Abstract
Intimal arteritis (the presence of v-lesions) in kidney transplant biopsy specimens is believed to have major prognostic and diagnostic significance. We assessed the relationship of v-lesions to prognosis in 703 indication biopsy specimens and used microarray-based molecular tests to re-examine the relationship of v-lesions to rejection. v-Lesions were noted in 49 specimens (7%) and were usually mild (v1). The presence of v-lesions had no effect on graft survival compared with the absence of v-lesions. Pathologists using current conventions almost always interpreted v-lesions as reflecting T cell–mediated rejection (TCMR), either pure or mixed with antibody-mediated rejection (ABMR). The molecular scores questioned the conventional diagnoses in 29 of 49 specimens (59%), including ten that were conventional TCMR with no molecular rejection and nine that were conventional TCMR mixed with pure ABMR molecularly. The presence of tubulointerstitial inflammation (i-t) meeting TCMR criteria allowed subclassification of v-lesion specimens into 21 i-t-v-lesion specimens and 28 isolated v-lesion specimens. Molecular TCMR scores were positive in 95% of i-t-v-lesion specimens but only 21% of isolated v-lesion specimens. Molecular ABMR scores were often positive in isolated v-lesion biopsies (46%). Time of biopsy after transplantation was critical for understanding isolated v-lesions: most early isolated v-lesion specimens had no molecular rejection and were DSA negative, whereas most isolated >1 year after transplantation had positive DSA and ABMR scores. Therefore, v-lesions in indication biopsy specimens do not affect prognosis and can reflect TCMR, ABMR, or no rejection. Time after transplantation, DSA, and accompanying inflammation provide probabilistic basis for interpreting v-lesions.
Keywords: kidney biopsy, kidney, kidney transplantation
Vascular rejection, meaning rejection with arteritis (v-lesions), has long been regarded as an ominous finding in renal transplant biopsies,1 but the mechanisms underlying the arterial lesions are problematic. The older literature on vascular rejection is difficult to interpret because in that era T cell–mediated rejection (TCMR) could not be distinguished from antibody-mediated rejection (ABMR) and because many patients had severe changes, such as panarteritis and transmural necrosis (v3-lesions),2 that are now rare in indication biopsies. Development of more effective immunosuppression on the basis of calcineurin inhibitors and mycophenolate3,4 led to major reductions in early rejection, especially TCMR,5 and consequently in v-lesions.6,7 As well, improved cross-matching reduced the incidence of early ABMR in sensitized patients. This data drift—effect of changing practices, diagnostic systems, and outcomes on the interpretation of test results—means that the mechanisms and significance of v-lesions gleaned from experience decades ago must be reinterpreted in the current era (i.e., in biopsies performed with current immunosuppression and cross-matching practices).
Although v-lesions have been known for many years to occur in some cases with ABMR,5,8–14 the histology consensus 2007 guidelines indicate that v-lesions can be used to diagnose TCMR.2 The 2013 Banff report acknowledged that v-lesions can occur in type 1 ABMR15 but did not change the guidelines for using v-lesions to diagnose TCMR. These guidelines for histologic diagnosis of TCMR, including the use of v-lesions for this purpose, are presently under consideration (Mark Haas, personal communication, 2014), leaving the 2007 guidelines in place for TCMR. The major issue is how to distinguish ABMR v-lesions from TCMR v-lesions, given that many ABMR patients are C4d-negative.16 ABMR occurs in two forms: type 1, early-onset in presensitized patients, usually with preexisting donor-specific HLA antibodies (DSAs) at the time of transplantation; and type 2, the common variety that begins to present in indication biopsies at the end of the first year, associated with de novo DSA formation.17 v-lesions were first recognized in type 1 ABMR18 and more recently in type 2 ABMR.1,11,18–24 Patients transplanted with preexisting DSAs and early type 1 ABMR often present with v3-lesions and have a poor prognosis,21,23–26 but the significance of v-lesions in the common type 2 ABMR appearing many years post-transplant needs to be clarified.27 The interpretation of v-lesions without tubulointerstitial inflammation (i-t), isolated v-lesions, has been particularly difficult to resolve. A comparison of 23 biopsies with isolated v-lesions to 23 matched biopsies with inflammation was unable to identify significant differences28 but underscored the concerns about ABMR.
The emergence of microarray-based tests for TCMR and ABMR offers an opportunity to re-examine the significance of v-lesions and their relationship to underlying disease states and to help resolve some uncertainty, such as the use of v-lesions to diagnose TCMR.29–32 The molecular TCMR and ABMR scores are highly correlated with conventional assessments, indicating that conventional and microarray tests independently detect the same underlying diseases.29–32 Therefore, the ABMR and TCMR scores provide an estimate of the probability of TCMR, ABMR, or both in biopsies independent of histologic diagnoses and can assess the association of lesions with TCMR or ABMR to assist the evolution of the diagnostic guidelines. This is supported by earlier analyses in which microarray-based TCMR scores were often very low in biopsies with isolated v-lesions (i.e., v-lesions without i-t meeting the conventional definition of TCMR).11,20,29,33–35
The goals of this study were to reassess the significance of v-lesions for prognosis and to use molecular tests to understand their relationship to TCMR and ABMR. We studied a reference set of 703 unselected transplant indication biopsies from consenting patients with histology, HLA antibody status, and microarray data, prospectively collected as a sample of troubled transplants in the prevalent international transplant population.17 The hypothesis was that, with microarray assessment as a guide, we could discover how to use associated findings, such as time post-transplant, DSA, and i-t to form a probabilistic basis for interpreting biopsies with v-lesions using conventional criteria, pending the wider availability of molecular tests. If so, this could help in the development of new guidelines for interpreting v-lesions.
