Abstract
T lymphocytes are established mediators of ischemia reperfusion (IR)–induced AKI, but traditional immune principles do not explain their mechanism of early action in the absence of alloantigen. Nrf2 is a transcription factor that is crucial for cytoprotective gene expression and is generally thought to have a key role in dampening IR-induced AKI through protective effects on epithelial cells. We proposed an alternative hypothesis that augmentation of Nrf2 in T cells is essential to mitigate oxidative stress during IR-induced AKI. We therefore generated mice with genetically amplified levels of Nrf2 specifically in T cells and examined the effect on antioxidant gene expression, T cell activation, cytokine production, and IR-induced AKI. T cell–specific augmentation of Nrf2 significantly increased baseline antioxidant gene expression. These mice had a high frequency of intrarenal CD25+Foxp3+ regulatory T cells and decreased frequencies of CD11b+CD11c+ and F4/80+ cells. Intracellular levels of TNF-α, IFN-γ, and IL-17 were significantly lower in CD4+ T cells with high Nrf2 expression. Mice with increased T cell expression of Nrf2 were significantly protected from functional and histologic consequences of AKI. Furthermore, adoptive transfer of high-Nrf2 T cells protected wild-type mice from IR injury and significantly improved their survival. These data demonstrate that T cell–specific activation of Nrf2 protects from IR-induced AKI, revealing a novel mechanism of tissue protection during acute injury responses.
Keywords: AKI, Nrf2-Keap1, T cell, oxidative stress, inflammation
Ischemia reperfusion (IR)–induced AKI is associated with significant mortality in native kidneys and worsens outcomes after kidney transplantation.1–3 Excessive oxidative stress, apoptosis, epithelial and endothelial cell dysfunction, and inflammation are among the important pathophysiologic mechanisms during ischemic AKI.4–7 Recent work demonstrates an important pathophysiologic role for T lymphocytes in AKI, but the underlying mechanisms are poorly understood.8–11 Traditional mechanisms of immune activation and responses through allo- or self-antigen are not known to occur during AKI. Some data suggest that excessive oxidative stress, such as during IR injury, can either activate various subsets of T cells or reduce T cell function and compromise T cell receptor (TCR) signaling.12,13 However, the role of oxidative stress involvement in T cells during AKI is unknown, as is the effect of T cell–specific augmentation of antioxidant responses.
Studies on mechanisms of AKI from a number of teams demonstrate an important role for Nrf2, a transcription factor that regulates the expression of multiple antioxidant and phase II metabolism genes.14 Nrf2 is a key mediator that mitigates both ischemic and nephrotoxic AKI, as well as various other oxidative stress–driven diseases.15,16 To date, Nrf2 has been shown to work in AKI through effects on resident renal epithelial cells. The transcriptional activity of Nrf2 is regulated by kelch-like ECH-associated protein 1 (Keap1), which retains Nrf2 in the cytoplasm and promotes its proteolytic degradation. We therefore hypothesized that T cell–specific Nrf2-mediated signaling was an important converging mechanism by which both T cells and Nrf2 regulate AKI. To test this hypothesis, we generated mice with genetic deletion of Keap1 using a T cell–specific Cre-loxP recombination strategy.
Our data demonstrate that T cell–specific activation of Nrf2 increases the baseline frequency of kidney CD25+Foxp3+ regulatory T cells (Tregs) and significantly attenuates proinflammatory cytokine production by CD4+ T lymphocytes in the kidney. Furthermore, mice with high Nrf2 in T cells had fewer CD11b+CD11c+ and F4/80+ cells in their kidneys. The high Nrf2 activity in T cells resulted in significant structural and functional protection against IR-induced AKI. Furthermore, T cells with activated Nrf2 were effective as cell therapy for AKI when adoptively transferred to wild-type (WT) mice. These results demonstrate a novel mechanism by which T cells mediate AKI and reveal an unexpected cell type by which Nrf2 modulates acute tissue injury.
Results
T Cell–Specific Deletion of Keap1 Increases Basal Antioxidant Response
To examine the role of Nrf2-regulated antioxidant response in T cells and its effect on ischemic AKI, we genetically deleted Keap1 from T cells by breeding mice with the lox-P allele of Keap1 (Keap1F/F) with CD4-Cre mice (Figure 1A). The CD4-Cre transgene brings about selective deletion of genes flanked by the lox-P sequence in thymocytes at the double-positive (CD4+CD8+) stage. The Cre is silenced in doubled negative (DN) (CD4−CD8−) and CD4−CD8+ mature thymocytes.17 This strategy resulted in the generation of Keap1F/FCD4-Cre mice (hereafter referred to as CD4-Keap1-KO) with successful deletion of exons 2 and 3 of Keap1, mostly in CD4+ and CD8+ T cells (Figure 1, B and C). We observed no signs of physiologic or phenotypic abnormalities due to this deletion in these mice. To assess the effect of Keap1 deletion on Nrf2 activity, we measured the expression of Nrf2-regulated antioxidant genes in purified T cells from the spleen by real-time PCR. Purified T cells from CD4-Keap1-KO mice showed significantly higher Nqo1 (P≤0.01), Ho-1 (P=0.05), and Gclc (P≤0.01) mRNA expression at baseline compared with Keap1F/F mice (Figure 1D). There was no difference in the expression of Nrf2 mRNA between CD4-Keap1-KO and Keap1F/F mice, indicating that disruption of Keap1 does not affect Nrf2 transcription. Furthermore, Keap1 deletion significantly increased (P≤0.001) nuclear Nrf2 and cytoplasmic Nqo1 protein levels in T cells isolated from CD4-Keap1-KO mice compared with Keap1F/F mice (Figure 1, E and F). The effect of Keap1 deletion on antioxidant gene expression in this study is corroborated by previous studies in non–T cell models.18,19
Figure 1.
