Abstract
Uniform vascular access guidelines for elderly patients may be inappropriate because of the competing risk of death, high rate of arteriovenous fistula (AVF) maturation failure, and poor vascular access outcomes in this population. However, the outcomes in elderly patients with advanced CKD who receive permanent vascular access before dialysis initiation are unclear. We identified a large nationally representative cohort of 3418 elderly patients (aged ≥70 years) with CKD undergoing predialysis AVF or arteriovenous graft (AVG) creation from 2004 to 2009, and assessed the frequencies of dialysis initiation, death before dialysis initiation, and dialysis-free survival for 2 years after vascular access creation. In all, 67% of patients with predialysis AVF and 71% of patients with predialysis AVG creation initiated dialysis within 2 years of access placement, but the overall risk of dialysis initiation was modified by patient age and race. Only one half of patients initiated dialysis with a functioning AVF or AVG; 46.8% of AVFs were created <90 days before dialysis initiation. Catheter dependence at dialysis initiation was more common in patients receiving predialysis AVF than in patients receiving AVG (46.0% versus 28.5%; P<0.001). In conclusion, most elderly patients with advanced CKD who received predialysis vascular access creation initiated dialysis within 2 years. As a consequence of late predialysis placement or maturation failure, almost one half of patients receiving AVFs initiated dialysis with a catheter. Insertion of an AVG closer to dialysis initiation may serve as a “catheter-sparing” approach and allow delay of permanent access placement in selected elderly patients with CKD.
Keywords: vascular access, arteriovenous fistula, arteriovenous graft
Approximately 80% of United States patients initiate hemodialysis with a central vein catheter (CVC), rather than a permanent vascular access, resulting in increased rates of infection, unnecessary hospitalizations, and higher mortality.1–6 Major efforts have been undertaken by the nephrology community to increase the use of permanent vascular access, particularly arteriovenous fistulas (AVFs). One major barrier to increasing the use of AVFs at dialysis initiation has been the paucity of evidence about the optimal timing of preemptive vascular access placement.7 If the AVF is created too late, the patient will likely start dialysis with a CVC. On the other hand, if the AVF is created too early, it may never be used because the patient dies before requiring dialysis or does not require initiation of dialysis for a prolonged period. The current guidelines from the Fistula First Breakthrough Initiative8 and 2006 Kidney Disease Outcomes Quality Initiative (KDOQI)9 recommend that “a fistula should be created at least 6 months or with sufficient lead time before the anticipated start of hemodialysis treatments for fistula maturation.” This timing allows for vascular access evaluation and additional time for revision to ensure that a working AVF is available at initiation of dialysis therapy.
There is even greater uncertainty about the optimal predialysis vascular access strategy in elderly patients with CKD. Applying uniform vascular access guidelines to elderly individuals may not be appropriate because of the competing risk of death,7,10 the high rate of AVFs that fail to mature,11 and poor vascular access outcomes in the elderly population.12 Thus, the objective of this study was to use a large representative United States population to identify the proportion of elderly patients with advanced CKD with predialysis AVF or arteriovenous graft (AVG) creation who survive to initiate dialysis, die before dialysis initiation, or survive dialysis free during 2 years of follow-up. In addition, we evaluated whether certain demographic and clinical factors affected the likelihood of dialysis initiation in this patient population, and we assessed the likelihood of initiating dialysis with the permanent access created before dialysis.
Results
Patient Characteristics
During the 6-year period between January 1, 2004, and December 31, 2009, 3418 elderly patients (aged ≥70 years) with CKD from the 5% Medicare sample received an AVF or AVG before initiating dialysis (Figure 1). Table 1 presents the baseline patient characteristics by demographics and comorbidities, which are stratified into three groups on the basis of the patient outcomes during the 2-year period after access surgery: (1) patients who initiated dialysis, (2) patients who died without initiating dialysis, and (3) patients who survived without requiring dialysis therapy. After predialysis vascular access creation, the median times to dialysis initiation, patient death, and dialysis-free patient survival were 84.5 days (interquartile range, 24–233), 132 days (interquartile range, 33–343), and 730 days, respectively. The mean age of patients in this study cohort was 78.0 years. The majority of patients were men (54.0%), white (74.7%), and had AVFs inserted as the initial vascular access (80.2%). A large proportion of patients at the time of vascular access insertion had diabetes (66.2%), ischemic heart disease (64.6%), peripheral vascular disease (63.4%), and congestive heart failure (40.6%); 12.4% of patients had depression. The mean comorbidity score for all patients was 8.2.
Figure 1.
Patient cascade of an elderly cohort with CKD with AVF/AVG creation before initiation of dialysis using 5% Medicare sample CKD-based cohort data set. HMO, health maintenance organization.
Table 1.