Results
Study Population
All 703 biopsies were classified using the current histology guidelines, including the 2008 TCMR guidelines still in effect,2,15 finding 49 biopsies with v-lesions (7%). Conventional assessment diagnosed TCMR in 47 biopsies (96%): 30 pure (61%) and 17 mixed with ABMR (35%) (Supplemental Table 1, Table 1). The only exceptions were two biopsies with polyoma virus nephropathy, where pathologists are often reluctant to diagnose TCMR.29 Most v-lesions were relatively mild: 36 biopsies were assessed as v1, one as v2, and only two as v3. Nine of the 10 failures of grafts with v-lesions were after biopsies with v1.
Table 1.
Association of conventional diagnosis with v-lesion grade and failures in 49 biopsies with v-lesions, identified in 703 indication biopsies from 564 kidneys
| Conventional Diagnosis | v1 (n=36) | v2 (n=11) | v3 (n=2) | Total (n=49) | No. Failed (No. in Each v-Lesion Grade) (n=10) |
|---|---|---|---|---|---|
| Pure TCMR | 24 | 5 | 1 | 30 (61%) | 5 (all v1) |
| Mixed | 10 | 6 | 1 | 17 (35%) | 5 (v1: n=4; v2: n=1) |
| No rejection | 2a | 0 | 0 | 2 (4%) | 0 |
| No. failed in each v-lesion grade | 9 | 1 | 0 | 10 |
Thirty biopsies were deleted from the analyses because no v-lesion was recorded. One biopsy previously called pure ABMR was recently reviewed and reclassified as TCMR, making these numbers slightly different from previous analyses.
Histologic lesions indicating TCMR are often not reported as rejection in biopsies with polyoma virus nephropathy because of uncertainty about whether the lesions reflect rejection versus virus effects (see text).
Relationship between v-Lesions in Biopsies and Risk of Progression To Graft Failure
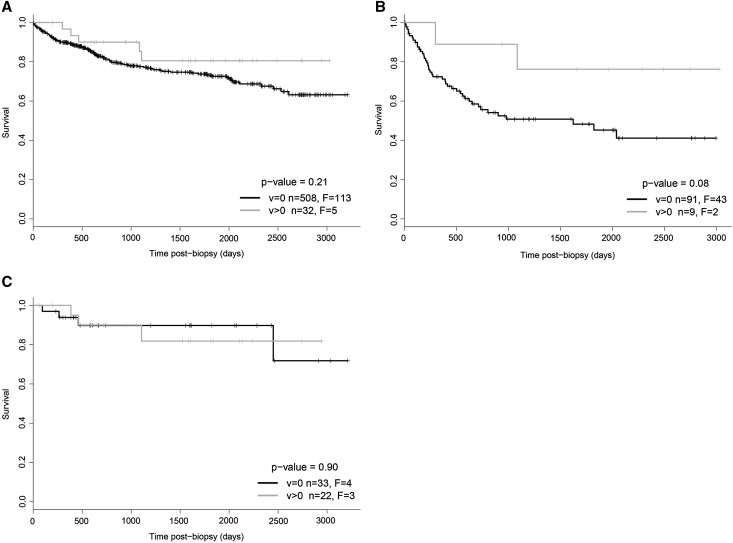
The presence of v-lesions did not increase the probability of progression to failure compared with kidneys with biopsies showing no v-lesions, whether examined in all biopsies (Figure 1A) or in biopsies conventionally diagnosed as ABMR and mixed rejection (Figure 1B) or pure TCMR (Figure 1C). In this analysis biopsies with no v-lesion assessment recorded were excluded.
Figure 1.
Kaplan–Meier curves for biopsies in v=0 and v>0 for 540 kidneys with one random biopsy per kidney, excluding biopsies with no recorded v-lesions. Because some patients had more than one biopsy, the survival analysis is presented by selecting one random biopsy per kidney from the 564 kidneys, from which the v-lesion positive and v-lesion negative biopsies were identified, and the biopsies in which no v assessment is recorded are excluded. (A) All 540 kidneys; (B) conventional ABMR and mixed rejection; and (C) conventional TCMR. Vertical ticks indicate censoring. F, failures; n, total number of kidneys per group.
The rarity of v3-lesions precluded assessment of their prognostic significance.
Distribution of v-Lesions over Time
The time post-transplant of biopsies with v-lesions varied from a few days to 13.7 years (Table 2): 28 in the first year, 10 between 1 and 5 years; and 11 after 5 years. The frequency of v-lesions was higher in biopsies <1 year post-transplant (9%) compared with biopsies >1 year (5%) post-transplant (Table 2).
Table 2.