Generation and characterization of CD4-Keap1-KO mice. (A) CD4-Cre mice are crossed with Keap1F/F mice to generate CD4-Keap1-KO mice. (B) Mice are genotyped to confirm the presence of the Cre and Keap1 floxed allele using CRE and floxed primers. Lanes in B represent the following: lane 1, 100-bp DNA ladder; lane 2, 324-bp internal positive control showing CD4-Cre–negative mice; lane 3, 324-bp internal positive control and 100-bp Cre showing CD4-Cre–positive mice; lane 4, 383-bp Keap1 floxed allele in Keap1F/F mice; and lane 5, 383-bp Keap1 floxed allele in CD4-Keap1-KO mice. (C) CD4-Cre–mediated deletion of exons 2 and 3 of Keap1 is further confirmed by using deletion-specific primers. Lanes in C represent the following: lane 1, 1-Kb DNA ladder; lane 2, 2954-bp WT Keap1 allele; and lane 3, 288-bp truncated Keap1 allele after deletion of exons 2 and 3. (D) Deletion of Keap1 significantly upregulates the expression of Nrf2 targets Nqo1 (P≤0.001), Ho-1 (P=0.05), and Gclc (P≤0.01) in T cells; however, there is no change in Nrf2 and Gclm mRNA levels. (E) Western blot analysis of Nrf2 and Nqo1 in nuclear and cytoplasmic fractions of T cells isolated from CD4-Keap1-KO (n=3) and Keap1F/F mice (n=3). (F) Quantification of Nrf2 and Nqo1 levels in nuclear and cytoplasmic fractions. Data represent the mean±SD. *P≤0.05; **P≤0.01; ***P≤0.001.
Nrf2 Augmentation Affects Immune Cell Recruitment, Activation, and Intracellular Cytokine Production By T Cells
We further compared phenotypic changes, activation status, and cytokine production in CD45+TCR+ cells isolated from the kidney, inguinal lymph node (LN), and thymus of CD4-Keap1-KO and Keap1F/F mice at baseline (no IR). Although CD4-Cre is expressed at the CD4+CD8+ stage, we included CD4−CD8− (DN) T cells in our analysis because they represent a major component of normal and ischemic kidneys.20,21 Furthermore, some of them may be derived from reverting CD4+CD8+ T cells.22,23 Flow cytometric analysis of CD45+TCR+CD4+ cells revealed a significantly higher percentage of CD25+Foxp3+ Tregs in kidneys of CD4-Keap1-KO mice compared with Keap1F/F mice (4.1%±0.4% versus 2.8%±0.7%; P=0.02) (Figure 2A). Furthermore, the percentage of CD11b+CD11c+ dendritic cells (DCs) (14.4%±2.2% versus 21.2%±3.5%; P=0.01) and F4/80+ macrophages (9.8%±2.6% versus 12.2%±2.8%; P≤0.01), among total CD45+ kidney mononuclear cells from baseline kidneys of CD4-Keap1-KO mice, was significantly lower compared with Keap1F/F controls (Figure 2, B and C, respectively). Percentages of CD11b+ (29.7%±6.6% versus 37.3%±12.5%) and CD11c+ (15.5%±4.6% versus 24.6%±7.9%) cells were not different between CD4-Keap1-KO kidneys and Keap1F/F mice (Supplemental Figure 1). We further examined the expression of CD69 to assess activation status of CD4, CD8, and double-negative (DN) T cells isolated from kidneys of CD4-Keap1-KO and Keap1F/F mice (Figure 2D). We observed significantly lower CD69 expression in CD4 (24.9%±5.6% versus 52.7%±21.4%; P=0.04) and CD8 (21.4%±5.7% versus 35.6%±10.8%; P=0.05) cells from CD4-Keap1-KO kidneys compared with Keap1F/F counterparts. Percentage of CD69+ DNT cells were comparable (7.4%±1.5 versus 7.5%±2.6) between CD4-Keap1-KO and Keap1F/F mice.
Figure 2.
Baseline characteristics of T cells in CD4-Keap1-KO mice. (A–C) T cell–specific augmentation of Nrf2 results in higher percentages of CD25+Foxp3+ Tregs (A) and lower percentages of CD11b+CD11c+ and F4/80+ cells in CD4-Keap1-KO kidneys at baseline compared with Keap1F/F kidneys (B and C). (D) The percentage of CD69+ CD4, CD8, and DNT cells is lower in kidneys of CD4-Keap1-KO mice than in Keap1F/F mice. (E) Percentages of CD4, CD8, and DNT cells for baseline intracellular TNF-α, IFN-γ, and IL-17 are lower in kidneys of CD4-Keap1-KO mice compared with Keap1F/F mice. Representative flow images show selected populations and corresponding graphs show average percentages from four independent experiments. Data represent the mean±SD. *P≤0.05; **P≤0.01. KMNC, kidney mononuclear cell.
We then studied the effect of Nrf2 activation on proinflammatory cytokine production by renal T cells by assessing intracellular levels of TNF-α, IFN-γ, and IL-17 in CD4, CD8, and DNT cells isolated from CD4-Keap1-KO and Keap1F/F kidneys (Figure 2E). Baseline levels of intracellular TNF-α (6.6%±1.9% versus 9.8%±1.3%; P=0.03), IFN-γ (9.0%±1.2% versus 12.6%±1.8%; P=0.01), and IL-17 (5.2%±0.9% versus 6.8%±0.4%; P=0.02) were significantly lower in CD4 T cells from CD4-Keap1-KO mice than in Keap1F/F mice. We observed a similar trend in CD8 and DNT cells isolated from CD4-Keap1-KO mice kidneys but those changes were not statistically significant.