Outcomes of elderly patients with CKD after first vascular access insertion, according to patient demographics, vascular access type, comorbidities, and year of vascular access insertion
| Covariate | Variablea | Initiated Dialysisb | Died before Dialysisb | Survived Dialysis Freeb | P Value |
|---|---|---|---|---|---|
| Study cohort | 3418 (100) | 2304 (67.4) | 515 (15.1) | 599 (17.5) | |
| Age at study start (yr) | |||||
| 70–<75 | 1047 (30.6) | 724 (69.1) | 127 (12.1) | 196 (18.7) | <0.001 |
| 75–<85 | 1914 (56.0) | 1306 (68.2) | 288 (15.0) | 320 (16.7) | |
| ≥85 | 457 (13.4) | 274 (60.0) | 100 (21.9) | 83 (18.2) | |
| Sex | |||||
| Men | 1845 (54.0) | 1255 (68.0) | 290 (15.7) | 300 (16.3) | 0.08 |
| Women | 1573 (46.0) | 1049 (66.7) | 225 (14.3) | 299 (19.0) | |
| Race | |||||
| White | 2552 (74.7) | 1676 (65.7) | 405 (15.9) | 471 (18.5) | 0.003 |
| Black | 597 (17.5) | 429 (71.9) | 72 (12.1) | 96 (16.1) | |
| Other/unknown | 269 (7.9) | 199 (74.0) | 38 (14.2) | 32 (11.9) | |
| Vascular access | |||||
| AVF | 2741 (80.2) | 1825 (66.6) | 382 (13.9) | 534 (19.5) | <0.001 |
| AVG | 677 (19.8) | 479 (70.8) | 133 (19.6) | 65 (9.6) | |
| Comorbidity score | |||||
| 0–4 | 829 (24.3) | 570 (68.8) | 48 (5.8) | 211 (25.5) | <0.001 |
| 5–10 | 1474 (43.1) | 1013 (68.7) | 208 (14.1) | 253 (17.2) | |
| 11–21 | 1115 (32.6) | 721 (64.7) | 259 (23.2) | 135 (12.2) | |
| Coexisting diseases | |||||
| Diabetes | 2263 (66.2) | 1555 (68.7) | 330 (14.6) | 378 (16.7) | 0.07 |
| Cancer | 925 (27.1) | 613 (66.3) | 160 (17.3) | 152 (16.4) | 0.07 |
| Ischemic heart disease | 2208 (64.6) | 1471 (66.6) | 376 (17.0) | 361 (16.4) | <0.001 |
| Chronic obstructive pulmonary disease | 1283 (37.5) | 837 (65.2) | 244 (19.0) | 202 (15.7) | <0.001 |
| Peripheral vascular disease | 2168 (63.4) | 1442 (66.5) | 373 (17.2) | 353 (16.3) | <0.001 |
| Cerebrovascular disease | 1368 (40.0) | 919 (67.2) | 240 (17.5) | 209 (15.3) | 0.001 |
| Depression | 423 (12.4) | 254 (60.0) | 99 (23.4) | 70 (16.5) | <0.001 |
| Dementia | 356 (10.4) | 219 (61.5) | 79 (22.2) | 58 (16.3) | 0.001 |
| Cardiac events | |||||
| Stroke | 129 (3.8) | 74 (57.4) | 39 (30.2) | 16 (12.4) | <0.001 |
| Myocardial infarction | 160 (4.7) | 90 (56.3) | 54 (33.8) | 16 (10.0) | <0.001 |
| Congestive heart failure | 1388 (40.6) | 915 (65.9) | 294 (21.2) | 179 (12.9) | <0.001 |
| Year of vascular access insertion | |||||
| 2004 | 569 (16.6) | 391 (68.7) | 105 (18.5) | 73 (12.8) | 0.01 |
| 2005 | 609 (17.8) | 410 (67.3) | 101 (16.6) | 98 (16.1) | |
| 2006 | 634 (18.5) | 430 (67.8) | 89 (14.0) | 115 (18.1) | |
| 2007 | 588 (17.2) | 376 (63.9) | 92 (15.6) | 120 (20.4) | |
| 2008 | 491 (14.4) | 344 (70.1) | 62 (12.6) | 85 (17.3) | |
| 2009 | 527 (15.4) | 353 (67.0) | 66 (12.5) | 108 (20.5) |
The cohort was followed for 2 years after first vascular access insertion until death, initiation of dialysis, or end of follow-up. P values were determined by Pearson’s chi-squared test. Patients’ comorbidities were determined by using one inpatient and/or two outpatient claims in the 5 years preceding the study index date. Cardiac events were determined 1 year preceding the study index date using primary inpatient diagnosis only.
Values are the number of patients with a given variable and the percentage of the total study cohort.
Values are the number of patients and percentage of patients for that row.