Incidence of intimal arteritis in 703 biopsies over time
| Biopsy Group | No. of Biopsies with v-Lesions by Time from Transplant to Biopsy | |||
|---|---|---|---|---|
| ≤1 y (n=298) | 1–5 y (n=190) | >5 y (n=215) | Total (n=703) | |
| No. with v-lesions (% of total for interval) | 28 (9) | 10 (5) | 11 (5) | 49 (7) |
| i-t-v-Lesions: v>0 and (i≥2 and t≥2) | 16 | 5 | 0 | 21 |
| Isolated v-lesions: v>0 and (i<2 or t<2) | 12 | 5 | 11 | 28 |
| Conventional diagnoses in v-lesion biopsies | ||||
| Pure TCMR | 24 | 3 | 3 | 30 |
| Mixed | 2 | 7 | 8 | 17 |
| No rejection | 2a | 0 | 0 | 2 |
Histologic lesions indicating TCMR are often not reported as rejection in biopsies with polyoma virus nephropathy because of uncertainty about whether the lesions reflect rejection versus virus effects (see text).
Twenty-one v-lesion biopsies had i-t, meeting the criteria for TCMR, and were subclassified as i-t-v-lesion biopsies (Table 2). The other 28 were classified as isolated v-lesion biopsies. The timing of i-t-v-lesion biopsies differed from that of isolated v-lesion biopsies: all 21 i-t-v-lesion biopsies were before 5 years, whereas the isolated v-lesion biopsies were distributed both before (n=17) and after 5 years (n=11). All 11 v-lesion biopsies after 5 years had isolated v-lesions.
Most v-lesion biopsies before 1 year were assigned a conventional diagnosis of pure TCMR, whereas most v-lesion biopsies after 1 year, when ABMR becomes common,36 were diagnosed conventionally as mixed rejection (i.e., TCMR plus ABMR).
Response To Treatment
Analysis of the effect of treatment postbiopsy was complicated by incomplete treatment records. Antirejection treatment after biopsy (high-dose steroids, sometimes followed by antithymocyte globulin) was recorded after 27 of the 49 biopsies with v-lesions (Supplemental Table 2). Patients with i-t-v-lesions had a high creatinine at the time of biopsy and a significant fall in creatinine at 1 month postbiopsy. Some patients with isolated v-lesions were recorded as receiving no treatment postbiopsy, unlike patients with i-t-v-lesion biopsies.
Relationship between Molecular Scores and v-Lesions
Molecular scores as an independent estimate of the probability of TCMR and ABMR were compared with the conventional assessment in the 49 v-lesion biopsies (Table 3). Using the published cutoffs (TCMR positive >0.1; ABMR positive >0.2),29,30 the molecular tests found that 18 had pure TCMR, 10 had pure ABMR, eight were mixed, and 13 had no rejection. Discrepancies between conventional and molecular assessment of rejection occurred in 29 of the 49 biopsies (59%): (1) 15 of 29 biopsies assessed conventionally as pure TCMR did not have pure TCMR molecularly: 10 had no rejection, and four were mixed; (2) 13 of 17 assessed conventionally as mixed rejection were not mixed molecularly: nine were pure ABMR, two were pure TCMR, and two had no rejection; and (3) one of two biopsies assessed as no rejection conventionally had pure TCMR molecularly.
Table 3.
Comparison between conventional and molecular diagnoses in 49 biopsies with v-lesions
| Diagnosis by Molecular Scores | Diagnosis by Conventional Assessment | Total (n=49) | ||
|---|---|---|---|---|
| Pure TCMR (n=30) | Mixed (n=17) | No Rejection (n=2) | ||
| Pure TCMR | 15a | 2 | 1 | 18a |
| Mixed | 4 | 4a | 0 | 8a |
| Pure ABMR | 1 | 9 | 0 | 10a |
| No rejection | 10 | 2 | 1a | 13a |
| Discrepancies | 15 | 13 | 1 | 29 |
Biopsies represent discrepancies or as otherwise indicated.
Agreement between the molecular and conventional assessment.
On the basis of molecular test cutoffs, the current conventions for v-lesion biopsies overdiagnosed pure TCMR (30 versus 15) and mixed (17 versus four). This caused many biopsies with no molecular TCMR to be assessed conventionally as having TCMR, including classifying some with molecular ABMR activity as mixed.
Heavy i-t in v-Lesion Biopsies Predicts Positive TCMR Scores
The presence of i-t meeting the conventional criteria for TCMR strongly predicted positive TCMR scores (Table 4). Twenty of 21 i-t-v-lesion biopsies (95%) (16 pure, three mixed, one no-TCMR) were TCMR score positive. Only six of 28 isolated v-lesion biopsies (21%) were TCMR score positive (three pure, three mixed).
Table 4.
Relationship between molecular TCMR and ABMR scores and tubulointerstitial lesions in 49 biopsies with v-lesions
| Molecular Assessment | Conventional Diagnosis in i-t-v-Lesions | Conventional Diagnosis in Isolated v-Lesions | Total (n=49) |
|---|---|---|---|
| All (n=21) | All (n=28) | ||
| TCMR score positive | 20 | 6 | 26 |
| ABMR score positive | 5 | 13 | 18 |
| No rejection | 1 | 12 | 13 |
In contrast, 13 of 28 isolated v-lesion biopsies (46%) were ABMR-score positive compared with five of 21 i-t-v-lesion biopsies (24%).
Features Associated with Molecular Scores in Isolated v-Lesion Biopsies
DSA
HLA antibody was tested at the time of biopsy in 46 of 49 isolated v-lesion biopsies and was present in 21 of 46 patients (Table 5). DSA was more common in isolated v-lesion biopsies (16 of 27; 57%) than in i-t-v-lesion biopsies (five of 19; 26%) (P=0.04).