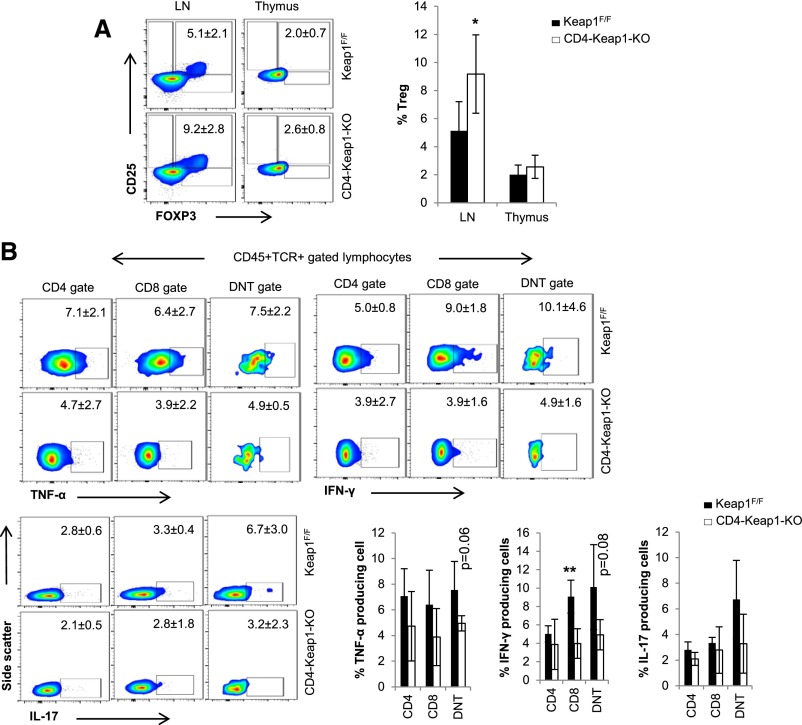
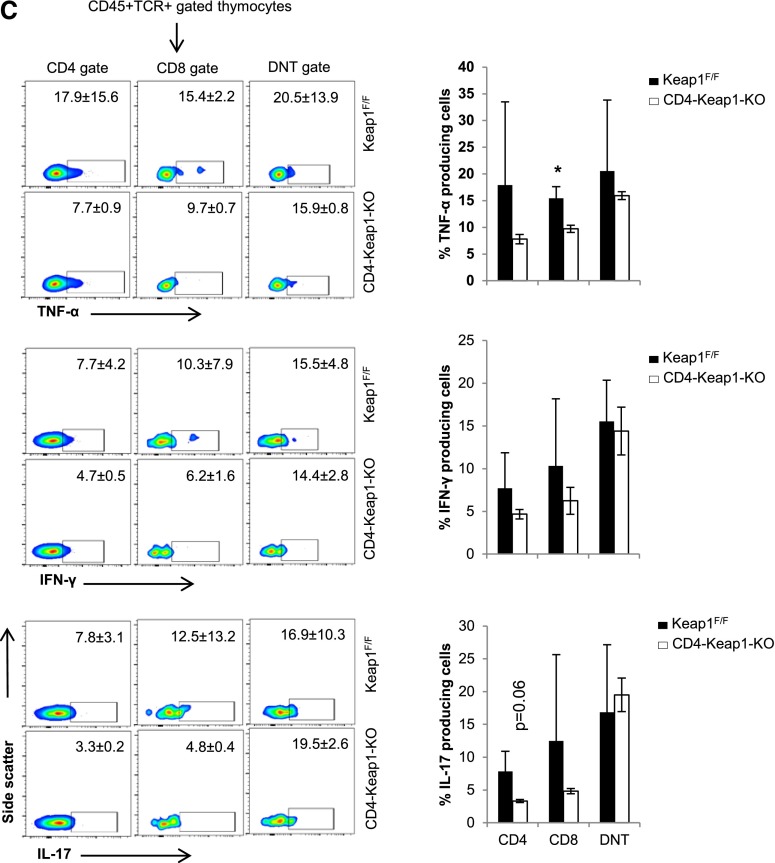
The frequency of CD4+CD25+FoxP3+ Tregs was higher in the LN (9.2%±2.3% versus 5.1%±2.1%; P=0.05) but was comparable in the thymus (2.6%±0.9% versus 2.0%±0.7%; P=0.42) of CD4-Keap1-KO mice compared with Keap1F/F mice (Figure 3A). The frequency of all Foxp3+ cells was significantly higher in the LN of CD4-Keap1-KO (4.8%±0.6% versus 2.6%±1.4%; P=0.03) but was comparable in the thymus (Supplemental Figure 2A). Baseline intracellular IFN-γ and TNF-α in CD8 T cells isolated from the LN and thymus of CD4-Keap1-KO mice were significantly attenuated (Figure 3, B and C, respectively). We did not observe any significant difference in the frequency of CD4, CD8, DNT, and double-positive populations in thymocytes between CD4-Keap1-KO and Keap1F/F mice, suggesting that T cell–specific augmentation of Nrf2 does not affect phenotypic diversity in T cell development (Supplemental Figure 2B).
Figure 3.
Frequency of Tregs and intracellular cytokines by lymphocytes isolated from inguinal LN and thymus at baseline. (A) The percentage of Tregs is significantly higher in the LN in CD4-Keap1-KO at baseline than in Keap1F/F mice. (B and C) Baseline intracellular TNF-α, IFN-γ, and IL-17 is lower in CD4, CD8, and DNT cells isolated from CD4-Keap1-KO LN (B) and thymus (C) than in Keap1F/F counterparts. Data represent the mean±SD. *P≤0.05; **P≤0.01.
T Cell–Specific Augmentation of Nrf2 Protects Kidneys from IR Injury
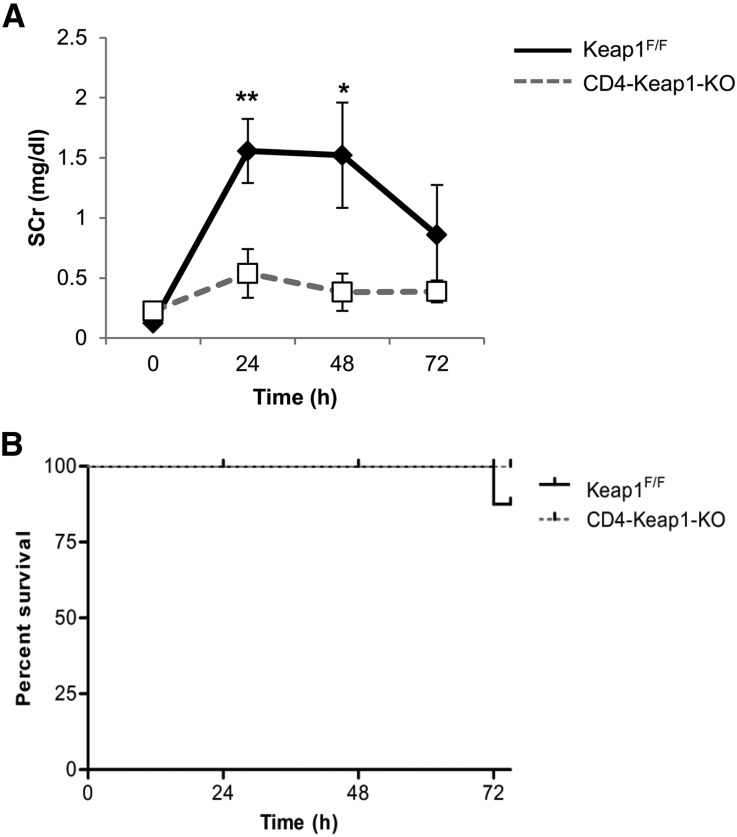
To further investigate the effect of T cell–specific Nrf2 activation on IR-induced AKI, we subjected CD4-Keap1-KO and Keap1F/F mice to a well established IRI model and evaluated structural and functional markers of kidney injury. We induced AKI by bilateral renal pedicle occlusion for 30 minutes followed by reperfusion. Increased antioxidant response in T cells in CD4-Keap1-KO mice resulted in significant protection from AKI compared with Keap1F/F mice. CD4-Keap1-KO mice exhibited significantly improved kidney function compared with Keap1F/F mice, indicated by reduced serum creatinine (SCr) levels at 24 hours (P≤0.01) and 48 hours (P≤0.05) after IR injury (Figure 4A). Furthermore, we observed no mortality in CD4-Keap1-KO mice, whereas approximately 20% of Keap1F/F mice died 72 hours after IR injury (Figure 4B).