Patient Outcomes after Predialysis Vascular Access Creation
Among the 3418 elderly patients with CKD receiving a vascular access before dialysis, 67.4% started dialysis, 15.1% died, and 17.5% survived without requiring dialysis by the end of the 2-year follow-up. Patient outcomes after vascular access creation differed significantly by age, race, comorbidities, and type of vascular access (Table 1). Patients aged between 70 and 74 years were more likely to initiate hemodialysis within 2 years of vascular access creation compared with those patients aged 75–84 years and ≥85 years (Table 1). When these age subgroups were adjusted in a proportional hazard model that treated death as a competing event, increasing age was associated with a decreased likelihood of initiating dialysis. However, only patients aged ≥85 years were less likely to start dialysis with death as a competing event compared with the reference group of patients (hazard ratio [HR], 0.83; 95% confidence interval [95% CI], 0.72 to 0.96; P=0.01) (Table 2).
Table 2.
Association of age, sex, race, Charlson Comorbidity Index score, and vascular access type on the likelihood of starting dialysis, using the Fine–Gray proportional model to adjust for the competing risk of deaths
| Covariate | Hazard Ratios (95% CIs) | P Value |
|---|---|---|
| Age (yr) | ||
| 70–<75 (reference) | ||
| 75–<85 | 0.97 (0.89 to 1.07) | 0.57 |
| ≥85 | 0.83 (0.72 to 0.96) | 0.01 |
| Sex | ||
| Men (reference) | ||
| Women | 0.94 (0.86 to 1.02) | 0.14 |
| Race | ||
| White (reference) | ||
| Black | 1.14 (1.02 to 1.27) | 0.02 |
| Other | 1.28 (1.10 to 1.50) | 0.002 |
| Comorbidity score | ||
| 0–4 (reference) | ||
| 5–10 | 1.09 (0.99 to 1.20) | 0.09 |
| 11–21 | 1.03 (0.93 to 1.15) | 0.56 |
| Vascular access type | ||
| AVF (reference) | ||
| AVG | 1.30 (1.16 to 1.45) | <0.001 |
Black patients were more likely to initiate dialysis after predialysis vascular access creation and were less likely to die before dialysis initiation compared with white patients (Table 1). When adjusted using a proportional hazards model that treated death as a competing event, black patients were more likely to start dialysis than die compared with white patients (HR, 1.14; 95% CI, 1.02 to 1.27; P=0.02) (Table 2).
The patients with the highest comorbidity scores (11–21) were least likely to initiate hemodialysis compared with those with lower scores (0–4 and 5–10) (Table 1). When adjusted using a proportional hazards model that treated death as a competing event, the comorbidity scores were no longer associated with likelihood of starting dialysis (Table 2).
Patients receiving an AVG were more likely to initiate dialysis after predialysis vascular access creation and were less likely to survive dialysis free compared with those receiving an AVF (Table 1). When adjusted using a proportional hazards model that treated death as a competing event, patients with predialysis AVG creation were more likely to initiate dialysis than die compared with those with predialysis AVF creation (HR, 1.30; 95% CI, 1.16 to 1.45; P<0.001) (Table 2).
Vascular Access Type and Patient Outcomes
Of the pre-ESRD accesses created, 80.2% were AVFs and 19.8% were AVGSs (Table 1). Among elderly patients with predialysis AVF creation, two thirds initiated dialysis, 13.9% died without starting dialysis, and 19.5% survived dialysis free (Table 1). Patients aged ≥85 years with predialysis AVF creation were less likely to initiate dialysis than die (HR, 0.79; 95% CI, 0.67 to 0.93; P=0.01) (Table 3). Among elderly patients with AVG creation, a slightly higher proportion, 70.8% initiated dialysis (Table 1). Among patients with AVG creation, age, sex, race, and comorbidity score did not influence the likelihood of initiating dialysis after adjusting for the competing risk of death (Table 3).
Table 3.