Table 5.
Relationship between DSA at the time of biopsy and conventional diagnosis in 46 v-lesion biopsies with known DSA status
| DSA Conventional | Isolated v-Lesion Biopsies | i-t-v-Lesion Biopsies | Total |
|---|---|---|---|
| DSA positive | 16 | 5 | 21 |
| DSA negative | 11 | 14 | 25 |
| Total | 27 | 19 | 46 |
Three of the 49 v-lesion biopsies had no HLA antibody assessment recorded. P=0.04, Fisher exact test.
Time of Biopsy Is Associated with DSA and Molecular Findings
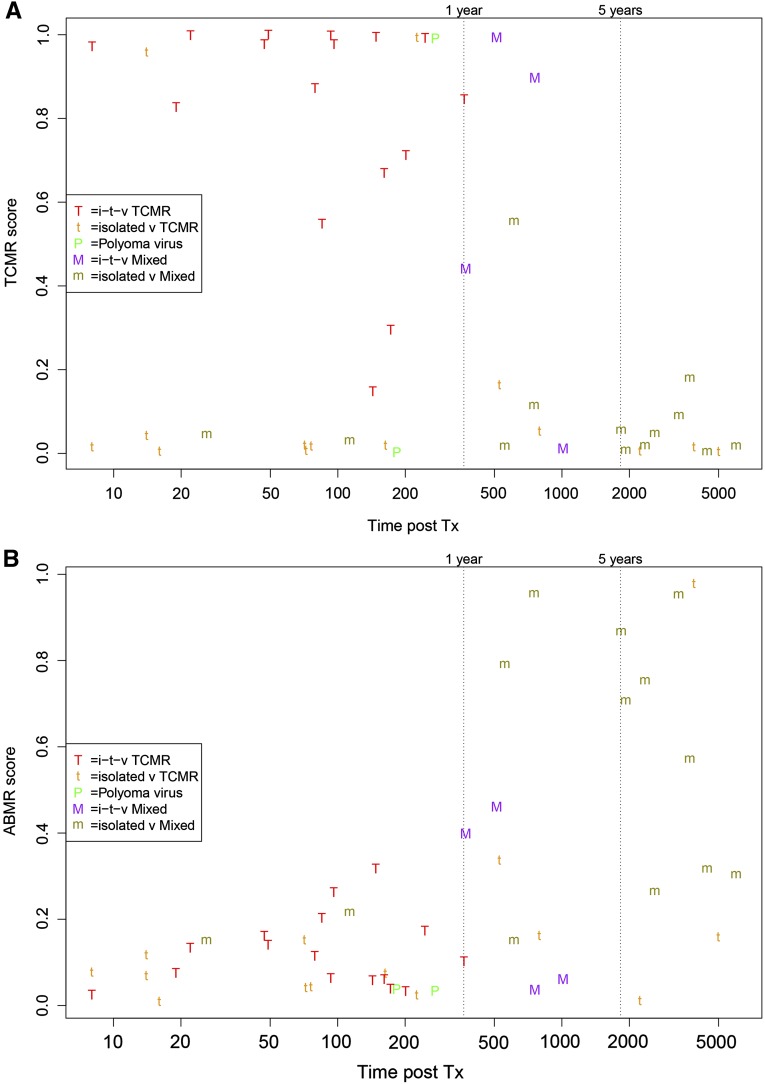
Figure 2 represents the relationship between molecular scores and the time post-transplant of all v-lesion biopsies. Most high TCMR scores were in biopsies before 500 days, with conventional diagnoses of i-t-v-lesion TCMR (Figure 2A). Most isolated v-lesion biopsies diagnosed as TCMR (t symbols) had low TCMR scores. Most late cases called mixed (m symbols) had low TCMR scores (Figure 2A) but high ABMR scores (Figure 2B).
Figure 2.
Relationship between molecular scores and the time of the biopsy post-transplant. The conventional diagnoses for i-t-v-lesion biopsies are indicated by the colored upper case symbols and for isolated v-lesion biopsies by the lower case symbols. (A) TCMR score versus time of biopsy post-transplant; and (B) ABMR score versus time of biopsy post-transplant. m, isolated v-lesion mixed; M, i-t-v-lesion mixed; P, polyoma virus nephropathy; t, isolated v-lesion TCMR; T, i-t-v-lesion TCMR; Tx, treatment.
Further analysis is presented in Table 6. DSA in isolated v-lesion biopsies was strongly related to time of biopsy post-transplant: only 17% completed at <1 year had DSA compared with 80% between 1 and 5 years and 91% at >5 years. The 12 isolated v-lesion biopsies completed at <1 year post-transplant presented early, at a mean of 82 days post-transplant, and nine had no molecular rejection. After 1 year, most isolated v-lesion biopsies had rejection. Between 1 and 5 years post-transplant, four of five had molecular rejection (two mixed, one TCMR, one ABMR). Beyond 5 years, nine of 11 biopsies had positive ABMR scores, but only one had a positive TCMR score, giving eight pure ABMR and one mixed, reflecting the previously reported rarity of TCMR activity after five years.17
Table 6.