Figure 4.
Effect of T cell–specific Keap1 deletion on IR-induced AKI. (A) Deletion of Keap1 from T cells in CD4-Keap1-KO mice (n=7) improves kidney function after bilateral IR injury compared with Keap1F/F mice (n=9). (B) There is no mortality in CD4-Keap1-KO mice; however, 20% of mice died in the control group 72 hours after IR injury. (C) Representative images of hematoxylin and eosin–stained kidney sections showing significantly fewer necrotic tubules and greater normal renal cortex and medullary tissue in CD4-Keap1-KO mice compared with Keap1F/F mice 24 and 72 hours after IR injury. (D) Dot plot showing the percent score for necrotic tubules and normal cortex and medulla for CD4-Keap1-KO (n=8–10) and Keap1F/F (n=9–11) mice 24 and 72 hours after IR injury. (E) Proinflammatory cytokine IFN-γ is lower in whole kidney lysates of CD4-Keap1-KO mice compared with Keap1F/F mice 72 hours after IR injury, whereas TNF-α, MCP-1, and IL-10 are not significantly different between the groups. Graphs represent the mean±SEM. *P≤0.05; **P≤0.01. MCP-1, monocyte chemoattractant protein-1. Original magnification, ×200 in C.
Histologic evaluation of kidney tissue assessed by an expert pathologist (L.C.R.) blinded to the mouse groups revealed significantly fewer necrotic tubules and more normal appearing tubules in cortical and outer medullary regions in CD4-Keap1-KO kidneys compared with Keap1F/F control kidneys (Figure 4, C and D). Proinflammatory cytokine analysis in the whole kidney lysate showed reduced levels of IFN-γ (21.2±1.8 versus 27.9±1.8; P=0.01). There was no significant difference in the other cytokines studied, including TNF-α (267.6±36 versus 400.1±53.5; P=0.07), monocyte chemoattractant protein-1 (136.9±5.9 versus 158.3±8.9; P=0.07), and the anti-inflammatory cytokine IL-10 (13±2.4 versus 8.8±1.1; P=0.1) (Figure 4E).
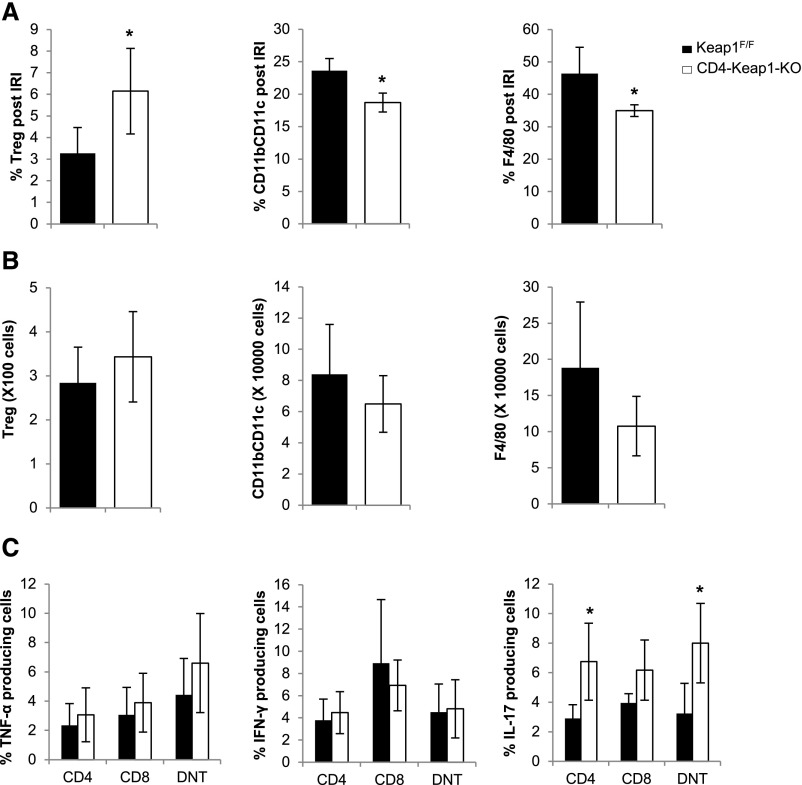
In an attempt to further understand the mechanism of structural and functional protection seen in CD4-Keap1-KO mice, we performed leukocyte phenotypic characterization and assessed intracellular cytokine levels in CD4-Keap1-KO and Keap1F/F mice after ischemic injury (Supplemental Figure 3). We observed a higher percentage of Tregs (6.1%±2% versus 3.3%±1.2%; P=0.04) and a lower percentage of CD11b+CD11c+ (18.7%±1.5% versus 23.6%±1.8%; P=0.03) and F4/80+ (34.9%±1.8% versus 46.4%±8.1%; P=0.03) cells in kidneys of CD4-Keap1-KO mice 24 hours after the induction of AKI (Figure 5A). Absolute numbers of Tregs were not significantly different (343.30±102.5 versus 284.1±80.9 cells) in post-IR kidneys of CD4-Keap1-KO mice, nor were CD11b+CD11c+ (6.4×104±1.8×103 versus 8.3×104±3.2×103 cells) and F4/80+ (1×105±4.1×103 versus 1.8×105±9.1×103 cells) compared with Keap1F/F mice (Figure 5B). Intracellular TNF-α and IFN-γ were comparable in CD4, CD8 and DNT cells isolated from CD4-Keap1-KO and Keap1F/F kidneys; however, intracellular IL-17 production was significantly higher from CD4 (6.7%±2.6% versus 2.9%±0.9%; P=0.03) and DNT (8%±2.7% versus 3.2%±2%; P=0.03) cells isolated from CD4-Keap1-KO kidneys (Figure 5C).