The likelihood of starting dialysis by vascular access type, using the Fine–Gray proportional model to adjust for the competing risk of death
| Covariate | AVF | AVG | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (yr) | ||||
| 70–<75 (reference) | ||||
| 75–<85 | 0.98 (0.88 to 1.08) | 0.65 | 0.96 (0.79 to 1.17) | 0.71 |
| ≥85 | 0.79 (0.67 to 0.93) | 0.01 | 0.97 (0.73 to 1.29) | 0.83 |
| Sex | ||||
| Men (reference) | ||||
| Women | 0.93 (0.84 to 1.02) | 0.12 | 0.97 (0.81 to 1.16) | 0.73 |
| Race | ||||
| White (reference) | ||||
| Black | 1.11 (0.98 to 1.26) | 0.10 | 1.16 (0.96 to 1.40) | 0.14 |
| Other | 1.36 (1.15 to 1.60) | 0.003 | 1.03 (0.72 to 1.48) | 0.88 |
| Comorbidity score | ||||
| 0–4 (reference) | ||||
| 5–10 | 1.09 (0.98 to 1.21) | 0.12 | 1.08 (0.87 to 1.35) | 0.47 |
| 11–21 | 1.12 (0.99 to 1.26) | 0.08 | 0.83 (0.66 to 1.05) | 0.13 |
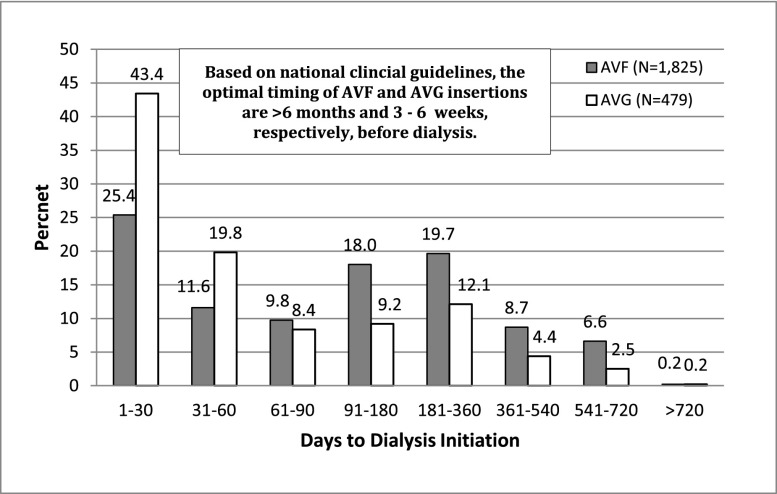
Figure 2 shows the distribution of time from vascular access creation to dialysis initiation for the 1825 patients with AVF creations and the 479 patients with AVG who began dialysis. Almost one half of AVFs (46.8%) were created <90 days before initiation of dialysis, 18.0% within 91 and 180 days, 19.7% with 181 and 360 days, and the remainder (15.5%) was created a year or more before dialysis. By contrast, the majority of AVGs (63.2%) were created within 60 days of dialysis initiation, 17.6% were created within 61 and 180 days, and 19.2% were created >180 days before dialysis.
Figure 2.
Time from predialysis vascular access creation to initiation of dialysis by type of vascular access.
Table 4 summarizes the vascular access used at the first dialysis session between 2006 and 2011, among the study patients with a previous permanent vascular access creation. In this analysis, 1381 patients had predialysis AVFs created and 277 patients had predialysis AVGs created. Among elderly patients with a predialysis AVF who survived to initiate dialysis, 47.7% used an AVF at dialysis initiation, whereas 33.2% used a catheter with a maturing AVF. Among those patients with predialysis AVGs created who survived to initiate dialysis, 54.5% initiated dialysis with an AVG, whereas 10.1% used a catheter with a healing AVG. Overall, among elderly patients with predialysis vascular access creation, the likelihood of initiating dialysis with a catheter was greater in those receiving an AVF versus AVG (46.0% versus 28.5%; P<0.001). Not surprisingly, longer predialysis nephrology care was associated with lower catheter dependence at initiation of dialysis for both AVF and AVG patients (Table 4). However, the proportion of patients with catheter dependence at initiation of dialysis was higher for those with predialysis AVFs, regardless of the duration of nephrology follow-up (65.4% versus 37.5% for patients with <4 months of nephrology follow-up; 51.7% versus 30.7% for those with 4–12 months of nephrology follow-up; and 41.4% versus 25.7% for those with >12 months of nephrology follow-up; P<0.001 for all comparisons) (Table 4).
Table 4.
Vascular access used at the first dialysis session by extent of predialysis nephrology care among patients with previous vascular access surgery by type of vascular access at initiation of dialysis
| Type of Predialysis Vascular Access Creation | Predialysis Nephrology Care (n) | AVF | AVG | CVC | Unknown | |||
|---|---|---|---|---|---|---|---|---|
| All | AVF-M | AVG-M | CVC Only | |||||
| AVG | 277 | 15.9 | 54.5 | 28.5 | 6.1 | 10.1 | 12.3 | 1.1 |
| Nephrology evaluation | ||||||||
| None or late (40) | 5.0 | 52.5 | 37.5 | 7.5 | 17.5 | 12.5 | 5.0 | |
| Intermediate (62) | 21.0 | 48.4 | 30.7 | 9.7 | 4.8 | 16.1 | 0 | |
| Early (175) | 16.6 | 57.1 | 25.7 | 4.6 | 10.3 | 10.9 | 0.6 | |
| AVF | 1381 | 47.7 | 3.9 | 46.0 | 33.2 | 1.0 | 11.8 | 2.3 |
| Nephrology evaluation | ||||||||
| None or late (179) | 30.7 | 1.1 | 65.4 | 47.5 | 1.7 | 16.2 | 2.8 | |
| Intermediate (213) | 44.6 | 3.3 | 51.7 | 37.6 | 1.4 | 12.7 | 0.5 | |
| Early (989) | 51.5 | 4.6 | 41.4 | 29.7 | 0.8 | 10.8 | 2.6 | |
Data are given as percentages unless otherwise specified. We excluded patients who initiated dialysis in 2004 and 2005, because information on vascular access use among dialysis patients was not collected before 2005. Time between the first evaluation by a nephrologist and the start of dialysis were categorized as none or late evaluation (0–≤4 months), intermediate evaluation (4–≤12 months), and early evaluation (12–24 months). CVC, central vein catheter; AVF-M, dialysis with central vein catheter with maturing arteriovenous fistula; AVG-M, dialysis with central vein catheter with healing arteriovenous graft.