Features of biopsies by time interval in 28 isolated v-lesion biopsies
| Biopsy Features | <1 y (n=12) | 1–5 y (n=5) | >5 y (n=11) |
|---|---|---|---|
| No. of DSA positive (% of n) | 2 (17) | 4 (80) | 10 (91) |
| Mean days from transplant to biopsy (interquartile range) | 82 (16–126) | 647 (556–751) | 3387 (2284–4162) |
| Molecular diagnosis | |||
| All TCMR score positive (pure plus mixed) | 3 | 3 | 1 |
| Pure TCMR | 2 | 1 | 0 |
| Mixed | 1 | 2 | 1 |
| Pure ABMR | 0 | 1 | 8 |
| All ABMR score positive (pure plus mixed) | 1 | 3 | 9 |
| No rejection | 9 | 1 | 2 |
i-t Inflammation in Isolated v-Lesion Biopsies
Although isolated v-lesion biopsies by definition did not have i- and t- lesions reaching the diagnostic thresholds for TCMR, many had mild i- or t-lesions recorded. This permitted the separation of the 28 biopsies with isolated v-lesions into two groups: 18 with i- or t-scores greater than zero and 10 with i- and t-scores of zero (Table 7). The isolated v-lesion biopsies with i- and t-scores of zero were usually ABMR score positive (seven out of 10) but rarely TCMR score positive (one out of 10), whereas those with i- and/or t-scores greater than zero were equally likely to have positive TCMR scores (five out of 18) or ABMR scores (six out of 18). Positive molecular ABMR scores were associated with microcirculation lesions (P=0.07), transplant glomerulopathy (P<0.002), and positive C4d staining in biopsies with isolated v-lesions (P=0.002), but positive TCMR scores were not (Supplemental Table 3).
Table 7.
Relationship of i-t to TCMR and ABMR scores in 28 biopsies with isolated v-lesions
| Molecular Scores | i-t in Isolated v-Lesion Biopsies | |
|---|---|---|
| i- or t-Lesions >0 (n=18) | i- and t-Lesions of 0 (n=10) | |
| TCMR>0.1 | 5 | 1c |
| TCMR<0.1 | 13 | 9 |
| P valuea | 0.37 | 0.37 |
| ABMR>0.2 | 6 | 7 |
| ABMR<0.2 | 12b | 3d |
| P valuea | 0.03 | 0.03 |
Fisher exact test.
Isolated v-lesion biopsies with i- or t-lesions greater than zero did not differ in the frequency of positive TCMR and ABMR scores (Fisher exact test, P=0.72).
This biopsy with ci3;ct3 (ci [interstitial fibrosis] score equal to 3, ct [tubular atrophy] score equal to 3) was given i- and t-lesion scores of zero, but this may not be reliable because of heavy scarring.
Isolated v-lesion biopsies with i- or t-lesions of zero had more positive ABMR scores than positive TCMR scores (Fisher exact test, P=0.02).
In summary, using molecular scores to estimate the probability of TCMR or ABMR, 95% of i-t-v-lesion biopsies had TCMR activity. Isolated v-lesion biopsies more often had no rejection or ABMR, not TCMR. DSA status, time post-transplant, and i-t inflammation helped to define subgroups with predictable phenotypes.
Discussion
To reassess the disease states associated with v-lesions and their consequences, we analyzed the conventional findings, outcomes, and microarray results in 49 kidney transplant biopsies with v-lesions. Very few biopsies had severe arteritis (v3), and there was no effect of v-lesions on survival compared with other kidney transplants undergoing indication biopsies. Because the current histology guidelines interpret v-lesions as TCMR activity, almost all biopsies were classified as TCMR or mixed. Twenty-one biopsies had i-t meeting the criteria for TCMR (i-t-v-lesion biopsies), but 28 did not (isolated v-lesion biopsies). Almost all i-t-v-lesion biopsies (95%) had positive molecular TCMR scores, whereas only 21% of isolated v-lesion biopsies had positive TCMR scores. Isolated v-lesion biopsies more often had positive ABMR scores (46%) than i-t-v-lesion biopsies (24%). The low TCMR and ABMR scores in many biopsies with isolated v-lesions indicate that v-lesions do not always imply rejection, especially in the first months after transplantation when isolated v-lesion biopsies were from patients with no HLA antibodies, and may reflect endothelial injury from the transplant process. In biopsies >1 year post-transplant, isolated v-lesions are largely in DSA-positive patients and reflect ABMR activity. The results suggest a new approach to the interpretation of v-lesions that acknowledges their complexity and provides a framework for considering such issues as risk of type 1 ABMR, time post-transplant, DSA, accompanying tubulointerstitial lesions, and when available the molecular phenotype.
The present results combined with the recent studies from populations highly enriched in type 1 ABMR26,37 permit a general view of the v-lesions in the renal transplant population. In the type 1 ABMR–enriched population, the ABMR score was prognostic as was donor age.26,37 v-lesions per se were not prognostic, but v3-lesions were associated with high failure rates.26 In unselected indication biopsies, v3-lesions are now very rare: two in 703 biopsies in this study, despite having almost 200 biopsies with various types of rejection. This study of unselected indication biopsies had virtually no early type 1 ABMR, reflecting the infrequency of transplanting DSA-positive recipients in the participating centers.17 As a result, the conventional ABMR and mixed biopsies almost all presented after 1 year post-transplant (median, 6 years), that is, with type 2 ABMR. Although type 2 ABMR has a major effect on survival compared with other diagnoses,17 v-lesions—at least the mild v1-lesions that are dominant in unselected indication biopsies—do not change the prognosis, unlike the type 1 ABMR with v3-lesions.