Figure 5.
Post-IR changes in kidney-infiltrating immune cells and cytokine production in CD4-Keap1-KO and Keap1F/F mice. (A) There is a significantly higher percentage of Tregs (P=0.04) and a lower percentage of CD11b+CD11c+ (P=0.02) and F4/80+ (P=0.03) cells in kidneys of CD4-Keap1-KO mice 24 hours after the induction of AKI. (B) Absolute numbers of Tregs (343.30±102.5 versus 284.1±80.9) and CD11b+CD11c+ (6.4×104±1.8×103 versus 8.3×104±3.2×103) and F4/80+ (1×105±4.1×103 versus 1.8×105±9.1×103) cells are not different between CD4-Keap1-KO and Keap1F/F mice at 24 hours after IR injury. (C) Intracellular IL-17 levels are higher in CD4, CD8, and DNT cells isolated 24 hours after IR from kidneys of CD4-Keap1-KO mice, whereas there is no difference in TNF-α and IFN-γ production. IRI, ischemia reperfusion injury. Data represent the mean±SD. *P≤0.05.
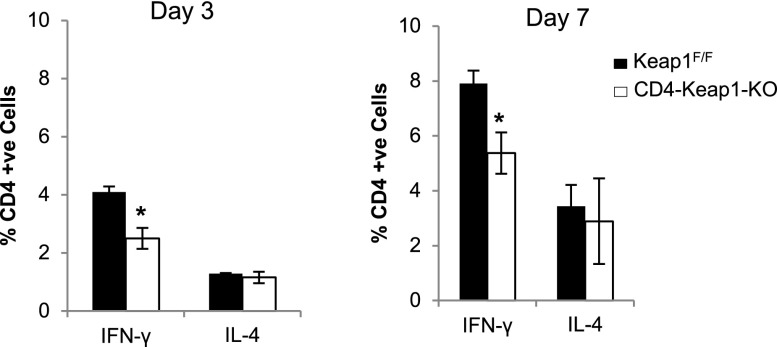
Augmentation of Nrf2 Decreases IFN-γ But Does Not Affect IL-4 Production by CD4+ T Cells
Based on the protection seen in our AKI model and in vivo intracellular data at baseline, we hypothesized that continuous Nrf2 activation in CD4-Keap1-KO mice resulted in T helper (Th) 2 type skewing in CD4+ T cells. Pharmacologic augmentation of Nrf2 has been shown to skew T cells toward the Th2 type that produces low levels of IFN-γ and high levels of IL-4.24 To test our hypothesis that T cell–specific Nrf2 activation by deleting Keap1 results in Th cell skewing, we purified CD4+ T cells from spleens of CD4-Keap1-KO and Keap1F/F mice and activated them in vitro with anti-CD3/CD28 antibodies under nonpolarizing conditions (without anti–IFN-γ and anti–IL-4) and measured intracellular levels of IFN-γ and IL-4 by flow cytometry. Consistent with our in vivo data and previously published data,24,25 we observed significantly fewer IFN-γ–producing CD4+ T cells at day 3 (P=0.03) and day 7 (P=0.05) in CD4-Keap1-KO mice compared with Keap1F/F counterparts. However, there was no difference in the IL-4–producing CD4+ T cell population in either CD4-Keap1-KO or Keap1F/F mice (Figure 6).
Figure 6.
In vitro activation of CD4+ T cells from spleens of CD4-Keap1-KO mice with anti-CD3/CD28 show attenuated IFN-γ production at day 3 (P=0.03) and day 7 (P=0.05) compared with Keap1F/F. There is no difference in IL-4–producing CD4+ T cell populations in either mouse. Data represent the mean±SD. *P≤0.05.
Adoptive Transfer of T Cells from CD4-Keap1-KO Mice Protects WT Mice from AKI and Improves Survival
To further test the hypothesis that T cell–specific activation of Nrf2 pathway protects from IR injury and to explore its clinical therapeutic relevance, we transferred T cells from CD4-Keap1-KO and Keap1F/F mice into WT C57Bl/6 mice by tail vein injection 24 hours before inducing AKI. The success of adoptive transfer was confirmed by establishing the presence of Carboxy fluorescein succinimidyl ester (CFSE) labeled T cells in peripheral blood of WT recipients before inducing AKI (Figure 7A). We observed a significant (P≤0.02) improvement in kidney function in WT mice receiving T cells from CD4-Keap1-KO mice, as indicated by reduced SCr levels (Figure 7B). Furthermore, adoptive transfer of T cells from CD4-Keap1-KO mice significantly (P≤0.01) improved survival of recipient WT mice after IR-induced AKI (Figure 7C).
Figure 7.
Effect of adoptive transfer of CD4-Keap1-KO T cells into WT (C57BL/6) mice (n=7–10). (A) The success of adoptive transfer is confirmed by establishing the presence of CFSE-labeled T cells in peripheral blood of WT recipients before inducing AKI. (B and C) Adoptive transfer of T cells from CD4-Keap1-KO mice significantly improves renal function (P=0.02) and improves survival (log-rank [Mantel–Cox] test, chi-squared P≤0.01) in WT mice after IR injury. Data represent the mean±SEM. *P≤0.05; **P≤0.01.
Discussion
T lymphocytes play an important pathophysiologic role in modulating ischemic and nephrotoxic AKI.8,9,26–30 T cells are present in the kidney during both ischemia and reperfusion, and thus are significantly exposed to various oxidant species that can modulate their function.9–13 In this study, we generated mice with genetically upregulated Nrf2 in T cells and tested them in an IR model of AKI. Our data demonstrate that T cell–specific augmentation of Nrf2 increases antioxidant response and affects phenotypic diversity, activation, and recruitment of immune cells and reduces intracellular cytokine production by T cells in the kidneys. Importantly, Nrf2 activation in T cells provides significant protection against IR-induced AKI and improves survival. Furthermore, adoptive transfer of Nrf2-activated T cells to WT mice improves outcomes from AKI.