Discussion
We analyzed the clinical outcomes in a large, nationally representative Medicare population of elderly patients (aged ≥70 years) receiving predialysis vascular access creation. Our major findings were as follows: (1) the large majority of elderly patients with advanced CKD in the United States who have predialysis vascular access surgery survive to initiate dialysis; (2) the majority of elderly patients with advanced CKD have predialysis AVFs created later than recommended by national guidelines and initiate dialysis with a catheter; (3) the likelihood of initiating dialysis after predialysis access surgery is modified by patient age and race; and (4) catheter dependence at initiation of dialysis is higher in elderly patients with CKD with predialysis AVF compared with those with predialysis AVG surgery, regardless of duration of predialysis nephrology follow-up.
The optimal timing of vascular access creation in patients with advanced CKD approaching ESRD remains challenging. The Fistula First Breakthrough Initiative8 and 2006 KDOQI9 recommend that “a fistula should be created at least 6 months or with sufficient lead time before the anticipated start of hemodialysis treatments for fistula maturation.” This allows for adequate time for an AVF to mature and necessary interventions to be performed to ensure successful AVF use at dialysis initiation. If the access is created too late, the patient is more likely to initiate dialysis with a catheter and to experience sepsis.13 If the access is created too early, it may not be needed, either because the patient dies before needing to start dialysis or does not progress to ESRD for a prolonged period of time. The decision-making process is particularly challenging in elderly patients with ESRD. The older the patient, the more likely he or she is to die during the follow-up period.7,14 Moreover, CKD progresses more slowly in older patients than younger patients.10 As a consequence of these two factors, the likelihood of initiating dialysis after vascular access creation decreases progressively with increasing patient age. For example, Oliver et al. reported that the likelihood of initiating dialysis during the 2 years after access creation among a cohort of Ontario patients was 88% in adult men aged <65 years compared with 65% in men aged ≥85 years.14 Similarly, O’Hare et al.7 evaluated a US Department of Veterans Affairs (largely male) population with advanced CKD, and created a hypothetical model in which all patients with advanced CKD were assumed to receive vascular access surgery. Using this model, they calculated the proportion of patients in whom AVF creation would be necessary (patient initiated dialysis within 2 years) or unnecessary (patient died without dialysis or survived dialysis free). The ratio of unnecessary to necessary access procedures increased progressively with older patient age and with vascular access surgery occurring at a higher eGFR.7 In contrast with this hypothetical model, the likelihood of patients with CKD actually undergoing predialysis access surgery decreased progressively with increasing patient age.7 These observations suggest that nephrologists are appropriately incorporating patient age in determining the timing of referral for vascular access.
Our present study confirms that the majority (67%) of elderly United States patients with advanced CKD who undergo predialysis vascular access creation initiate dialysis during a 2-year follow-up. These findings are remarkably consistent with two previous Canadian studies that focused on patients of all ages with CKD. Weber et al. in a single-center study from Vancouver, reported that 70% of patients with who underwent AVF creation started dialysis within 2 years.15 Likewise, Oliver et al., in a regional study from Ontario, reported that 81% of all patients with CKD initiated dialysis within 2 years of AVF creation.14 Our observation that initiation of dialysis was more likely during follow-up in patients receiving predialysis AVG versus AVF may reflect in part the practice of placing an AVG in patients with lower eGFR.
In the process of trying to balance the desire to avoid unnecessary predialysis access in elderly patients with CKD who will not require dialysis with the need to have a functioning access in those who will initiate dialysis, it is inevitable that a subset of patients will have their AVF created too late. Oliver et al. reported that 30% of their patients of all ages with CKD underwent AVF surgery <90 days before ESRD. In this study of elderly patients with CKD, we found that a higher proportion (47%) had their AVF created within 90 days of dialysis initiation.16 Given the relatively long maturation time of AVF and the frequent need for interventions to promote AVF maturation,17 a substantial proportion of patients with late AVF surgery will initiate dialysis with a catheter with its attendant complications. Notably, in our study, nearly one half of all patients with AVF creation initiated dialysis with a catheter.