The use of molecular tests to guide improvements in the interpretation of the conventional tests is on the basis of the high concordance between the two platforms, with no assumption that either is always correct. Iterative use of the available platforms offers many possibilities for improving understanding of disease phenotypes. For example, we took advantage of this in the past by identifying the molecular changes in biopsies with C4d-positive ABMR, and then showing that these changes were also found in many biopsies that were C4d negative,16,36 a major step in understanding C4d-negative ABMR. Focusing on outliers and discrepancies and seeking consensus is important because conventional and molecular phenotyping both remain imperfect. It is also both likely and desirable that combining these approaches will reveal important new rejection subclasses and variants.
Although the presence of v-lesions did not add risk in this study, v-lesions are an important piece of evidence and must trigger a review of the conventional evidence and if possible the molecular evidence. Untreated rejection (TCMR, type 1 ABMR, type 2 ABMR) is a threat to the graft, but unnecessary treatment is a threat to the health of the patient through complications such as cancer, post-transplant lymphoproliferative disease, and infections. The decision to use expensive and potentially risky treatments should be on the basis of all reliable sources of information.
The main limitation of this study lies in the small number of v-lesion biopsies, reflecting the paucity of v-lesions and particularly severe arteritis in contemporary indication biopsies. The 49 biopsies with v-lesions, mostly v1, permit an estimate of the effect of v-lesions, but a much larger study would be desirable to draw conclusions, such as the effect of v3-lesions on graft survival. Some disparities between the conventional and molecular tests will be resolved by a new generation of molecular classifiers for rejection in development,38 which may subclassify ABMR. Moreover, in the early biopsies with v-lesions in DSA-negative patients and negative TCMR scores, we cannot exclude a second type of TCMR with unknown molecular features or that molecular studies are not detecting a form of TCMR that lacks i-t. However, it seems more likely that these early v-lesions reflect endothelial injury from the transplantation process distinct from alloimmunity.
Our conclusion that v-lesions have no independent effect on risk for graft failure compared with controls differs from two recent retrospective studies analyzing v-lesions in biopsies from earlier eras39,40 when aggressive TCMR was more common, ABMR was poorly recognized, and HLA antibody detection was less advanced. One study of biopsies from as early as 1999 included three selected groups, with HLA antibody data missing in most subjects.39 Kidneys with v-lesions had poorer later survival than the selected control group, but the controls had more live donors, more protocol biopsies, and less delayed graft function than the study patients. The other analysis, which reported lower survival in TCMR biopsies when they had v-lesions, studied biopsies as far back as 1996.40 Both studies focused on biopsies taken in the first months post-transplant and as such are difficult to compare with the present prospective studies because of differences in eras, case mixes, times post-transplant and because of lack of rigorous assessment of HLA antibody and ABMR. More detail on the phenotype of the late failures would be welcome to clarify the mechanisms involved, namely, whether early severe TCMR leads to late emergence of ABMR. In summary, comparison of these studies with our prospective study suggests that the arteritis in the more severe TCMR episodes in earlier eras actually did impair late survival, but this effect has been lost with improved immunosuppression and less severe TCMR.
Until molecular assessments are available, our results can guide the development of a new approach to interpreting v-lesion biopsies that takes into account the full phenotype, particularly time post-transplant, DSA, and tubulointerstitial changes. (This is for patients with no high risk for type 1 ABMR, which requires a separate assessment of probabilities.26,37) We suggest the following:
Biopsies with i-t-v-lesions, which are usually <5 years post-transplant, almost certainly reflect TCMR activity but are occasionally mixed. Evidence for associated ABMR (and nonadherence41) should be sought, particularly in all biopsies >1 year post-transplant.
-
Biopsies with isolated v-lesions should be assigned a differential diagnosis on the basis of DSA, time, and presence or absence of mild tubulointerstitial lesions:
Before 1 year, and particularly in the first few months, isolated v-lesions in DSA-negative patients usually do not reflect rejection, but the possibility of TCMR must be considered, particularly if there are some i- and t-lesions.
After 1 year post transplant, isolated v-lesions usually mean rejection. Many are DSA positive and have ABMR, but some can reflect TCMR, particularly <5 years post-transplant.
Isolated v-lesions should not be used to diagnose TCMR except in DSA-negative patients who have at least some i-t.
Recognition that v-lesions have been seriously misinterpreted for >20 years, with potential for consequences to patients in terms of incorrect treatment, suggests that clinicians and pathologists should be moving toward a more probability-based assessment system, acknowledging that lesions must be interpreted in the light of factors such as time of biopsy post-transplant, DSA, and associated lesions. Molecular studies will add another dimension to increase precision,15 but until these are widely available, conventional assessment can be on the basis of guidelines that reflect the lessons learned from the present reference set to reduce the probability of errors.
Concise Methods
Patient and Biopsy Population
The 703 biopsies were collected in prospective multicenter studies of biopsies performed between late 2005 and 2012, as already described: 403 from the Genome Canada study and 300 from the International Collaborative Microarray Study (ClinicalTrials.gov no. NCT01299168).29–32 The population has been recently published.17 In reviewing the data for this article, one data error was found, resulting in one patient entered as conventionally diagnosed ABMR being changed to TCMR. All renal transplant biopsies were performed for clinical indications after informed consent, in protocols approved by institutional review boards. Forty-nine biopsies from 46 kidneys had v-lesions, out of 703 biopsies from 564 kidneys.