We observed many differences in CD4-Keap1-KO mice compared with Keap1F/F mice that may be responsible for the protection seen in our experimental AKI model. Frequency of Tregs was significantly higher in CD4-Keap1-KO mice, whereas CD11b+CD11c+ DCs and F4/80+ macrophages were significantly reduced at baseline. Similar trends were observed for these cell types at 24 hours after IR injury, whereas Treg frequency remained significantly higher in CD4-Keap1-KO mice. These cell types are known to affect IR-induced kidney injury and repair. Multiple studies have demonstrated that Tregs promote postischemic kidney preconditioning and repair, suppress rejection, and induce allograft tolerance in kidney transplantation.30–33 Alternately, DCs and macrophages have been shown to worsen ischemic injury through various mechanisms and depletion of these cells protects the kidney from IR injury.34–38 Although numbers of DCs and macrophages increased after IR compared with baseline kidneys, their number was low in kidneys of CD4-Keap1-KO mice in this study. Attenuated DC and macrophage numbers in kidneys of CD4-Keap1-KO mice could be a direct effect of higher numbers of Tregs that regulate DCs and macrophage recruitment in the ischemic tissue.30 In addition, an increased Treg frequency in lymphoid tissue may provide a mechanism to attenuate inflammation during kidney IRI. Furthermore, T cells from CD4-Keap1-KO mice produced fewer proinflammatory cytokines than Keap1F/F mice. Although the exact mechanism by which T cell–specific Nrf2 activation ameliorates proinflammatory cytokine secretion is not clear, pharmacologic activation of Nrf2 with tert-butylhydroquinone and butylated hydroxyanisole was shown to skew T cells toward a Th2 type phenotype and suppress TNF-α and IFN-γ production after CD3/CD28 activation.24,25 We did not observe any Th2 skewing per se; nonetheless, purified CD4+ T cells from CD4-Keap1-KO mice produced less IFN-γ after in vitro CD3/CD28 activation, which is in concordance with our in vivo intracellular cytokine data at baseline and after IR injury. Li et al. recently demonstrated that activation of DCs with adenosine protects from AKI through modulation of natural killer (NK) T cell function and by attenuating IFN-γ secretion, accompanied by increased IL-10 levels and subsequently reduced postischemic inflammation.39 Because we observed reduced IFN-γ in post-IR kidneys of CD4-Keap1-KO mice, similar downstream effects along with phenotypic changes during AKI could be responsible for the protection from IR injury observed in this study. Furthermore, adoptive transfer experiments demonstrate that these T cells exert a strong protective effect given that they were transferred to WT mice with normal Nrf2 levels in T cells.
The pathogenesis of IR injury is complex and there is likely intricate crosstalk between multiple immune cells via production of cytokines, chemokines, oxygen free radicals, complement, and coagulant factors that accentuates tissue damage. Both NADPH oxidase and mitochondrial reactive oxygen species play critical pathophysiologic roles in AKI,40–43 but how T cell–specific Nrf2 augmentation affects these processes is not clear from this study. Recent studies demonstrate that engagement of TCR induces rapid production of reactive oxygen species and further modifies T cell signal transduction and gene expression.44,45 Oxidative stress further promotes T cell differentiation toward the Th2 phenotype under polarizing conditions.46 Additional data demonstrate that redox modulation suppresses CD8 T cell response to alloantigen and the TCR transgenic CD8 T cell responds to its cognate antigen by inhibiting proliferation, proinflammatory cytokine synthesis, and cytotoxic T lymphocyte effector mechanisms.47 In vitro–derived Th1 and Th2 clones or T cells derived from autoimmune thyroiditis have been shown to expand and produce cytokines in an oxidative environment.48 Furthermore, T cells are a heterogeneous group of cells with diverse functions and their interaction with DCs and macrophages during an ischemic event dictates the injury outcome. In this study, we observed attenuation of proinflammatory cytokines by CD4 and CD8 T cells, as well as by DNT cells. However, there are subtle differences in how different cytokines are regulated in the different lymphocytes by the Keap1 deletion. The response by DNT cells is particularly interesting because we did not predict them to be affected by CD4-Cre–mediated deletion of Keap1.17 This may be an effect of their interaction with other immune cells and over all cytokine milieu. Furthermore, a proportion of DNT cells may represent the reverting double-positive (CD4+ CD8+) T cells.22,23 Therefore, T cell–specific activation of Nrf2-regulated antioxidant response appears to help in the maintenance of a low proinflammatory environment and optimal T cell function that subsequently results in reduced oxidative and inflammatory tissue injury. It is important to note that Nrf2-independent effects of Keap1 deletion in T lymphocytes may also be involved. Keap1 acts as an adapter protein for the E3 ubiquitin ligase complex that directs multiple proteins, including Nrf2, for proteasomal degradation. In addition, Keap1 has complex interactions with many other proteins that regulate NF-κB activation, T cell proliferation, integrin expression, and perforin production in NK cells. Keap1 has been linked to inflammation, autophagy, and apoptosis.49–51 Further experiments are warranted to identify Nrf2-independent effects of Keap1 deletion in T cells.
In summary, augmented antioxidant response in CD4-Keap1-KO mice increased the basal Treg population, reduced numbers of CD11b+CD11c+ and F4/80+ cells and promoted an anti-inflammatory environment in the kidney. These results demonstrate that basal Nrf2 levels in T cells have widespread effects on immune cell activation and injury outcome after an ischemic event. Despite the promise of Nrf2 targeting for kidney diseases, the search for an optimal Nrf2 activator is ongoing.52–54 Our data demonstrate that adoptively transferred T cells from CD4-Keap1-KO mice produced a strong protective effect in the WT mice; thus, activation of Nrf2 in T cells holds promise for immune cell therapy.
Concise Methods
Generation and Characterization of CD4-Keap1-KO Mice
T cell–specific Keap1-deficient (referred to as CD4-Keap1-KO) mice were generated by crossbreeding Keap1F/F mice with CD4-Cre mice. Keap1 floxed mice used for these studies were kindly provided by Dr. Shyam Biswal and have been completely characterized.18,19 CD4-Cre mice were purchased from Taconics (Hudson, NY). In these CD4-Cre mice, the transgene is under the control of the CD4 promoter/enhancer/silencer, which first allows expression of CD4-Cre in thymocytes at the double-positive (CD4+CD8+) stage. The silencer region extinguishes transgene expression at the DN (CD4−CD8−) stage as well as in the CD4−CD8+ stage. The mice were genotyped to confirm the presence of the Cre transgene, flox status, and deletion of Keap1 exons 2 and 3 with PCR using primer sets shown in Supplemental Table 1.
