was assessed by injecting increasing doses of Ang II (0.03, 0.1, and 0.3 µg/kg) as previously described into the internal jugular vein while RBF was continuously monitored. Data are expressed as percent change in mean RBF from baseline value determined just before injection.
Metabolic Balance Studies
A separate group of mice were individually placed in specially designed metabolic cages (Hatteras Instruments, Cary, NC)5,50 and fed 10 g/d gel diet containing nutrients, water, and 0.1% w/w sodium (Nutra-Gel; Bio-Serv, Frenchtown, NJ). After 5 days of baseline collections, mice were implanted with osmotic minipumps infusing Ang II (1000 ng/kg per min) and returned to metabolic cages for 5 more days. Urinary sodium content was measured daily and was determined using an IL943 Automatic Flame photometer (Instrumentation Laboratory, Lexington, MA). Sodium balance was determined by subtracting the total amount of sodium ingested daily by the total amount of sodium excreted in the urine over a 24-hour period.
Determination of Urinary Norepinephrine Content
Norepinephrine content was extracted from the urine and quantitated by enzyme immunoassay (Norepinephrine ELISA, 17-NORHU-E01.1; ALPCO Diagnostics, Salem, NH) according to the manufacturer’s instructions.
Statistical Analyses
The values for each parameter within a group are expressed as mean±SEM. For comparisons between groups with normally distributed data, statistical significance was assessed using a an unpaired t test. For comparisons within groups statistical significance was assessed using a paired t test. For comparisons between groups with non-normally distributed data, the Mann–Whitney U test was used. A P value <0.05 was considered statistically significant unless otherwise indicated. Normality was determined using the Shapiro–Wilk test. A one-way ANOVA with Bonferroni multiple comparison test was used when multiple interventions were tested.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research, National Institutes of Health grants HL056122 and P30-DK096493 (to T.M.C.); a Career Development Award IK2BX002240 from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs Office of Research and Development (to M.A.S.); a Scientist Development Award 13SDG13990017 from the American Heart Association (to M.A.S.); a Chair’s Research Award from the Duke Department of Medicine (to M.A.S.); and a grant from the Institute for Medical Research at the Durham VAMC (to M.A.S.).
SMMHC-ERT2-Cre mice were provided by Prof. Dr. Stefan Offermanns, Department of Pharmacology, Max-Planck-Institute for Heart and Lung Research, Ludwigstr. Bad Nauheim, Germany.
The views expressed in this article are those of the authors and do not necessarily represent the policy or position of the United States Department of Veteran Affairs or the United States government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080816/-/DCSupplemental.
References
- 1.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, LIFE Study Group : Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 359: 995–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, Powers B, Samsa GP, Gray RN: Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med 148: 16–29, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM: Classical renin-angiotensin system in kidney physiology. Compr Physiol 4: 1201–1228, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD: Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol 301: R1067–R1077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilluy C, Brégeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G: The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med 16: 183–190, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME: High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A 105: 6702–6707, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ: Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18: 1429–1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earley L, Schrier R: Intrarenal control of sodium excretion by hemodynamics and physical factors. In: Handbook of Physiology: Renal Physiology, Washington, DC, American Physiological Society, 1973, pp 721–762 [Google Scholar]
- 12.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Sparks MA, Makhanova NA, Griffiths RC, Snouwaert JN, Koller BH, Coffman TM: Thromboxane receptors in smooth muscle promote hypertension, vascular remodeling, and sudden death. Hypertension 61: 166–173, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Oliverio MI, Best CF, Smithies O, Coffman TM: Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension 35: 550–554, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Weinberger MH: Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep 8: 166–170, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS: A mouse model of angiotensin II slow pressor response: Role of oxidative stress. J Am Soc Nephrol 13: 2860–2868, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM: Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A 92: 3521–3525, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM: Angiotensin II responses in AT1A receptor-deficient mice: A role for AT1B receptors in blood pressure regulation. Am J Physiol 272: F515–F520, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD: The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res 83: 1047–1058, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD: Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest 106: 103–106, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan X, Oliverio MI, Coffman TM, Arendshorst WJ: Renal vascular reactivity in mice: AngII-induced vasoconstriction in AT1A receptor null mice. J Am Soc Nephrol 10: 2620–2630, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Basso N, Terragno NA: History about the discovery of the renin-angiotensin system. Hypertension 38: 1246–1249, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Page IH, Helmer OM: A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med 71: 29–42, 1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touyz RM, Schiffrin EL: Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000 [PubMed] [Google Scholar]
- 25.Kai H, Fukui T, Lassègue B, Shah A, Minieri CA, Griendling KK: Prolonged exposure to agonist results in a reduction in the levels of the Gq/G11 alpha subunits in cultured vascular smooth muscle cells. Mol Pharmacol 49: 96–104, 1996 [PubMed] [Google Scholar]
- 26.Schiffrin EL: Vascular remodeling in hypertension: Mechanisms and treatment. Hypertension 59: 367–374, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Mendelsohn ME: In hypertension, the kidney is not always the heart of the matter. J Clin Invest 115: 840–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyton AC: Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19[Suppl]: I2–I8, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki T, Delea CS, Bartter FC, Smith H: The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 64: 193–198, 1978 [DOI] [PubMed] [Google Scholar]
- 30.London GM, Levenson JA, London AM, Simon AC, Safar ME: Systemic compliance, renal hemodynamics, and sodium excretion in hypertension. Kidney Int 26: 342–350, 1984 [DOI] [PubMed] [Google Scholar]
- 31.Hollenberg NK, Adams DF: The renal circulation in hypertensive disease. Am J Med 60: 773–784, 1976 [DOI] [PubMed] [Google Scholar]
- 32.Martino JA, Earley LE: The effects of infusion of water on renal hemodynamics and the tubular reabsorption of sodium. J Clin Invest 46: 1229–1238, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martino JA, Earley LE: Demonstration of a role of physical factors as determinants of the natriuretic response to volume expansion. J Clin Invest 46: 1963–1978, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowley AW, Jr, Roman RJ: The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996 [PubMed] [Google Scholar]
- 35.Carter AJ, Gardiner DG, Burges RA: Natriuretic activity of amlodipine, diltiazem, and nitrendipine in saline-loaded anesthetized dogs. J Cardiovasc Pharmacol 12[Suppl 7]: S34–S38, 1988 [DOI] [PubMed] [Google Scholar]
- 36.May CN, Evans RG: Frontiers in research series: Neural, hormonal and renal interactions in long-term blood pressure control II. Introduction. Clin Exp Pharmacol Physiol 37: 272–273, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Touyz RM: Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: Role of angiotensin II. Exp Physiol 90: 449–455, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Harrison-Bernard LM, Cook AK, Oliverio MI, Coffman TM: Renal segmental microvascular responses to ANG II in AT1A receptor null mice. Am J Physiol Renal Physiol 284: F538–F545, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Chen Y, Dirksen WP, Morris M, Periasamy M: AT1b receptor predominantly mediates contractions in major mouse blood vessels. Circ Res 93: 1089–1094, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Kooner JS, May CN, Peart S, Mathias CJ: Separation of peripheral and central cardiovascular actions of angiotensin II. Am J Physiol 273: H2620–H2626, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL: Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res 90: 617–624, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y: Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology 146: 3481–3489, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM: Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension 57: 577–585, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson RD, Stricklett P, Gustafson C, Stevens A, Ausiello D, Brown D, Kohan DE: Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am J Physiol 275: C216–C226, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS: Structure and expression of a smooth muscle cell-specific gene, SM22 alpha. J Biol Chem 270: 13460–13469, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall SE, D’Alecy LG, Chen YE: Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation 119: 2161–2169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y, Zhang H, Yu L, Gunel M, Boggon TJ, Chen H, Min W: Stabilization of VEGFR2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Sci Signal 3: ra26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM: Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A 95: 15496–15501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butz GM, Davisson RL: Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics 5: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM: Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 43: 364–369, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.