Conventional (Histology) Classification and Molecular Classification
The biopsies were all assessed by local pathologists using the Banff guidelines, recording lesions and diagnoses, particularly the Banff 2007 guidelines for TCMR2 and the Banff 2013 guidelines,15 and acknowledging the existence of C4d-negative ABMR and v-lesions in ABMR.17,42 The principle was to capture what the local center felt was the diagnosis on the basis of lesions and DSA, but updated with the newer guidelines. HLA antibody assessment was by local standard of care as reported previously.17
Of the 703 biopsies analyzed, 49 presented v-lesions (v-score >0). On the basis of presence or absence of i-t meeting the criteria for the diagnosis of TCMR, we divided the v-lesion biopsies into two categories: isolated v-lesion biopsies and i-t-v-lesion biopsies. Each could be either pure or mixed, giving rise to v-lesion TCMR (pure or mixed) and i-t-v-lesion TCMR (pure or mixed), respectively.
Molecular scores used the published cutoffs (TCMR positive >0.1, ABMR positive >0.2), which created four molecular classes: pure molecular TCMR (TCMR>0.1, ABMR<0.2), pure molecular ABMR (ABMR>0.2, TCMR<0.1), molecular mixed (ABMR>0.2, TCMR>0.1), and no molecular rejection (ABMR<0.2, TCMR<0.1).29,30
Data Analyses
Data analyses were performed using GraphPad Prism 5 statistical software package; chi-squared or Fisher exact tests were used to compare certain variables.
Histology and Microarray Assessment
As previously outlined,29 biopsies were processed for histology (periodic acid–Schiff and trichrome) as per local standard of care.
One 18-gauge biopsy core was placed immediately in RNALater and stored at –20°C. RNA extraction, labeling, and hybridization to the HG U133 Plus 2.0 GeneChip (Affymetrix, Santa Clara, CA) were carried out according to the manufacturer’s protocols. Microarrays were scanned using the GeneArrayScanner (Affymetrix) and processed with GeneChip Operating Software Version 1.4.0 (Affymetrix). Detailed protocols for microarray processing are available in the Affymetrix Technical Manual (www.affymetrix.com).
Disclosures
P.F. Halloran holds shares in Transcriptome Sciences Inc., a company with an interest in molecular diagnostics. The other authors have no competing financial interests.
Supplementary Material
Acknowledgments
We are grateful to Drs. Konrad Famulski and Jeff Reeve for critical review of the manuscript.
This research has been supported by funding and/or resources from Novartis Pharma AG, and in the past by Genome Canada, the University of Alberta Hospital Foundation, Roche Molecular Systems, Hoffmann-La Roche Canada Ltd., Canada Foundation for Innovation, the Alberta Ministry of Advanced Education and Technology, the Roche Organ Transplant Research Foundation, and Astellas. Dr. Halloran held a Canada Research Chair in Transplant Immunology until 2008 and currently holds the Muttart Chair in Clinical Immunology. The Spanish Society of Nephrology provided a scholarship to Dr. Israel D.R. Salazar for study at foreign institutions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111064/-/DCSupplemental.
References
- 1.Jeannet M, Pinn VW, Flax MH, Winn HJ, Russell PS: Humoral antibodies in renal allotransplantation in man. N Engl J Med 282: 111–117, 1970 [DOI] [PubMed] [Google Scholar]
- 2.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Coelho T, Tredger M, Dhawan A: Current status of immunosuppressive agents for solid organ transplantation in children. Pediatr Transplant 16: 106–122, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Sellarés J, de Freitas DG, Mengel M, Sis B, Hidalgo LG, Matas AJ, Kaplan B, Halloran PF: Inflammation lesions in kidney transplant biopsies: Association with survival is due to the underlying diseases. Am J Transplant 11: 489–499, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Sollinger HW, Belzer FO, Deierhoi MH, Diethelm AG, Gonwa TA, Kauffman RS, Klintmalm GB, McDiarmid SV, Roberts J, Rosenthal JT, Tomlanovich SJ: RS-61443 (mycophenolate mofetil). A multicenter study for refractory kidney transplant rejection. Ann Surg 216: 513–518, discussion 518–519, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sollinger HW, U.S. Renal Transplant Mycophenolate Mofetil Study Group : Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 60: 225–232, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Magil A, Rubin J, Ladewig L, Johnson M, Goldstein MB, Bear RA: Renal biopsy in acute allograft rejection. Significance of moderate vascular lesions in long-term graft survival. Nephron 26: 180–183, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Matas AJ, Sibley R, Mauer M, Sutherland DE, Simmons RL, Najarian JS: The value of needle renal allograft biopsy. I. A retrospective study of biopsies performed during putative rejection episodes. Ann Surg 197: 226–237, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooijmans-Coutinho MF, Hermans J, Schrama E, Ringers J, Daha MR, Bruijn JA, van der Woude FJ: Interstitial rejection, vascular rejection, and diffuse thrombosis of renal allografts. Predisposing factors, histology, immunohistochemistry, and relation to outcome. Transplantation 61: 1338–1344, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Ishida H, Shirakawa H, Omoto K, Tsunoyama K, Tokumoto T, Tanabe K: Clinicopathological analysis of acute vascular rejection cases after renal transplantation. Clin Transplant 24[Suppl 22]: 22–26, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Haas M, Kraus ES, Samaniego-Picota M, Racusen LC, Ni W, Eustace JA: Acute renal allograft rejection with intimal arteritis: Histologic predictors of response to therapy and graft survival. Kidney Int 61: 1516–1526, 2002 [DOI] [PubMed] [Google Scholar]