Mouse Model of AKI
An established mouse model of renal IR injury was used. All animal experiments were performed using Johns Hopkins University Institutional Animal Care and Use Committee–approved protocols. Animals were anesthetized with sodium pentobarbital (Voshell’s Pharmacy, Baltimore, MD) at a dose of 75 mg/kg (intraperitoneal injection). The mice were put on a heating pad (45°C) during the procedure and core body temperature was maintained at approximately 37°C. Left and right renal pedicles were bluntly dissected after laparotomy and ischemia was induced by placing a nontraumatic microvascular clip (Roboz, Gaithersburg, MD) on each renal pedicle for 30 minutes. During the procedure, mice were well hydrated by infusing warm saline (37°C–40°C) directly into the peritoneal cavity. The kidneys were allowed to reperfuse by removing the microvascular clips, wounds were sutured, and animals were allowed to recover on the heating pad. Once awake, the mice were transferred to a clean cage and housed in the animal facility at room temperature with food and water ad libitum.
Assessment of Renal Function
Blood samples were obtained from the tail before (0 hours) and 24, 48, and 72 hours after kidney IR injury to collect serum. SCr was measured as a marker of renal function by a Cobas Mira Plus automated analyzer system (Roche) by using creatinine measurement reagents (Pointe Scientific Inc., Canton, MI).
Histologic Evaluation of Kidney Injury
Upon euthanasia, the kidneys were harvested and cut into three equal transverse pieces. One piece from each kidney was fixed with 10% buffered formalin phosphate and embedded with paraffin for histologic evaluation. The remaining two kidney pieces were either snap-frozen with liquid nitrogen or stored in RNAlater solution (Life Technologies, Grand Island, NY) for molecular studies. Tissue sections (5 µm) were stained with hematoxylin and eosin. A renal pathologist (L.C.R.) at Johns Hopkins Hospital, who was blinded to the experimental groups, scored the percentage of necrotic tubules out of total tubules in each of at least 10 high-power fields in the cortex and outer medulla, and the average percentage of tubular necrosis in all fields was presented as the renal tubular injury score of each mouse.
Antioxidant Gene Expression Analyses
Total RNA (1 µg) from purified T cells was isolated with the RNeasy mini kit (Qiagen, Valencia, CA) and reverse transcribed using a high-capacity cDNA synthesis kit (Life Technologies). A gene-specific TaqMan primer and probe sets were used to assess transcriptional status of Nrf2, Nqo1, Ho-1, Gclm, and Gclc in Quantstudio 12K flex real-time PCR (Life Technologies). The absolute expression values for each gene were normalized to that of β-actin and the relative gene expression values calculated.
Assessment of Kidney Inflammation
Levels of IFN-γ, TNF-α, monocyte chemoattractant protein-1, and IL-10 were assessed by the Bio-Plex multiple cytokine kit (Bio-Rad, Hercules, CA) to evaluate inflammation of kidney tissue. The total protein concentration of each sample was determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL) and was used to normalize the measured cytokine levels.
Phenotypic Characterization and Intracellular Cytokine Analyses
Phenotypic characterization and intracellular cytokine analysis in kidney-infiltrating CD45+ T cells, inguinal LN lymphocytes, and thymocytes were performed in normal and postischemic mice. The following fluorochrome-conjugated mAbs to mouse antigens were used to construct four different panels (Supplemental Table 2) for flow cytometry analysis: CD45-APC-Cy7 (BioLegend, San Diego, CA), TCR-Pacific Blue/APC (Invitrogen/BioLegend), CD4-PE-Cy7/PE (BioLegend/BD Biosciences, Franklin Lakes, NJ), CD8-PerCP (BioLegend), CD25-APC (eBioscience, San Diego, CA), CD19-APC (BioLegend), NK1.1-APC (eBioscience), Foxp3-PE (eBioscience), IFN-γ–PE (BD Biosciences, Franklin Lakes, NJ), F4/80+-PE (eBioscience), CD69-FITC (BD Biosciences), CD11b+-FITC (BD Biosciences), TNF-α–FITC (BD Biosciences), Ly-6G(Gr1)-FITC (eBioscience), CD11c+-PE-Cy7 (eBioscience), and IL-17–BV-605 (BioLegend). Briefly, kidney mononuclear cells were isolated using density gradient centrifugation (Percoll) as previously described55 and CD45+ cells were enriched using CD45 microbeads (Miltenyi Biotech, San Diego, CA). Freshly isolated lymphocytes from the kidney (approximately 5×106 cells) LN (1×106 cells), and thymus (1×106 cells) were stimulated with PMA (5 ng/ml) and ionomycin (500 ng/ml) before staining for surface markers and intracellular cytokines. Labeled samples were analyzed with the LSRII flow cytometer (BD Biosciences). Controls (fluorescence minus one) were used to correctly identify and gate cell populations during analysis using FlowJo software (TreeStar Inc., Ashland, OR).
CD4+ T Cell Activation In Vitro
CD4+ T cells were isolated with the CD4+ T cell isolation kit (Miltenyi Biotech). Briefly, approximately 1×106 cells/ml per well were plated in a 24-well plate precoated with CD3/CD28 (1 µg/ml) and IL-2 (20 IU/ml). At days 3 and 7, intracellular levels of IFN-γ and IL-4 were analyzed by flow cytometry as described earlier.
Adoptive Transfer of T Cells
T cells were isolated from mouse spleen using the Pan-T cells isolation kit (Miltenyi Biotech) and approximately 15×106 T cells were adoptively transferred to WT C57Bl/6 mice (The Jackson Laboratory, Bar Harbor, ME) by tail vein injection. T cells were CFSE labeled to confirm the success of tail vain injection. Twenty-four hours after T cell transfer, the mice underwent IR-induced AKI. The presence of transferred CFSE-labeled T cells was confirmed in recipient blood before IR surgery.
Immunoblotting
T cells were isolated from CD4-Keap1-KO (n=3) and Keap1F/F mice (n=3) as described earlier, and nuclear and cytoplasmic extracts were prepared using the NE PER kit (Thermo Fisher Scientific). For Western blot analysis, a total of 50 µg cytoplasmic extract and 20 µg nuclear extract from each sample were separated on a 10% SDS-PAGE, and the membranes were probed with antibodies specific for Nrf2 (Santa Cruz Biotechnology, Santa Cruz, CA) and Nqo1 (NeoBioLab, Woburn, MA). β-actin (Sigma-Aldrich, St. Louis, MO) and Lamin B (Santa Cruz Biotechnology) were used as the loading controls. The blots were developed using an enhanced chemiluminescence kit (HyGlo; Denville Scientific Inc., Metuchen, NJ) and band intensities were measured using ImageJ Software (National Institutes of Health, Bethesda, MD).