One potential solution to this dilemma is to wait longer (closer to clinically requiring dialysis) before creating vascular access in elderly patients with CKD, but preferentially create an AVG, rather than an AVF, in many of these patients. Given the shorter time to cannulation of AVG,18 and the less frequent need for interventions before successful cannulation, more liberal AVG surgery in this population may be considered a catheter-sparing procedure.19 In this study, 63% of patients receiving an AVG underwent surgery within an ideal time frame (within 60 days) to optimize initiation of dialysis without a catheter. Notably, a substantially lower proportion of patients with predialysis AVG than those with predialysis AVF used a catheter at initiation of dialysis (28.5% versus 46.0%). Two recent observations lend further support to implementing a clinical strategy of preferentially placing an AVG in older patients with advanced CKD. First, in patients with CKD who are aged ≥80 years, those with AVF creation have no significant survival benefit compared with those with predialysis AVG creation,20 thus minimizing some of the potential benefits of AVFs in the elderly population. Second, earlier creation of AVF in elderly patients with CKD (>6–9 months) is not associated with better AVF success at dialysis initiation, but results in more predialysis interventional access procedures.21
We also evaluated the duration of predialysis nephrology care among patients with predialysis AVF and AVG creation. Among those with AVFs and AVGs created, 72% and 63%, respectively, had early nephrology follow-up (between 12 and 24 months before dialysis initiation). These results suggest that the large majority of elderly patients with advanced CKD have adequate predialysis nephrology follow-up. However, even among patients with predialysis AVF creation with early nephrology follow-up, 41% still initiated dialysis with a catheter.
Of interest, a subset of elderly patients undergoing predialysis vascular access creation had a different type of permanent access upon dialysis initiation. Thus, among patients who received a predialysis AVF, an AVG was in use or present as a secondary (maturing) access in 4.9% when dialysis was started. Presumably, these were patients whose AVF failed to mature, and they subsequently received a predialysis AVG. Conversely, among patients who received a predialysis AVG, an AVF was in use or present as a secondary (maturing) access in 22% when dialysis was started. One possible explanation is that the initial AVG caused dilation of the proximal veins, thereby permitting a subsequent AVF creation when the AVG failed.
The strengths of our study include the large, nationally representative sample size derived from a random 5% Medicare sample to evaluate predialysis AVF creation, which may provide broadly generalizable results. Furthermore, this data set provides a representative data sample of United States patients with advanced CKD who progress to ESRD with accurate linkage to US Renal Data System (USRDS) data to identify primary outcomes of interest. We were able to measure actual AVF use upon dialysis initiation, information not reported in previous studies such as that by Oliver et al.14
Our study also has some limitations. First, we were not able to collect information about the patients’ serum creatinine, GFR, or rate of progression of CKD. However, predialysis permanent vascular accesses are usually not created until a patient reaches advanced CKD (stages 4 or 5). Second, the administrative data sets used did not include information that might affect the timing and type of predialysis vascular access, such preoperative vascular mapping, surgeon experience, or patient preferences. Third, we did not collect data on patient mortality or vascular access outcomes after dialysis initiation. However, our major goal was to study the events before dialysis initiation. Finally, there may be residual confounding because of patient characteristics that were not available in the data sets used for this study, and were therefore not incorporated into the statistical models.
In conclusion, the majority of elderly United States patients with advanced CKD receiving a predialysis permanent vascular access initiate dialysis within 2 years of access surgery. Although the large majority of patients with predialysis permanent vascular access creation have >1 year of nephrology care before initiating dialysis, a substantial proportion of those receiving an AVF undergo the surgery too late to optimize the likelihood of initiating dialysis with a mature AVF. Thus, in a subset of elderly patients with advanced CKD, the preferred strategy may be to place an AVG as a “catheter-sparing” strategy.
Concise Methods
Data Sources
We utilized the 1999–2011 Medicare 5% sample CKD-based cohort data set, a nationally representative sample of Medicare patients with CKD, to conduct this study. Specifically, the CKD patient, physician/supplier, and institutional claims were used to obtain data on hospitalization, surgery, comorbidities, and outpatient encounters. Insurance status and death were determined from the CKD payer sequence and master file, respectively. The CKD master file contains two patient identification (ID) numbers. One is a unique patient ID (FIVEP_ID) used in the 5% Medicare sample population. The second number (USRDS_ID) is the unique USRDS ID that identifies patients enrolled in the ESRD program and receiving dialysis. Only patients with ESRD are assigned the second number. Patients with CKD who initiated dialysis were linked by the USRDS ID to the US Centers for Medicare and Medicaid Services (CMS) Form 2728, which is required for all newly diagnosed patients with ESRD. Starting in 2005, the CMS Form 2728, filled out upon initiation of dialysis, began collecting data regarding the initial type of vascular access used for dialysis.