- 13.van Saase JL, van der Woude FJ, Thorogood J, Hollander AA, van Es LA, Weening JJ, van Bockel JH, Bruijn JA: The relation between acute vascular and interstitial renal allograft rejection and subsequent chronic rejection. Transplantation 59: 1280–1285, 1995 [PubMed] [Google Scholar]
- 14.Schroeder TJ, Weiss MA, Smith RD, Stephens GW, First MR: The efficacy of OKT3 in vascular rejection. Transplantation 51: 312–315, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M, Matas A, Picton M, de Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G, Reeve J: Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients [published online ahead of print November 6, 2014]. J Am Soc Nephrol 10.1681/ASN.2014060588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halloran PF, Schlaut J, Solez K, Srinivasa NS: The significance of the anti-class I response. II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation 53: 550–555, 1992 [PubMed] [Google Scholar]
- 19.Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF: Pathologic features of acute renal allograft rejection associated with donor-specific antibody, Analysis using the Banff grading schema. Transplantation 61: 1586–1592, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Tanabe T, Shirakawa H, Omoto K, Ishida H, Tanabe K: Acute vascular rejection after renal transplantation and isolated v-lesion. Clin Transplant 26[Suppl 24]: 2–8, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Haas M: Pathologic features of antibody-mediated rejection in renal allografts: An expanding spectrum. Curr Opin Nephrol Hypertens 21: 264–271, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Brown CC, Sebire NJ, Wittenhagen P, Shaw O, Marks SD: Clinical significance of isolated v lesions in paediatric renal transplant biopsies: Muscular arteries required to refute the diagnosis of acute rejection. Transpl Int 27: 170–175, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Sis B, Einecke G, Chang J, Hidalgo LG, Mengel M, Kaplan B, Halloran PF: Cluster analysis of lesions in nonselected kidney transplant biopsies: Microcirculation changes, tubulointerstitial inflammation and scarring. Am J Transplant 10: 421–430, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Herzenberg AM, Gill JS, Djurdjev O, Magil AB: C4d deposition in acute rejection: An independent long-term prognostic factor. J Am Soc Nephrol 13: 234–241, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X: Antibody-mediated vascular rejection of kidney allografts: A population-based study. Lancet 381: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Nankivell BJ: Antibody-mediated vascular rejection: Relation to causation. Lancet 381: 275–277, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Bröcker V, Hirzallah M, Gwinner W, Bockmeyer CL, Wittig J, Zell S, Agustian PA, Schwarz A, Ganzenmüller T, Zilian E, Immenschuh S, Becker JU: Histopathological and clinical findings in renal transplants with Banff type II and III acute cellular rejection without tubulointerstitial infiltrates. Virchows Arch 464: 203–211, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Reeve J, Sellarés J, Mengel M, Sis B, Skene A, Hidalgo L, de Freitas DG, Famulski KS, Halloran PF: Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am J Transplant 13: 645–655, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, Halloran PF: Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 13: 971–983, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Halloran PF, Pereira AB, Chang J, Matas A, Picton M, De Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G, Reeve J: Potential impact of microarray diagnosis of T cell-mediated rejection in kidney transplants: The INTERCOM study. Am J Transplant 13: 2352–2363, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Halloran PF, Pereira AB, Chang J, Matas A, Picton M, De Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G, Reeve J: Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: An international prospective study (INTERCOM). Am J Transplant 13: 2865–2874, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Mueller TF, Reeve J, Jhangri GS, Mengel M, Jacaj Z, Cairo L, Obeidat M, Todd G, Moore R, Famulski KS, Cruz J, Wishart D, Meng C, Sis B, Solez K, Kaplan B, Halloran PF: The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant 8: 78–85, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Reeve J, Einecke G, Mengel M, Sis B, Kayser N, Kaplan B, Halloran PF: Diagnosing rejection in renal transplants: A comparison of molecular- and histopathology-based approaches. Am J Transplant 9: 1802–1810, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T, Verine J, Aubert O, Dubleumortier S, Duong van Huyen JP, Jouven X, Glotz D, Legendre C, Halloran PF: Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol 25: 2267–2277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeve J, Famulski K, Halloran PF, and the INTERCOM Group : Microarray gene expression for predicting histo-clinical variables in kidney transplant biopsies [Abstract]. Am J Transplant 14(S3): 890, 2014 [Google Scholar]
- 39.Sis B, Bagnasco SM, Cornell LD, Randhawa P, Haas M, Lategan B, Magil AB, Herzenberg AM, Gibson IW, Kuperman M, Sasaki K, Kraus ES, the Banff Working Group : Isolated endarteritis and kidney transplant survival: A multicenter collaborative study [published online ahead of print November 7, 2014]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu KY, Budde K, Schmidt D, Neumayer HH, Rudolph B: Acute cellular rejection with isolated v-lesions is not associated with more favorable outcomes than vascular rejection with more tubulointerstitial inflammations. Clin Transplant 28: 410–418, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.