Statistical Analyses
Data are presented as the mean±SEM or SD, and are compared by a paired, two-tailed t test for a single comparison between two groups. Cumulative survival was analyzed by the log-rank (Mantel–Cox) test. Statistical significance of difference was defined as a P value ≤0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Shyam Biswal for providing the Keap1F/F mice.
This work was funded and supported by the National Institutes of Health (Grant R01-DK084445) and a generous gift from Mr. Rogelio Miro of Panama.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Oxidative Stress and Metabolism: The NF–Erythroid 2 p45–Related Factor 2:Kelch–like ECH–Associated Protein 1 System and Regulatory T Lymphocytes in Ischemic AKI,” on pages 2893–2895.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014100978/-/DCSupplemental.
References
- 1.Clarkson MR, Friedewald JJ, Eustace JA, Rabb H: Acute kidney injury. In: Brenner & Rector’s The Kidney, edited by Brenner BM, 8th Ed., Philadelphia, Saunders, Elsevier, 2008, pp 943–986 [Google Scholar]
- 2.Srisawat N, Kellum JA: Acute kidney injury: Definition, epidemiology, and outcome. Curr Opin Crit Care 17: 548–555, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Jang HR, Ko GJ, Wasowska BA, Rabb H: The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 87: 859–864, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Kinsey GR, Okusa MD: Role of leukocytes in the pathogenesis of acute kidney injury. Crit Care 16: 214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron, Exp Nephrol 109: e102–e107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao X, Hu Y, Quirós PM, Wei Q, López-Otín C, Dong Z: OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol 306: F1318–F1326, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, Tang WW: Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 279: F525–F531, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Rabb H, Womer KL: Ischemia-reperfusion and immediate T cell responses. Cell Immunol 248: 4–11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinsey GR, Okusa MD: Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens 23: 9–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cemerski S, van Meerwijk JP, Romagnoli P: Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur J Immunol 33: 2178–2185, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Cemerski S, Cantagrel A, Van Meerwijk JP, Romagnoli P: Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem 277: 19585–19593, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kensler TW, Wakabayashi N, Biswal S: Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H: Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76: 277–285, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Saito H: Toxico-pharmacological perspective of the Nrf2-Keap1 defense system against oxidative stress in kidney diseases. Biochem Pharmacol 85: 865–872, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Pérez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB: A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15: 763–774, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S: Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol 42: 524–536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S: Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 184: 928–938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, Soloski MJ, Rabb H: Normal mouse kidneys contain activated and CD3+CD4- CD8- double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martina MN, Bandapalle S, Rabb H, Hamad AR: Isolation of double negative αβ T cells from the kidney. J Vis Exp 16: 1–6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juvet SC, Zhang L: Double negative regulatory T cells in transplantation and autoimmunity: Recent progress and future directions. J Mol Cell Biol 4: 48–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Yang W, Degauque N, Tian Y, Mikita A, Zheng XX: New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood 109: 4071–4079, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockwell CE, Zhang M, Fields PE, Klaassen CD: Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol 188: 1630–1637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwell CE, Klaassen CD: Inhibition of IFN production by the Nrf2 activators, tBHQ and BHA, in activated murine T cells. FASEB J 22: 1139.4, 2008 [Google Scholar]
- 26.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H: Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol 285: F87–F94, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Linfert D, Chowdhry T, Rabb H: Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 23: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H: Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int 69: 233–238, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD: Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int 77: 771–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Bestard O, Cruzado JM, Rama I, Torras J, Gomà M, Serón D, Moreso F, Gil-Vernet S, Grinyó JM: Presence of FoxP3+ regulatory T cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol 19: 2020–2026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dummer CD, Carpio VN, Gonçalves LF, Manfro RC, Veronese FV: FOXP3+ regulatory T cells: From suppression of rejection to induction of renal allograft tolerance. Transpl Immunol 26: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Dong X, Bachman LA, Miller MN, Nath KA, Griffin MD: Dendritic cells facilitate accumulation of IL-17 T cells in the kidney following acute renal obstruction. Kidney Int 74: 1294–1309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi E, Suzuki T, Yamamoto M: Roles nrf2 plays in myeloid cells and related disorders. Oxid Med Cell Longev 2013: 529219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK: Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD: Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J Clin Invest 122: 3931–3942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK: Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int 63: 179–185, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS: T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol 5: 818–827, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS: Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38: 225–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan M, Brooks C, Liu F, Sun L, Dong Z: Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83: 568–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams MS, Kwon J: T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med 37: 1144–1151, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Larbi A, Kempf J, Pawelec G: Oxidative stress modulation and T cell activation. Exp Gerontol 42: 852–858, 2007 [DOI] [PubMed] [Google Scholar]
- 46.King MR, Ismail AS, Davis LS, Karp DR: Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J Immunol 176: 2765–2772, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Sklavos MM, Tse HM, Piganelli JD: Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med 45: 1477–1486, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Frossi B, De Carli M, Piemonte M, Pucillo C: Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol Immunol 45: 58–64, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Stępkowski TM, Kruszewski MK: Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med 50: 1186–1195, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Skopeliti M, Iconomidou VA, Derhovanessian E, Pawelec G, Voelter W, Kalbacher H, Hamodrakas SJ, Tsitsilonis OE: Prothymosin alpha immunoactive carboxyl-terminal peptide TKKQKTDEDD stimulates lymphocyte reactions, induces dendritic cell maturation and adopts a beta-sheet conformation in a sequence-specific manner. Mol Immunol 46: 784–792, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Ueda H, Fujita R, Yoshida A, Matsunaga H, Ueda M: Identification of prothymosin-alpha1, the necrosis-apoptosis switch molecule in cortical neuronal cultures. J Cell Biol 176: 853–862, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, Winterberg P, Chen B, Agarwal A, Lu CY: Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Renal Physiol 300: F1180–F1192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB: The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789–4798, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Liu M, Reddy NM, Higbee EM, Potteti HR, Noel S, Racusen L, Kensler TW, Sporn MB, Reddy SP, Rabb H: The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int 85: 134–141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H: Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.