Study Population
We identified 7887 Medicare patients aged ≥70 years with a predialysis vascular access surgery performed between January 1, 2004, and December 31, 2009, using Common Procedural Terminology–4 codes 36818, 36819, 36820, 36825, and 36821 for AVF and 36830 for AVG creation. These codes have been widely used in other USRDS studies on vascular access.1,16,21–25 The date of vascular access creation was deemed the study index date. A vascular access surgery was determined to be a “first predialysis” surgery if there was no history of an earlier vascular access procedure in the 5 years before the study index date. Patients enrolled in a health maintenance organization were excluded (n=186) because we have no records of their health service utilization. We further excluded 4283 patients who started dialysis before or on the same day as the access surgery. Our final cohort includes 3418 older Medicare patients who had a predialysis vascular access created before dialysis initiation (Figure 1). A 5-year baseline before the index date (similar to one used by Oliver et al.14) was used to ensure a first predialysis vascular access surgery and to collect severity of illness and comorbidity information. A 2-year follow-up after the index date was used to examine study outcomes. Patients were followed from predialysis vascular access creation until initiation of dialysis, death, or end of 2-year follow-up.
Study Outcomes
Our primary outcome was the proportion of elderly patients with advanced CKD with AVF or AVG creation that survived to initiate dialysis, died before dialysis initiation, or survived dialysis free 2 years after vascular access creation. Our secondary analyses included the following: (1) the effect of demographic and clinical factors on the primary study outcomes, (2) the time intervals between predialysis vascular access creation and dialysis initiation, and (3) the type of vascular access used at the time of dialysis initiation by extent of predialysis nephrology care.
Variables of Interest
Patient demographics (age, sex, and race) were determined at the time of the index date. Comorbidities were determined by one inpatient diagnosis code (either primary or secondary) and/or two outpatient diagnosis codes in the year before the index date and included diabetes, cancer, ischemic heart disease, chronic obstructive pulmonary disease, peripheral vascular disease, cerebral vascular disease, depression, and dementia. Cardiac comorbidities (e.g., stroke, myocardial infarction, and congestive heart failure) were identified based on a primary reason for hospitalization during the baseline year. Comorbidities were selected based upon published literature indicating a substantial influence on mortality among patients with late-stage CKD.26,27 We used a comorbidity index developed and validated for dialysis patients, which outperformed the more widely used Charlson Comorbidity Index28 in both predictive ability and inference.29,30 At dialysis initiation, data from CMS Form 2728 on the type of vascular access used was collected, including AVF, AVG, catheter only, catheter with maturing AVF, and catheter with healing AVG. In a recent study, vascular access data as reported by CMS Form 2728 was determined to be valid and reliable for use in research studies.31 Evaluation by a nephrologist before dialysis initiation was determined by using physician supplier claims in the 2 years preceding dialysis. Patients who initiated dialysis before 2004 and 2005 were excluded because information on vascular access was not fully collected before 2005. Time between the first evaluation by a nephrologist and the start of dialysis were categorized as none or late evaluation (0–≤4 months), intermediate evaluation (4–≤12 months), and early evaluation (12–24 months).32
Statistical Analyses
Patients were classified into three groups according to whether they experienced an outcome of interest (dialysis or death): (1) AVF/AVG created, patient survived and initiated dialysis within 2 years; (2) AVF/AVG created, patient died within 2 years of AVF/AVG and did not start dialysis; and (3) AVF/AVG created, patient survived and no dialysis initiation within 2 years of AVF/AVG creation (e.g., nonprogressors). Summary statistics are presented as percentages for categorical data and means±SDs. Differences in baseline characteristics were compared using chi-squared tests. Because our cohort was exposed to mutually exclusive, competing risks of dialysis initiation and death before dialysis, the traditional Kaplan–Meier method and Cox model for survival analysis do not yield valid results.33,34 Using a method proposed by Fine and Gray,34 we applied a semiparametric Cox proportional hazards model that estimates the risk of starting dialysis compared with remaining in predialysis (nonprogression); in this model, death is treated as a competing event. We used SAS macro %CIF35 to estimate cumulative probability of starting dialysis over time. Cumulative incidence functions are also compared across different age, comorbid, and vascular access groups using Gray’s test.36 To assess the effect of covariates on time to dialysis initiation, we fit a proportional subdistribution hazards model34 by using an SAS macro37 that is specific for survival data subject to competing risks. The model is adjusted for age, sex, race, comorbidity score, and type of vascular access insertion. All analyses assume a two-sided, type 1 error probability set at <0.05.
Disclosures
T.L. is the chairman of the Data Safety Monitoring Board for the Bioconnect OPEN study. M.A. is a consultant for CorMedix and Gore.
Acknowledgments
T.L. is supported by an American Society of Nephrology Carl W. Gottschalk Scholar Grant. M.T. is supported by a grant from the National Institute of Aging (R21-AG043516). M.A. is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK085027).
The data reported herein were supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as the official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Lacson E, Jr, Wang W, DeVries C, Leste K, Hakim RM, Lazarus M, Pulliam J: Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 58: 235–242, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Lacson E, Jr, Wang W, Lazarus JM, Hakim RM: Change in vascular access and hospitalization risk in long-term hemodialysis patients. Clin J Am Soc Nephrol 5: 1996–2003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue H, Lacson E, Jr, Wang W, Curhan GC, Brunelli SM: Choice of vascular access among incident hemodialysis patients: A decision and cost-utility analysis. Clin J Am Soc Nephrol 5: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury BD, Chen F, Furniss A, Pisoni RL, Keen M, Mapes D, Krishnan M: Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis 53: 804–814, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 7.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: Should age be a consideration? Kidney Int 71: 555–561, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Fistula First Breakthrough Initiative: Fistula First National Access Improvements Initiative. Available at: http://esrdncc.org/ffcl/. Accessed June 3, 2014
- 9.Vascular Access 2006 Work Group : Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 10.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hod T, Desilva RN, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS: Factors predicting failure of AV “fistula first” policy in the elderly. Hemodial Int 18: 507–515, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Richardson AI, 2nd, Leake A, Schmieder GC, Biuckians A, Stokes GK, Panneton JM, Glickman MH: Should fistulas really be first in the elderly patient? J Vasc Access 10: 199–202, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE: Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 15: 1936–1942, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM: Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol 7: 466–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber CL, Djurdjev O, Levin A, Kiaii M: Outcomes of vascular access creation prior to dialysis: Building the case for early referral. ASAIO J 55: 355–360, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Danese MD, Liu Z, Griffiths RI, Dylan M, Yu HT, Dubois R, Nissenson AR: Catheter use is high even among hemodialysis patients with a fistula or graft. Kidney Int 70: 1482–1485, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, Chen K, Piera L, Saito A, Young EW: Timing of first cannulation and vascular access failure in haemodialysis: An analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant 19: 2334–2340, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Shingarev R, Maya ID, Barker-Finkel J, Allon M: Arteriovenous graft placement in predialysis patients: A potential catheter-sparing strategy. Am J Kidney Dis 58: 243–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS: Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297–1304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hod T, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS: Arteriovenous fistula placement in the elderly: When is the optimal time? J Am Soc Nephrol 26: 448–456, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solid CA, Carlin C: Timing of arteriovenous fistula placement and Medicare costs during dialysis initiation. Am J Nephrol 35: 498–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas SE, Feldman HI: Synthetic vascular hemodialysis access versus native arteriovenous fistula: A cost-utility analysis. Ann Surg 255: 181–186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands J, Perry M: Where are all the AV fistulas? Semin Dial 15: 146–148, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Collins AJ, Roberts TL, St Peter WL, Chen SC, Ebben J, Constantini E: United States Renal Data System assessment of the impact of the National Kidney Foundation-Dialysis Outcomes Quality Initiative guidelines. Am J Kidney Dis 39: 784–795, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Gullion CM, Keith DS, Nichols GA, Smith DH: Impact of comorbidities on mortality in managed care patients with CKD. Am J Kidney Dis 48: 212–220, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen SC, Bakris GL, McCullough PA: Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55[Suppl 2]: S23–S33, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Seliger SL: Comorbidity and confounding in end-stage renal disease. Kidney Int 77: 83–85, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Solid CA, Collins AJ, Ebben JP, Chen SC, Faravardeh A, Foley RN, Ishani A: Agreement of reported vascular access on the medical evidence report and on Medicare claims at hemodialysis initiation. BMC Nephrol 15: 30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Koller MT, Raatz H, Steyerberg EW, Wolbers M: Competing risks and the clinical community: Irrelevance or ignorance? Stat Med 31: 1089–1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 35.Lin G, So Y, Johnston G: Analyzing survival data with competing risks using SAS software. In: Proceedings of SAS Global Forum 2012, Cary, NC, SAS Institute Inc., 2012 [Google Scholar]
- 36.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16: 1141–1154, 1988 [Google Scholar]
- 37.Kohl M, Heinze G: PSHREG: A SAS Macro for Proportional and Nonproportional Subdistribution Hazards Regression with Competing Risk Data, Vienna, Austria, Medical University of Vienna Center for Medical Statistics, Informatics and Intelligent Systems, 2012 [Google Scholar]